The Cell-Penetrating Peptide GV1001 Enhances Bone Formation via Pin1-Mediated Augmentation of Runx2 and Osterix Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmid, Antibodies, and Reagents
2.2. Cell Culture and Transient Transfection
2.3. Biotin Conjugation Reaction
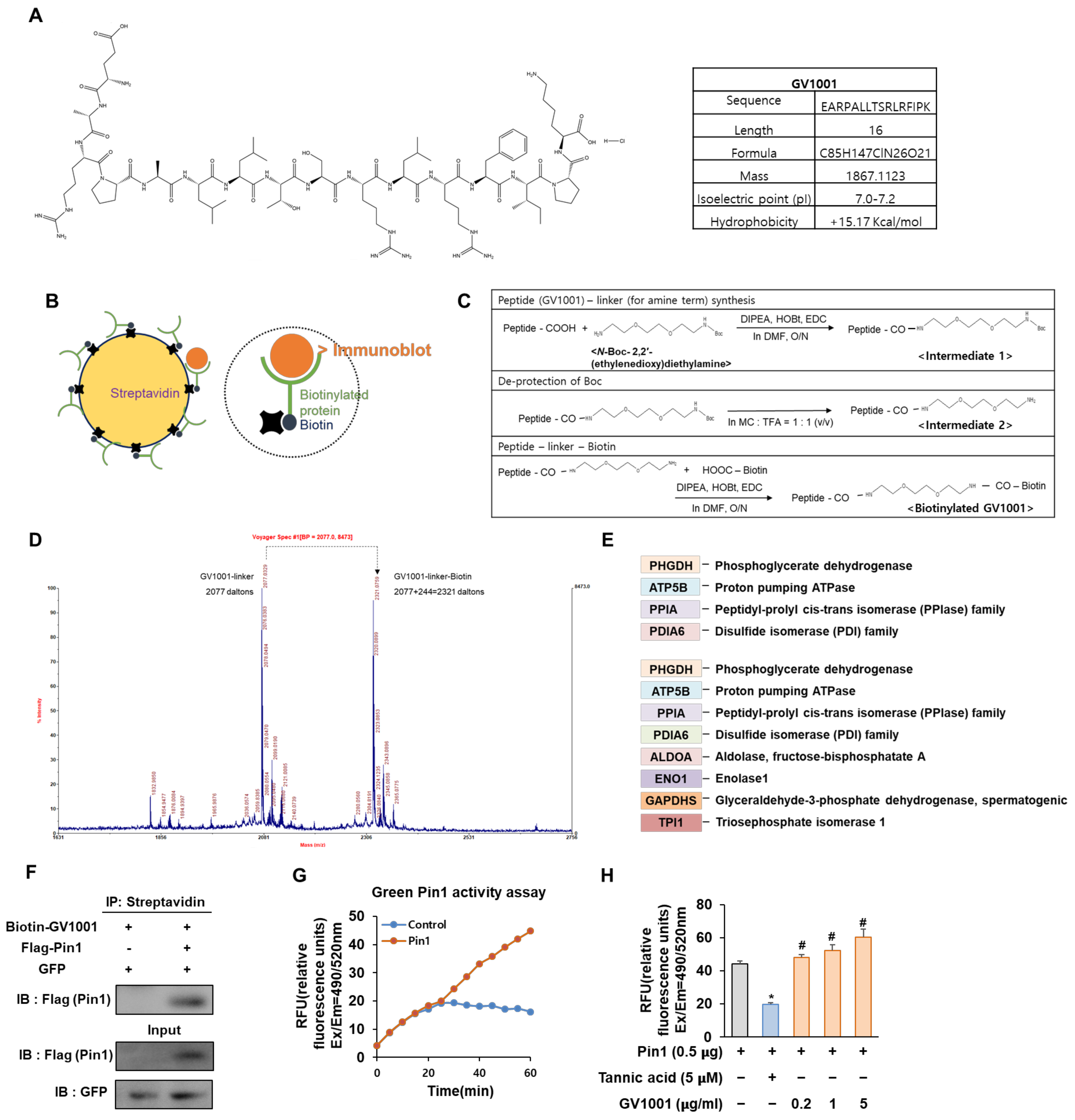
2.4. Determination of GV1001-Binding Proteins by Matrix-Assisted Laser Desorption Ionization Mass Spectrometry (MALDI-MS)
2.5. Pin1 Activity Assay
2.6. Alkaline Phosphatase (ALP) and Alizarin Red S (ARS) Staining
2.7. RNA Extraction and Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.8. Immunoblotting (IB) and Co-Immunoprecipitation (Co-IP)
2.9. Luciferase Reporter Assay
2.10. Enzyme-Linked Immunosorbent Assay (ELISA) for OCN Detection
2.11. Animal Models and Treatments
2.12. Micro-Computed Tomography (µCT)
2.13. Statistical Analysis
3. Results
3.1. Biotinylated GV1001 Associates with Peptidyl-Prolyl Isomerase A (PPIA) and GV1001 Regulates Pin1 Activity by Direct Interaction
3.2. GV1001 Promotes Osteoblast Differentiation after Stimulation of C2C12 Cells with BMP4
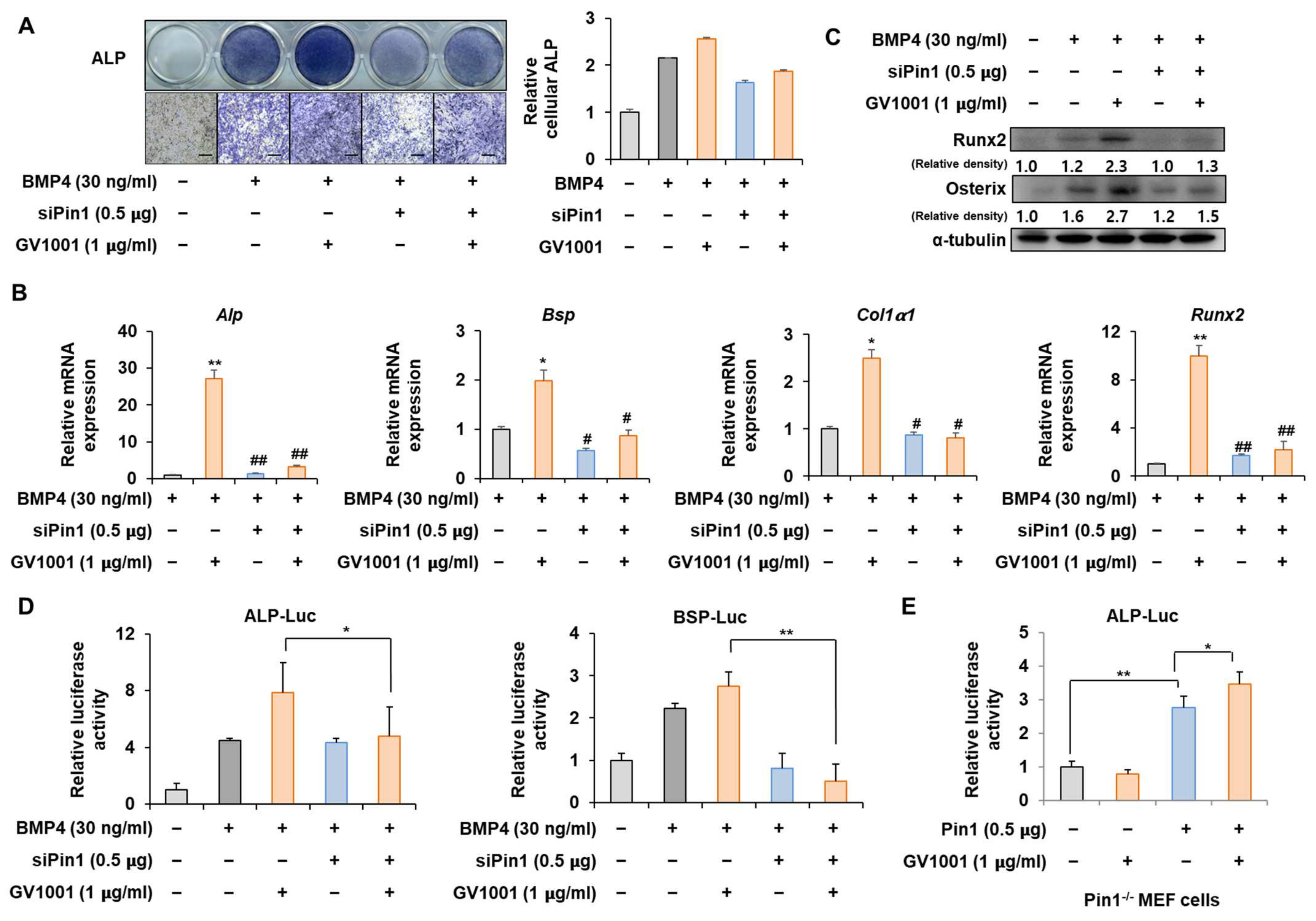
3.3. Knockdown of Pin1 Partially Reduced the Osteogenic Activity of GV1001
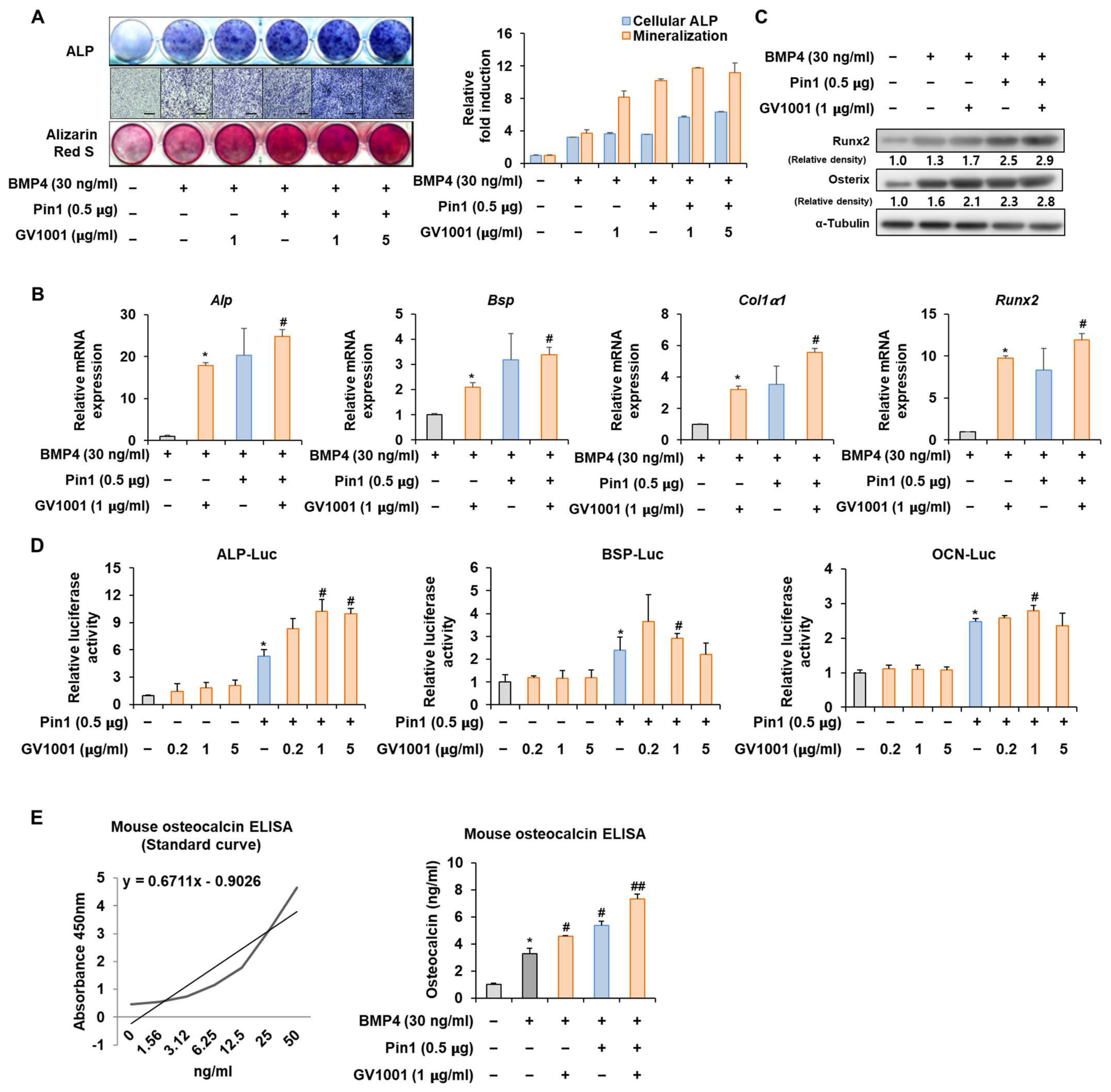
3.4. GV1001 Regulates Pin1-Mediated Osteoblast Differentiation
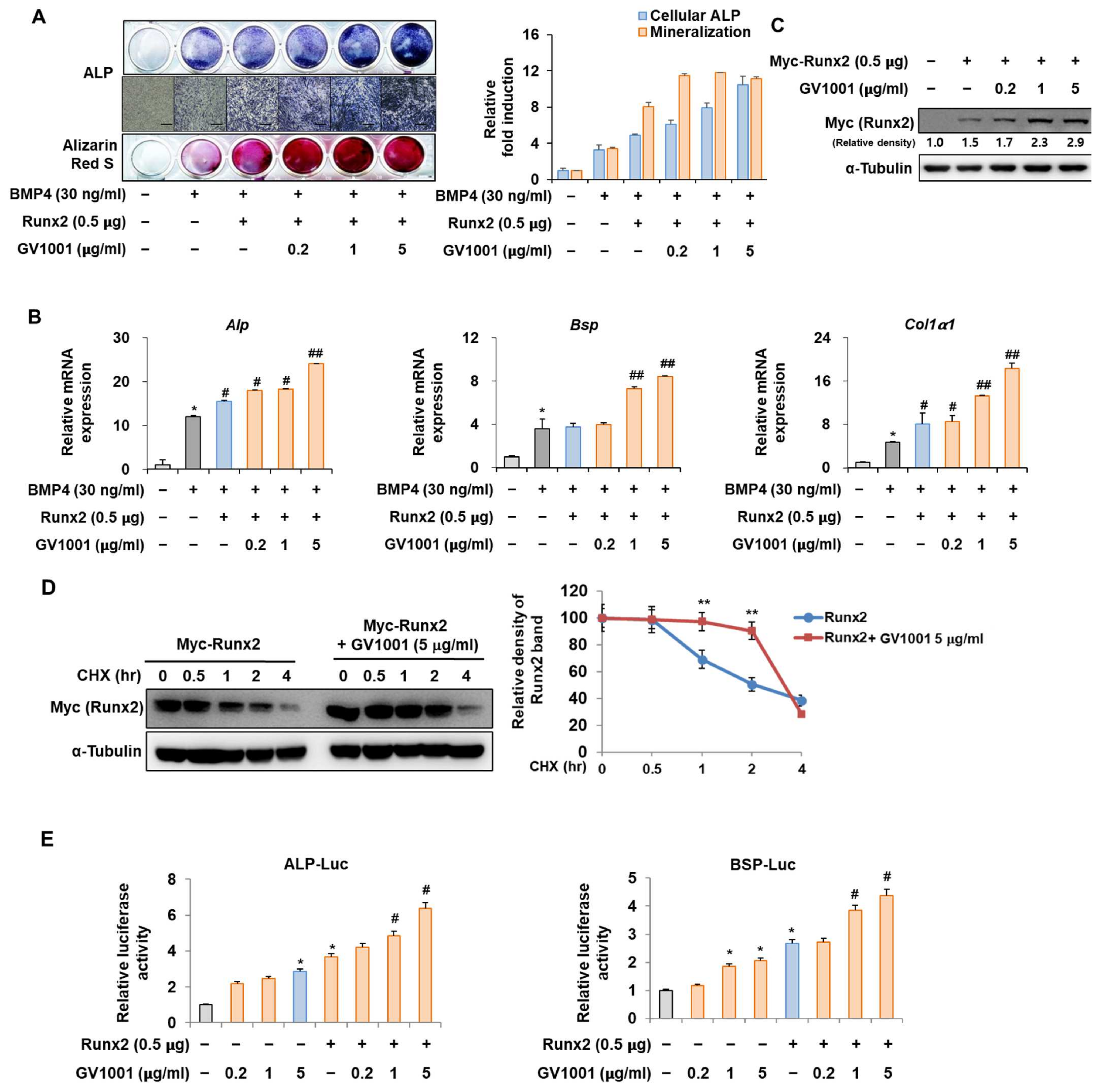
3.5. GV1001 Enhances the Osteoblast Differentiation via Stabilization of the Runx2 Protein
