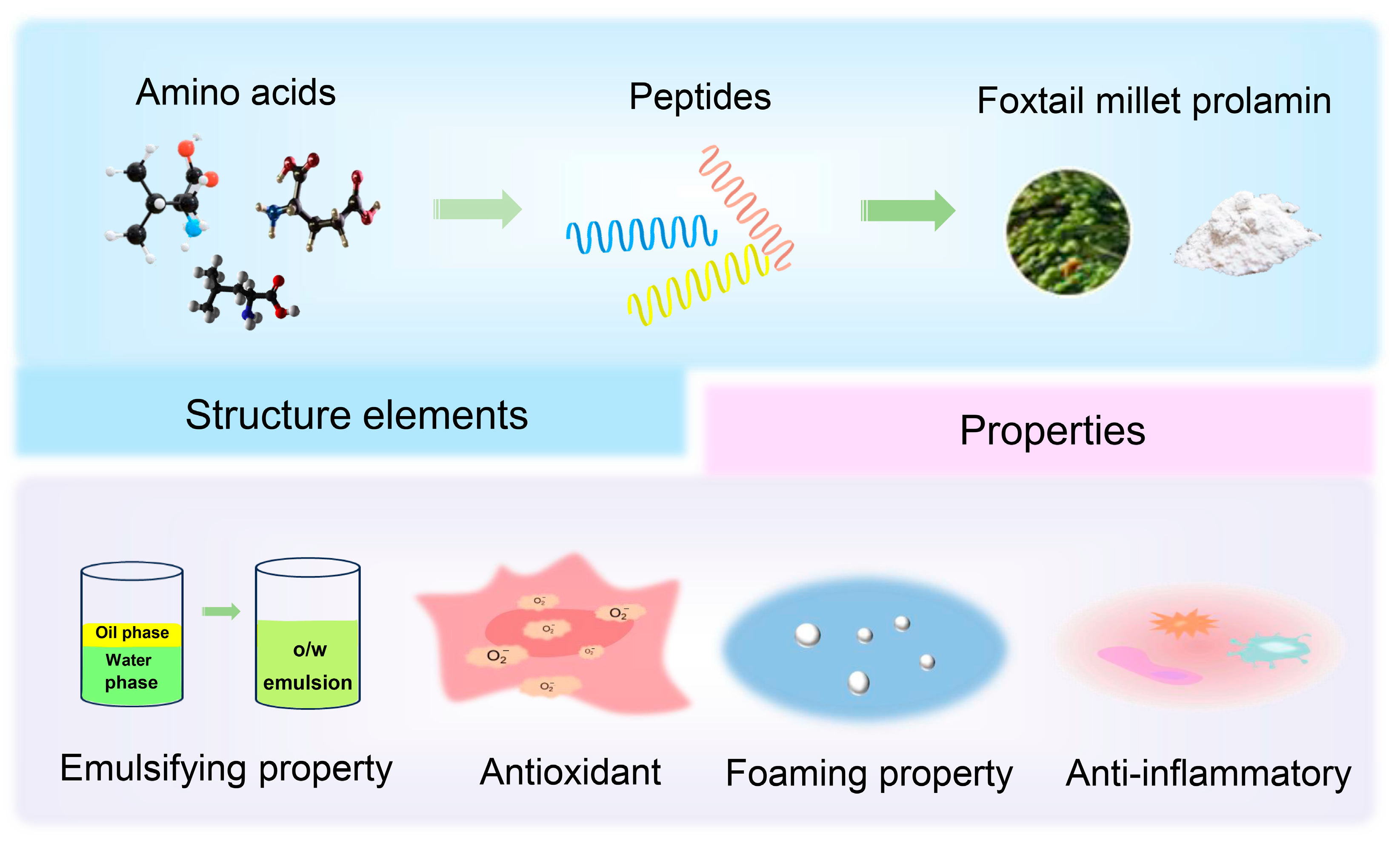
Structure, Functional Properties, and Applications of Foxtail Millet Prolamin: A Review
Abstract
1. Introduction
2. The Structure of Foxtail Millet Prolamin
2.1. Amino Acid Composition
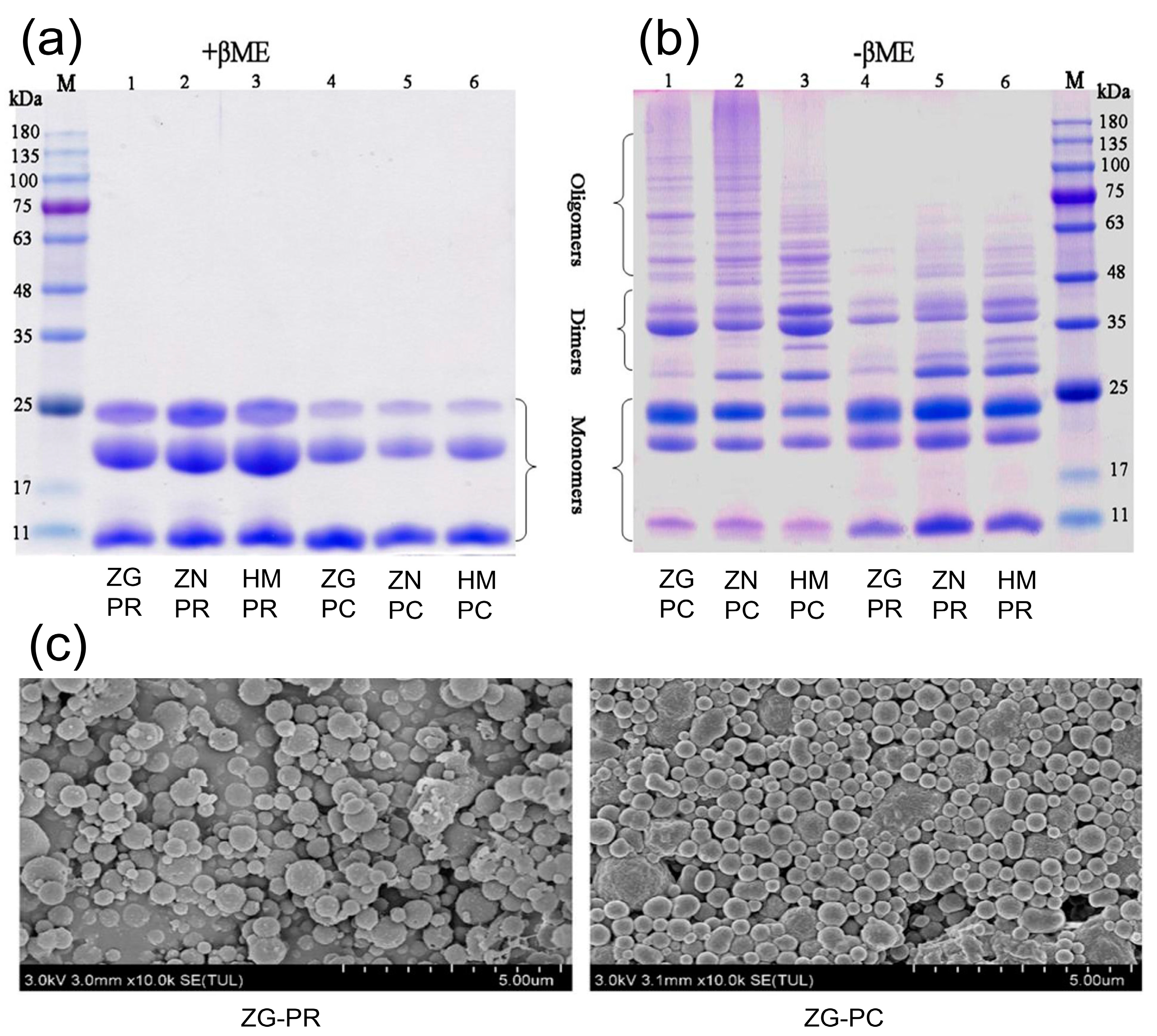
2.2. Folding Blocks
2.3. Spatial Structure
3. Characteristics of Foxtail Millet Prolamin
3.1. Physicochemical Properties
3.2. Functional Properties
3.2.1. Emulsifying Properties
3.2.2. Foaming Properties
3.3. Biological Activities
3.3.1. Anti-Oxidant Activities
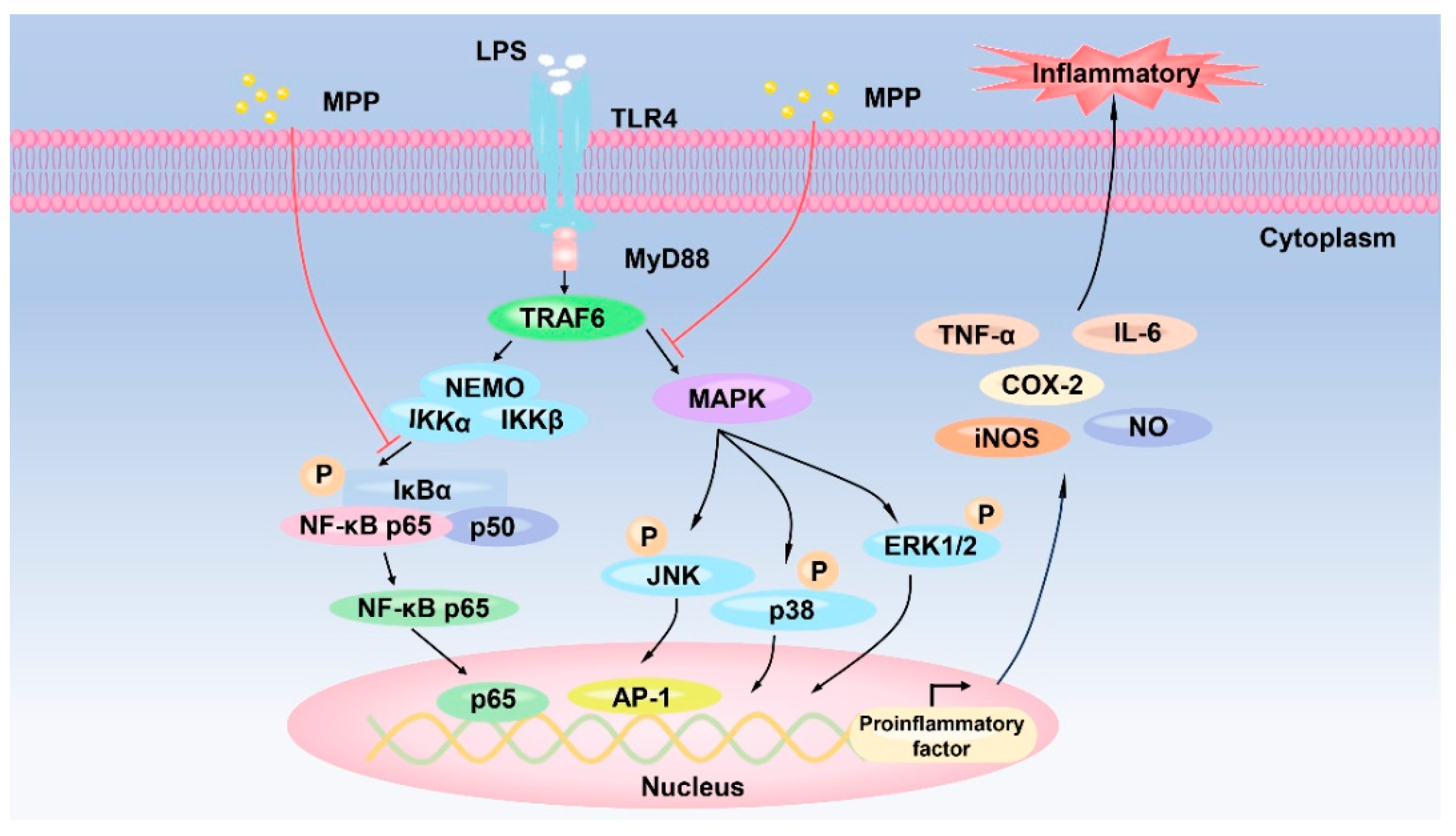
3.3.2. Anti-Inflammatory Activities
3.3.3. Other Biological Activities
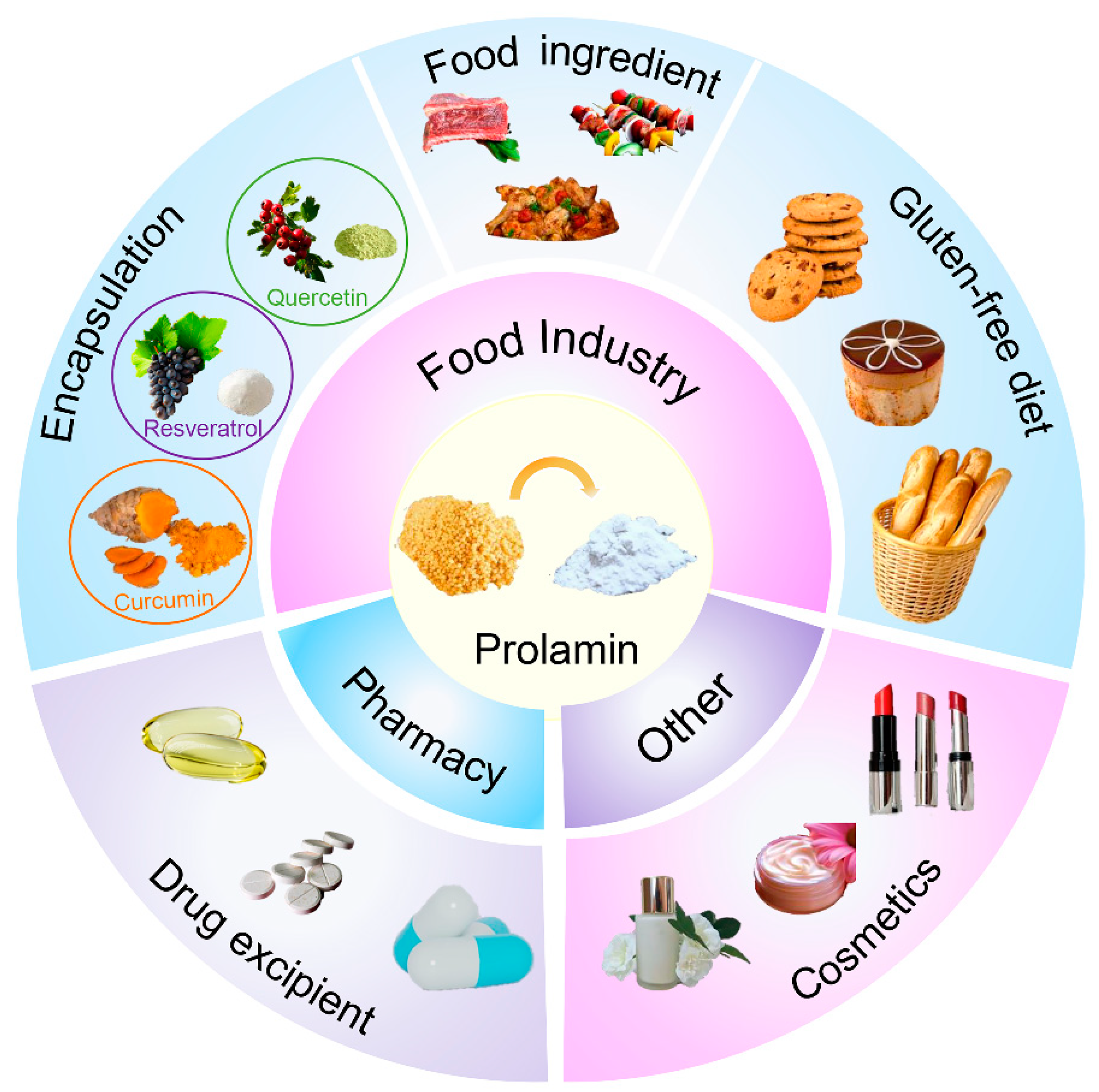
4. Applications of Foxtail Millet Prolamin
4.1. Development of Foxtail Millet Prolamin in Food-Related Fields
4.1.1. Gluten-Free Products
4.1.2. Utilization of Foxtail Millet Prolamin as a Food Ingredient
4.1.3. Protein Nanoparticles for Encapsulation
4.2. Role of Foxtail Millet Prolamin in the Pharmaceutical Field
5. Challenges and Prospects
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meherunnahar, M.; Hoque, M.M.; Satter, M.A.; Ahmed, T.; Chowdhury, R.S.; Aziz, S. Effect of cooking characteristics, amino acid consistency, and functional properties of composite noodles made from foxtail millet. Meas. Food 2024, 13, 100138. [Google Scholar] [CrossRef]
- Saleh, A.S.M.; Zhang, Q.; Chen, J.; Shen, Q. Millet Grains: Nutritional Quality, Processing, and Potential Health Benefits. Compr. Rev. Food Sci. Food Saf. 2013, 12, 281–295. [Google Scholar] [CrossRef]
- Sachdev, N.; Goomer, S.; Singh, L.R. Foxtail millet: A potential crop to meet future demand scenario for alternative sustainable protein. J. Sci. Food Agric. 2021, 101, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Austin, D.F. Fox-tail millets (Setaria: Poaceae)—Abandoned food in two hemispheres. Econ. Bot. 2006, 60, 143–158. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Y.; Xiang, J.; Zheng, B.; Yuan, Y.; Luo, D.; Fan, J. Comparative evaluation on phenolic profiles, antioxidant properties and α-glucosidase inhibitory effects of different milling fractions of foxtail millet. J. Cereal Sci. 2021, 99, 103217. [Google Scholar] [CrossRef]
- Yang, T.; Ma, S.; Liu, J.; Sun, B.; Wang, X. Influences of four processing methods on main nutritional components of foxtail millet: A review. Grain Oil Sci. Technol. 2022, 5, 156–165. [Google Scholar] [CrossRef]
- Sarabhai, S.; Tamilselvan, T.; Prabhasankar, P. Role of enzymes for improvement in gluten-free foxtail millet bread: It’s effect on quality, textural, rheological and pasting properties. LWT 2021, 137, 110365. [Google Scholar] [CrossRef]
