Comparative Cardioprotective Effectiveness: NOACs vs. Nattokinase—Bridging Basic Research to Clinical Findings
Abstract
1. Introduction
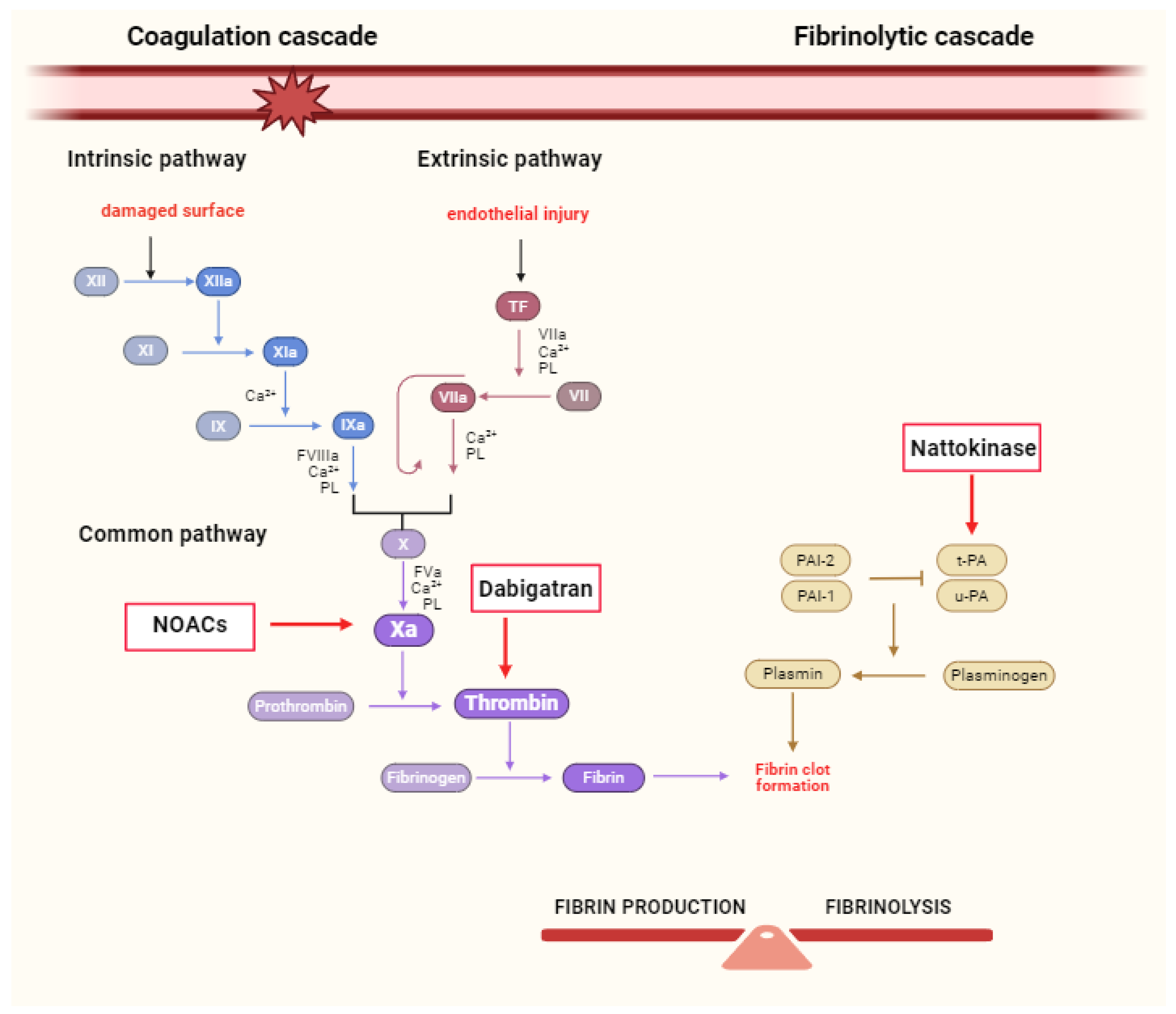
2. The Role of NOACs in Coagulation Cascade
2.1. Inhibition of FXa as a Therapeutic Strategy
2.2. Thrombin Inhibition as a Therapeutic Strategy
3. NOACs through the Scope of Large Clinical Trials
4. Nattokinase—A Promising Agent for CVD Treatment
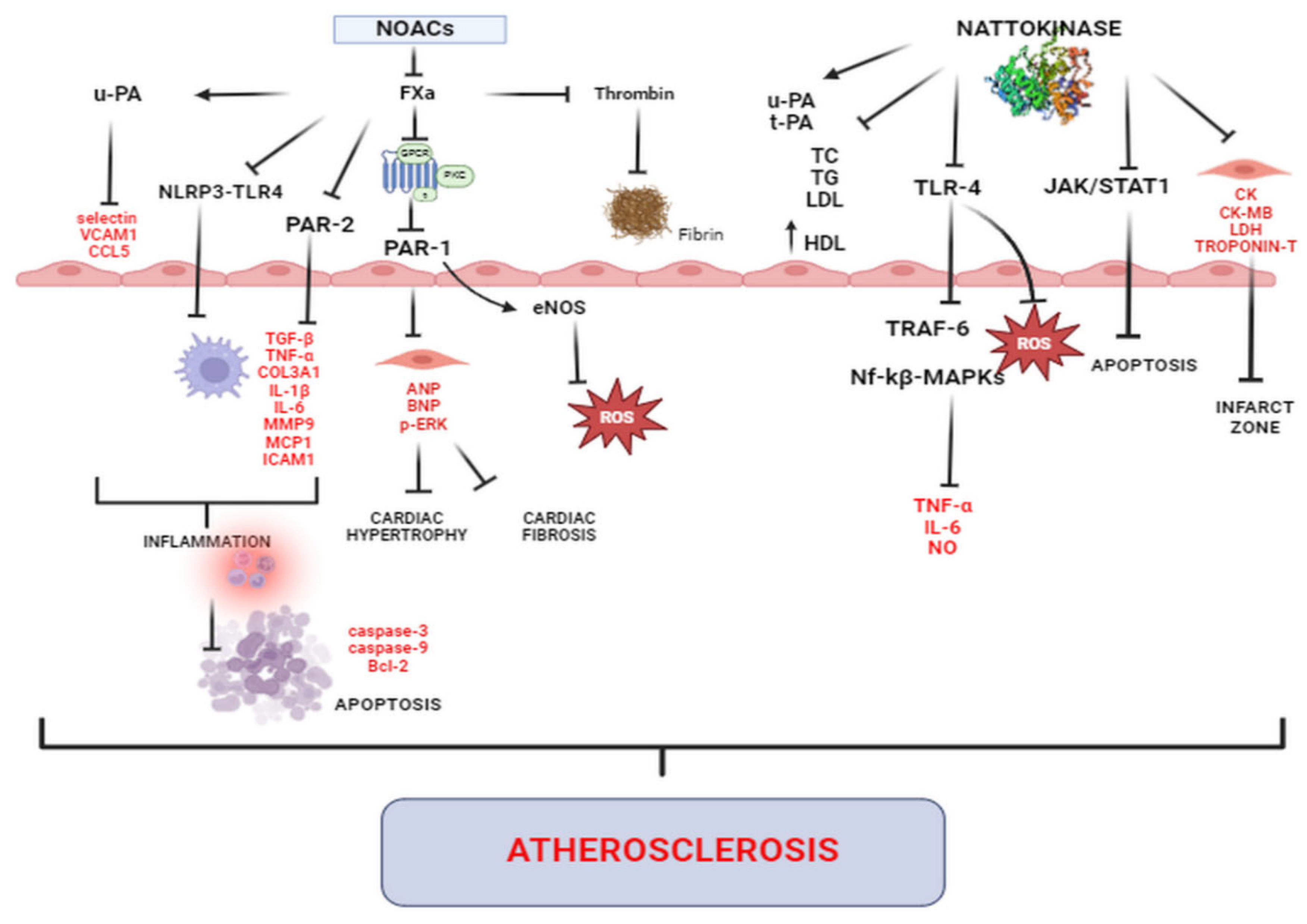
5. Cardioprotection beyond Anticoagulation—Evidence from Basic and Clinical Research
5.1. Effects on Myocardial Structure and Function
5.2. Antihypertensive Effects
5.3. Antifibrotic and Antihypertrophic Effects
5.4. Anti-Inflammatory, Anti-Atherosclerotic Effects and Effects on Vasculature and Endothelial Function
5.5. Antioxidative Effects
5.6. Anti-Apoptotic Effects
6. Concluding Remarks and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farinha, J.M.; Jones, I.D.; Lip, G.Y.H. Optimizing adherence and persistence to non-vitamin K antagonist oral anticoagulant therapy in atrial fibrillation. Eur. Heart. J. Suppl. 2022, 24 (Suppl. A), A42–A55. [Google Scholar] [CrossRef]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart. J 2016, 37, 2893–2962. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart. J 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Polzin, A.; Dannenberg, L.; Thienel, M.; Orban, M.; Wolff, G.; Hohlfeld, T.; Zeus, T.; Kelm, M.; Petzold, T. Noncanonical Effects of Oral Thrombin and Factor Xa Inhibitors in Platelet Activation and Arterial Thrombosis. Thromb. haemost 2021, 121, 122–130. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Cannon, C.P.; Bhatt, D.L.; Oldgren, J.; Lip, G.Y.H.; Ellis, S.G.; Kimura, T.; Maeng, M.; Merkely, B.; Zeymer, U.; Gropper, S.; et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N. Engl. J. Med. 2017, 377, 1513–1524. [Google Scholar] [CrossRef]
- Artang, R.; Rome, E.; Nielsen, J.D.; Vidaillet, H.J. Meta-analysis of randomized controlled trials on risk of myocardial infarction from the use of oral direct thrombin inhibitors. Am. J. Cardiol. 2013, 112, 1973–1979. [Google Scholar] [CrossRef]
- Weng, Y.; Yao, J.; Sparks, S.; Wang, K.Y. Nattokinase: An Oral Antithrombotic Agent for the Prevention of Cardiovascular Disease. Int. J. Mol. Sci. 2017, 18, 523. [Google Scholar] [CrossRef]
- Sumi, H.; Hamada, H.; Tsushima, H.; Mihara, H.; Muraki, H. A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto; a typical and popular soybean food in the Japanese diet. Experientia 1987, 43, 1110–1111. [Google Scholar] [CrossRef]
- Yang, M.; Mei, Y.; Liang, Y. Effect of nattokinase extraction on anti-thrombosis function. Food. Sci. Technol. 2013, 38, 197–200. [Google Scholar]
- Kurosawa, Y.; Nirengi, S.; Homma, T.; Esaki, K.; Ohta, M.; Clark, J.F.; Hamaoka, T. A single-dose of oral nattokinase potentiates thrombolysis and anti-coagulation profiles. Sci. Rep. 2015, 5, 11601. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; McGowan, E.M.; Ren, N.; Lal, S.; Nassif, N.; Shad-Kaneez, F.; Qu, X.; Lin, Y. Nattokinase: A Promising Alternative in Prevention and Treatment of Cardiovascular Diseases. Biomark. Insights 2018, 13, 1177271918785130. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Toh, C.H. Rethinking coagulation: From enzymatic cascade and cell-based reactions to a convergent model involving innate immune activation. Blood 2023, 142, 2133–2145. [Google Scholar] [CrossRef] [PubMed]
- Green, D. Coagulation cascade. Hemodial. Int. 2006, 10, S2–S4. [Google Scholar] [CrossRef] [PubMed]
- Furie, B.; Furie, B.C. The molecular basis of blood coagulation. Cell 1988, 53, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Posma, J.J.; Grover, S.P.; Hisada, Y.; Owens, A.P., 3rd; Antoniak, S.; Spronk, H.M.; Mackman, N. Roles of Coagulation Proteases and PARs (Protease-Activated Receptors) in Mouse Models of Inflammatory Diseases. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Mackman, N.; Bergmeier, W.; Stouffer, G.A.; Weitz, J.I. The multifaceted role of the coagulation cascade in the regulation of angiogenesis. Nat. Rev. Mol. Cell Biol. 2021, 22, 190–205. [Google Scholar]
- Tjarnlund-Wolf, A.; Brogren, H.; Lo, E.H.; Wang, X. Plasminogen activator inhibitor-1 and thrombotic cerebrovascular diseases. Stroke 2012, 43, 2833–2839. [Google Scholar] [CrossRef] [PubMed]
- Mihalko, E.; Brown, A.C. Clot Structure and Implications for Bleeding and Thrombosis. Semin. Thrombosis Hemost. 2020, 46, 96–104. [Google Scholar] [CrossRef]
- Camire, R.M. Blood coagulation factor X: Molecular biology, inherited disease, and engineered therapeutics. J. Thromb. Thrombolysis 2021, 52, 383–390. [Google Scholar] [CrossRef]
- Hertzberg, M. Biochemistry of factor X. Blood. Rev. 1994, 8, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.G.; Nesheim, M.E.; Church, W.R.; Haley, P.; Krishnaswamy, S. Surface-dependent reactions of the vitamin K-dependent enzyme complexes. Blood 1990, 76, 1–16. [Google Scholar] [CrossRef]
- Mackman, N.; Tilley, R.E.; Key, N.S. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1687–1693. [Google Scholar] [CrossRef] [PubMed]
- Kakar, P.; Watson, T.; Lip, G.Y.H. Drug evaluation: Rivaroxaban, an oral, direct inhibitor of activated factor X. Curr. Opin. Investig. Drugs 2007, 8, 256–265. [Google Scholar] [PubMed]
- Kubitza, D.; Becka, M.; Voith, B.; Zuehlsdorf, M.; Wensing, G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin. Pharmacol. Ther. 2005, 78, 412–421. [Google Scholar] [CrossRef]
- Jiménez, D.; Yusen, R.D.; Ramacciotti, E. Apixaban: An oral direct factor-xa inhibitor. Adv. Ther. 2012, 29, 187–201. [Google Scholar] [CrossRef]
- Byon, W.; Garonzik, S.; Boyd, R.A.; Frost, C.E. Apixaban: A Clinical Pharmacokinetic and Pharmacodynamic Review. Clin. Pharmacokinet. 2019, 58, 1265–1279. [Google Scholar] [CrossRef]
- Raghavan, N.; Frost, C.E.; Yu, Z.; He, K.; Zhang, H.; Humphreys, W.G.; Pinto, D.; Chen, S.; Bonacorsi, S.; Wong, P.C.; et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug. Metab. Dispos. 2009, 37, 74–81. [Google Scholar] [CrossRef]