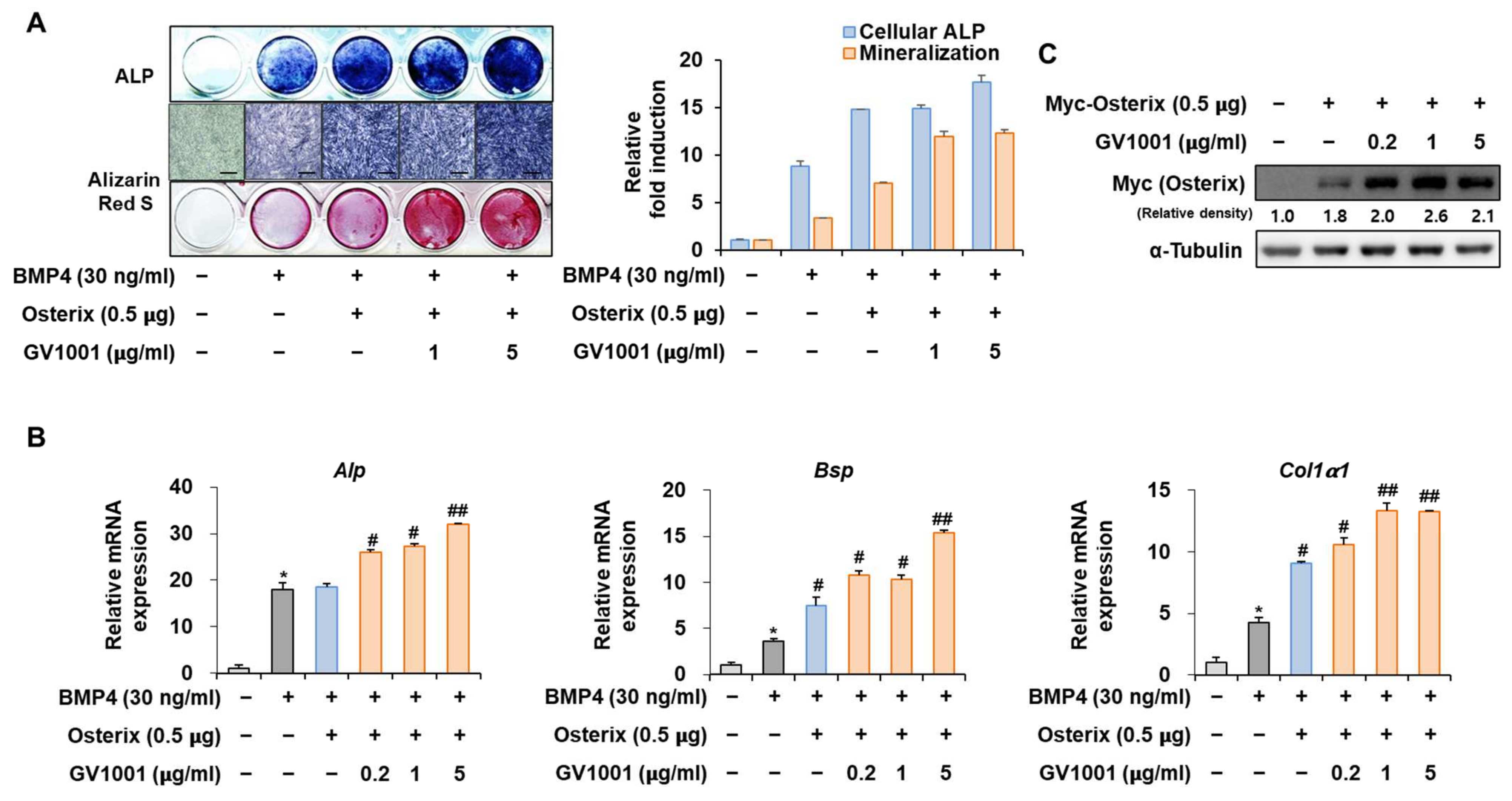
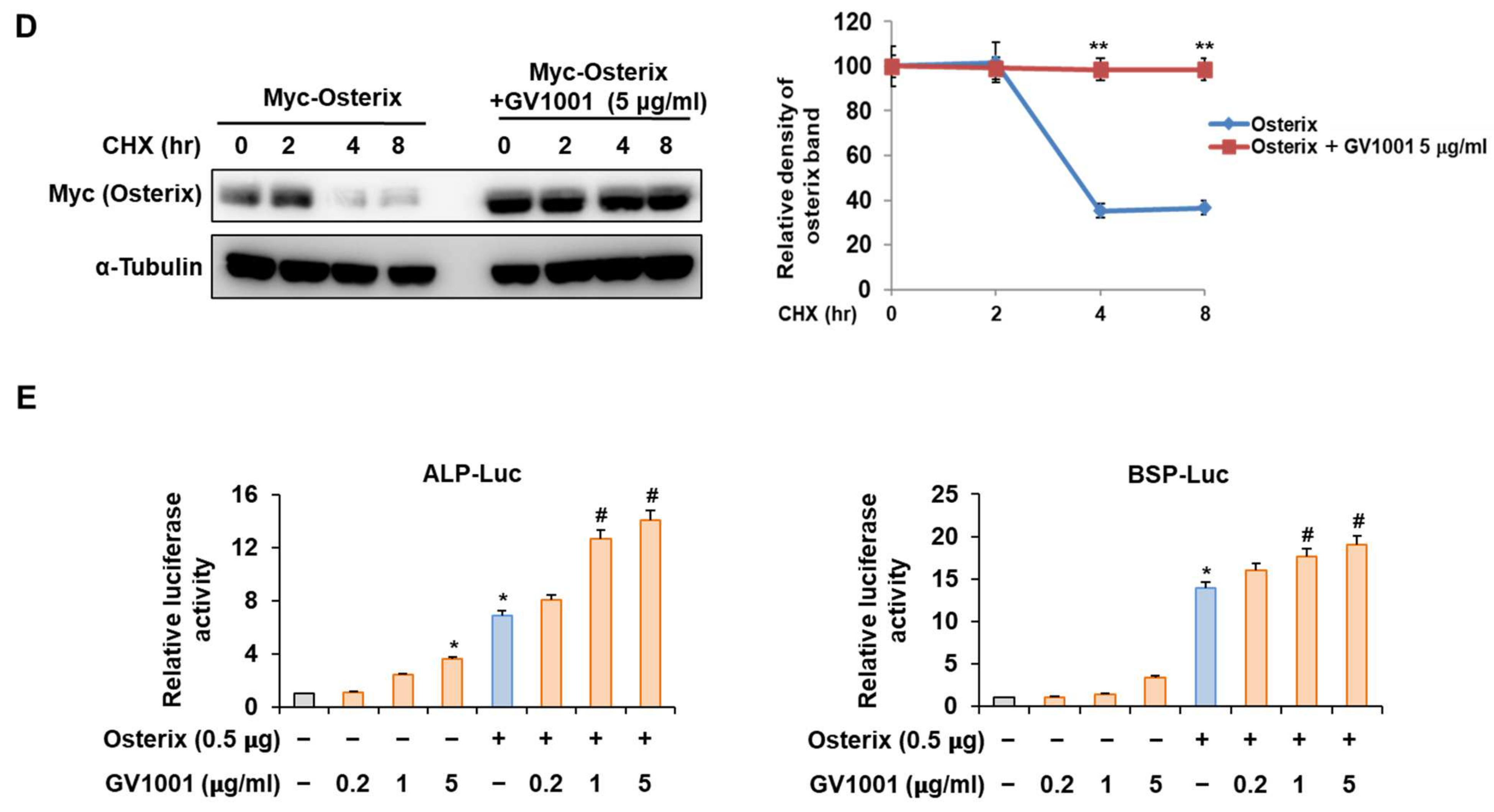
3.6. GV1001 Also Increases the Osteoblast Differentiation by Stabilization of Osterix Protein
3.7. GV1001 Reduces OVX-Induced Bone Destruction in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, T.; Das, N.; Imran, S. The Prevention and Therapy of Osteoporosis: A Review on Emerging Trends from Hormonal Therapy to Synthetic Drugs to Plant-Based Bioactives. J. Diet. Suppl. 2019, 16, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Ganji, R.; Moghbeli, M.; Sadeghi, R.; Bayat, G.; Ganji, A. Prevalence of osteoporosis and osteopenia in men and premenopausal women with celiac disease: A systematic review. Nutr. J. 2019, 18, 9. [Google Scholar] [CrossRef]
- Zleik, N.; Weaver, F.; Harmon, R.L.; Le, B.; Radhakrishnan, R.; Jirau-Rosaly, W.D.; Craven, B.C.; Raiford, M.; Hill, J.N.; Etingen, B.; et al. Prevention and management of osteoporosis and osteoporotic fractures in persons with a spinal cord injury or disorder: A systematic scoping review. J. Spinal Cord Med. 2019, 42, 735–759. [Google Scholar] [CrossRef] [PubMed]
- Lewiecki, E.M.; Miller, P.D.; Harris, S.T.; Bauer, D.C.; Davison, K.S.; Dian, L.; Hanley, D.A.; McClung, M.R.; Yuen, C.K.; Kendler, D.L. Understanding and communicating the benefits and risks of denosumab, raloxifene, and teriparatide for the treatment of osteoporosis. J. Clin. Densitom. 2014, 17, 490–495. [Google Scholar] [CrossRef]
