Bacterial Purine Nucleoside Phosphorylases from Mesophilic and Thermophilic Sources: Characterization of Their Interaction with Natural Nucleosides and Modified Arabinofuranoside Analogues
Abstract
1. Introduction
2. Materials and Methods
2.1. Equipment
2.2. Chemicals
2.3. Enzymes
2.4. Purification of PNP from Escherichia coli
2.5. Purification of PNP I and II from Thermus thermophilus HB27
2.6. Activity Measurements
2.7. Kinetic Methods
2.8. Synthesis of 9-(β-D-Arabinofuranosyl)Hypoxanthine
3. Results and Discussion
3.1. Comparison of Substrate Specificity of Mesophilic and Thermophilic Enzymes towards Natural Substrates and Their Arabino-Derivatives
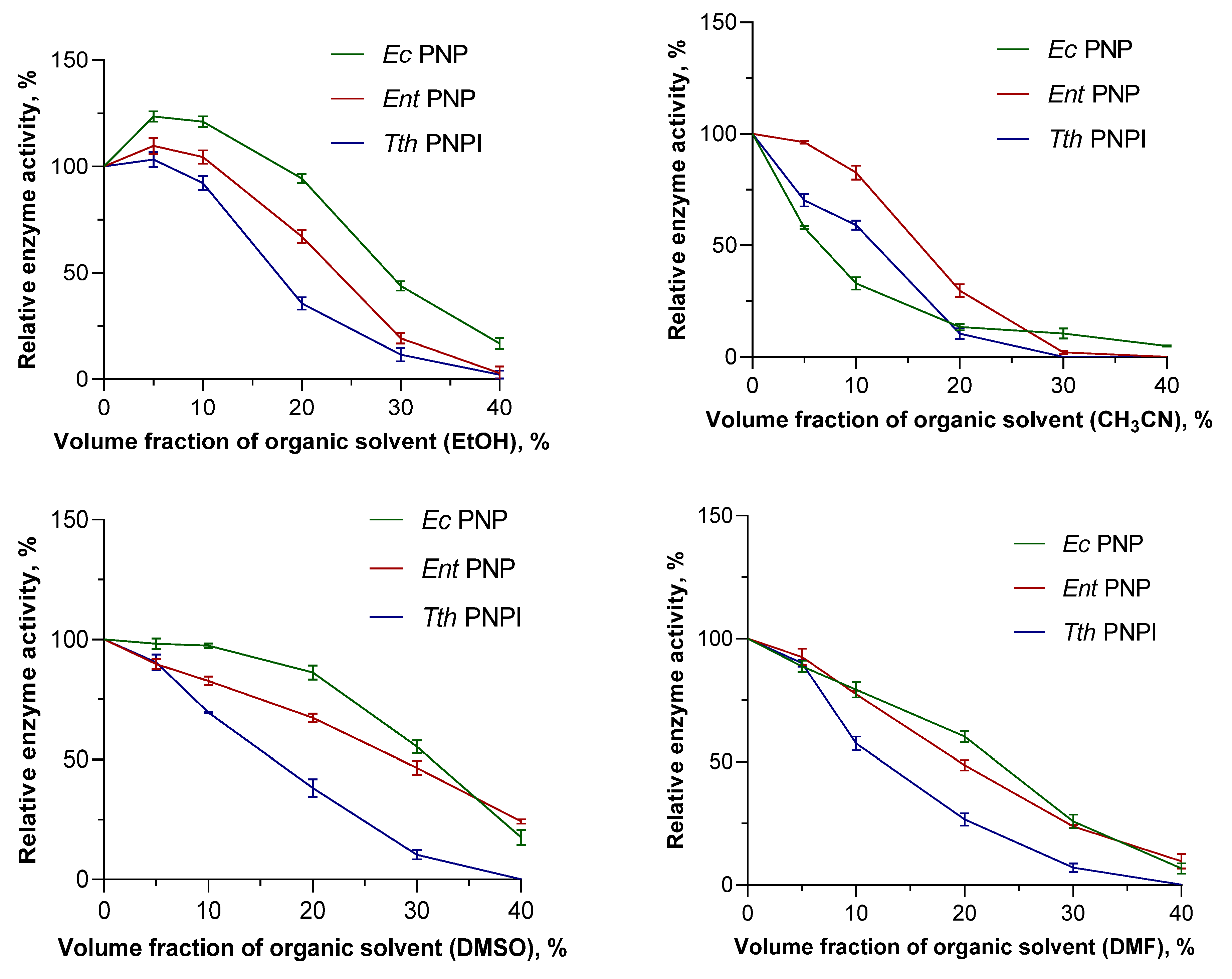
3.2. Effect of Organic Solvents on the Activity of Mesophilic and Thermophilic PNPs
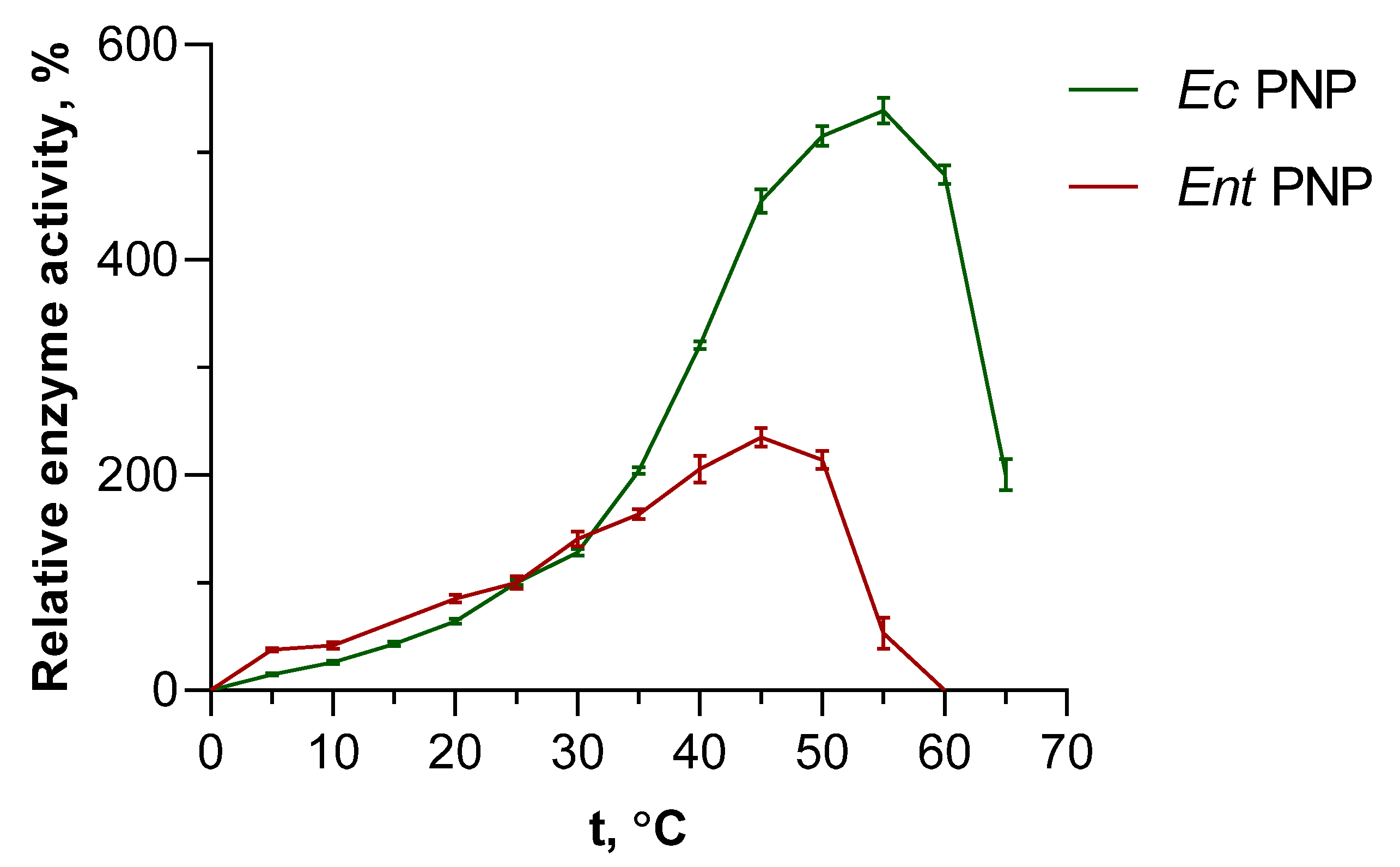
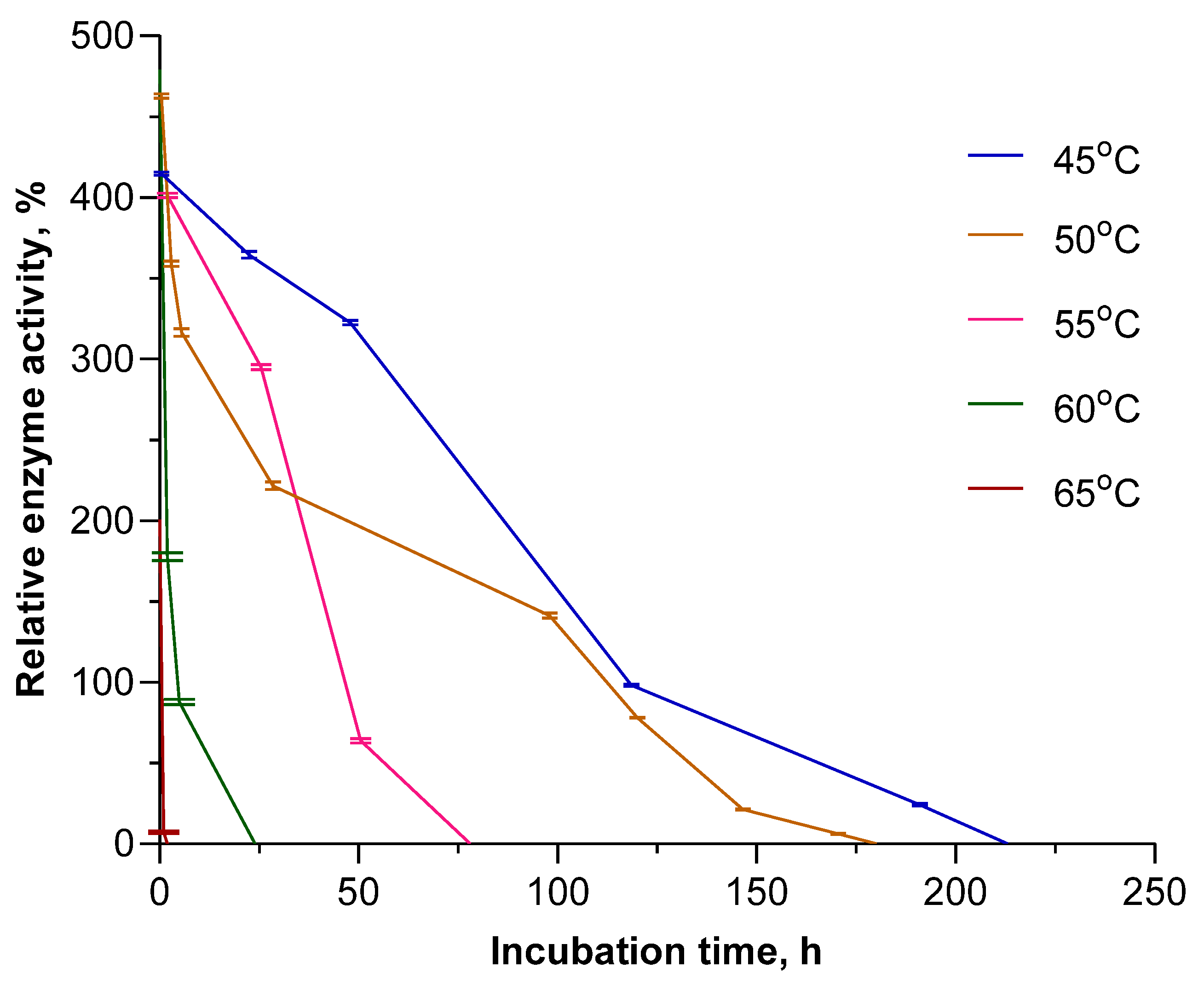
3.3. Influence of Temperature on the Activity of PNPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bzowska, A.; Kulikowska, E.; Shugar, D. Purine nucleoside phosphorylases: Properties, functions, and clinical aspects. Pharmacol. Ther. 2000, 88, 349–425. [Google Scholar] [CrossRef] [PubMed]
- Yehia, H.; Kamel, S.; Paulick, K.; Wagner, A.; Neubauer, P. Substrate spectra of nucleoside phosphorylases and their potential in the production of pharmaceutically active compounds. Curr. Pharm. Des. 2017, 23, 6913–6935. [Google Scholar] [CrossRef] [PubMed]
- Bennett, E.M.; Li, C.; Allan, P.W.; Parker, W.B.; Ealick, S.E. Structural basis for substrate specificity of Escherichia coli purine nucleoside phosphorylase. J. Biol. Chem. 2003, 278, 47110–47118. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, S.C.; Miller, G.J. Advances in Biocatalytic and Chemoenzymatic Synthesis of Nucleoside Analogues. Expert Opin. Drug Discov. 2022, 17, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lucas, J. Biocatalysis: An Eco-Friendly Scenario for the Manufacturing of APIs. Int. J. Mol. Sci. 2023, 24, 4474. [Google Scholar] [CrossRef]