- Gupta, N.; Srivastava, A.K.; Pandey, V.N. Biodiversity and Nutraceutical Quality of Some Indian Millets. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2012, 82, 265–273. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, X.; Wang, G.; Wang, H.; Liu, J.; Zhao, W.; Zhang, Y. Crude Fat Content and Fatty Acid Profile and Their Correlations in Foxtail Millet. Cereal Chem. 2015, 92, 455–459. [Google Scholar] [CrossRef]
- Zhang, F.; Fu, Y.; Liu, Z.; Shen, Q. Comparison of the characteristics of prolamins among foxtail millet varieties with different palatability: Structural, morphological, and physicochemical properties. Int. J. Biol. Macromol. 2021, 186, 194–205. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.-C.; Liu, Y.; Qian, L.-H.; Zhang, Y.-H.; Li, H.-J. Single/co-encapsulation capacity and physicochemical stability of zein and foxtail millet prolamin nanoparticles. Colloids Surf. B Biointerfaces 2022, 217, 112685. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.-X.; Mata, A.; Corke, H.; Gan, R.-Y.; Fang, Y. Physicochemical and pH-dependent functional properties of proteins isolated from eight traditional Chinese beans. Food Hydrocoll. 2021, 112, 106288. [Google Scholar] [CrossRef]
- Parameswaran, K.P.; Thayumanavan, B. Homologies between prolamins of different minor millets. Plant Foods Hum. Nutr. 1995, 48, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sahu, J.K.; Choudhary, A.; Meenu, M.; Bansal, V. High intensity ultrasound (HIU)-induced functionalization of foxtail millet protein and its fractions. Food Hydrocoll. 2023, 134, 108083. [Google Scholar] [CrossRef]
- Kumar, K.K.; Parameswaran, K.P. Characterisation of storage protein from selected varieties of foxtail millet (Setaria italica (L) Beauv). J. Sci. Food Agric. 1998, 77, 535–542. [Google Scholar] [CrossRef]
- Guo, X.; Tang, X.; Zhang, M.; Ma, X.; Wang, J.; Liang, H. New progress in the deep understanding of the biocake layer property: Combined effect of neglected protein secondary structure, morphology, and mechanism. Water Res. 2024, 250, 121038. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, Z.; Wang, H.; Zhang, F.; Guo, S.; Shen, Q. Comparison of the generation of α-glucosidase inhibitory peptides derived from prolamins of raw and cooked foxtail millet: In vitro activity, de novo sequencing, and in silico docking. Food Chem. 2023, 411, 135378. [Google Scholar] [CrossRef] [PubMed]
- Marcellino, L.; Junior, C.; Gander, E. Characterization of Pearl Millet Prolamins. Protein Pept. Lett. 2002, 9, 237–244. [Google Scholar] [CrossRef]
- Ji, Z.; Feng, R.; Mao, J. Separation and identification of antioxidant peptides from foxtail millet (Setaria italica) prolamins enzymatic hydrolysate. Cereal Chem. 2019, 96, 981–993. [Google Scholar] [CrossRef]