- Feigerlova, E.; Demarquet, L.; Gueant, J.L. One carbon metabolism and bone homeostasis and remodeling: A review of experimental research and population studies. Biochimie 2016, 126, 115–123. [Google Scholar] [CrossRef]
- Guralp, O. Effects of vitamin E on bone remodeling in perimenopausal women: Mini review. Maturitas 2014, 79, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, S.; Li, X.; Zhang, X.; Yan, M. Structure, function and molecular design strategies of antibacterial peptide SMAP-29: A review. Sheng Wu Gong Cheng Xue Bao Chin. J. Biotechnol. 2011, 27, 846–859. [Google Scholar]
- Owji, H.; Nezafat, N.; Negahdaripour, M.; Hajiebrahimi, A.; Ghasemi, Y. A comprehensive review of signal peptides: Structure, roles, and applications. Eur. J. Cell Biol. 2018, 97, 422–441. [Google Scholar] [CrossRef]
- Lazurova, I.; Pura, M.; Wagnerova, H.; Tajtakova, M.; Sedlakova, M.; Tomas, L.; Payer, J.; Hruzikova, P.; Vanuga, P.; Podoba, J.; et al. Effect of growth hormone replacement therapy on plasma brain natriuretic peptide concentration, cardiac morphology and function in adults with growth hormone deficiency. Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2010, 118, 172–176. [Google Scholar] [CrossRef]
- Lee, A.C.; Harris, J.L.; Khanna, K.K.; Hong, J.H. A Comprehensive Review on Current Advances in Peptide Drug Development and Design. Int. J. Mol. Sci. 2019, 20, 2383. [Google Scholar] [CrossRef]