- Fur Furugohri, T.; Isobe, K.; Honda, Y.; Kamisato-Matsumoto, C.; Sugiyama, N.; Nagahara, T.; Morishima, Y.; Shibano, T. DU-176b, a potent and orally active factor Xa inhibitor: In vitro and in vivo pharmacological profiles. J. Thromb. Haemost. 2008, 6, 1542–1549. [Google Scholar] [CrossRef]
- Stacy, Z.A.; Call, W.B.; Hartmann, A.P.; Peters, G.L.; Richter, S.K. Edoxaban: A Comprehensive Review of the Pharmacology and Clinical Data for the Management of Atrial Fibrillation and Venous Thromboembolism. Cardiol. Ther. 2016, 5, 1–18. [Google Scholar] [CrossRef]
- Hughes, G.J.; Hilas, O. Edoxaban: An investigational factor Xa inhibitor. Pharm. Therapeutics 2014, 39, 686–715. [Google Scholar]
- Lee, C.J.; Ansell, J.E. Direct thrombin inhibitors. Br. J. Clin. Pharmacol. 2011, 72, 581–592. [Google Scholar] [CrossRef]
- Keisu, M.; Andersson, T.B. Drug-induced liver injury in humans: The case of ximelagatran. Handb. Exp. Pharmacol. 2010, 196, 407–418. [Google Scholar]
- Blech, S.; Ebner, T.; Ludwig-Schwellinger, E.; Stangier, J.; Roth, W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug. Metab. Dispos. 2008, 36, 386–399. [Google Scholar] [CrossRef]
- Hammwöhner, M.; Goette, A. Ten years of non-vitamin K antagonists oral anticoagulants for stroke prevention in atrial fibrillation: Is warfarin obsolete? Eur. Heart. J. Suppl. 2020, 22, O28–O41. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef]
- Schulman, S.; Kearon, C.; Kakkar, A.K.; Mismetti, P.; Schellong, S.; Eriksson, H.; Baanstra, D.; Schnee, J.; Goldhaber, S.Z.; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N. Engl. J. Med. 2009, 361, 2342–2352. [Google Scholar] [CrossRef]
- Bauersachs, R.; Berkowitz, S.D.; Brenner, B.; Buller, H.R.; Decousus, H.; Gallus, A.S.; Lensing, A.W.; Misselwitz, F.; Prins, M.H.; Raskob, G.E.; et al. Oral rivaroxaban for symptomatic venous thromboembolism. N. Engl. J. Med. 2010, 363, 2499–2510. [Google Scholar]
- Cohen, A.T.; Bauersachs, R. Rivaroxaban and the EINSTEIN clinical trial programme. Blood. Coagul. Fibrinolysis 2019, 30, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Büller, H.R.; Décousus, H.; Grosso, M.A.; Mercuri, M.; Middeldorp, S.; Prins, M.H.; Raskob, G.E.; Schellong, S.M.; Schwocho, L.; Segers, A.; et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N. Engl. J. Med. 2013, 369, 1406–1415. [Google Scholar] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; BlomstromLundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of CardioThoracic Surgery (EACTS). Eur. Heart. J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- da Silva, R.M. Novel oral anticoagulants in non-valvular atrial fibrillation. Cardiovasc. Hematol. Agents. Med. Chem. 2014, 12, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Giugliano, R.P.; De Caterina, R. Non-Vitamin K Antagonist Oral Anticoagulants for Mechanical Heart Valves: Is the Door Still Open? Circulation 2018, 138, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Ingason, A.B.; Hreinsson, J.P.; Agustsson, A.S.; Lund, S.H.; Rumba, E.; Palsson, D.A.; Reynisson, I.E.; Gudmundsdottir, B.R.; Onundarson, P.T.; Bjornsson, E.S. Comparison of the effectiveness and safety of direct oral anticoagulants: A nationwide propensity score-weighted study. Blood Adv. 2023, 7, 2564–2572. [Google Scholar] [CrossRef] [PubMed]
- Mujer, M.T.P.; Rai, M.P.; Atti, V.; Dimaandal, I.L.; Chan, A.S.; Shrotriya, S.; Gundabolu, K.; Dhakal, P. An Update on the Reversal of Non-Vitamin K Antagonist Oral Anticoagulants. Adv. Hematol. 2020, 2020, 7636104. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.B.; Pahade, A.; Chawla, R. Novel reversal agents and laboratory evaluation for direct-acting oral anticoagulants (DOAC): An update. Indian. J. Anaesth. 2019, 63, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Wada, K.; Tamura, T.; Konishi, K.; Goto, Y.; Koda, S.; Kawachi, T.; Tsuji, M.; Nakamura, K. Dietary soy and natto intake and cardiovascular disease mortality in Japanese adults: The Takayama study. Am. J. Clin. Nutr. 2017, 105, 426–431. [Google Scholar] [CrossRef]
- Fujita, M.; Hong, K.; Ito, Y.; Fujii, R.; Kariya, K.; Nishimuro, S. Thrombolytic effect of nattokinase on a chemically induced thrombosis model in rat. Biol. Pharm. Bull. 1995, 18, 1387–1391. [Google Scholar] [CrossRef]
- Sumi, H.; Yanagisawa, Y.; Yatagai, C.; Saito, J. Natto Bacillus as an oral fibrinolytic agent: Nattokinase activity and the ingestion effect of Bacillus subtilis natto. Food. Sci. Technol. Res. 2004, 10, 17–20. [Google Scholar] [CrossRef]
- Kamiya, S.; Hagimori, M.; Ogasawara, M.; Arakawa, M. In vivo evaluation method of the effect of nattokinase on carrageenan-induced tail thrombosis in a rat model. Acta. Haematol. 2010, 124, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Omura, K.; Hitosugi, M.; Zhu, X.; Ikeda, M.; Maeda, H.; Tokudome, S. A newly derived protein from Bacillus subtilis natto with both antithrombotic and fibrinolytic effects. J. Pharmacol. Sci. 2005, 99, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Urano, T.; Ihara, H.; Umemura, K.; Suzuki, Y.; Oike, M.; Akita, S.; Tsukamoto, Y.; Suzuki, I.; Takada, A. The profibrinolytic enzyme subtilisin NAT purified from Bacillus subtilis cleaves and inactivates plasminogen activator inhibitor type 1. J. Biol. Chem. 2001, 276, 24690–24696. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Hou, L.; Hu, M.; Gao, Y.; Tian, Z.; Fan, B.; Li, S.; Wang, F. Recent Advances in Nattokinase-Enriched Fermented Soybean Foods: A Review. Foods 2022, 11, 1867. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kim, T.S.; Cai, J.; Kim, J.; Kim, Y.; Shin, K.; Kim, K.S.; Park, S.K.; Lee, S.P.; Choi, E.K.; et al. Nattokinase improves blood flow by inhibiting platelet aggregation and thrombus formation. Lab. Anim, Res. 2013, 29, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Hsia, C.H.; Shen, M.C.; Lin, J.S.; Wen, Y.K.; Hwang, K.L.; Cham, T.M.; Yang, N.C. Nattokinase decreases plasma levels of fibrinogen, factor VII, and factor VIII in human subjects. Nutr. Res. 2009, 29, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Yuan, B.; Wang, M.; Liu, J.; Wang, Z. Nattokinase: Insights into Biological Activity, Therapeutic Applications, and the Influence of Microbial Fermentation. Fermentation 2023, 9, 950. [Google Scholar] [CrossRef]