- Pribic, R.; Vanstokkum, I.H.M.; Chapman, D.; Haris, P.I.; Bloemendal, M. Protein Secondary Structure from Fourier Transform Infrared and/or Circular Dichroism Spectra. Anal. Biochem. 1993, 214, 366–378. [Google Scholar] [CrossRef]
- Stathopulos, P.B.; Scholz, G.A.; Hwang, Y.-M.; Rumfeldt, J.A.O.; Lepock, J.R.; Meiering, E.M. Sonication of proteins causes formation of aggregates that resemble amyloid. Protein Sci. 2004, 13, 3017–3027. [Google Scholar] [CrossRef] [PubMed]
- Long, G.; Ji, Y.; Pan, H.; Sun, Z.; Li, Y.; Qin, G. Characterization of Thermal Denaturation Structure and Morphology of Soy Glycinin by FTIR and SEM. Int. J. Food Prop. 2015, 18, 763–774. [Google Scholar] [CrossRef]
- Du, Q.; Li, H.; Tu, M.; Wu, Z.; Zhang, T.; Liu, J.; Ding, Y.; Zeng, X.; Pan, D. Legume protein fermented by lactic acid bacteria: Specific enzymatic hydrolysis, protein composition, structure, and functional properties. Colloids Surf. B Biointerfaces 2024, 238, 113929. [Google Scholar] [CrossRef]
- Ochoa-Rivas, A.; Nava-Valdez, Y.; Serna-Saldívar, S.O.; Chuck-Hernández, C. Microwave and Ultrasound to Enhance Protein Extraction from Peanut Flour under Alkaline Conditions: Effects in Yield and Functional Properties of Protein Isolates. Food Bioprocess Technol. 2017, 10, 543–555. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.-Y.; Wu, Y.-C.; Gong, P.-X.; Li, H.-J. Foxtail millet prolamin as an effective encapsulant deliver curcumin by fabricating caseinate stabilized composite nanoparticles. Food Chem. 2022, 367, 130764. [Google Scholar] [CrossRef] [PubMed]
- Jog, R.; Burgess, D.J. Pharmaceutical Amorphous Nanoparticles. J. Pharm. Sci. 2017, 106, 39–65. [Google Scholar] [CrossRef]
- Ito, K.; Inaba, K. The disulfide bond formation (Dsb) system. Curr. Opin. Struct. Biol. 2008, 18, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Bamdad, F.; Wu, J.; Chen, L. Effects of enzymatic hydrolysis on molecular structure and antioxidant activity of barley hordein. J. Cereal Sci. 2011, 54, 20–28. [Google Scholar] [CrossRef]
- Li, R.; Xiong, Y.L. Ultrasound-induced structural modification and thermal properties of oat protein. LWT 2021, 149, 111861. [Google Scholar] [CrossRef]
- Jhan, F.; Gani, A.; Noor, N.; Shah, A. Nanoreduction of Millet Proteins: Effect on Structural and Functional Properties. ACS Food Sci. Technol. 2021, 1, 1418–1427. [Google Scholar] [CrossRef]
- Ji, Z.; Mao, J.; Chen, S.; Mao, J. Antioxidant and anti-inflammatory activity of peptides from foxtail millet (Setaria italica) prolamins in HaCaT cells and RAW264.7 murine macrophages. Food Biosci. 2020, 36, 100636. [Google Scholar] [CrossRef]
