Sirt1: An Increasingly Interesting Molecule with a Potential Role in Bone Metabolism and Osteoporosis
Abstract
1. Introduction
2. Bone Formation and the Pathogenesis of Osteoporosis
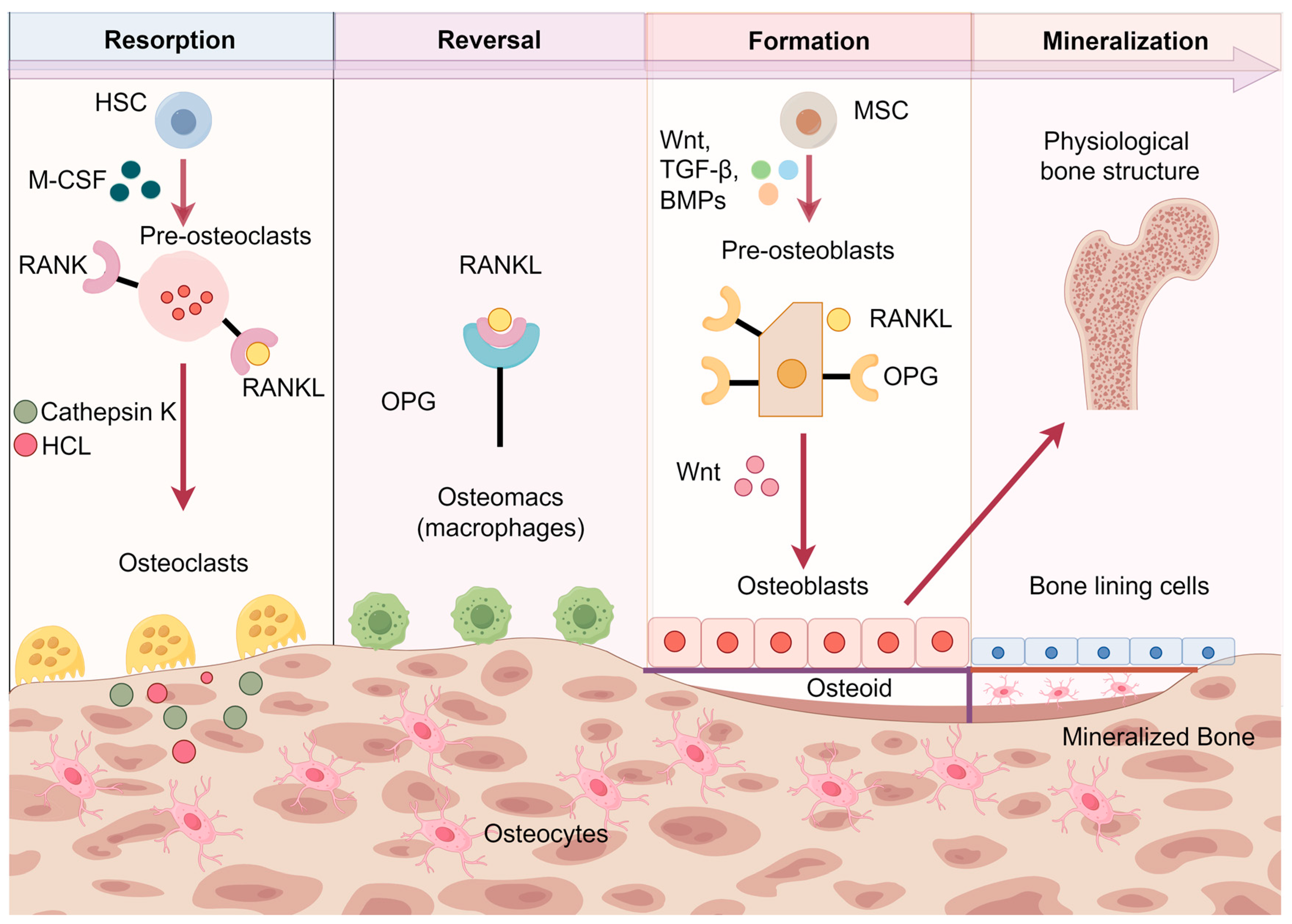
2.1. Bone Turnover and Formation
2.2. Pathogenesis of Osteoporosis
3. SIRT Family
| Sirtuin | Subcellular Localization | Enzyme Activity | Functions | References |
|---|---|---|---|---|
| SIRT1 | Nucleus and cytoplasm | Deacetylase | Cell survival, metabolism regulation, oxidative stress response, inflammatin, mitochondrial biogenesis, life span regulation | [68,69,74,78,87,88,89,90] |
| SIRT2 | Nucleus and cytoplasm | Deacetylase | Neurodegeneration, cell cycle regulation, tumor suppression/promotion | [91,92,93,94,95] |
| SIRT3 | Nucleus, cytoplasm and mitochondria | Deacetylase | Protection against oxidative stress, tumor suppression, mitochondrial metabolism | [96,97,98,99,100] |
| SIRT4 | Mitochondria | Deacetylase ADP-ribosylase Lipoamidase | Tumor suppression, amino acid catabolism, mitochondrial metabolism | [101,102,103,104] |
| SIRT5 | Mitochondria | Deacetylase Desuccinylase Demalonylase | Apoptosis, urea cycle, amino acid metabolism, fatty acid metabolism | [68,69,85,105,106,107] |
| SIRT6 | Nucleus | Deacetylase ADP-ribosylase | DNA repair, genome stability, glucose and lipid metabolism, inflammation | [108,109,110,111] |
| SIRT7 | Nucleus | Deacetylase | Cell cycle regulation, ribosome biogenesis, rRNA transcription | [112,113,114,115] |
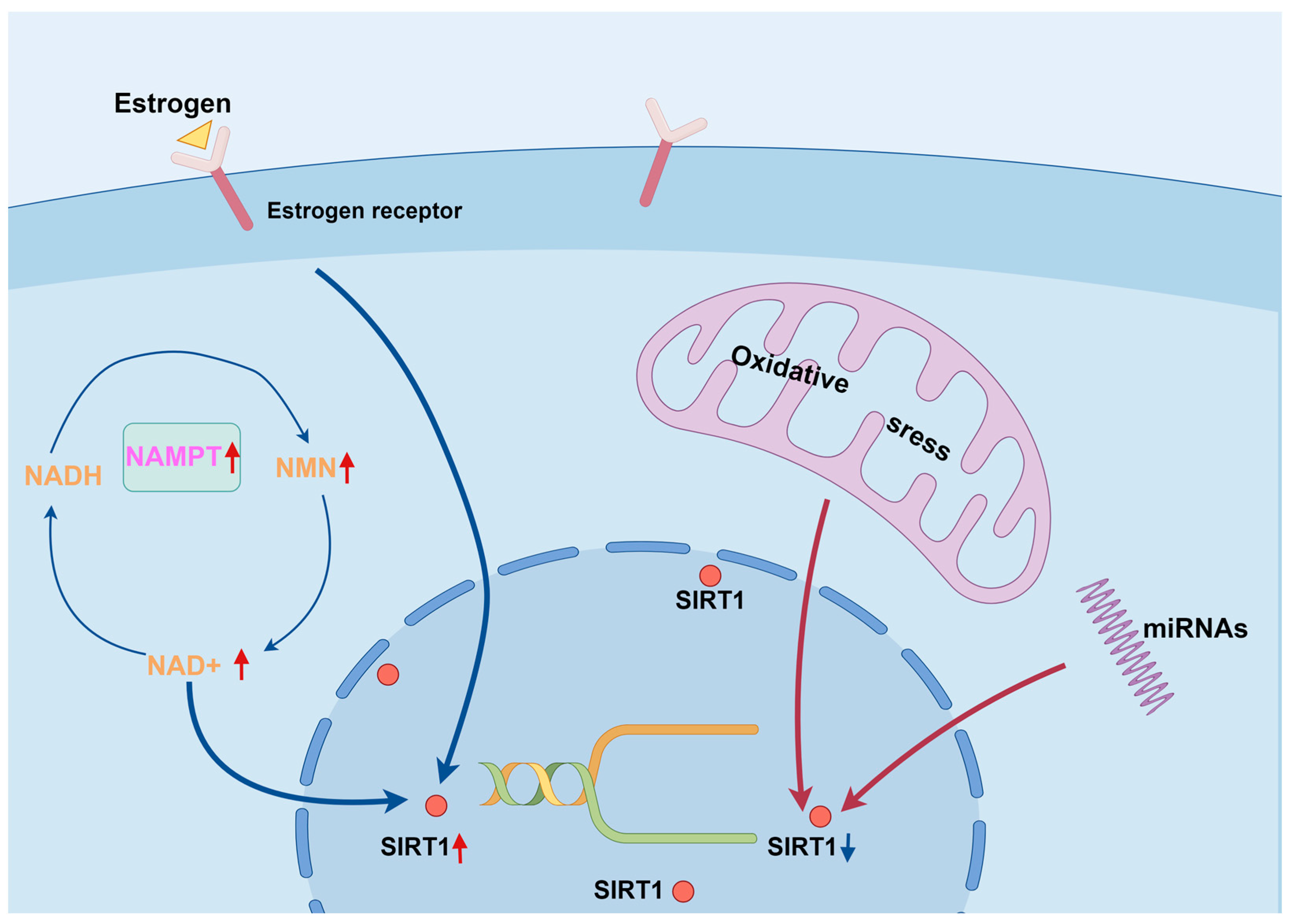
4. The Physiological Functions of SIRT1
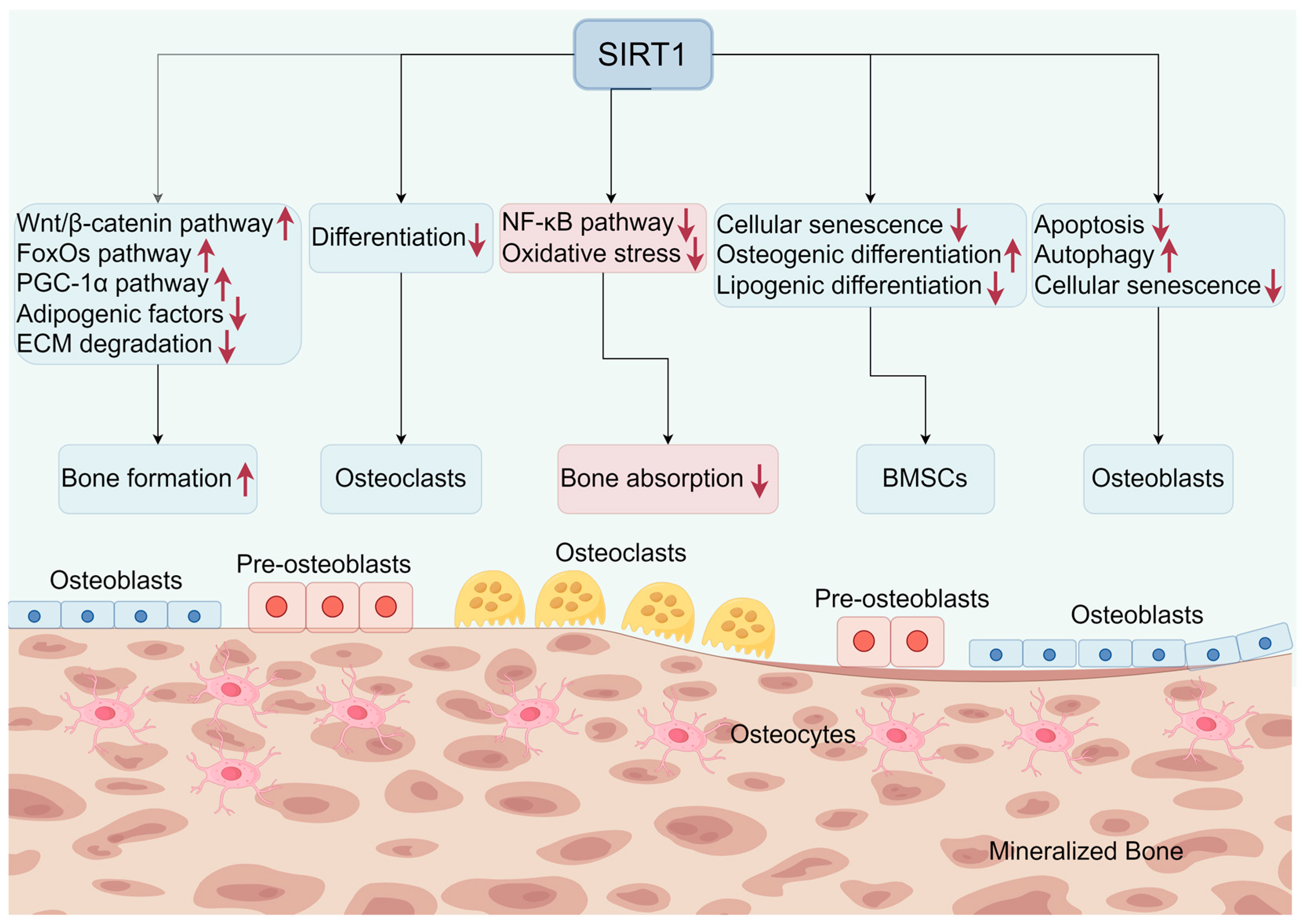
5. The Role of SIRT1 in Osteoblasts, Osteoclasts, Bone Marrow Mesenchymal Cells, and Osteocytes
5.1. SIRT1 and Osteoblasts
5.2. SIRT1 and Osteoclasts
5.3. SIRT1 and Bone Marrow Mesenchymal Cells
5.4. SIRT1 and Osteocytes
6. The Role of SIRT1 in the Pathogenesis of Different Types of Osteoporosis
7. The Application of SIRT1 Agonists in Osteoporosis
8. Summary and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. WHO Study Group. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Garnero, P.; Delmas, P.D. Osteoporosis. Endocrinol. Metab. Clin. N. Am. 1997, 26, 913–936. [Google Scholar] [CrossRef]
- Adami, S.; Ortolani, S.; Wasnich, R. Evaluation of therapeutic efficacy in osteoporosis. Osteoporos. Int. 1995, 5, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Kupai, K.; Kang, H.L.; Pósa, A.; Csonka, Á.; Várkonyi, T.; Valkusz, Z. Bone Loss in Diabetes Mellitus: Diaporosis. Int. J. Mol. Sci. 2024, 25, 7269. [Google Scholar] [CrossRef]
- McCloskey, E.; Tan, A.T.H.; Schini, M. Update on fracture risk assessment in osteoporosis. Curr. Opin. Endocrinol. Diabetes Obes. 2024, 31, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Harada, S.; Rodan, G.A. Control of osteoblast function and regulation of bone mass. Nature 2003, 423, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Raisz, L.G. Pathogenesis of osteoporosis: Concepts, conflicts, and prospects. J. Clin. Investig. 2005, 115, 3318–3325. [Google Scholar] [CrossRef]
- Gao, M.; Gao, W.; Papadimitriou, J.M.; Zhang, C.; Gao, J.; Zheng, M. Exosomes-the enigmatic regulators of bone homeostasis. Bone Res. 2018, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Waykar, T.R.; Mandlik, S.K.; Mandlik, D.S. Sirtuins: Exploring next-gen therapeutics in the pathogenesis osteoporosis and associated diseases. Immunopharmacol. Immunotoxicol. 2024, 46, 277–301. [Google Scholar] [CrossRef]
- North American Menopause, S. Management of osteoporosis in postmenopausal women: 2006 position statement of The North American Menopause Society. Menopause 2006, 13, 340–367. [Google Scholar]
- Favero, V.; Eller-Vainicher, C.; Chiodini, I. Secondary Osteoporosis: A Still Neglected Condition. Int. J. Mol. Sci. 2023, 24, 8558. [Google Scholar] [CrossRef] [PubMed]
- Sobh, M.M.; Abdalbary, M.; Elnagar, S.; Nagy, E.; Elshabrawy, N.; Abdelsalam, M.; Asadipooya, K.; El-Husseini, A. Secondary Osteoporosis and Metabolic Bone Diseases. J. Clin. Med. 2022, 11, 2382. [Google Scholar] [CrossRef] [PubMed]
- Ebeling, P.R.; Nguyen, H.H.; Aleksova, J.; Vincent, A.J.; Wong, P.; Milat, F. Secondary Osteoporosis. Endocr. Rev. 2022, 43, 240–313. [Google Scholar] [PubMed]
- El-Gazzar, A.; Högler, W. Mechanisms of Bone Fragility: From Osteogenesis Imperfecta to Secondary Osteoporosis. Int. J. Mol. Sci. 2021, 22, 625. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, L.; Biamonte, F.; Pepe, J.; Cipriani, C.; Minisola, S. Understanding and managing secondary osteoporosis. Expert Rev. Endocrinol. Metab. 2019, 14, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Sheu, A.; Diamond, T. Secondary osteoporosis. Aust. Prescr. 2016, 39, 85–87. [Google Scholar]
- Mirza, F.; Canalis, E. Management of endocrine disease: Secondary osteoporosis: Pathophysiology and management. Eur. J. Endocrinol. 2015, 173, R131–R151. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.Y.; Lu, X.T.; Hou, M.L.; Cao, T.; Tian, Z. Sirtuin1-p53: A potential axis for cancer therapy. Biochem. Pharmacol. 2023, 212, 115543. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef]
- Alves-Fernandes, D.K.; Jasiulionis, M.G. The Role of SIRT1 on DNA Damage Response and Epigenetic Alterations in Cancer. Int. J. Mol. Sci. 2019, 20, 3153. [Google Scholar] [CrossRef] [PubMed]
- Molina-Serrano, D.; Kyriakou, D.; Kirmizis, A. Histone Modifications as an Intersection Between Diet and Longevity. Front. Genet. 2019, 10, 192. [Google Scholar] [CrossRef]
- Nogueiras, R.; Habegger, K.M.; Chaudhary, N.; Finan, B.; Banks, A.S.; Dietrich, M.O.; Horvath, T.L.; Sinclair, D.A.; Pfluger, P.T.; Tschöp, M.H. Sirtuin 1 and sirtuin 3: Physiological modulators of metabolism. Physiol. Rev. 2012, 92, 1479–1514. [Google Scholar] [CrossRef]
- Daenthanasanmak, A.; Iamsawat, S.; Chakraborty, P.; Nguyen, H.D.; Bastian, D.; Liu, C.; Mehrotra, S.; Yu, X.Z. Targeting Sirt-1 controls GVHD by inhibiting T-cell allo-response and promoting Treg stability in mice. Blood 2019, 133, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Z.; Wang, F.; Gao, P.; Pei, J.F.; Liu, Y.; Xu, T.T.; Tang, X.; Fu, W.Y.; Lu, J.; Yan, Y.F.; et al. Age-Associated Sirtuin 1 Reduction in Vascular Smooth Muscle Links Vascular Senescence and Inflammation to Abdominal Aortic Aneurysm. Circ. Res. 2016, 119, 1076–1088. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Kfir, E.; Artsi, H.; Levin, A.; Abramowitz, E.; Bajayo, A.; Gurt, I.; Zhong, L.; D’Urso, A.; Toiber, D.; Mostoslavsky, R.; et al. Sirt1 is a regulator of bone mass and a repressor of Sost encoding for sclerostin, a bone formation inhibitor. Endocrinology 2011, 152, 4514–4524. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.R.; Perrien, D.S.; Fleming, N.; Nyman, J.S.; Ono, K.; Connelly, L.; Moore, M.M.; Lwin, S.T.; Yull, F.E.; Mundy, G.R.; et al. Silent information regulator (Sir)T1 inhibits NF-κB signaling to maintain normal skeletal remodeling. J. Bone Min. Res. 2013, 28, 960–969. [Google Scholar] [CrossRef]