- Oba, M.; Rongduo, W.; Saito, A.; Okabayashi, T.; Yokota, T.; Yasuoka, J.; Sato, Y.; Nishifuji, K.; Wake, H.; Nibu, Y.; et al. Natto extract, a Japanese fermented soybean food, directly inhibits viral infections including SARS-CoV-2 in vitro. Biochem. Biophys. Res. Commun. 2021, 570, 21–25. [Google Scholar] [CrossRef]
- Tanikawa, T.; Kiba, Y.; Yu, J.; Hsu, K.; Chen, S.; Ishii, A.; Yokogawa, T.; Suzuki, R.; Inoue, Y.; Kitamura, M. Degradative Effect of Nattokinase on Spike Protein of SARS-CoV-2. Molecules 2022, 27, 5405. [Google Scholar] [CrossRef]
- Nakanishi, N.; Kaikita, K.; Ishii, M.; Oimatsu, Y.; Mitsuse, T.; Ito, M.; Yamanaga, K.; Fujisue, K.; Kanazawa, H.; Sueta, D.; et al. Cardioprotective Effects of Rivaroxaban on Cardiac Remodeling After Experimental Myocardial Infarction in Mice. Circ. Rep. 2020, 2, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, Z.; Chen, W.; Zheng, H.; Si, D.; Zhang, W. Rivaroxaban, a direct inhibitor of coagulation factor Xa, attenuates adverse cardiac remodeling in rats by regulating the PAR-2 and TGF-β1 signaling pathways. PeerJ 2023, 11, e16097. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Kolpakov, M.A.; Hooshdaran, B.; Schappell, W.; Wang, T.; Eguchi, S.; Elliott, K.J.; Tilley, D.G.; Rao, A.K.; Andrade-Gordon, P.; et al. Cardiac Expression of Factor X Mediates Cardiac Hypertrophy and Fibrosis in Pressure Overload. JACC. Basic. Transl. Sci. 2020, 5, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Bode, M.F.; Auriemma, A.C.; Grover, S.P.; Hisada, Y.; Rennie, A.; Bode, W.D.; Vora, R.; Subramaniam, S.; Cooley, B.; Andrade-Gordon, P.; et al. The factor Xa inhibitor rivaroxaban reduces cardiac dysfunction in a mouse model of myocardial infarction. Thromb. Res. 2018, 167, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Mueller, P.; Yang, F.; Yang, L.; Morris, A.; Smyth, S.S. Direct thrombin inhibition with dabigatran attenuates pressure overload-induced cardiac fibrosis and dysfunction in mice. Thromb. Res. 2017, 159, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernández, J.; Almengló, C.; Babarro, B.; Iglesias-Rey, R.; García-Caballero, T.; Fernández, Á.L.; Souto-Bayarri, M.; González-Juanatey, J.R.; Álvarez, E. Edoxaban treatment in a post-infarction experimental model. Eur. J. Pharmacol. 2024, 962, 176216. [Google Scholar] [CrossRef] [PubMed]
- Tsujino, Y.; Sakamoto, T.; Kinoshita, K.; Nakatani, Y.; Yamaguchi, Y.; Kataoka, N.; Nishida, K.; Kinugawa, K. Edoxaban suppresses the progression of atrial fibrosis and atrial fibrillation in a canine congestive heart failure model. Heart Vessels 2019, 34, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Yang, X.; Pan, M.; Sun, J.; Ke, H.; Zhang, C.; Geng, H. Apixaban attenuates ischemia-induced myocardial fibrosis by inhibition of Gq/PKC signaling. BBRC 2018, 500, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Abe, I.; Fukui, A.; Saito, S.; Miyoshi, M.; Aoki, K.; Shinohara, T.; Teshima, Y.; Yufu, K.; Takahashi, N. Possible role of rivaroxaban in attenuating pressure-overload-induced atrial fibrosis and fibrillation. J. Cardiol. 2018, 71, 310–319. [Google Scholar] [CrossRef]
- Gencpinar, T.; Bilen, C.; Kemahli, B.; Kacar, K.; Akokay, P.; Bayrak, S.; Erdal, C. Effects of rivaroxaban on myocardial mitophagy in the rat heart. Turk. Gogus. Kalp. Damar. Cerrahisi. Derg. 2023, 31, 301–308. [Google Scholar] [CrossRef]
- Tran, H.T.; Mai, T.P.; Nguyen, L.H.; Nguyen, T.H.; Bui, S.S.; Van Vu, A.; Do, H.T.; Trinh, Q.V. Myocardial infarction model induced by isoproterenol in rats and potential cardiovascular protective effect of a nattokinase-containing hard capsule. Phytomed. Plus 2023, 3, 100472. [Google Scholar]
- Lee, B.H.; Lai, Y.S.; Wu, S.C. Antioxidation, angiotensin converting enzyme inhibition activity, nattokinase, and antihypertension of Bacillus subtilis (natto)-fermented pigeon pea. J. Food. Drug. Anal. 2015, 23, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kondo, K.; Matsumoto, Y.; Zhao, B.Q.; Otsuguro, K.; Maeda, T.; Tsukamoto, Y.; Urano, T.; Umemura, K. Dietary supplementation of fermented soybean, natto, suppresses intimal thickening and modulates the lysis of mural thrombi after endothelial injury in rat femoral artery. Life. Sci. 2003, 73, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K.; Nakaya, N.; Kawasaki, Y.; Matsue, H. Antioxidative functions of natto, a kind of fermented soybeans: Effect on LDL oxidation and lipid metabolism in cholesterol-fed rats. J. Agric. Food. Chem. 2002, 50, 3597–3601. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, Y.; Zhang, Y.; Xu, F.; Chen, J.; Duan, L.; Zhang, T.; Wang, J.; Zhang, F. Breaking the vicious loop between inflammation, oxidative stress and coagulation, a novel anti-thrombus insight of nattokinase by inhibiting LPS-induced inflammation and oxidative stress. Redox. Biol. 2020, 32, 101500. [Google Scholar] [CrossRef] [PubMed]
- Shiraga, S.; Adamus, G. Mechanism of CAR syndrome: Anti-recoverin antibodies are the inducers of retinal cell apoptotic death via the caspase 9- and caspase 3-dependent pathway. J. Neuroimmunol. 2002, 132, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Yu, L.; Liu, K.; Yu, Z.; Zhang, Q.; Zou, F.; Liu, B. Mechanisms of Nattokinase in protection of cerebral ischemia. Eur. J. Pharmacol. 2014, 745, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Douxfils, J.; Buckinx, F.; Mullier, F.; Minet, V.; Rabenda, V.; Reginster, J.Y.; Hainaut, P.; Bruyère, O.; Dogné, J.M. Dabigatran etexilate and risk of myocardial infarction, other cardiovascular events, major bleeding, and all-cause mortality: A systematic review and meta-analysis of randomized controlled trials. J. Am. Heart. Assoc. 2014, 3, e000515. [Google Scholar] [CrossRef]
- Carmo, J.; Moscoso Costa, F.; Ferreira, J.; Mendes, M. Dabigatran in real-world atrial fibrillation. Meta-analysis of observational comparison studies with vitamin K antagonists. Thromb. Haemost. 2016, 116, 754–763. [Google Scholar]