- Sireesha, Y.; Kasetti, R.B.; Nabi, S.A.; Swapna, S.; Apparao, C. Antihyperglycemic and hypolipidemic activities of Setaria italica seeds in STZ diabetic rats. Pathophysiology 2011, 18, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res. Int. 2011, 44, 2742–2750. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Rana, J.C.; Kaur, A. Relationship between physicochemical and functional properties of amaranth (Amaranthus hypochondriacus) protein isolates. Int. J. Food Sci. Technol. 2014, 49, 541–550. [Google Scholar] [CrossRef]
- Gani, A.; ul Ashraf, Z.; Noor, N.; Ahmed Wani, I. Ultrasonication as an innovative approach to tailor the apple seed proteins into nanosize: Effect on protein structural and functional properties. Ultrason. Sonochemistry 2022, 86, 106010. [Google Scholar] [CrossRef]
- Agboola, S.; Ng, D.; Mills, D. Characterisation and functional properties of Australian rice protein isolates. J. Cereal Sci. 2005, 41, 283–290. [Google Scholar] [CrossRef]
- Dickinson, E.; Galazka, V.B. Emulsion stabilization by ionic and covalent complexes of β-lactoglobulin with polysaccharides. Food Hydrocoll. 1991, 5, 281–296. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, N. Studies on functional, thermal and pasting properties of flours from different chickpea (Cicer arietinum L.) cultivars. Food Chem. 2005, 91, 403–411. [Google Scholar] [CrossRef]
- Bejosano, F.P.; Corke, H. Properties of protein concentrates and hydrolysates from Amaranthus and Buckwheat. Ind. Crops Prod. 1999, 10, 175–183. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Yao, L.; Qin, F.; Hou, G.; Chen, B.; Jin, L.; Deng, J.; Shen, Y. Characterisation, physicochemical and functional properties of protein isolates from Amygdalus pedunculata Pall seeds. Food Chem. 2020, 311, 125888. [Google Scholar] [CrossRef]
- Liochev, S.I. Reactive oxygen species and the free radical theory of aging. Free Radic. Biol. Med. 2013, 60, 1–4. [Google Scholar] [CrossRef]
- Wu, S.; Wang, X.; Qi, W.; Guo, Q. Bioactive protein/peptides of flaxseed: A review. Trends Food Sci. Technol. 2019, 92, 184–193. [Google Scholar] [CrossRef]
- Wong, F.-C.; Xiao, J.; Wang, S.; Ee, K.-Y.; Chai, T.-T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci. Technol. 2020, 99, 44–57. [Google Scholar] [CrossRef]
- Wang, X.; Fu, J.; Bhullar, K.S.; Chen, B.; Liu, H.; Zhang, Y.; Wang, C.; Liu, C.; Su, D.; Ma, X.; et al. Identification, in silico selection, and mechanistic investigation of antioxidant peptides from corn gluten meal hydrolysate. Food Chem. 2024, 446, 138777. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, M.; Fan, H.; Wu, J. Emerging proteins as precursors of bioactive peptides/hydrolysates with health benefits. Curr. Opin. Food Sci. 2022, 48, 100914. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Kim, S.-K. Antioxidant Properties of a Radical-Scavenging Peptide Purified from Enzymatically Prepared Fish Skin Gelatin Hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef]