- Godfrin-Valnet, M.; Khan, K.A.; Guillot, X.; Prati, C.; Baud, L.; Abbas, W.; Toussirot, E.; Wendling, D.; Herbein, G. Sirtuin 1 activity in peripheral blood mononuclear cells of patients with osteoporosis. Med. Sci. Monit. Basic Res. 2014, 20, 142–145. [Google Scholar] [CrossRef] [PubMed]
- He, X.; He, J.; Shi, Y.; Pi, C.; Yang, Y.; Sun, Y.; Ma, C.; Lin, L.; Zhang, L.; Li, Y.; et al. Nicotinamide phosphoribosyltransferase (Nampt) may serve as the marker for osteoblast differentiation of bone marrow-derived mesenchymal stem cells. Exp. Cell Res. 2017, 352, 45–52. [Google Scholar] [CrossRef]
- Elbaz, A.; Rivas, D.; Duque, G. Effect of estrogens on bone marrow adipogenesis and Sirt1 in aging C57BL/6J mice. Biogerontology 2009, 10, 747–755. [Google Scholar] [CrossRef]
- Louvet, L.; Leterme, D.; Delplace, S.; Miellot, F.; Marchandise, P.; Gauthier, V.; Hardouin, P.; Chauveau, C.; Ghali Mhenni, O. Sirtuin 1 deficiency decreases bone mass and increases bone marrow adiposity in a mouse model of chronic energy deficiency. Bone 2020, 136, 115361. [Google Scholar] [CrossRef] [PubMed]
- El-Haj, M.; Gurt, I.; Cohen-Kfir, E.; Dixit, V.; Artsi, H.; Kandel, L.; Yakubovsky, O.; Safran, O.; Dresner-Pollak, R. Reduced Sirtuin1 expression at the femoral neck in women who sustained an osteoporotic hip fracture. Osteoporos. Int. 2016, 27, 2373–2378. [Google Scholar] [CrossRef] [PubMed]
- Notelovitz, M. Osteoporosis: Screening, prevention, and management. Fertil. Steril. 1993, 59, 707–725. [Google Scholar] [CrossRef] [PubMed]
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef]
- Abdallah, B.M.; Jafari, A.; Zaher, W.; Qiu, W.; Kassem, M. Skeletal (stromal) stem cells: An update on intracellular signaling pathways controlling osteoblast differentiation. Bone 2015, 70, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Ohata, Y.; Ozono, K. Bone and Stem Cells. The mechanism of osteogenic differentiation from mesenchymal stem cell. Clin. Calcium 2014, 24, 501–508. [Google Scholar] [PubMed]
- Hu, L.; Chen, W.; Qian, A.; Li, Y.P. Wnt/β-catenin signaling components and mechanisms in bone formation, homeostasis, and disease. Bone Res. 2024, 12, 39. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, W.; Masson, A.; Li, Y.P. Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis. Cell Discov. 2024, 10, 71. [Google Scholar] [CrossRef]
- Li, S.Y.; Xue, S.T.; Li, Z.R. Osteoporosis: Emerging targets on the classical signaling pathways of bone formation. Eur. J. Pharmacol. 2024, 973, 176574. [Google Scholar] [CrossRef]
- Zou, Z.; Liu, W.; Cao, L.; Liu, Y.; He, T.; Peng, S.; Shuai, C. Advances in the occurrence and biotherapy of osteoporosis. Biochem. Soc. Trans. 2020, 48, 1623–1636. [Google Scholar] [CrossRef]
- Abdallah, B.M. Marrow adipocytes inhibit the differentiation of mesenchymal stem cells into osteoblasts via suppressing BMP-signaling. J. Biomed. Sci. 2017, 24, 11. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; de Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef] [PubMed]
- Elango, J.; Sanchez, C.; de Val, J.; Henrotin, Y.; Wang, S.; Motaung, K.; Guo, R.; Wang, C.; Robinson, J.; Regenstein, J.M.; et al. Cross-talk between primary osteocytes and bone marrow macrophages for osteoclastogenesis upon collagen treatment. Sci. Rep. 2018, 8, 5318. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Huang, B.; Gu, Y.; Luo, Y.; Zhi, X.; Hu, Y.; Zhang, H.; Gu, Z.; Cui, J.; et al. Targeting actin-bundling protein L-plastin as an anabolic therapy for bone loss. Sci. Adv. 2020, 6, eabb7135. [Google Scholar] [CrossRef] [PubMed]
- Vasikaran, S.D.; Chubb, S.A. The use of biochemical markers of bone turnover in the clinical management of primary and secondary osteoporosis. Endocrine 2016, 52, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, I.; dell’Isola, F.; Andreaus, U.; Alzahrani, F.; Hayat, T.; Lekszycki, T. On mechanically driven biological stimulus for bone remodeling as a diffusive phenomenon. Biomech. Model. Mechanobiol. 2019, 18, 1639–1663. [Google Scholar] [CrossRef] [PubMed]
- Stepan, J.J.; Hruskova, H.; Kverka, M. Update on Menopausal Hormone Therapy for Fracture Prevention. Curr. Osteoporos. Rep. 2019, 17, 465–473. [Google Scholar] [CrossRef]
- Manolagas, S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef]
- Rodan, G.A.; Martin, T.J. Therapeutic approaches to bone diseases. Science 2000, 289, 1508–1514. [Google Scholar] [CrossRef]
- Edwards, J.R.; Mundy, G.R. Advances in osteoclast biology: Old findings and new insights from mouse models. Nat. Rev. Rheumatol. 2011, 7, 235–243. [Google Scholar] [CrossRef]
- Golob, A.L.; Laya, M.B. Osteoporosis: Screening, prevention, and management. Med. Clin. N. Am. 2015, 99, 587–606. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Q.; Wang, Y.; Li, L.; Bu, H.; Bao, J. Senescence of mesenchymal stem cells (Review). Int. J. Mol. Med. 2017, 39, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Herath, M.; Langdahl, B.; Ebeling, P.R.; Milat, F. Challenges in the diagnosis and management of glucocorticoid-induced osteoporosis in younger and older adults. Clin. Endocrinol. 2022, 96, 460–474. [Google Scholar] [CrossRef] [PubMed]
- Ohnaka, K.; Tanabe, M.; Kawate, H.; Nawata, H.; Takayanagi, R. Glucocorticoid suppresses the canonical Wnt signal in cultured human osteoblasts. Biochem. Biophys. Res. Commun. 2005, 329, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Chen, L.R.; Chen, K.H. Osteoporosis Due to Hormone Imbalance: An Overview of the Effects of Estrogen Deficiency and Glucocorticoid Overuse on Bone Turnover. Int. J. Mol. Sci. 2022, 23, 1376. [Google Scholar] [CrossRef] [PubMed]
- Callaway, D.A.; Jiang, J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Min. Metab. 2015, 33, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, H.; Mani, D.; Singh, D.; Gupta, A. The underlying pathophysiology and therapeutic approaches for osteoporosis. Med. Res. Rev. 2018, 38, 2024–2057. [Google Scholar] [CrossRef] [PubMed]
- Pouresmaeili, F.; Kamalidehghan, B.; Kamarehei, M.; Goh, Y.M. A comprehensive overview on osteoporosis and its risk factors. Ther. Clin. Risk Manag. 2018, 14, 2029–2049. [Google Scholar] [CrossRef]