- De Clercq, E.; Li, G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef]
- Galmarini, C.M.; Mackey, J.R.; Dumontet, C. Nucleoside analogues and nucleobases in cancer treatment. Lancet Oncol. 2002, 3, 415–424. [Google Scholar] [CrossRef]
- Thomson, J.M.; Lamont, I.L. Nucleoside analogues as antibacterial agents. Front. Microbiol. 2019, 10, 952. [Google Scholar] [CrossRef]
- Zenchenko, A.A.; Drenichev, M.S.; Il’icheva, I.A.; Mikhailov, S.N. Antiviral and Antimicrobial Nucleoside Derivatives: Structural Features and Mechanisms of Action. Mol. Biol. 2021, 55, 786–812. [Google Scholar] [CrossRef]
- De Jonghe, S.; Herdewijn, P. An overview of marketed nucleoside and nucleotide analogs. Curr. Protoc. 2022, 2, e376. [Google Scholar] [CrossRef]
- Westarp, S.; Kaspar, F.; Neubauer, P.; Kurreck, A. Industrial potential of the enzymatic synthesis of nucleoside analogs: Existing challenges and perspectives. Curr. Opin. Biotechnol. 2022, 78, 102829. [Google Scholar] [CrossRef]
- Esipov, R.S.; Gurevich, A.I.; Chuvikovsky, D.V.; Chupova, L.A.; Muravyova, T.I.; Miroshnikov, A.I. Overexpression of Escherichia coli genes encoding nucleoside phosphorylases in the pET/Bl21(DE3) system yields active recombinant enzymes. Protein Expr. Purif. 2002, 24, 56–60. [Google Scholar] [CrossRef]
- Fateev, I.V.; Kostromina, M.A.; Abramchik, Y.A.; Eletskaya, B.Z.; Mikheeva, O.O.; Lukoshin, D.D.; Zayats, E.A.; Berzina, M.Y.; Dorofeeva, E.V.; Paramonov, A.S.; et al. Multi-Enzymatic Cascades in the Synthesis of Modified Nucleosides: Comparison of the Thermophilic and Mesophilic Pathways. Biomolecules 2021, 11, 586. [Google Scholar] [CrossRef] [PubMed]
- Timofeev, V.I.; Fateev, I.V.; Kostromina, M.A.; Abramchik, Y.A.; Konstantinova, I.D.; Volkov, V.V.; Lykoshin, D.D.; Mikheeva, O.O.; Muravieva, T.I.; Esipov, R.S.; et al. The comparative analysis of the properties and structures of purine nucleoside phosphorylases from thermophilic bacterium Thermus thermophilus HB27. J. Biomol. Struct. Dyn. 2022, 40, 3626–3641. [Google Scholar] [CrossRef]