- Alemán, A.; Giménez, B.; Pérez-Santin, E.; Gómez-Guillén, M.C.; Montero, P. Contribution of Leu and Hyp residues to antioxidant and ACE-inhibitory activities of peptide sequences isolated from squid gelatin hydrolysate. Food Chem. 2011, 125, 334–341. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Nonresolving Inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef]
- Saleem, S. Targeting MAPK signaling: A promising approach for treating inflammatory lung disease. Pathol. Res. Pract. 2024, 254, 155122. [Google Scholar] [CrossRef]
- Capece, D.; Verzella, D.; Flati, I.; Arboretto, P.; Cornice, J.; Franzoso, G. NF-κB: Blending metabolism, immunity, and inflammation. Trends Immunol. 2022, 43, 757–775. [Google Scholar] [CrossRef]
- Tavares, L.P.; Negreiros-Lima, G.L.; Lima, K.M.; E Silva, P.M.R.; Pinho, V.; Teixeira, M.M.; Sousa, L.P. Blame the signaling: Role of cAMP for the resolution of inflammation. Pharmacol. Res. 2020, 159, 105030. [Google Scholar] [CrossRef]
- Zhu, B.; He, H.; Hou, T. A Comprehensive Review of Corn Protein-derived Bioactive Peptides: Production, Characterization, Bioactivities, and Transport Pathways. Compr. Rev. Food Sci. Food Saf. 2018, 18, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Jhan, F.; Gani, A.; Shah, A.; Ashwar, B.A.; Bhat, N.A.; Ganaie, T.A. Gluten-free minor cereals of Himalayan origin: Characterization, nutraceutical potential and utilization as possible anti-diabetic food for growing diabetic population of the world. Food Hydrocoll. 2021, 113, 106402. [Google Scholar] [CrossRef]
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A paradigm of proactive medicine. Eur. J. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Reaz, A.H.; Abedin, M.J.; Mohammad Abdullah, A.T.; Satter, M.A.; Farzana, T. Physicochemical and structural impact of CMC-hydrocolloids on the development of gluten-free foxtail millet biscuits. Heliyon 2023, 9, e17176. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gao, J.; Cao, G.; Guo, S.; Lu, D.; Hu, B.; Yang, Z.; Tong, Y.; Wen, C. The properties of potato gluten-free doughs: Comparative and combined effects of propylene glycol alginate and hydroxypropyl methyl cellulose or flaxseed gum. Int. J. Food Eng. 2023, 19, 61–71. [Google Scholar] [CrossRef]