- Liu, J.; Gray, W.D.; Davis, M.E.; Luo, Y. Peptide- and saccharide-conjugated dendrimers for targeted drug delivery: A concise review. Interface Focus 2012, 2, 307–324. [Google Scholar] [CrossRef] [PubMed]
- Kyte, J.A. Cancer vaccination with telomerase peptide GV1001. Expert Opin. Investig. Drugs 2009, 18, 687–694. [Google Scholar] [CrossRef]
- Park, Y.H.; Jung, A.R.; Kim, G.E.; Kim, M.Y.; Sung, J.W.; Shin, D.; Cho, H.J.; Ha, U.S.; Hong, S.H.; Kim, S.W.; et al. GV1001 inhibits cell viability and induces apoptosis in castration-resistant prostate cancer cells through the AKT/NF-κB/VEGF pathway. J. Cancer 2019, 10, 6269–6277. [Google Scholar] [CrossRef]
- Kim, J.W.; Yadav, D.K.; Kim, S.J.; Lee, M.Y.; Park, J.M.; Kim, B.S.; Kim, M.H.; Park, H.G.; Kang, K.W. Anti-cancer effect of GV1001 for prostate cancer: Function as a ligand of GnRHR. Endocr. Relat. Cancer 2019, 26, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Fanis, P.; Neocleous, V.; Papapetrou, I.; Phylactou, L.A.; Skordis, N. Gonadotropin-Releasing Hormone Receptor (GnRHR) and Hypogonadotropic Hypogonadism. Int. J. Mol. Sci. 2023, 24, 15965. [Google Scholar] [CrossRef] [PubMed]
- Gennari, L.; Merlotti, D.; De Paola, V.; Calabro, A.; Becherini, L.; Martini, G.; Nuti, R. Estrogen receptor gene polymorphisms and the genetics of osteoporosis: A HuGE review. Am. J. Epidemiol. 2005, 161, 307–320. [Google Scholar] [CrossRef]