- Chan, Y.H.; Kuo, C.T.; Yeh, Y.H.; Chang, S.H.; Wu, L.S.; Lee, H.F.; Tu, H.T.; See, L.C. Thromboembolic, Bleeding, and Mortality Risks of Rivaroxaban and Dabigatran in Asians with Nonvalvular Atrial Fibrillation. J. Am. Coll. Cardiol. 2016, 68, 1389–1401. [Google Scholar] [CrossRef]
- Graham, D.J.; Reichman, M.E.; Wernecke, M.; Hsueh, Y.H.; Izem, R.; Southworth, M.R.; Wei, Y.; Liao, J.; Goulding, M.R.; Mott, K.; et al. Stroke, bleeding, and mortality risks in elderly Medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern. Med. 2016, 176, 1662–1671. [Google Scholar] [CrossRef] [PubMed]
- Blin, P.; Dureau-Pournin, C.; Cottin, Y.; Bénichou, J.; Mismetti, P.; Abouelfath, A.; Lassalle, R.; Droz, C.; Moore, N. Comparative Effectiveness and Safety of Standard or Reduced Dose Dabigatran vs. Rivaroxaban in Nonvalvular Atrial Fibrillation. Clin. Pharmacol. Ther. 2019, 105, 1439–1455. [Google Scholar] [CrossRef] [PubMed]
- Achilles, A.; Mohring, A.; Dannenberg, L.; Grandoch, M.; Hohlfeld, T.; Fischer, J.W.; Levkau, B.; Kelm, M.; Zeus, T.; Polzin, A. Dabigatran enhances platelet reactivity and platelet thrombin receptor expression in patients with atrial fibrillation. J. Thromb. Haemost. 2017, 15, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Olivier, C.B.; Weik, P.; Meyer, M.; Weber, S.; Anto-Michel, N.; Diehl, P.; Zhou, Q.; Geisen, U.; Bode, C.; Moser, M. TRAP-induced platelet aggregation is enhanced in cardiovascular patients receiving dabigatran. Thromb. Res. 2016, 138, 63–68. [Google Scholar] [CrossRef]
- Petzold, T.; Thienel, M.; Konrad, I.; Schubert, I.; Regenauer, R.; Hoppe, B.; Lorenz, M.; Eckart, A.; Chandraratne, S.; Lennerz, C.; et al. Oral thrombin inhibitor aggravates platelet adhesion and aggregation during arterial thrombosis. Sci. Transl. Med. 2016, 8, 367ra168. [Google Scholar] [CrossRef] [PubMed]
- Antoniak, S.; Pawlinski, R.; Mackman, N. Protease-activated receptors and myocardial infarction. IUBMB. Life 2011, 63, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Antoniak, S.R.M.; Spring, D.; Bullard, T.A.; Verrier, E.D.; Blaxall, B.C.; Mackman, N.; Pawlinski, R. Protease-activated receptor 2 deficiency reduces cardiac ischemia/reperfusion injury. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2136–2142. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Ramachandran, R.; Hollenberg, M.D.; Muruve, D.A. Proteinase-activated receptor-2 transactivation of epidermal growth factor receptor and transforming growth factor-beta receptor signaling pathways contributes to renal fibrosis. J. Biol. Chem. 2013, 288, 37319–37331. [Google Scholar] [CrossRef]
- Witte, D.; Zeeh, F.; Gadeken, T.; Gieseler, F.; Rauch, B.H.; Settmacher, U.; Kaufmann, R.; Lehnert, H.; Ungefroren, H. Proteinase-activated receptor 2 is a novel regulator of TGF-beta signaling in pancreatic cancer. J. Clin. Med. 2016, 5, 111. [Google Scholar] [CrossRef]
- Narita, M.; Hanada, K.; Kawamura, Y.; Ichikawa, H.; Sakai, S.; Yokono, Y.; Senoo, M.; Narita, N.; Shimada, M.; Osanai, T.; et al. RIV attenuates cardiac hypertrophy by inhibiting protease-activated receptor-2 signaling in renin-overexpressing hypertensive mice. Hypertens. Res. 2021, 44, 1261–1273. [Google Scholar] [CrossRef]
- Song, K.; Wang, Y.; Sheng, J.; Ma, C.; Li, H. Effects of dabigatran regulates no-reflow phenomenon in acute myocardial infarction mice through anti-inflammatory and anti-oxidative activities and connective tissue growth factor expression. Mol. Med. Rep. 2018, 17, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Delbeck, M.; Nickel, K.F.; Perzborn, E.; Ellinghaus, P.; Strassburger, J.; Kast, R.; Laux, V.; Schäfer, S.; Schermuly, R.T.; von Degenfeld, G. A role for coagulation factor Xa in experimental pulmonary arterial hypertension. Cardiovasc. Res. 2011, 92, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Yurista, S.R.; Silljé, H.H.W.; Nijholt, K.T.; Dokter, M.M.; van Veldhuisen, D.J.; de Boer, R.A.; Westenbrink, B.D. Factor Xa Inhibition with Apixaban Does Not Influence Cardiac Remodelling in Rats with Heart Failure After Myocardial Infarction. Cardiovasc. Drug. Ther. 2021, 35, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Grajek, S.; Kałużna-Oleksy, M.; Siller-Matula, J.M.; Grajek, M.; Michalak, M. Non-Vitamin K Antagonist Oral Anticoagulants and Risk of Myocardial Infarction in Patients with Atrial Fibrillation with or without Percutaneous Coronary Interventions: A Meta-Analysis. J. Pers. Med. 2021, 11, 1013. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; White, H.D.; Jaffe, A.S.; Katus, H.A.; Apple, F.S.; Lindahl, B.; Morrow, D.A.; Chaitman, B.A.; Clemmensen, P.M.; et al. Third universal definition of myocardial infarction. Eur. Heart. J. 2012, 33, 2551–2567. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.K.; Bergheanu, S.C.; Hasan-Ali, H.; Liem, S.S.; van der Laarse, A.; Wolterbeek, R.; Atsma, D.E.; Schalij, M.J.; Jukema, J.W. Usefulness of peak troponin-T to predict infarct size and long-term outcome in patients with first acute myocardial infarction after primary percutaneous coronary intervention. Am. J. Cardiol. 2009, 103, 779–784. [Google Scholar] [CrossRef]
- Sechi, L.A.; Novello, M.; Colussi, G.; Di Fabio, A.; Chiuch, A.; Nadalini, E.; Casanova-Borca, A.; Uzzau, A.; Catena, C. Relationship of plasma renin with a prothrombotic state in hypertension: Relevance for organ damage. Am. J. Hypertens. 2008, 21, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Sechi, L.A.; Zingaro, L.; Catena, C.; Casaccio, D.; De Marchi, S. Relationship of fibrinogen levels and hemostatic abnormalities with organ damage in hypertension. Hypertension 2000, 36, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Bager, J.E.; Hjerpe, P.; Schiöler, L.; Bengtsson Boström, K.; Kahan, T.; Ödesjö, H.; Jood, K.; Hasselström, J.; Ljungman, C.; Manhem, K.; et al. Blood pressure levels and risk of haemorrhagic stroke in patients with atrial fibrillation and oral anticoagulants: Results from The Swedish Primary Care Cardiovascular Database of Skaraborg. J. Hypertens. 2021, 39, 1670–1677. [Google Scholar] [CrossRef]