- Abdollahzadeh, A.; Vazifedoost, M.; Didar, Z.; Haddadkhodaprast, M.H.; Armin, M. Comparison of the effect of hydroxyl propyl methyl cellulose, pectin, and concentrated raisin juice on gluten-free bread based on rice and foxtail millet flour. Food Sci. Nutr. 2024, 12, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, S.; Xu, M.; Min, F.; Yu, T.; Yuan, J.; Gao, J.; Chen, H.; Wu, Y. Elm (Ulmus pumila L.) bark flour as a gluten substitute in gluten-free whole foxtail millet bread. J. Food Sci. Technol. 2023, 60, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- López, D.N.; Galante, M.; Robson, M.; Boeris, V.; Spelzini, D. Amaranth, quinoa and chia protein isolates: Physicochemical and structural properties. Int. J. Biol. Macromol. 2018, 109, 152–159. [Google Scholar] [CrossRef]
- Fu, Y.; Yin, R.; Liu, Z.; Niu, Y.; Guo, E.; Cheng, R.; Diao, X.; Xue, Y.; Shen, Q. Hypoglycemic Effect of Prolamin from Cooked Foxtail Millet (Setaria italic) on Streptozotocin-Induced Diabetic Mice. Nutrients 2020, 12, 3452. [Google Scholar] [CrossRef]
- Sharma, N.; Niranjan, K. Foxtail millet: Properties, processing, health benefits, and uses. Food Rev. Int. 2018, 34, 329–363. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Yao, H.; Zhou, J.; Duan, Y.; Zhang, H.; Ma, H. Advances in renewable plant-derived protein source: The structure, physicochemical properties affected by ultrasonication. Ultrason. Sonochemistry 2019, 53, 83–98. [Google Scholar] [CrossRef]
- Sahni, P.; Sharma, S. Quality characteristics, amino acid composition, and bioactive potential of wheat cookies protein-enriched with unconventional legume protein isolates. Qual. Assur. Saf. Crops Foods 2023, 15, 1–10. [Google Scholar] [CrossRef]
- Monteiro, P.V.; Virupaksha, T.K.; Rao, D.R. Proteins of Italian millet: Amino acid composition, solubility fractionation and electrophoresis of protein fractions. J. Sci. Food Agric. 1982, 33, 1072–1079. [Google Scholar] [CrossRef]
- Joye, I.; Julian McClements, D. Biopolymer-Based Delivery Systems: Challenges and Opportunities. Curr. Top. Med. Chem. 2016, 16, 1026–1039. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.-C.; Gong, P.-X.; Zhang, Y.-H.; Li, H.-J. Chondroitin sulfate deposited on foxtail millet prolamin/caseinate nanoparticles to improve physicochemical properties and enhance cancer therapeutic effects. Food Funct. 2022, 13, 5343–5352. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.-C.; Qian, L.-H.; Zhang, Y.-H.; Gong, P.-X.; Liu, W.; Li, H.-J. Fabrication of foxtail millet prolamin/caseinate/chitosan hydrochloride composite nanoparticles using antisolvent and pH-driven methods for curcumin delivery. Food Chem. 2023, 404, 134604. [Google Scholar] [CrossRef]