- Shaw, V.E.; Naisbitt, D.J.; Costello, E.; Greenhalf, W.; Park, B.K.; Neoptolemos, J.P.; Middleton, G.W. Current status of GV1001 and other telomerase vaccination strategies in the treatment of cancer. Expert Rev. Vaccines 2010, 9, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Roth, B. Penetration of parenterally administered rifampicin into bone tissue. Chemotherapy 1984, 30, 358–365. [Google Scholar] [CrossRef]
- Piga, I.; Magni, F.; Smith, A. The journey towards clinical adoption of MALDI-MS-based imaging proteomics: From current challenges to future expectations. FEBS Lett. 2024, 598, 621–634. [Google Scholar] [CrossRef]
- Choi, J.H.; Han, Y.; Kim, Y.A.; Jin, S.W.; Lee, G.H.; Jeong, H.M.; Lee, H.S.; Chung, Y.C.; Lee, Y.C.; Kim, E.J.; et al. Platycodin D Inhibits Osteoclastogenesis by Repressing the NFATc1 and MAPK Signaling Pathway. J. Cell. Biochem. 2017, 118, 860–868. [Google Scholar] [CrossRef]
- Azhar, M.A.; Wright, M.; Kamal, A.; Nagy, J.; Miller, A.D. Biotin-c10-AppCH2ppA is an effective new chemical proteomics probe for diadenosine polyphosphate binding proteins. Bioorganic Med. Chem. Lett. 2014, 24, 2928–2933. [Google Scholar] [CrossRef] [PubMed]
- Galigniana, M.D. Peptidyl-Prolyl Isomerase Activity of Immunophilins Could Be the Mere Consequence of Protein Complex Organization. Bioessays 2020, 42, e2000073. [Google Scholar] [CrossRef]
- Kim, W.J.; Islam, R.; Kim, B.S.; Cho, Y.D.; Yoon, W.J.; Baek, J.H.; Woo, K.M.; Ryoo, H.M. Direct Delivery of Recombinant Pin1 Protein Rescued Osteoblast Differentiation of Pin1-Deficient Cells. J. Cell. Physiol. 2017, 232, 2798–2805. [Google Scholar] [CrossRef] [PubMed]