- Vilaseca, M.; García-Calderó, H.; Lafoz, E.; García-Irigoyen, O.; Avila, M.A.; Reverter, J.C.; Bosch, J.; Hernández-Gea, V.; Gracia-Sancho, J.; García-Pagán, J.C. The anticoagulant rivaroxaban lowers portal hypertension in cirrhotic rats mainly by deactivating hepatic stellate cells. Hepatology 2017, 65, 2031–2044. [Google Scholar] [CrossRef]
- Sanchez, A.P.; Turon, F.; Martinez-Gonzalez, J.; Fortea, J.I.; Hernandez-Guerra, M.; Alvardo-Tapias, E.A.; Pons, M.; Magaz, M.; Elba, L.H.; Alvarez-Navascues, C.; et al. Rivaroxaban improves survival and decompensation in cirrhotic patients with moderate liver dysfunction: Double-oblind, placebo-controlled trial. J. Hepatol. 2023, 78, S2–S3. [Google Scholar] [CrossRef]
- Ichikawa, H.; Shimada, M.; Narita, M.; Narita, I.; Kimura, Y.; Tanaka, M.; Osanai, T.; Okumura, K.; Tomita, H. Rivaroxaban, a Direct Factor Xa Inhibitor, Ameliorates Hypertensive Renal Damage Through Inhibition of the Inflammatory Response Mediated by Protease-Activated Receptor Pathway. J. Am. Heart. Assoc. 2019, 8, e012195. [Google Scholar] [CrossRef] [PubMed]
- Ware, K.M.; Vance, J.C.; Muni, N.; Hebert, L.A.; Satoskar, A.A.; Nadasdy, G.; Ivanov, I.; Nadasdy, T.; Rovin, B.H.; Brodsky, S.V. Oral warfarin and the thrombin inhibitor dabigatran increase blood pressure in rats: Hidden danger of anticoagulants? Am. J. Hypertens. 2015, 28, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Joung, H.J.; Lee, J.M.; Woo, J.S.; Kim, W.S.; Kim, K.S.; Lee, K.H.; Kim, W. Evaluation of the vascular protective effects of new oral anticoagulants in high-risk patients with atrial fibrillation (PREFER-AF): Study protocol for a randomized controlled trial. Trials 2016, 17, 422. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Volpe, M.; Savoia, C. Endothelial Dysfunction in Hypertension: Current Concepts and Clinical Implications. Front. Med. 2022, 8, 798958. [Google Scholar] [CrossRef] [PubMed]
- Gorzelak-Pabiś, P.; Broncel, M.; Pawlos, A.; Wojdan, K.; Gajewski, A.; Chałubiński, M.; Woźniak, E. Dabigatran: Its protective effect against endothelial cell damage by oxysterol. Biomed. Pharmacother. 2022, 147, 112679. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, A.; Hanagata, H.; Kawamura, Y.; Yanagida, F. Anti-hypertensive substances in fermented soybean, natto. Plant. Food. Hum. Nutr. 1995, 47, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Gum, S.N.; Paik, J.K.; Lim, H.H.; Kim, K.C.; Ogasawara, K.; Inoue, K.; Park, S.; Jang, Y.; Lee, J.H. Effects of nattokinase on blood pressure: A randomized, controlled trial. Hypertens. Res. 2008, 31, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.S.; Lenninger, M.; Ero, M.P.; Benson, K.F. Consumption of nattokinase is associated with reduced blood pressure and von Willebrand factor, a cardiovascular risk marker: Results from a randomized, double-blind, placebo-controlled, multicenter North American clinical trial. Integr. Blood. Press. Control 2016, 9, 95–104. [Google Scholar] [CrossRef]
- Murakami, K.; Yamanaka, N.; Ohnishi, K.; Fukayama, M.; Yoshino, M. Inhibition of angiotensin I converting enzyme by subtilisin NAT (nattokinase) in natto, a Japanese traditional fermented food. Food. Funct. 2012, 3, 674–678. [Google Scholar] [CrossRef]
- Ibe, S.; Yoshida, K.; Kumada, K.; Tsurushiin, S.; Furusho, T.; Otobe, K. Antihypertensive effects of natto, a traditional Japanese fermented food, in spontaneously hypertensive rats. Food. Sci. Technol. Res. 2009, 15, 199–202. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Guo, Y.; Meng, X.; Li, Y.; Xia, C.; Meng, L.; Dong, M.; Wang, F. Beneficial Effect of Edoxaban on Preventing Atrial Fibrillation and Coagulation by Reducing Inflammation via HBG1/HBD Biomarkers. Front. Pharmacol. 2022, 13, 904317. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.A.; Braunwald, E. Ventricular remodeling after myocardial infarction: Experimental observations and clinical implications. Circulation 1990, 81, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Hama, N.; Itoh, H.; Shirakami, G.; Nakagawa, O.; Suga, S.; Ogawa, Y.; Masuda, I.; Nakanishi, K.; Yoshimasa, T.; Hashimoto, Y.; et al. Rapid ventricular induction of brain natriuretic peptide gene expression in experimental acute myocardial infarction. Circulation 1995, 92, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Saito, Y.; Kuwahara, K.; Harada, M.; Kishimoto, I.; Ogawa, Y.; Kawakami, R.; Nakagawa, Y.; Nakanishi, M.; Nakao, K. Angiotensin II-induced ventricular hypertrophy and extracellular signal-regulated kinase activation are suppressed in mice overexpressing brain natriuretic peptide in circulation. Hypertens. Res. 2003, 26, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pei, P.; Zhou, H.; Xie, Y.; Yang, S.; Shen, W.; Hu, L.; Zhang, Y.; Liu, T.; Yang, K. Nattokinase-Mediated Regulation of Tumor Physical Microenvironment to Enhance Chemotherapy, Radiotherapy, and CAR-T Therapy of Solid Tumor. ACS. Nano 2023, 17, 7475–7486. [Google Scholar] [CrossRef] [PubMed]
- Björkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef] [PubMed]
- Mega, J.L.; Braunwald, E.; Wiviott, S.D.; Bassand, J.P.; Bhatt, D.L.; Bode, C.; Burton, P.; Cohen, M.; Cook-Bruns, N.; Fox, K.A.; et al. Rivaroxaban in patients with a recent acute coronary syndrome. NEJM 2012, 366, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gallego, C.G.; Badimon, L.; Badimon, J.J. Direct and specific inhibition of factor Xa: An emerging therapeutic strategy for atherothrombotic disease. Eur. Heart. J. Suppl. 2014, 16, A56–A60. [Google Scholar] [CrossRef][Green Version]
- Borensztajn, K.; Stiekema, J.; Nijmeijer, S.; Reitsma, P.H.; Peppelenbosch, M.P.; Spek, C.A. Factor Xa stimulates proinflammatory and profibrotic responses in fibroblasts via protease-activated receptor-2 activation. Am. J. Pathol. 2008, 172, 309–320. [Google Scholar] [CrossRef]