- Tanaka, K.; Yamaguchi, T.; Kanazawa, I.; Sugimoto, T. Effects of high glucose and advanced glycation end products on the expressions of sclerostin and RANKL as well as apoptosis in osteocyte-like MLO-Y4-A2 cells. Biochem. Biophys. Res. Commun. 2015, 461, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, C.; Colangelo, L.; Santori, R.; Renella, M.; Mastrantonio, M.; Minisola, S.; Pepe, J. The Interplay between Bone and Glucose Metabolism. Front. Endocrinol. 2020, 11, 122. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Lu, L.; Yu, X. The relationship between bone marrow adipose tissue and bone metabolism in postmenopausal osteoporosis. Cytokine Growth Factor. Rev. 2020, 52, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Reginster, J.Y.; Burlet, N. Osteoporosis: A still increasing prevalence. Bone 2006, 38 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef]
- Khosla, S.; Hofbauer, L.C. Osteoporosis treatment: Recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017, 5, 898–907. [Google Scholar] [CrossRef]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar] [PubMed]
- Black, D.M.; Rosen, C.J. Clinical Practice. Postmenopausal Osteoporosis. N. Engl. J. Med. 2016, 374, 254–262. [Google Scholar] [CrossRef]
- Dai, H.; Sinclair, D.A.; Ellis, J.L.; Steegborn, C. Sirtuin activators and inhibitors: Promises, achievements, and challenges. Pharmacol. Ther. 2018, 188, 140–154. [Google Scholar] [CrossRef]
- Jiao, F.; Gong, Z. The Beneficial Roles of SIRT1 in Neuroinflammation-Related Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 6782872. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Auwerx, J. The role of sirtuins in the control of metabolic homeostasis. Ann. N. Y. Acad. Sci. 2009, 1173 (Suppl. S1), E10–E19. [Google Scholar] [CrossRef]
- Yamamoto, H.; Schoonjans, K.; Auwerx, J. Sirtuin functions in health and disease. Mol. Endocrinol. 2007, 21, 1745–1755. [Google Scholar] [CrossRef]
- Ivy, J.M.; Klar, A.J.; Hicks, J.B. Cloning and characterization of four SIR genes of Saccharomyces cerevisiae. Mol. Cell Biol. 1986, 6, 688–702. [Google Scholar]
- Kennedy, B.K.; Austriaco, N.R., Jr.; Zhang, J.; Guarente, L. Mutation in the silencing gene SIR4 can delay aging in S. cerevisiae. Cell 1995, 80, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Kaeberlein, M.; McVey, M.; Guarente, L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999, 13, 2570–2580. [Google Scholar] [CrossRef] [PubMed]
- Tissenbaum, H.A.; Guarente, L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 2001, 410, 227–230. [Google Scholar] [CrossRef]
- Vaziri, H.; Dessain, S.K.; Ng Eaton, E.; Imai, S.I.; Frye, R.A.; Pandita, T.K.; Guarente, L.; Weinberg, R.A. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 2001, 107, 149–159. [Google Scholar] [CrossRef]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. Embo J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Haigis, M.C.; Mostoslavsky, R.; Haigis, K.M.; Fahie, K.; Christodoulou, D.C.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; Karow, M.; Blander, G.; et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell 2006, 126, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Deng, C.X.; Mostoslavsky, R. Recent progress in the biology and physiology of sirtuins. Nature 2009, 460, 587–591. [Google Scholar] [CrossRef]
- McCord, R.A.; Michishita, E.; Hong, T.; Berber, E.; Boxer, L.D.; Kusumoto, R.; Guan, S.; Shi, X.; Gozani, O.; Burlingame, A.L.; et al. SIRT6 stabilizes DNA-dependent protein kinase at chromatin for DNA double-strand break repair. Aging 2009, 1, 109–121. [Google Scholar] [CrossRef]
- Haigis, M.C.; Sinclair, D.A. Mammalian sirtuins: Biological insights and disease relevance. Annu. Rev. Pathol. 2010, 5, 253–295. [Google Scholar] [CrossRef]
- Kincaid, B.; Bossy-Wetzel, E. Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front. Aging Neurosci. 2013, 5, 48. [Google Scholar] [CrossRef]
- Rardin, M.J.; He, W.; Nishida, Y.; Newman, J.C.; Carrico, C.; Danielson, S.R.; Guo, A.; Gut, P.; Sahu, A.K.; Li, B.; et al. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab. 2013, 18, 920–933. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shi, L.; Yang, S.; Yan, R.; Zhang, D.; Yang, J.; He, L.; Li, W.; Yi, X.; Sun, L.; et al. SIRT7 is a histone desuccinylase that functionally links to chromatin compaction and genome stability. Nat. Commun. 2016, 7, 12235. [Google Scholar] [CrossRef] [PubMed]
- Guarente, L.; Picard, F. Calorie restriction--the SIR2 connection. Cell 2005, 120, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Donmez, G.; Guarente, L. Aging and disease: Connections to sirtuins. Aging Cell 2010, 9, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Mouchiroud, L.; Houtkooper, R.H.; Moullan, N.; Katsyuba, E.; Ryu, D.; Cantó, C.; Mottis, A.; Jo, Y.S.; Viswanathan, M.; Schoonjans, K.; et al. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell 2013, 154, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Brace, C.S.; Rensing, N.; Cliften, P.; Wozniak, D.F.; Herzog, E.D.; Yamada, K.A.; Imai, S. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013, 18, 416–430. [Google Scholar] [CrossRef]
- Shan, P.; Fan, G.; Sun, L.; Liu, J.; Wang, W.; Hu, C.; Zhang, X.; Zhai, Q.; Song, X.; Cao, L.; et al. SIRT1 Functions as a Negative Regulator of Eukaryotic Poly(A)RNA Transport. Curr. Biol. 2017, 27, 2271–2284.e2275. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Shen, M.; Kuang, L.; Yang, K.; Wu, S.; Liu, X.; Wang, Y.; Wang, Y. SIRT1/SREBPs-mediated regulation of lipid metabolism. Pharmacol. Res. 2024, 199, 107037. [Google Scholar] [CrossRef] [PubMed]
- Rakshe, P.S.; Dutta, B.J.; Chib, S.; Maurya, N.; Singh, S. Unveiling the interplay of AMPK/SIRT1/PGC-1α axis in brain health: Promising targets against aging and NDDs. Ageing Res. Rev. 2024, 96, 102255. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Hong, T.; Chen, X.; Cui, L. SIRT2: Controversy and multiple roles in disease and physiology. Ageing Res. Rev. 2019, 55, 100961. [Google Scholar] [CrossRef] [PubMed]
- Donmez, G.; Outeiro, T.F. SIRT1 and SIRT2: Emerging targets in neurodegeneration. EMBO Mol. Med. 2013, 5, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Sola-Sevilla, N.; Puerta, E. SIRT2 as a potential new therapeutic target for Alzheimer’s disease. Neural Regen. Res. 2024, 19, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, P.; Hu, C. The role of SIRT2 in cancer: A novel therapeutic target. Int. J. Cancer 2020, 147, 3297–3304. [Google Scholar] [CrossRef] [PubMed]