- Chen, G.; Dong, S.; Chen, Y.; Gao, Y.; Zhang, Z.; Li, S. Complex coacervation of zein-chitosan via atmospheric cold plasma treatment: Improvement of encapsulation efficiency and dispersion stability. Food Hydrocoll. 2020, 107, 105943. [Google Scholar] [CrossRef]
- Wei, Y.; Yu, Z.; Lin, K.; Sun, C.; Dai, L.; Yang, S.; Mao, L.; Yuan, F.; Gao, Y. Fabrication and characterization of resveratrol loaded zein-propylene glycol alginate-rhamnolipid composite nanoparticles: Physicochemical stability, formation mechanism and in vitro digestion. Food Hydrocoll. 2019, 95, 336–348. [Google Scholar] [CrossRef]
- Chen, X.; Yu, C.; Wang, J.-H.; Wu, Y.-C.; Ma, Y.; Li, H.-J. Fabrication of curcumin−loaded foxtail millet prolamin−based nanoparticle: Impact of curdlan sulfate on the particle properties. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131893. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.-C.; Gong, P.-X.; Li, H.-J. Co-assembly of foxtail millet prolamin-lecithin/alginate sodium in citric acid–potassium phosphate buffer for delivery of quercetin. Food Chem. 2022, 381, 132268. [Google Scholar] [CrossRef]
- Lordan, S.; Smyth, T.J.; Soler-Vila, A.; Stanton, C.; Ross, R.P. The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013, 141, 2170–2176. [Google Scholar] [CrossRef]
- Rana, S.S.; Tiwari, S.; Gupta, N.; Tripathi, M.K.; Tripathi, N.; Singh, S.; Bhagyawant, S.S. Validating the Nutraceutical Significance of Minor Millets by Employing Nutritional–Antinutritional Profiling. Life 2023, 13, 1918. [Google Scholar] [CrossRef]
| Source of Seed | Characteristics | Description | References |
|---|---|---|---|
| Dhan Foundation India (Madhya Pradesh, India) | Particle size | The lowest level of 1.001 μm after HIU treatment | [14] |
| The Chifeng Academy of Agriculture and Animal Science (Chifeng, the Inner Mongolia Autonomous Region, China) | Surface charge (zeta potential) | −18~−24 mV among different foxtail millet variety −3.0~−8.6 mV appeared near the isoelectric point at pH = 5 | [10] [29] |
| The Chifeng Academy of Agriculture and Animal Science (Chifeng, the Inner Mongolia Autonomous Region, China) | Surface hydrophobicity (H0) | PR: 6031.63~6728.02 PC: 3996.22~4905.13 | [10] |
| Dhan Foundation India (Madhya Pradesh, India) | Solubility | Poorly soluble in water alone, soluble in aqueous ethanol | [14] |
| The Chifeng Academy of Agriculture and Animal Science (Chifeng, the Inner Mongolia Autonomous Region, China) | Water holding capacity (WHO) | 1.87~2.30 g/g before cooking 0.54~0.93 g/g after cooking | [10] |
| Thermal stability | Td = 84.68–86.24 °C ∆H = 16.14~22.29 J/g | ||
| Dhan Foundation India (Madhya Pradesh, India) | Emulsifying property | EAI = 5.5~14.22 m2/g ESI = 27.96~46.02 min | [14] [30] |