- Hara, T.; Fukuda, D.; Tanaka, K.; Higashikuni, Y.; Hirata, Y.; Yagi, S.; Soeki, T.; Shimabukuro, M.; Sata, M. Inhibition of activated factor X by rivaroxaban attenuates neointima formation after wire-mediated vascular injury. Eur. J. Pharmacol. 2018, 820, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Borst, O.; Münzer, P.; Alnaggar, N.; Geue, S.; Tegtmeyer, R.; Rath, D.; Droppa, M.; Seizer, P.; Heitmeier, S.; Heemskerk, J.W.M.; et al. Inhibitory mechanisms of very low-dose rivaroxaban in non-ST-elevation myocardial infarction. Blood. Adv. 2018, 2, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Posthuma, J.J.; Posma, J.J.N.; van Oerle, R.; Leenders, P.; van Gorp, R.H.; Jaminon, A.M.G.; Mackmal, N.; Heitmeier, S.; Schurgers, L.J.; ten Cate, H.; et al. Targeting coagulation factor Xa promotes regression of advanced atherosclerosis in apolipoprotein-E deficient mice. Sci. Rep. 2019, 9, 3909. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.P.; Coughlin, T.; Fleifil, S.M.; Posma, J.J.N.; Spronk, H.H.M.; Heitmeier, S.; Owens, A.P.; Mackman, N. Effect of combining aspirin and rivaroxaban on atherosclerosis in mice. Atherosclerosis 2022, 345, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, M.; Bellmunt, S.; Cosín-Sales, J.; García-Moll, X.; Riera Mestre, A.; Almendro-Delia, M.; Hernández, J.L.; Lozano, F.; Mazón, P.; Suarez Fernández, C. Role of rivaroxaban in the prevention of atherosclerotic events. Expert. Rev. Clin. Pharmacol. 2019, 12, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Win, T.T.; Nakanishi, R.; Osawa, K.; Li, D.; Susaria, S.S.; Jayawardena, E.; Hamal, S.; Kim, M.; Broersen, A.; Kitslaar, P.H.; et al. Apixaban versus warfarin in evaluation of progression of atherosclerotic and calcified plaques (prospective randomized trial). Am. Heart. J. 2019, 212, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Aldana-Bitar, J.; Moore, J.; Manubolu, V.S.; Dahal, S.; Verghese, D.; Lakshmanan, S.; Hussein, L.; Crabtree, T.; Jonas, R.; Min, J.K.; et al. Plaque Progression Differences between Apixaban and Rivaroxaban in Patients with Atrial Fibrillation Measured with Cardiac Computed Tomography and Plaque Quantification. Am. J. Ther. 2023, 30, e313–e320. [Google Scholar] [CrossRef] [PubMed]
- Millenaar, D.; Bachmann, P.; Böhm, M.; Custodis, F.; Schirmer, S.H. Effects of edoxaban and warfarin on vascular remodeling: Atherosclerotic plaque progression and collateral artery growth. Vasc. Pharmacol. 2020, 127, 106661. [Google Scholar] [CrossRef] [PubMed]
- Sanda, T.; Yoshimura, M.; Hyodo, K.; Ishii, H.; Yamashita, T. Effects of Long-term Thrombin Inhibition (Dabigatran Etexilate) on Spontaneous Thrombolytic Activity during the Progression of Atherosclerosis in ApoE-/--LDLR-/- Double-Knockout Mice. Korean. Circ. J. 2020, 50, 804–816. [Google Scholar] [CrossRef]
- Feldmann, K.; Grandoch, M.; Kohlmorgen, C.; Valentin, B.; Gerfer, S.; Nagy, N.; Hartwig, S.; Lehr, S.; Fender, A.C.; Fischer, J.W. Decreased M1 macrophage polarization in dabigatran-treated Ldlr-deficient mice: Implications for atherosclerosis and adipose tissue inflammation. Atherosclerosis 2019, 287, 81–88. [Google Scholar] [CrossRef]
- Hezi-Yamit, A.; Wong, P.W.; Bien-Ly, N.; Komuves, L.G.; Prasad, K.S.; Phillips, D.R.; Sinha, U. Synergistic induction of tissue factor by coagulation factor Xa and TNF: Evidence for involvement of negative regulatory signaling cascades. Proc. Natl. Acad. Sci. USA 2005, 102, 12077–12082. [Google Scholar] [CrossRef]
- Nystedt, S.; Ramakrishnan, V.; Sundelin, J. The proteinase-activated receptor 2 is induced by inflammatory mediators in human endothelial cells. Comparison with the thrombin receptor. J. Biol. Chem. 1996, 271, 14910–14915. [Google Scholar] [CrossRef] [PubMed]
- Sanada, F.; Taniyama, Y.; Muratsu, J.; Otsu, R.; Shimizu, H.; Rakugi, H.; Morishita, R. IGF Binding Protein-5 Induces Cell Senescence. Front. Endocrinol. 2018, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Anderson, M.D.; Sutton, T.A. Pathophysiology of acute kidney injury. Compr. Physiol. 2012, 2, 1303–1353. [Google Scholar]
- Atzemian, N.; Kareli, D.; Ragia, G.; Manolopoulos, V.G. Distinct pleiotropic effects of direct oral anticoagulants on cultured endothelial cells: A comprehensive review. Front. Pharmacol. 2023, 14, 1244098. [Google Scholar] [CrossRef]
- Álvarez, E.; Paradela-Dobarro, B.; Raposeiras-Roubín, S.; González-Juanatey, J.R. Protective, repairing and fibrinolytic effects of rivaroxaban on vascular endothelium. Br. J. Clin. Pharmacol. 2018, 84, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, R.; Dirrichs, T.; Kneizeh, K.; Reinartz, S.; Frank, D.; Brachmann, J.; Schroeder, J.; Schurgers, L.; Göttsch, C.; Keszei, A.; et al. Influence of rivaroxaban compared to vitamin K antagonist treatment upon development of cardiovascular calcification in patients with atrial fibrillation and/or pulmonary embolism. Clin. Cardiol. 2022, 45, 352–358. [Google Scholar] [CrossRef]
- Di Lullo, L.; Lavalle, C.; Magnocavallo, M.; Mariani, M.V.; Della Rocca, D.G.; Severino, P.; Di Iorio, B.R.; Russo, D.; Summaria, F.; Forleo, G.B.; et al. New evidence of direct oral anticoagulation therapy on cardiac valve calcifications, renal preservation and inflammatory modulation. Int. J. Cardiol. 2021, 345, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Katoh, H.; Nozue, T.; Michishita, I. Anti-inflammatory effect of factor-Xa inhibitors in Japanese patients with atrial fibrillation. Heart. Vessels 2017, 32, 1130–1136. [Google Scholar] [CrossRef]
- Martins, G.L.; Duarte, R.C.F.; Vieira, É.L.M.; Rocha, N.P.; Figueiredo, E.L.; Silveira, F.R.; Caiaffa, J.R.S.; Lanna, R.P.; Carvalho, M.D.G.; Palotás, A.; et al. Comparison of Inflammatory Mediators in Patients with Atrial Fibrillation Using Warfarin or Rivaroxaban. Front. Cardiovasc. Med. 2020, 7, 114. [Google Scholar] [CrossRef]
- Kirchhof, P.; Ezekowitz, M.D.; Purmah, Y.; Schiffer, S.; Meng, I.L.; Camm, A.J.; Hohnloser, S.H.; Schulz, A.; Wosnitza, M.; Cappato, R. Effects of Rivaroxaban on Biomarkers of Coagulation and Inflammation: A Post Hoc Analysis of the X-VeRT Trial. TH Open 2020, 4, e20–e32. [Google Scholar] [CrossRef] [PubMed]