- Manjula, R.; Anuja, K.; Alcain, F.J. SIRT1 and SIRT2 Activity Control in Neurodegenerative Diseases. Front. Pharmacol. 2020, 11, 585821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ye, J.; Zhu, S.; Han, B.; Liu, B. Context-dependent role of SIRT3 in cancer. Trends Pharmacol. Sci. 2024, 45, 173–190. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Shen, L. Mitochondrial proteins in heart failure: The role of deacetylation by SIRT3. Pharmacol. Res. 2021, 172, 105802. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Siyuan, Z.; Xing, C.; Ruxiu, L. SIRT3 regulates mitochondrial function: A promising star target for cardiovascular disease therapy. Biomed. Pharmacother. 2024, 170, 116004. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, Q.; Shi, J.; Zhou, S. Regulation of SIRT3 on mitochondrial functions and oxidative stress in Parkinson’s disease. Biomed. Pharmacother. 2020, 132, 110928. [Google Scholar] [CrossRef]
- Mishra, Y.; Kaundal, R.K. Role of SIRT3 in mitochondrial biology and its therapeutic implications in neurodegenerative disorders. Drug Discov. Today 2023, 28, 103583. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, Y.; Principe, D.R.; Zou, X.; Vassilopoulos, A.; Gius, D. SIRT3 and SIRT4 are mitochondrial tumor suppressor proteins that connect mitochondrial metabolism and carcinogenesis. Cancer Metab. 2014, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Min, Z.; Gao, J.; Yu, Y. The Roles of Mitochondrial SIRT4 in Cellular Metabolism. Front. Endocrinol. 2018, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhu, G. Sirtuin-4 (SIRT4), a therapeutic target with oncogenic and tumor-suppressive activity in cancer. Onco Targets Ther. 2018, 11, 3395–3400. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Wang, F.; Chen, X.; Wang, C.; Wang, J.; Liu, T.; Li, Y.; He, B. SIRT4 is the last puzzle of mitochondrial sirtuins. Bioorg. Med. Chem. 2018, 26, 3861–3865. [Google Scholar] [CrossRef]
- Kumar, S.; Lombard, D.B. Functions of the sirtuin deacylase SIRT5 in normal physiology and pathobiology. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 311–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, H.; Zha, X. Overview of SIRT5 as a potential therapeutic target: Structure, function and inhibitors. Eur. J. Med. Chem. 2022, 236, 114363. [Google Scholar] [CrossRef] [PubMed]
- Fabbrizi, E.; Fiorentino, F.; Carafa, V.; Altucci, L.; Mai, A.; Rotili, D. Emerging Roles of SIRT5 in Metabolism, Cancer, and SARS-CoV-2 Infection. Cells 2023, 12, 852. [Google Scholar] [CrossRef]
- Chang, A.R.; Ferrer, C.M.; Mostoslavsky, R. SIRT6, a Mammalian Deacylase with Multitasking Abilities. Physiol. Rev. 2020, 100, 145–169. [Google Scholar] [CrossRef]
- Vitiello, M.; Zullo, A.; Servillo, L.; Mancini, F.P.; Borriello, A.; Giovane, A.; Della Ragione, F.; D’Onofrio, N.; Balestrieri, M.L. Multiple pathways of SIRT6 at the crossroads in the control of longevity, cancer, and cardiovascular diseases. Ageing Res. Rev. 2017, 35, 301–311. [Google Scholar] [CrossRef]
- Kugel, S.; Mostoslavsky, R. Chromatin and beyond: The multitasking roles for SIRT6. Trends Biochem. Sci. 2014, 39, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Chen, H.; Liu, H.; Zhang, W.; Zhou, J. Emerging roles of SIRT6 in human diseases and its modulators. Med. Res. Rev. 2021, 41, 1089–1137. [Google Scholar] [CrossRef] [PubMed]
- Raza, U.; Tang, X.; Liu, Z.; Liu, B. SIRT7: The seventh key to unlocking the mystery of aging. Physiol. Rev. 2024, 104, 253–280. [Google Scholar] [CrossRef] [PubMed]
- Ianni, A.; Kumari, P.; Tarighi, S.; Braun, T.; Vaquero, A. SIRT7: A novel molecular target for personalized cancer treatment? Oncogene 2024, 43, 993–1006. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Mizumoto, T.; Yoshizawa, T. The Emerging Role of SIRT7 in Glucose and Lipid Metabolism. Cells 2023, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Tang, H.; Tu, B.; Zhu, W.G. SIRT7: A sentinel of genome stability. Open Biol. 2021, 11, 210047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, Q.; Zeng, Z.; Wu, J.; Zhang, Y.; Chen, Z. Sirt1 Inhibits Oxidative Stress in Vascular Endothelial Cells. Oxid. Med. Cell. Longev. 2017, 2017, 7543973. [Google Scholar] [CrossRef]
- Cheng, H.L.; Mostoslavsky, R.; Saito, S.; Manis, J.P.; Gu, Y.; Patel, P.; Bronson, R.; Appella, E.; Alt, F.W.; Chua, K.F. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc. Natl. Acad. Sci. USA 2003, 100, 10794–10799. [Google Scholar] [CrossRef]
- Brooks, C.L.; Gu, W. How does SIRT1 affect metabolism, senescence and cancer? Nat. Rev. Cancer 2009, 9, 123–128. [Google Scholar] [CrossRef]
- Vaquero, A.; Scher, M.; Lee, D.; Erdjument-Bromage, H.; Tempst, P.; Reinberg, D. Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol. Cell 2004, 16, 93–105. [Google Scholar] [CrossRef]
- Motta, M.C.; Divecha, N.; Lemieux, M.; Kamel, C.; Chen, D.; Gu, W.; Bultsma, Y.; McBurney, M.; Guarente, L. Mammalian SIRT1 represses forkhead transcription factors. Cell 2004, 116, 551–563. [Google Scholar] [CrossRef]
- Pruitt, K.; Zinn, R.L.; Ohm, J.E.; McGarvey, K.M.; Kang, S.H.; Watkins, D.N.; Herman, J.G.; Baylin, S.B. Inhibition of SIRT1 reactivates silenced cancer genes without loss of promoter DNA hypermethylation. PLoS Genet. 2006, 2, e40. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.; Han, L.; Bartell, S.M.; Kim, H.N.; Gubrij, I.; de Cabo, R.; O’Brien, C.A.; Manolagas, S.C.; Almeida, M. Sirtuin1 (Sirt1) promotes cortical bone formation by preventing β-catenin sequestration by FoxO transcription factors in osteoblast progenitors. J. Biol. Chem. 2014, 289, 24069–24078. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Luo, J. SIRT1 and p53, effect on cancer, senescence and beyond. Biochim. Biophys. Acta 2010, 1804, 1684–1689. [Google Scholar] [CrossRef] [PubMed]
- Mayoral, R.; Osborn, O.; McNelis, J.; Johnson, A.M.; Oh, D.Y.; Izquierdo, C.L.; Chung, H.; Li, P.; Traves, P.G.; Bandyopadhyay, G.; et al. Adipocyte SIRT1 knockout promotes PPARγ activity, adipogenesis and insulin sensitivity in chronic-HFD and obesity. Mol. Metab. 2015, 4, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Auwerx, J. Targeting sirtuin 1 to improve metabolism: All you need is NAD(+)? Pharmacol. Rev. 2012, 64, 166–187. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Lai, Y.L.; Lin, S.D.; Li, X.T.; Fu, Y.C.; Xu, W.C. SIRT1 interacts with metabolic transcriptional factors in the pancreas of insulin-resistant and calorie-restricted rats. Mol. Biol. Rep. 2013, 40, 3373–3380. [Google Scholar] [CrossRef] [PubMed]