- Almendros, M.; Berenguer, J.; Sinisterra, J.V. Thermus thermophilus nucleoside phosphorylases active in the synthesis of nucleoside analogues. Appl. Environ. Microbiol. 2012, 78, 3128–3135. [Google Scholar] [CrossRef] [PubMed]
- Mikleušević, G.; Štefanić, Z.; Narczyk, M.; Wielgus-Kutrowska, B.; Bzowska, A.; Luić, M. Validation of the catalytic mechanism of Escherichia coli purine nucleoside phosphorylase by structural and kinetic studies. Biochimie 2011, 93, 1610–1622. [Google Scholar] [CrossRef]
- Zhou, X.; Szeker, K.; Janocha, B.; Böhme, T.; Albrecht, D.; Mikhailopulo, I.A.; Neubauer, P. Recombinant purine nucleoside phosphorylases from thermophiles: Preparation, properties and activity towards purine and pyrimidine nucleosides. FEBS J. 2013, 280, 1475–1490. [Google Scholar] [CrossRef]
- Utagawa, T.; Morisawa, H.; Yamanaka, S.; Yamazaki, A.; Yoshinaga, F.; Hirose, Y. Microbial synthesis of purine arabinosides and their biological activity. Agr. Biol. Chem. 1985, 49, 2167–2171. [Google Scholar] [CrossRef]
- Reist, E.J.; Benitez, A.; Goodman, L.; Baker, B.R.; Lee, W.W. Potential Anticancer Agents LXXVI. Synthesis of Purine Nucleosides of β-D-Arabinofuranose. J. Org. Chem. 1962, 27, 3274–3279. [Google Scholar] [CrossRef]
- Lewkowicz, E.S.; Iribarren, A.M. Nucleoside phosphorylases. Curr. Org. Chem. 2006, 10, 1197–1215. [Google Scholar] [CrossRef]
- Yalkowsky, S.H.; He, Y.; Jain, P. Handbook of Aqueous Solubility Data, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 137, 143, 144, 700. [Google Scholar] [CrossRef]
- Zhou, X.; Szeker, K.; Jiao, L.Y.; Oestreich, M.; Mikhailopulo, I.A.; Neubauer, P. Synthesis of 2,6-dihalogenated purine nucleosides by thermostable nucleoside phosphorylases. Adv. Synth. Catal. 2015, 357, 1237–1244. [Google Scholar] [CrossRef]