- Pistrosch, F.; Matschke, J.B.; Schipp, D.; Schipp, B.; Henkel, E.; Weigmann, I.; Sradnick, J.; Bornstein, S.R.; Birkenfeld, A.L.; Hanefeld, M. Rivaroxaban compared with low-dose aspirin in individuals with type 2 diabetes and high cardiovascular risk: A randomised trial to assess effects on endothelial function, platelet activation and vascular biomarkers. Diabetologia 2021, 64, 2701–2712. [Google Scholar] [CrossRef] [PubMed]
- Torramade-Moix, S.; Palomo, M.; Vera, M.; Jerez, D.; Moreno-Castaño, A.B.; Zafar, M.U.; Rovira, J.; Diekmann, F.; Garcia-Pagan, J.C.; Escolar, G.; et al. Apixaban Downregulates Endothelial Inflammatory and Prothrombotic Phenotype in an In Vitro Model of Endothelial Dysfunction in Uremia. Cardiovasc. Drugs. Ther. 2021, 35, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Nakase, T.; Moroi, J.; Ishikawa, T. Anti-inflammatory and antiplatelet effects of non-vitamin K antagonist oral anticoagulants in acute phase of ischemic stroke patients. Clin. Transl. Med. 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Berk, B.C.; Weintraub, W.S.; Alexander, R.W. Elevation of C-reactive protein in “active” coronary artery disease. Am. J. Cardiol. 1990, 65, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kondo, K.; Ichise, H.; Tsukamoto, Y.; Urano, T.; Umemura, K. Dietary supplementation with fermented soybeans suppresses intimal thickening. Nutrition 2003, 19, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Chen, K.T.; Lee, T.H.; Wang, C.H.; Kuo, Y.W.; Chiu, Y.H.; Hsieh, C.L.; Wu, C.J.; Chang, Y.L. Effects of natto extract on endothelial injury in a rat model. Acta. Med. Okayama 2010, 64, 399–406. [Google Scholar] [PubMed]
- Ren, N.N.; Chen, H.J.; Li, Y.; Mcgowan, G.W.; Lin, Y.G. A clinical study on the effect of nattokinase on carotid artery atherosclerosis and hyperlipidaemia]. Zhonghua Yi Xue Za Zhi 2017, 97, 2038–2042. [Google Scholar]
- Chiu, H.W.; Chou, C.L.; Lee, K.T.; Shih, C.C.; Huang, T.H.; Sung, L.C. Nattokinase attenuates endothelial inflammation through the activation of SRF and THBS1. Int. J. Biol. Macromol. 2024, 268, 131779. [Google Scholar] [CrossRef]
- Hodis, H.N.; Mack, W.J.; Meiselman, H.J.; Kalra, V.; Liebman, H.; Hwang-Levine, J.; Dustin, L.; Kono, N.; Mert, M.; Wenby, R.B.; et al. Nattokinase atherothrombotic prevention study: A randomized controlled trial. Clin. Hemorheol. Microcirc. 2021, 78, 339–353. [Google Scholar] [CrossRef]
- Park, K.J.; Kang, J.I.; Kim, T.S.; Yeo, I.H. The antithrombotic and fibrinolytic effect of natto in hypercholesterolemia rats. Prev. Nutr. Food. Sci. 2012, 17, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Lim, Y.; Kim, A.J. Korean red ginseng combined with nattokinase ameliorates dyslipidemia and the area of aortic plaques in high cholesterol-diet fed rabbits. Food. Sci. Biotechnol. 2014, 23, 283–287. [Google Scholar] [CrossRef]
- Croce, K.; Libby, P. Intertwining of thrombosis and inflammation in atherosclerosis. Curr. Opin. Hematol. 2007, 14, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, F.; Kubik, L.; Oblak, A.; Lore, N.I.; Cigana, C.; Lanzetta, R. Activation of human toll-like receptor 4 (TLR4)center dot myeloid differentiation factor 2 (MD-2) by hypoacylated lipopolysaccharide from a clinical isolate of Burkholderia cenocepacia. J. Biol. Chem. 2015, 290, 21305–21319. [Google Scholar] [CrossRef]
- Yu, Y.; Ge, N.L.; Xie, M.; Sun, W.J.; Burlingame, S.; Pass, A.K. Phosphorylation of Thr-178 and Thr-184 in the TAK1 T-loop is required for interleukin (IL)-1-mediated optimal NF kappa B and AP-1 activation as well as IL-6 gene expression. J. Biol. Chem. 2008, 283, 24497–24505. [Google Scholar] [CrossRef] [PubMed]
- Sorescu, D.; Weiss, D.; Lassegue, B.; Clempus, R.E.; Szocs, K.; Sorescu, G.P. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 2002, 105, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, Q.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive oxygen species: Key regulators in vascular health and diseases. Br. J. Pharmacol. 2018, 175, 1279–1292. [Google Scholar] [CrossRef]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef]
- Masselli, E.; Pozzi, G.; Vaccarezza, M.; Mirandola, P.; Galli, D.; Vitale, M.; Carubbi, C.; Gobbi, G. ROS in Platelet Biology: Functional Aspects and Methodological Insights. Int. J. Mol. Sci. 2020, 21, 4866. [Google Scholar] [CrossRef]
- Ohashi, M.; Runge, M.S.; Faraci, F.M.; Heistad, D.D. MnSOD deficiency increases endothelial dysfunction in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2331–2336. [Google Scholar] [CrossRef]
- Ohkura, N.; Hiraishi, S.; Itabe, H.; Hamuro, T.; Kamikubo, Y.; Takano, T.; Matsuda, J.; Horie, S. Oxidized phospholipids in oxidized low-density lipoprotein reduce the activity of tissue factor pathway inhibitor through association with its carboxy-terminal region. Antioxid. Redox. Signal 2004, 6, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zennadi, R. Oxidative Stress and Thrombosis during Aging: The Roles of Oxidative Stress in RBCs in Venous Thrombosis. Int. J. Mol. Sci. 2020, 21, 4259. [Google Scholar] [CrossRef] [PubMed]
- Mihm, M.J.; Yu, F.; Carnes, C.A.; Reiser, P.J.; McCarthy, P.M.; Van Wagoner, D.R.; Bauer, J.A. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation 2001, 104, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Santulli, G.; Reiken, S.R.; Yuan, Q.; Osborne, B.W.; Chen, B.X.; Marks, A.R. Mitochondrial oxidative stress promotes atrial fibrillation. Sci. Rep. 2015, 5, 11427. [Google Scholar] [CrossRef] [PubMed]
- Imam, F.; Al-Harbi, N.O.; Khan, M.R.; Qamar, W.; Alharbi, M.; Alshamrani, A.A.; Alhamami, H.N.; Alsaleh, N.B.; Alharbi, K.S. Protective Effect of RIVA Against Sunitinib-Induced Cardiotoxicity by Inhibiting Oxidative Stress-Mediated Inflammation: Probable Role of TGF-β and Smad Signaling. Cardiovas. Toxicol. 2020, 20, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, N.O.; Imam, F.; Alharbi, M.M.; Khan, M.R.; Qamar, W.; Afzal, M.; Algahtani, M.; Alobaid, S.; Alfardan, A.S.; Alshammari, A.; et al. Role of rivaroxaban in sunitinib-induced renal injuries via inhibition of oxidative stress-induced apoptosis and inflammation through the tissue nacrosis factor-α induced nuclear factor-κappa B signaling pathway in rats. J. Thromb. Thrombolysis 2020, 50, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Abdelzaher, W.Y.; Mohammed, H.H.; Welson, N.N.; Batiha, G.E.; Baty, R.S.; Abdel-Aziz, A.M. Rivaroxaban Modulates TLR4/Myd88/NF-Kβ Signaling Pathway in a Dose-Dependent Manner with Suppression of Oxidative Stress and Inflammation in an Experimental Model of Depression. Front. Pharmacol. 2021, 12, 715354. [Google Scholar] [CrossRef] [PubMed]