- Thakran, S.; Sharma, P.; Attia, R.R.; Hori, R.T.; Deng, X.; Elam, M.B.; Park, E.A. Role of sirtuin 1 in the regulation of hepatic gene expression by thyroid hormone. J. Biol. Chem. 2013, 288, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, M.E.; Yang, X.; Jardine, K.; He, X.; Jacobsen, K.X.; Staines, W.A.; Harper, M.E.; McBurney, M.W. The Sirt1 deacetylase modulates the insulin-like growth factor signaling pathway in mammals. Mech. Ageing Dev. 2005, 126, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Côté, C.D.; Rasmussen, B.A.; Duca, F.A.; Zadeh-Tahmasebi, M.; Baur, J.A.; Daljeet, M.; Breen, D.M.; Filippi, B.M.; Lam, T.K. Resveratrol activates duodenal Sirt1 to reverse insulin resistance in rats through a neuronal network. Nat. Med. 2015, 21, 498–505. [Google Scholar] [CrossRef]
- Zainabadi, K.; Liu, C.J.; Caldwell, A.L.M.; Guarente, L. SIRT1 is a positive regulator of in vivo bone mass and a therapeutic target for osteoporosis. PLoS ONE 2017, 12, e0185236. [Google Scholar] [CrossRef]
- Chen, H.; Hu, X.; Yang, R.; Wu, G.; Tan, Q.; Goltzman, D.; Miao, D. SIRT1/FOXO3a axis plays an important role in the prevention of mandibular bone loss induced by 1,25(OH)(2)D deficiency. Int. J. Biol. Sci. 2020, 16, 2712–2726. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Kang, X.; Zhao, L.; Xu, M.; Xie, T.; Li, H.; Li, F.; Qian, Z.; Ma, Z.; Zhang, Y.; et al. Cartilage Ablation of Sirt1 Causes Inhibition of Growth Plate Chondrogenesis by Hyperactivation of mTORC1 Signaling. Endocrinology 2019, 160, 3001–3017. [Google Scholar] [CrossRef] [PubMed]
- Shtaif, B.; Bar-Maisels, M.; Gabet, Y.; Hiram-Bab, S.; Yackobovitch-Gavan, M.; Phillip, M.; Gat-Yablonski, G. Cartilage -specific knockout of Sirt1 significantly reduces bone quality and catch-up growth efficiency. Bone 2020, 138, 115468. [Google Scholar] [CrossRef] [PubMed]
- McBurney, M.W.; Yang, X.; Jardine, K.; Hixon, M.; Boekelheide, K.; Webb, J.R.; Lansdorp, P.M.; Lemieux, M. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol. Cell Biol. 2003, 23, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Kolthur-Seetharam, U.; Teerds, K.; de Rooij, D.G.; Wendling, O.; McBurney, M.; Sassone-Corsi, P.; Davidson, I. The histone deacetylase SIRT1 controls male fertility in mice through regulation of hypothalamic-pituitary gonadotropin signaling. Biol. Reprod. 2009, 80, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Khawar, M.B.; Liu, C.; Gao, F.; Gao, H.; Liu, W.; Han, T.; Wang, L.; Li, G.; Jiang, H.; Li, W. Sirt1 regulates testosterone biosynthesis in Leydig cells via modulating autophagy. Protein Cell 2021, 12, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Dvir-Ginzberg, M.; Gagarina, V.; Lee, E.J.; Hall, D.J. Regulation of cartilage-specific gene expression in human chondrocytes by SirT1 and nicotinamide phosphoribosyltransferase. J. Biol. Chem. 2008, 283, 36300–36310. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Ikeda, Y.; Miyauchi, T.; Uchikado, Y.; Akasaki, Y.; Ohishi, M. Estrogen-SIRT1 Axis Plays a Pivotal Role in Protecting Arteries against Menopause-Induced Senescence and Atherosclerosis. J. Atheroscler. Thromb. 2020, 27, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhou, X.; Liu, Y.; Tan, S.; Li, Y. The Role of Sirtuin-1 in Immune Response and Systemic Lupus Erythematosus. Front. Immunol. 2021, 12, 632383. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Yang, F.; Ning, G.; Zhen, L.; Wu, L.; Zheng, Y.; Zhang, Q.; Lin, D.; Xie, C.; et al. Nicotinamide mononucleotide promotes osteogenesis and reduces adipogenesis by regulating mesenchymal stromal cells via the SIRT1 pathway in aged bone marrow. Cell Death Dis. 2019, 10, 336. [Google Scholar] [CrossRef]
- Li, Y.; He, X.; Li, Y.; He, J.; Anderstam, B.; Andersson, G.; Lindgren, U. Nicotinamide phosphoribosyltransferase (Nampt) affects the lineage fate determination of mesenchymal stem cells: A possible cause for reduced osteogenesis and increased adipogenesis in older individuals. J. Bone Min. Res. 2011, 26, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Fulco, M.; Cen, Y.; Zhao, P.; Hoffman, E.P.; McBurney, M.W.; Sauve, A.A.; Sartorelli, V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev. Cell 2008, 14, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, J.; Yu, Q.; Gao, Y.; Zheng, Y.; Han, L.; Ding, X. Regulation of yak longissimus lumborum energy metabolism and tenderness by the AMPK/SIRT1 signaling pathways during postmortem storage. PLoS ONE 2022, 17, e0277410. [Google Scholar] [CrossRef] [PubMed]
- Brandauer, J.; Vienberg, S.G.; Andersen, M.A.; Ringholm, S.; Risis, S.; Larsen, P.S.; Kristensen, J.M.; Frøsig, C.; Leick, L.; Fentz, J.; et al. AMP-activated protein kinase regulates nicotinamide phosphoribosyl transferase expression in skeletal muscle. J. Physiol. 2013, 591, 5207–5220. [Google Scholar] [CrossRef]
- Bai, P.; Cantó, C.; Oudart, H.; Brunyánszki, A.; Cen, Y.; Thomas, C.; Yamamoto, H.; Huber, A.; Kiss, B.; Houtkooper, R.H.; et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011, 13, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Karbasforooshan, H.; Roohbakhsh, A.; Karimi, G. SIRT1 and microRNAs: The role in breast, lung and prostate cancers. Exp. Cell Res. 2018, 367, 1–6. [Google Scholar] [CrossRef]
- Carnevale, I.; Pellegrini, L.; D‘Aquila, P.; Saladini, S.; Lococo, E.; Polletta, L.; Vernucci, E.; Foglio, E.; Coppola, S.; Sansone, L.; et al. SIRT1-SIRT3 Axis Regulates Cellular Response to Oxidative Stress and Etoposide. J. Cell. Physiol. 2017, 232, 1835–1844. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Crosstalk between Oxidative Stress and SIRT1: Impact on the Aging Process. Int. J. Mol. Sci. 2013, 14, 3834–3859. [Google Scholar] [CrossRef]
- Yoon, D.S.; Choi, Y.; Lee, J.W. Cellular localization of NRF2 determines the self-renewal and osteogenic differentiation potential of human MSCs via the P53-SIRT1 axis. Cell Death Dis. 2016, 7, e2093. [Google Scholar] [CrossRef]
- Huang, W.; Shang, W.L.; Wang, H.D.; Wu, W.W.; Hou, S.X. Sirt1 overexpression protects murine osteoblasts against TNF-α-induced injury in vitro by suppressing the NF-κB signaling pathway. Acta Pharmacol. Sin. 2012, 33, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Abed, É.; Delalandre, A.; Lajeunesse, D. Beneficial effect of resveratrol on phenotypic features and activity of osteoarthritic osteoblasts. Arthritis Res. Ther. 2017, 19, 151. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wang, Z.; Jin, J.; Wang, Y.; Bao, N.; Gao, Q.; Zhao, J. SIRT1 protects osteoblasts against particle-induced inflammatory responses and apoptosis in aseptic prosthesis loosening. Acta Biomater. 2017, 49, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, S.I.; Moon, Y.J.; Kim, K.M.; Lee, K.B.; Park, B.H.; Jang, K.Y.; Kim, J.R. Overexpression of SIRT1 prevents hypoxia-induced apoptosis in osteoblast cells. Mol. Med. Rep. 2017, 16, 2969–2975. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Yao, Z.; Zhang, S.; Zhang, W.; Zhou, W. Upregulation of SIRT1 inhibits H2O2-induced osteoblast apoptosis via FoxO1/β-catenin pathway. Mol. Med. Rep. 2018, 17, 6681–6690. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.C.; Xu, Q.; Li, H.; Qin, Y.F.; Song, L.C.; Wang, C.G.; Cui, W.H.; Zheng, Z.; Yan, D.W.; Li, Z.J.; et al. NADPH Oxidase Isoforms Are Involved in Glucocorticoid-Induced Preosteoblast Apoptosis. Oxid. Med. Cell. Longev. 2019, 2019, 9192413. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Sun, X.; Ma, X.; Qin, Z.; Gao, X.; Kang, X.; Li, H.; Sun, H. SIRT1 maintains bone homeostasis by regulating osteoblast glycolysis through GOT1. Cell Mol. Life Sci. 2024, 81, 204. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wang, Z.; Gao, J.; Han, D.; Zhang, L.; Chen, P.; Luo, G.; Han, B. SIRT1 suppresses p53-dependent apoptosis by modulation of p21 in osteoblast-like MC3T3-E1 cells exposed to fluoride. Toxicol. Vitr. 2019, 57, 28–38. [Google Scholar] [CrossRef]