- Chen, J.; Chen, G.; Cheng, C.; Cong, Y.; Li, X.; Zhao, H. Equilibrium solubility, dissolution thermodynamics and preferential solvation of adenosine in aqueous solutions of N, N-dimethylformamide, N-methyl-2-pyrrolidone, dimethylsulfoxide and propylene glycol. J. Chem. Thermodyn. 2017, 115, 52–62. [Google Scholar] [CrossRef]
- Burton, R.C.; Davey, R.J.; Sadiq, G.; Auffret, A.; Cioffi, M.; Hunter, C.A. The nucleation of inosine: The impact of solution chemistry on the appearance of polymorphic and hydrated crystal forms. Faraday Discuss 2007, 136, 179–193. [Google Scholar] [CrossRef]
- Atalah, J.; Cáceres-Moreno, P.; Espina, G.; Blamey, J.M. Thermophiles and the applications of their enzymes as new biocatalysts. Bioresour. Technol. 2019, 280, 478–488. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J.; Chu, J.; Jiang, T.; Qin, S.; Gao, Z.; He, B. Engineering Substrate Promiscuity of Nucleoside Phosphorylase Via an Insertions–Deletions Strategy. J. Am. Chem. Soc. Au 2024, 4, 454–464. [Google Scholar] [CrossRef]
- Xie, X.; Huo, W.; Xia, J.; Xu, Q.; Chen, N. Structure–activity relationship of a cold-adapted purine nucleoside phosphorylase by site-directed mutagenesis. Enzym. Microb. Technol. 2012, 51, 59–65. [Google Scholar] [CrossRef]
- Renata, H.; Wang, Z.J.; Arnold, F.H. Expanding the Enzyme Universe: Accessing Non-Natural Reactions by Mechanism-Guided Directed Evolution. Angew. Chem. Int. Ed. 2015, 54, 3351–3367. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Tong, X.; Wang, J.; Wu, B.; Chu, J.; Jian, Y.; He, B. Reshaping the binding pocket of purine nucleoside phosphorylase for improved production of 2-halogenated-2′-deoxyadenosines. Catal. Sci. Technol. 2021, 11, 4439–4446. [Google Scholar] [CrossRef]
- Lapponi, M.J.; Rivero, C.W.; Zinni, M.A.; Britos, C.N.; Trelles, J.A. New developments in nucleoside analogues biosynthesis: A review. J. Mol. Catal. B Enzym. 2016, 133, 218–233. [Google Scholar] [CrossRef]