- Shafiey, S.I.; Abo-Saif, A.A.; Abo-Youssef, A.M.; Mohamed, W.R. Protective effects of rivaroxaban against cisplatin-induced testicular damage in rats: Impact on oxidative stress, coagulation, and p-NF-κB/VCAM-1 signaling. Food. Chem. Toxicol. 2022, 169, 113419. [Google Scholar] [CrossRef]
- Caliskan, A.; Yavuz, C.; Karahan, O.; Yazici, S.; Guclu, O.; Demirtas, S.; Mavitas, B. Factor-Xa inhibitors protect against systemic oxidant damage induced by peripheral-ischemia reperfusion. J. Thromb. Thrombolysis 2014, 37, 464–468. [Google Scholar] [CrossRef]
- Ellinghaus, P.; Perzborn, E.; Hauenschild, P.; Gerdes, C.; Heitmeier, S.; Visser, M.; Summer, H.; Laux, V. Expression of pro-inflammatory genes in human endothelial cells: Comparison of rivaroxaban and dabigatran. Thromb. Res. 2016, 142, 44–51. [Google Scholar] [CrossRef]
- Maeda, M.; Tsuboi, T.; Hayashi, T. An Inhibitor of Activated Blood Coagulation Factor X Shows Anti-Endothelial Senescence and Anti-Atherosclerotic Effects. J. Vasc. Res. 2019, 56, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Abedalqader, N.N.; Rababa’h, A.M.; Ababneh, M. The protective effect of rivaroxaban with or without aspirin on inflammation, oxidative stress, and platelet reactivity in isoproterenol-induced cardiac injury in rats. Naunyn. Schmiedebergs. Arch. Pharmacol. 2023, 396, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, A.; Deng, J.; Yang, J.; Chen, Q.; Chen, G.; Ye, M.; Lin, D. Rivaroxaban down-regulates pyroptosis and the TLR4/NF-κB/NLRP3 signaling pathway to promote flap survival. Int. Immunopharmacol. 2024, 128, 111568. [Google Scholar] [CrossRef] [PubMed]
- Favere, K.; Bosman, P.L.M.; Delputte, H.W.; Favoreel, E.M.; Van Craenenbroeck, J.; De Sutter, I.; Witvrouwen, G.R.Y.; De Meyer, H.; Heidbuchel, P.D.F. Guns, A systematic literature review on the effects of exercise on human Toll-like receptor expression. Exerc. Immunol. Rev. 2021, 27, 84–124. [Google Scholar] [PubMed]
- El-Shitany, N.A.; Eid, B.G. Icariin modulates carrageenan-induced acute inflammation through HO-1/Nrf2 and NF-kB signaling pathways. Biomed. Pharmacother. 2019, 120, 109567. [Google Scholar] [CrossRef] [PubMed]
- Newmeyer, D.D.; Bossy-Wetzel, E.; Kluck, R.M.; Wolf, B.B.; Beere, H.M.; Green, D.R. Bcl-xL does not inhibit the function of Apaf-1. Cell. Death. Differ. 2000, 7, 402–407. [Google Scholar] [CrossRef]
- Ahmed, H.H.; Nevein, N.F.; Karima, A.; Hamza, A.H. Miracle enzymes serrapeptase and nattokinase mitigate neuroinflammation and apoptosis associated with Alzheimer’s disease in experimental model. WJPPS 2013, 3, 876–891. [Google Scholar]
| Animal Model | Drug(s)/Dose(s)/Duration | Proposed Molecular Mechanism(s) | Cardioprotective Effect(s) | Ref. |
|---|---|---|---|---|
| MI mouse model | rivaroxaban 138.5 ± 50.3 mg/kg/day or vehicle 2 weeks |
|
| [61] |
| MI rat model | rivaroxaban 3 mg/kg/day or PAR-2 10 μg/kg/day 4 weeks |
|
| [62] |
| TAC mouse model | rivaroxaban 1 mg/kg/day or vehicle 3 weeks |
|
| [63] |
| MI-induced HF mouse model | rivaroxaban 80 mg/kg/day or placebo 4 weeks |
|
| [64] |
| PO mouse model | dabigatran 10 mg/gm or placebo 5 weeks |
|
| [65] |
| MI rat model | edoxaban 20 mg/kg/day or vehicle 4 weeks |
|
| [66] |
| Congestive HF canine model | edoxaban 2 mg/kg/day or placebo 19 days |
|
| [67] |
| AF mouse model | rivaroxaban 0.01 mg/kg/day or edoxaban 0.03 mg/kg/day or placebo 2 weeks |
|
| [68] |
| Myocardial ischemia mouse model | apixaban 30 or 60 mg/g/day or vehicle 4 weeks |
|
| [68] |
| TAC mouse model | rivaroxaban 30 mg/kg/day or placebo 2 weeks |
|
| [69] |
| Myocardial ischemia rat model | rivaroxaban 2 mg/kg/day or placebo 28 days |
|
| [70] |
| Animal Model | Drug(s)/Dose(s)/Duration | Proposed Molecular Mechanism(s) | Protective Effect(s) | Ref. |
|---|---|---|---|---|
| MI-induced HF mouse model | NK capsules 200 mg 1 mL/kg/day or vehicle 30 days |
|
| [71] |
| SHR model | NK water extract 100 mg/kg/day or captopril 15 mg/kg/day 8 weeks |
|
| [72] |
| In vitro model | Incubation of endothelial cells with NK extract for 48 h at 37 °C or vehicle |
|
| [73] |
| Hypercholesterolemic rats | water soluble NK fraction or placebo 3 weeks |
|
| [74] |
| LPS-induced glomerular thrombosis in mice | NK 3000, 6000 or 9000 FU/kg or placebo 1 h before LPS |
|
| [75] |
| Alzheimer’s rat model | NK 360 and 720 FU/kg or serrapeptase 10,800 and 21,600 U/kg 45 days |
|
| [76] |
| MCAO rat model | NK 9.4 mg/d 4 h, 24 h or 48 h after reperfusion injury or model control group |
|
| [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muric, M.; Nikolic, M.; Todorovic, A.; Jakovljevic, V.; Vucicevic, K. Comparative Cardioprotective Effectiveness: NOACs vs. Nattokinase—Bridging Basic Research to Clinical Findings. Biomolecules 2024, 14, 956. https://doi.org/10.3390/biom14080956
Muric M, Nikolic M, Todorovic A, Jakovljevic V, Vucicevic K. Comparative Cardioprotective Effectiveness: NOACs vs. Nattokinase—Bridging Basic Research to Clinical Findings. Biomolecules. 2024; 14(8):956. https://doi.org/10.3390/biom14080956
Chicago/Turabian StyleMuric, Maja, Marina Nikolic, Andreja Todorovic, Vladimir Jakovljevic, and Ksenija Vucicevic. 2024. "Comparative Cardioprotective Effectiveness: NOACs vs. Nattokinase—Bridging Basic Research to Clinical Findings" Biomolecules 14, no. 8: 956. https://doi.org/10.3390/biom14080956
APA StyleMuric, M., Nikolic, M., Todorovic, A., Jakovljevic, V., & Vucicevic, K. (2024). Comparative Cardioprotective Effectiveness: NOACs vs. Nattokinase—Bridging Basic Research to Clinical Findings. Biomolecules, 14(8), 956. https://doi.org/10.3390/biom14080956