- Qu, H.; Li, T.; Jin, H.; Zhang, S.; He, B. Silent Mating Type Information Regulation 2 Homolog (SIRT1) Influences Osteogenic Proliferation and Differentiation of MC3T3-E1 Cells via Regulation of miR-132-3p. Med. Sci. Monit. 2019, 25, 2289–2295. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, T.; Wang, Y.; Guo, L. The Role and Mechanism of SIRT1 in Resveratrol-regulated Osteoblast Autophagy in Osteoporosis Rats. Sci. Rep. 2019, 9, 18424. [Google Scholar] [CrossRef]
- Zhou, D.; Ran, Y.; Yu, R.; Liu, G.; Ran, D.; Liu, Z. SIRT1 regulates osteoblast senescence through SOD2 acetylation and mitochondrial dysfunction in the progression of Osteoporosis caused by Cadmium exposure. Chem. Biol. Interact. 2023, 382, 110632. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Falsetti, I.; Bilia, A.R.; Vincenzini, M.T.; Brandi, M.L.; Iantomasi, T. Blueberry Juice Antioxidants Protect Osteogenic Activity against Oxidative Stress and Improve Long-Term Activation of the Mineralization Process in Human Osteoblast-Like SaOS-2 Cells: Involvement of SIRT1. Antioxidants 2020, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.L.; Xin, H.; Yan, X.N.; Huang, J.; Zhang, Y.P.; Du, H. 17β-Estradiol improves osteoblastic cell function through the Sirt1/NF-κB/MMP-8 pathway. Climacteric 2020, 23, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mei, R.; Hao, S.; Luo, P.; Wang, P.; Almatari, Y.; Guo, L.; Guo, L. Up-regulation of SIRT1 induced by 17beta-estradiol promotes autophagy and inhibits apoptosis in osteoblasts. Aging 2021, 13, 23652–23671. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Chen, Y.; Lin, Y.; Hu, Z.; Li, L.; Huang, H.; Lin, J. SIRT1 Asn346 sugar chain promoting collagen deacetylation protective effect on osteoblasts under stress. Biochem. Biophys. Res. Commun. 2023, 682, 148–155. [Google Scholar] [CrossRef]
- Gao, J.; Feng, Z.; Wang, X.; Zeng, M.; Liu, J.; Han, S.; Xu, J.; Chen, L.; Cao, K.; Long, J.; et al. SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress. Cell Death Differ. 2018, 25, 229–240. [Google Scholar] [CrossRef]
- Leslie, W.D.; Morin, S.N. Osteoporosis epidemiology 2013: Implications for diagnosis, risk assessment, and treatment. Curr. Opin. Rheumatol. 2014, 26, 440–446. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, Y.M.; Park, J.S.; Lee, S.K.; Kim, E.C. SIRT1 modulates high-mobility group box 1-induced osteoclastogenic cytokines in human periodontal ligament cells. J. Cell Biochem. 2010, 111, 1310–1320. [Google Scholar] [CrossRef]
- Shakibaei, M.; Buhrmann, C.; Mobasheri, A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-kappaB ligand (RANKL) activation of NF-kappaB signaling and inhibit osteoclastogenesis in bone-derived cells. J. Biol. Chem. 2011, 286, 11492–11505. [Google Scholar] [CrossRef]
- Gurt, I.; Artsi, H.; Cohen-Kfir, E.; Hamdani, G.; Ben-Shalom, G.; Feinstein, B.; El-Haj, M.; Dresner-Pollak, R. The Sirt1 Activators SRT2183 and SRT3025 Inhibit RANKL-Induced Osteoclastogenesis in Bone Marrow-Derived Macrophages and Down-Regulate Sirt3 in Sirt1 Null Cells. PLoS ONE 2015, 10, e0134391. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, S.W.; Kim, H.Y.; Lee, S.Y.; Lee, W.S.; Hong, K.W.; Kim, C.D. Suppression of RANKL-induced osteoclast differentiation by cilostazol via SIRT1-induced RANK inhibition. Biochim. Biophys. Acta 2015, 1852, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Han, L.; Iyer, S.; de Cabo, R.; Zhao, H.; O’Brien, C.A.; Manolagas, S.C.; Almeida, M. Sirtuin1 Suppresses Osteoclastogenesis by Deacetylating FoxOs. Mol. Endocrinol. 2015, 29, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Gong, K.; Yang, H.; Li, Y.; Jiang, T.; Zeng, Z.; Cao, Z.; Pan, X. SIRT1 suppresses high glucose and palmitate-induced osteoclast differentiation via deacetylating p66Shc. Mol. Cell Endocrinol. 2018, 474, 97–104. [Google Scholar] [CrossRef]
- Zhang, L.; Bao, D.; Li, P.; Lu, Z.; Pang, L.; Chen, Z.; Guo, H.; Gao, Z.; Jin, Q. Particle-induced SIRT1 downregulation promotes osteoclastogenesis and osteolysis through ER stress regulation. Biomed. Pharmacother. 2018, 104, 300–306. [Google Scholar] [CrossRef]
- Yan, S.; Miao, L.; Lu, Y.; Wang, L. Sirtuin 1 inhibits TNF-α-mediated osteoclastogenesis of bone marrow-derived macrophages through both ROS generation and TRPV1 activation. Mol. Cell. Biochem. 2019, 455, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Xu, H.; Liu, S.; Li, Z.; Zhou, J.; Ding, F.; Zhang, X.; Wang, Y.; Jin, Y.; Wang, Q. Apoptotic extracellular vesicles alleviate Pg-LPS induced inflammatory responses of macrophages via AMPK/SIRT1/NF-κB pathway and inhibit osteoclast formation. J. Periodontol. 2022, 93, 1738–1751. [Google Scholar] [CrossRef]
- Bäckesjö, C.M.; Li, Y.; Lindgren, U.; Haldosén, L.A. Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. Cells Tissues Organs 2009, 189, 93–97. [Google Scholar] [CrossRef]
- Tseng, P.C.; Hou, S.M.; Chen, R.J.; Peng, H.W.; Hsieh, C.F.; Kuo, M.L.; Yen, M.L. Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. J. Bone Min. Res. 2011, 26, 2552–2563. [Google Scholar] [CrossRef]
- Shakibaei, M.; Shayan, P.; Busch, F.; Aldinger, C.; Buhrmann, C.; Lueders, C.; Mobasheri, A. Resveratrol mediated modulation of Sirt-1/Runx2 promotes osteogenic differentiation of mesenchymal stem cells: Potential role of Runx2 deacetylation. PLoS ONE 2012, 7, e35712. [Google Scholar] [CrossRef]
- Simic, P.; Zainabadi, K.; Bell, E.; Sykes, D.B.; Saez, B.; Lotinun, S.; Baron, R.; Scadden, D.; Schipani, E.; Guarente, L. SIRT1 regulates differentiation of mesenchymal stem cells by deacetylating β-catenin. EMBO Mol. Med. 2013, 5, 430–440. [Google Scholar] [CrossRef]