- Brennan-Speranza, T.C.; Conigrave, A.D. Osteocalcin: An osteoblast-derived polypeptide hormone that modulates whole body energy metabolism. Calcif. Tissue Int. 2015, 96, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, Y.H.; Kim, Y.J.; Choi, H.S.; Yeo, C.Y.; Lee, K.Y. Prolyl isomerase Pin1 enhances osteoblast differentiation through Runx2 regulation. FEBS Lett. 2013, 587, 3640–3647. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jeong, H.M.; Han, Y.; Cheong, H.; Kang, B.Y.; Lee, K.Y. Prolyl isomerase Pin1 regulates the osteogenic activity of Osterix. Mol. Cell. Endocrinol. 2015, 400, 32–40. [Google Scholar] [CrossRef]
- Marie, P.J.; Kassem, M. Osteoblasts in osteoporosis: Past, emerging, and future anabolic targets. Eur. J. Endocrinol. 2011, 165, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gallacher, S.J.; Dixon, T. Impact of treatments for postmenopausal osteoporosis (bisphosphonates, parathyroid hormone, strontium ranelate, and denosumab) on bone quality: A systematic review. Calcif. Tissue Int. 2010, 87, 469–484. [Google Scholar] [CrossRef]
- Choonara, B.F.; Choonara, Y.E.; Kumar, P.; Bijukumar, D.; du Toit, L.C.; Pillay, V. A review of advanced oral drug delivery technologies facilitating the protection and absorption of protein and peptide molecules. Biotechnol. Adv. 2014, 32, 1269–1282. [Google Scholar] [CrossRef]