- Taran, S.A.; Verevkina, K.N.; Feofanov, S.A.; Miroshnikov, A.I. Enzymatic transglycosylation of natural and modified nucleosides by immobilized thermostable nucleoside phosphorylases from Geobacillus stearothermophilus. Russ. J. Bioorg. Chem. 2009, 35, 739–745. [Google Scholar] [CrossRef]
- Eletskaya, B.Z.; Berzina, M.Y.; Fateev, I.V.; Kayushin, A.L.; Dorofeeva, E.V.; Lutonina, O.I.; Zorina, E.A.; Antonov, K.V.; Paramonov, A.S.; Muzyka, I.S.; et al. Enzymatic Synthesis of 2-Chloropurine Arabinonucleosides with Chiral Amino Acid Amides at the C6 Position and an Evaluation of Antiproliferative Activity In Vitro. Int. J. Mol. Sci. 2023, 24, 6223. [Google Scholar] [CrossRef] [PubMed]
- Karshikoff, A.; Ladenshtein, R. Proteins from thermophilic and mesophilic organisms essentially do not differ in packing. Prot. Eng. 1998, 11, 867–872. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kamel, S.; Thiele, I.; Neubauer, P.; Wagner, A. Thermophilic nucleoside phosphorylases: Their properties, characteris-tics and applications. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140304. [Google Scholar] [CrossRef]
- Mordkovich, N.N.; Antipov, A.N.; Okorokova, N.A.; Safonova, T.N.; Polyakov, K.M.; Veiko, V.P. The Nature of Thermal Stability of Prokaryotic Nucleoside Phosphorylases. Appl. Biochem. Microbiol. 2020, 56, 662–670. [Google Scholar] [CrossRef]
- Szeker, K.; Zhou, X.; Schwab, T.; Casanueva, A.; Cowan, D.; Mikhailopulo, I.A.; Neubauer, P. Comparative investigations on thermostable pyrimidine nucleoside phosphorylases from Geobacillus thermoglucosidasius and Thermus thermophilus. J. Mol. Catal. B Enzym. 2012, 84, 27–34. [Google Scholar] [CrossRef]
- Parks, R.E., Jr.; Agarwal, R.P. Purine Nucleoside Phosphorylase. In The Enzymes, 3rd ed.; Boyer, P.D., Ed.; Academic Press: Los Angeles, CA, USA, 1972; Volume 7, pp. 483–514. [Google Scholar]
- Kalckar, H.M. Differential spectrophotometry of purine compounds by means of specific enzymes. I. Determination of hydroxypurine compounds. J. Biol. Chem. 1947, 167, 429–443. [Google Scholar] [CrossRef] [PubMed]