- Yoon, D.S.; Choi, Y.; Jang, Y.; Lee, M.; Choi, W.J.; Kim, S.H.; Lee, J.W. SIRT1 directly regulates SOX2 to maintain self-renewal and multipotency in bone marrow-derived mesenchymal stem cells. Stem Cells 2014, 32, 3219–3231. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Qiao, W.; Zhou, B.; Hu, Z.; Yan, Q.; Wu, J.; Wang, R.; Zhang, Q.; Miao, D. Overexpression of Sirt1 in mesenchymal stem cells protects against bone loss in mice by FOXO3a deacetylation and oxidative stress inhibition. Metabolism 2018, 88, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Ye, C.; Chen, M.; Li, W.; Gao, X.; He, R.; Zheng, Q.; Zhang, W. Bergenin Activates SIRT1 as a Novel Therapeutic Agent for Osteogenesis of Bone Mesenchymal Stem Cells. Front. Pharmacol. 2019, 10, 618. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Gong, K.; Yang, H.S.; Li, Y.G.; Jiang, T.; Zeng, Z.M.; Cao, Z.R.; Pan, X.M. MiR-449 overexpression inhibits osteogenic differentiation of bone marrow mesenchymal stem cells via suppressing Sirt1/Fra-1 pathway in high glucose and free fatty acids microenvironment. Biochem. Biophys. Res. Commun. 2018, 496, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.; Ren, W.; Zhou, Y.; Ye, Z.; Tan, W.S. β-mercaptoethanol promotes osteogenesis of human mesenchymal stem cells via sirt1-ERK pathway. Cytotechnology 2020, 72, 695–706. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Z.; Wu, J.; Mei, Y.; Zhang, Q.; Zhang, H.; Miao, D.; Sun, W. Sirt1 Promotes Osteogenic Differentiation and Increases Alveolar Bone Mass via Bmi1 Activation in Mice. J. Bone Min. Res. 2019, 34, 1169–1181. [Google Scholar] [CrossRef]
- Chen, W.; Chen, X.; Chen, A.C.; Shi, Q.; Pan, G.; Pei, M.; Yang, H.; Liu, T.; He, F. Melatonin restores the osteoporosis-impaired osteogenic potential of bone marrow mesenchymal stem cells by preserving SIRT1-mediated intracellular antioxidant properties. Free Radic. Biol. Med. 2020, 146, 92–106. [Google Scholar] [CrossRef]
- Chen, H.; Liu, X.; Zhu, W.; Chen, H.; Hu, X.; Jiang, Z.; Xu, Y.; Wang, L.; Zhou, Y.; Chen, P.; et al. SIRT1 ameliorates age-related senescence of mesenchymal stem cells via modulating telomere shelterin. Front. Aging Neurosci. 2014, 6, 103. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Ding, Z.; Luo, Q.; Ju, Y.; Song, G. Exogenous NAD(+) Postpones the D-Gal-Induced Senescence of Bone Marrow-Derived Mesenchymal Stem Cells via Sirt1 Signaling. Antioxidants 2021, 10, 254. [Google Scholar] [CrossRef]
- Kou, Y.; Rong, X.; Tang, R.; Zhang, Y.; Yang, P.; Liu, H.; Ma, W.; Li, M. Eldecalcitol prevented OVX-induced osteoporosis through inhibiting BMSCs senescence by regulating the SIRT1-Nrf2 signal. Front. Pharmacol. 2023, 14, 1067085. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, K.; Deng, X.; Li, X. Salvianolic acid C promotes osteogenic differentiation of bone marrow mesenchymal stem cells in osteoporotic rats through activation of AMPK/SIRT1 pathway. Int. J. Rheum. Dis. 2023, 26, 1571–1578. [Google Scholar] [CrossRef]
- Intemann, J.; De Gorter, D.J.J.; Naylor, A.J.; Dankbar, B.; Wehmeyer, C. Importance of osteocyte-mediated regulation of bone remodelling in inflammatory bone disease. Swiss Med. Wkly. 2020, 150, w20187. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Senda, T.; Kubo, K.Y. The osteocyte plays multiple roles in bone remodeling and mineral homeostasis. Med. Mol. Morphol. 2015, 48, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R. The osteocyte: Key player in regulating bone turnover. RMD Open 2015, 1, e000049. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Marcucci, G.; Pierucci, F.; Bruno, G.; Di Cesare Mannelli, L.; Ghelardini, C.; Brandi, M.L.; Iantomasi, T.; Meacci, E.; Vincenzini, M.T. Blueberry juice protects osteocytes and bone precursor cells against oxidative stress partly through SIRT1. FEBS Open Bio 2019, 9, 1082–1096. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Yang, Y.S.; Xie, J.; Lee, O.; Kim, J.; Hong, J.; Boldyreff, B.; Filhol, O.; Chun, H.; Greenblatt, M.B.; et al. Regulation of sclerostin by the SIRT1 stabilization pathway in osteocytes. Cell Death Differ. 2022, 29, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.; Massoumi, R.; Alliston, T. CYLD, a mechanosensitive deubiquitinase, regulates TGFβ signaling in load-induced bone formation. Bone 2020, 131, 115148. [Google Scholar] [CrossRef] [PubMed]
- Rangaswami, H.; Marathe, N.; Zhuang, S.; Chen, Y.; Yeh, J.C.; Frangos, J.A.; Boss, G.R.; Pilz, R.B. Type II cGMP-dependent protein kinase mediates osteoblast mechanotransduction. J. Biol. Chem. 2009, 284, 14796–14808. [Google Scholar] [CrossRef]
- Armstrong, V.J.; Muzylak, M.; Sunters, A.; Zaman, G.; Saxon, L.K.; Price, J.S.; Lanyon, L.E. Wnt/beta-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor alpha. J. Biol. Chem. 2007, 282, 20715–20727. [Google Scholar] [CrossRef]
- Papachroni, K.K.; Karatzas, D.N.; Papavassiliou, K.A.; Basdra, E.K.; Papavassiliou, A.G. Mechanotransduction in osteoblast regulation and bone disease. Trends Mol. Med. 2009, 15, 208–216. [Google Scholar] [CrossRef]
- Kulkarni, R.N.; Bakker, A.D.; Gruber, E.V.; Chae, T.D.; Veldkamp, J.B.; Klein-Nulend, J.; Everts, V. MT1-MMP modulates the mechanosensitivity of osteocytes. Biochem. Biophys. Res. Commun. 2012, 417, 824–829. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Bellido, T. The osteocyte as a signaling cell. Physiol. Rev. 2022, 102, 379–410. [Google Scholar] [CrossRef]
- Tresguerres, F.G.F.; Torres, J.; López-Quiles, J.; Hernández, G.; Vega, J.A.; Tresguerres, I.F. The osteocyte: A multifunctional cell within the bone. Ann. Anat. 2020, 227, 151422. [Google Scholar] [CrossRef]
- Neve, A.; Corrado, A.; Cantatore, F.P. Osteocytes: Central conductors of bone biology in normal and pathological conditions. Acta Physiol. 2012, 204, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shibata, Y.; Zhu, T.; Zhou, J.; Zhang, J. Osteocytes in bone aging: Advances, challenges, and future perspectives. Ageing Res. Rev. 2022, 77, 101608. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Z.; Yang, J.; Wang, Y.; Li, K.; Huang, B.; Yan, B.; Wang, T.; Li, M.; Zou, Z.; et al. Osteocyte TSC1 promotes sclerostin secretion to restrain osteogenesis in mice. Open Biol. 2019, 9, 180262. [Google Scholar] [CrossRef] [PubMed]
- Rawal, K.; Purohit, K.M.; Patel, T.P.; Karont, N.; Gupta, S. Resistin mitigates stemness and metabolic profile of human adipose-derived mesenchymal stem cells via insulin resistance. Cytokine 2021, 138, 155374. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.J.; Zhang, T.N.; Chen, H.H.; Yu, X.F.; Lv, J.L.; Liu, Y.Y.; Liu, Y.S.; Zheng, G.; Zhao, J.Q.; Wei, Y.F.; et al. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar] [PubMed]