| Foaming property | Foaming capacity value is 7.32% Foaming stability value is 57.89% | ||
| Shanxi province, China | Anti-oxidant activity | DPPH radical scavenging activity is 149 μM TE/g protein ORCA value is 1180 μMTE/g | [19] |
| Shanxi province, China | Anti-inflammatory activity | – | [31] |
| Dong fang liang Life Technology Co., Ltd. (Datong, Shanxi province, China) | Anti-diabetic activity | Improve glucose homeostasis disorders; Alleviating triglyceride accumulation; α-glucosidase inhibitory activity | [17] |
| Tirupati (Andhra Pradesh, India) | Anti-hyperlipidemic effect | – | [32] |
| Co-Delivery | Bioactive Compounds | Methods | Loading Capacity (LC) and Encapsulation Efficiency (EE) | Improvement of Properties | References |
|---|---|---|---|---|---|
| Lecithin; Alginate | Quercetin | Anti-solvent | LC = 4.4% EE = 95.7% | pH stability; Re-dispersibility; Bio-accessibility; Cell-uptake capacity; Controlled release behavior | [71] |
| Curdlan sulfate; Sodium caseinate | Curcumin | pH-driven | EE = 85.9% | Sustained release performance; Storage stabilities; Bioactivity | [70] |
| Sodium caseinate | Puerarian; Resveratrol; Diosmetin; Curcumin | Polarity mediation | LC = 7.4~9.2% EE = 60.7~91.9% | Storage stability; Long-term storage stability; Bioavailability | [11] |
| Caseinate | Curcumin | Antisolvent/ evaporation | EE = 71.3~98.4% | Prevent the degradation of curcumin during heat treatment; Antioxidant; Anti-tumor | [25] |
| Caseinate chitosan hydrochloride | Curcumin | Antisolvent pH-driven | EE = 85.6% | pH stability; Sustained and controlled release behavior | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zhang, G.; Liang, W.; Tian, J.; Sun, S.; Zhang, X.; Lv, X.; Guo, P.; Qu, A.; Wu, Z. Structure, Functional Properties, and Applications of Foxtail Millet Prolamin: A Review. Biomolecules 2024, 14, 913. https://doi.org/10.3390/biom14080913
Zhang W, Zhang G, Liang W, Tian J, Sun S, Zhang X, Lv X, Guo P, Qu A, Wu Z. Structure, Functional Properties, and Applications of Foxtail Millet Prolamin: A Review. Biomolecules. 2024; 14(8):913. https://doi.org/10.3390/biom14080913
Chicago/Turabian StyleZhang, Wen, Guijun Zhang, Wenjing Liang, Jiayi Tian, Shuhao Sun, Xinping Zhang, Xinyi Lv, Peibo Guo, Ao Qu, and Zijian Wu. 2024. "Structure, Functional Properties, and Applications of Foxtail Millet Prolamin: A Review" Biomolecules 14, no. 8: 913. https://doi.org/10.3390/biom14080913
APA StyleZhang, W., Zhang, G., Liang, W., Tian, J., Sun, S., Zhang, X., Lv, X., Guo, P., Qu, A., & Wu, Z. (2024). Structure, Functional Properties, and Applications of Foxtail Millet Prolamin: A Review. Biomolecules, 14(8), 913. https://doi.org/10.3390/biom14080913