| Substrate | Concentration | λobs (nm) | ε or Δε (M−1cm−1) | Method * | Enzyme |
|---|---|---|---|---|---|
| Ino | 400 μM | 293 | 12,000 | a | EcPNP, EntPNP |
| 15 mM | 278 for b; 260 for c | 1100 for b; 7900Hx/7100Ino for c | b and c | TthPNP I | |
| Ado | 200 μM | 274 | 1020 | b | EcPNP |
| 4 mM | 260 | 13,300Ade/14,900Ado | c | TthPNP II | |
| Ara-Hx | 4 mM | 243 | 9600Hx/11,300araHx | d | EcPNP, EntPNP, TthPNP I |
| Ara-A | 1.5 mM | 260 | 13,300Ade/14,700araA | e | EcPNP, TthPNP II |
| Compound | Solubility, mM, 25 °C | Solubility, mM, Elevated Temperature (T, °C) | |
|---|---|---|---|
| Adenine | 6.6–8.0 | 13.9 (38) | 180.5 (100) |
| Adenosine | 19.2 | 58.8 (50) * | |
| Guanine | 0.039 | 0.265 (40) | |
| Guanosine | 1.82 | 107.3 (100) | |
| Hypoxanthine | 5.29 | 108 (100) | |
| Inosine | 78.4 ** | 605 (70) ** | |
| Enzyme Characteristics | EcPNP | EntPNP | TthPNP | |
|---|---|---|---|---|
| I | II | |||
| Natural substrates | ||||
| Specificity to Ino | + | + | + | -- |
| Specificity to Ado | + | -- | -- | + |
| Phosphorolysis of Ino | ||||
| Activity (mM/min per mg) * | 100% (7.27) | 100% (8.37) | 100% (67.4) | -- |
| KM, µM | 76 ± 21 1 | 107 ± 21 | 894 ± 196 1 | -- |
| kcat calc, s−1 ** | 29.2 | 19.1 | 192.6 | -- |
| kcat calc / KM ** | 0.38 | 0.18 | 0.22 | -- |
| Phosphorolysis of Ado | ||||
| Activity (mM/min per mg) * | 626% (45.5) | -- | -- | 100% (157) |
| KM, µM | 21 ± 5 1 | -- | -- | 383 ± 132 1 |
| kcat calc, s−1 ** | 137.7 | -- | -- | 482.7 |
| kcat calc/KM ** | 6.6 | -- | -- | 1.3 |
| Arabinose derivatives | ||||
| Specificity to ara-Hx | + | + | + | -- |
| Specificity to ara-A | + | -- | -- | + |
| Phosphorolysis of ara-Hx | ||||
| Activity (mM/min per mg) * | 2.6% (0.186) | 0.036% (0.00304) | 0.22% (0.150) | -- |
| KM, µM | 1810 ± 371 | 2898 ± 831 | ≥21,500 *** | -- |
| kcat calc (kcat obs), s−1 ** | 0.709 (0.479) | 0.0079 (0.0044) | ≥2.42 (0.391) *** | -- |
| kcat calc/KM ** | 3.9 ×·10−4 | 2.7 ×·10−6 | ~1.1 × 10−4 *** | -- |