- Rasha, F.; Mims, B.M.; Castro-Piedras, I.; Barnes, B.J.; Grisham, M.B.; Rahman, R.L.; Pruitt, K. The Versatility of Sirtuin-1 in Endocrinology and Immunology. Front. Cell Dev. Biol. 2020, 8, 589016. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Zhao, H.; Qin, J.; Qi, H.; Zu, F.; Zhou, Y.; Zhang, Y. gCTRP3 inhibits oophorectomy-induced osteoporosis by activating the AMPK/SIRT1/Nrf2 signaling pathway in mice. Mol. Med. Rep. 2024, 30, 133. [Google Scholar] [CrossRef]
- Ameen, O.; Yassien, R.I.; Naguib, Y.M. Activation of FoxO1/SIRT1/RANKL/OPG pathway may underlie the therapeutic effects of resveratrol on aging-dependent male osteoporosis. BMC Musculoskelet. Disord. 2020, 21, 375. [Google Scholar] [CrossRef]
- Hong, W.; Wei, Z.; Qiu, Z.; Li, Z.; Fu, C.; Ye, Z.; Xu, X. Atorvastatin promotes bone formation in aged apoE(–/–) mice through the Sirt1-Runx2 axis. J. Orthop. Surg. Res. 2020, 15, 303. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Bao, M.; Tang, M.; He, X.; Yao, X.; Li, L. Low magnitude vibration alleviates age-related bone loss by inhibiting cell senescence of osteogenic cells in naturally senescent rats. Aging 2021, 13, 12031–12045. [Google Scholar] [CrossRef]
- Kim, H.N.; Ponte, F.; Warren, A.; Ring, R.; Iyer, S.; Han, L.; Almeida, M. A decrease in NAD(+) contributes to the loss of osteoprogenitors and bone mass with aging. NPJ Aging Mech. Dis. 2021, 7, 8. [Google Scholar] [CrossRef]
- Zeng, J.; Xiao, Q.; Li, X.; Chen, J. Advanced oxidation protein products aggravate age-related bone loss by increasing sclerostin expression in osteocytes via ROS-dependent downregulation of Sirt1. Int. J. Mol. Med. 2021, 47, 108. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Zhang, L.; Yang, X. Ferulic acid, a natural polyphenol, protects against osteoporosis by activating SIRT1 and NF-κB in neonatal rats with glucocorticoid-induced osteoporosis. Biomed. Pharmacother. 2019, 120, 109205. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.X.; Tao, J. Nicotinamide mononucleotide attenuates glucocorticoid-induced osteogenic inhibition by regulating the SIRT1/PGC-1α signaling pathway. Mol. Med. Rep. 2020, 22, 145–154. [Google Scholar] [CrossRef]
- Liu, W.; Li, G.; Li, J.; Chen, W. Long noncoding RNA TRG-AS1 protects against glucocorticoid-induced osteoporosis in a rat model by regulating miR-802-mediated CAB39/AMPK/SIRT-1/NF-κB axis. Hum. Cell 2022, 35, 1424–1439. [Google Scholar] [CrossRef]
- Xiao, J.; Li, W.; Li, G.; Tan, J.; Dong, N. STK11 overexpression prevents glucocorticoid-induced osteoporosis via activating the AMPK/SIRT1/PGC1α axis. Hum. Cell 2022, 35, 1045–1059. [Google Scholar] [CrossRef]
- Jin, C.; Tan, K.; Yao, Z.; Lin, B.H.; Zhang, D.P.; Chen, W.K.; Mao, S.M.; Zhang, W.; Chen, L.; Lin, Z.; et al. A Novel Anti-Osteoporosis Mechanism of VK2: Interfering with Ferroptosis via AMPK/SIRT1 Pathway in Type 2 Diabetic Osteoporosis. J. Agric. Food Chem. 2023, 71, 2745–2761. [Google Scholar] [CrossRef]
- Wang, C.; Chen, R.; Zhu, X.; Zhang, X.; Lian, N. METTL14 alleviates the development of osteoporosis in ovariectomized mice by upregulating m(6)A level of SIRT1 mRNA. Bone 2023, 168, 116652. [Google Scholar] [CrossRef] [PubMed]
- Artsi, H.; Cohen-Kfir, E.; Gurt, I.; Shahar, R.; Bajayo, A.; Kalish, N.; Bellido, T.M.; Gabet, Y.; Dresner-Pollak, R. The Sirtuin1 activator SRT3025 down-regulates sclerostin and rescues ovariectomy-induced bone loss and biomechanical deterioration in female mice. Endocrinology 2014, 155, 3508–3515. [Google Scholar] [CrossRef] [PubMed]
- Mercken, E.M.; Mitchell, S.J.; Martin-Montalvo, A.; Minor, R.K.; Almeida, M.; Gomes, A.P.; Scheibye-Knudsen, M.; Palacios, H.H.; Licata, J.J.; Zhang, Y.; et al. SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell 2014, 13, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.X.; van Tits, L.J.; Lohmann, C.; Arsiwala, T.; Winnik, S.; Tailleux, A.; Stein, S.; Gomes, A.P.; Suri, V.; Ellis, J.L.; et al. The Sirt1 activator SRT3025 provides atheroprotection in Apoe-/- mice by reducing hepatic Pcsk9 secretion and enhancing Ldlr expression. Eur. Heart J. 2015, 36, 51–59. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, A.J.; Scicluna, B.P.; Moerland, P.D.; Lin, J.; Jacobson, E.W.; Vlasuk, G.P.; van der Poll, T. The Selective Sirtuin 1 Activator SRT2104 Reduces Endotoxin-Induced Cytokine Release and Coagulation Activation in Humans. Crit. Care Med. 2015, 43, e199–e202. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.M.; Shandala, T.; Nguyen, L.; Muhlhausler, B.S.; Chen, K.M.; Howe, P.R.; Xian, C.J. Effects of resveratrol supplementation on bone growth in young rats and microarchitecture and remodeling in ageing rats. Nutrients 2014, 6, 5871–5887. [Google Scholar] [CrossRef]
- Feng, J.; Liu, S.; Ma, S.; Zhao, J.; Zhang, W.; Qi, W.; Cao, P.; Wang, Z.; Lei, W. Protective effects of resveratrol on postmenopausal osteoporosis: Regulation of SIRT1-NF-κB signaling pathway. Acta Biochim. Biophys. Sin. 2014, 46, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Gambino, R.; Ponzo, V.; Cioffi, I.; Goitre, I.; Evangelista, A.; Ciccone, G.; Cassader, M.; Procopio, M. Effects of resveratrol on bone health in type 2 diabetic patients. A double-blind randomized-controlled trial. Nutr. Diabetes 2018, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Luo, W.; Wang, B.; Wang, X.; Gong, P.; Xiong, Y. Resveratrol promotes osteogenesis via activating SIRT1/FoxO1 pathway in osteoporosis mice. Life Sci. 2020, 246, 117422. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Xiao, H.; Liu, Z.; Teng, F.; Yang, A.; Geng, B.; Sheng, X.; Xia, Y. Sirt1: An Increasingly Interesting Molecule with a Potential Role in Bone Metabolism and Osteoporosis. Biomolecules 2024, 14, 970. https://doi.org/10.3390/biom14080970
Chen Y, Xiao H, Liu Z, Teng F, Yang A, Geng B, Sheng X, Xia Y. Sirt1: An Increasingly Interesting Molecule with a Potential Role in Bone Metabolism and Osteoporosis. Biomolecules. 2024; 14(8):970. https://doi.org/10.3390/biom14080970
Chicago/Turabian StyleChen, Yi, Hefang Xiao, Zirui Liu, Fei Teng, Ao Yang, Bin Geng, Xiaoyun Sheng, and Yayi Xia. 2024. "Sirt1: An Increasingly Interesting Molecule with a Potential Role in Bone Metabolism and Osteoporosis" Biomolecules 14, no. 8: 970. https://doi.org/10.3390/biom14080970
APA StyleChen, Y., Xiao, H., Liu, Z., Teng, F., Yang, A., Geng, B., Sheng, X., & Xia, Y. (2024). Sirt1: An Increasingly Interesting Molecule with a Potential Role in Bone Metabolism and Osteoporosis. Biomolecules, 14(8), 970. https://doi.org/10.3390/biom14080970