| Phosphorolysis of ara-A | ||||
| Activity (mM/min per mg) * | 1.8% (0.815) | -- | -- | 0.057% (0.0902) |
| KM, µM | ≥1260 *** | -- | -- | ≥1015 *** |
| kcat calc (kcat obs), s−1 ** | ≥3.46 (2.10) *** | -- | -- | ≥0.420 (0.271) *** |
| kcat calc/KM ** | ~2.7 ×·10−3 *** | -- | -- | ~4.1 ×·10−4 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bychek, I.A.; Zenchenko, A.A.; Kostromina, M.A.; Khisamov, M.M.; Solyev, P.N.; Esipov, R.S.; Mikhailov, S.N.; Varizhuk, I.V. Bacterial Purine Nucleoside Phosphorylases from Mesophilic and Thermophilic Sources: Characterization of Their Interaction with Natural Nucleosides and Modified Arabinofuranoside Analogues. Biomolecules 2024, 14, 1069. https://doi.org/10.3390/biom14091069
Bychek IA, Zenchenko AA, Kostromina MA, Khisamov MM, Solyev PN, Esipov RS, Mikhailov SN, Varizhuk IV. Bacterial Purine Nucleoside Phosphorylases from Mesophilic and Thermophilic Sources: Characterization of Their Interaction with Natural Nucleosides and Modified Arabinofuranoside Analogues. Biomolecules. 2024; 14(9):1069. https://doi.org/10.3390/biom14091069
Chicago/Turabian StyleBychek, Irina A., Anastasia A. Zenchenko, Maria A. Kostromina, Marat M. Khisamov, Pavel N. Solyev, Roman S. Esipov, Sergey N. Mikhailov, and Irina V. Varizhuk. 2024. "Bacterial Purine Nucleoside Phosphorylases from Mesophilic and Thermophilic Sources: Characterization of Their Interaction with Natural Nucleosides and Modified Arabinofuranoside Analogues" Biomolecules 14, no. 9: 1069. https://doi.org/10.3390/biom14091069
APA StyleBychek, I. A., Zenchenko, A. A., Kostromina, M. A., Khisamov, M. M., Solyev, P. N., Esipov, R. S., Mikhailov, S. N., & Varizhuk, I. V. (2024). Bacterial Purine Nucleoside Phosphorylases from Mesophilic and Thermophilic Sources: Characterization of Their Interaction with Natural Nucleosides and Modified Arabinofuranoside Analogues. Biomolecules, 14(9), 1069. https://doi.org/10.3390/biom14091069