- Mander, K.; Harford-Wright, E.; Lewis, K.M.; Vink, R. Advancing drug therapy for brain tumours: A current review of the pro-inflammatory peptide Substance P and its antagonists as anti-cancer agents. Recent Pat. CNS Drug Discov. 2014, 9, 110–121. [Google Scholar] [CrossRef]
- Banno, Y.; Sasaki, S.; Kamata, M.; Kunitomo, J.; Miyamoto, Y.; Abe, H.; Taya, N.; Oi, S.; Watanabe, M.; Urushibara, T.; et al. Design and synthesis of a novel series of orally active, selective somatostatin receptor 2 agonists for the treatment of type 2 diabetes. Bioorganic Med. Chem. 2017, 25, 5995–6006. [Google Scholar] [CrossRef] [PubMed]
- Busch, R.S.; Kane, M.P. Combination SGLT2 inhibitor and GLP-1 receptor agonist therapy: A complementary approach to the treatment of type 2 diabetes. Postgrad. Med. 2017, 129, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, J.; Li, Y.; Zhou, Y.; Cui, Y.; Yang, G.; Hong, Y. Rap1A Regulates Osteoblastic Differentiation via the ERK and p38 Mediated Signaling. PLoS ONE 2015, 10, e0143777. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Song, Y.M.; Cho, T.H.; Kim, J.Y.; Weber, F.E.; Hwang, S.J. Synergistic action of static stretching and BMP-2 stimulation in the osteoblast differentiation of C2C12 myoblasts. J. Biomech. 2009, 42, 2721–2727. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Peng, H.; Corsi, K.; Usas, A.; Olshanski, A.; Huard, J. Differential effect of BMP4 on NIH/3T3 and C2C12 cells: Implications for endochondral bone formation. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2005, 20, 1611–1623. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Cbfa1/Runx2, an essential transcription factor for the regulation of osteoblast differentiation. Nihon Rinsho. Jpn. J. Clin. Med. 2002, 60 (Suppl. S3), 91–97. [Google Scholar]
- Sinha, K.M.; Zhou, X. Genetic and molecular control of osterix in skeletal formation. J. Cell. Biochem. 2013, 114, 975–984. [Google Scholar] [CrossRef]
- Matsubara, T.; Kida, K.; Yamaguchi, A.; Hata, K.; Ichida, F.; Meguro, H.; Aburatani, H.; Nishimura, R.; Yoneda, T. BMP2 regulates Osterix through Msx2 and Runx2 during osteoblast differentiation. J. Biol. Chem. 2008, 283, 29119–29125. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kim, Y.J.; Jeong, H.M.; Jin, Y.H.; Yeo, C.Y.; Lee, K.Y. Akt enhances Runx2 protein stability by regulating Smurf2 function during osteoblast differentiation. FEBS J. 2014, 281, 3656–3666. [Google Scholar] [CrossRef]
- Salingcarnboriboon, R.A.; Pavasant, P.; Noda, M. Cbl-b enhances Runx2 protein stability and augments osteocalcin promoter activity in osteoblastic cell lines. J. Cell. Physiol. 2010, 224, 743–747. [Google Scholar] [CrossRef]
- Yoon, W.J.; Islam, R.; Cho, Y.D.; Woo, K.M.; Baek, J.H.; Uchida, T.; Komori, T.; van Wijnen, A.; Stein, J.L.; Lian, J.B.; et al. Pin1-mediated Runx2 modification is critical for skeletal development. J. Cell. Physiol. 2013, 228, 2377–2385. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Yang, H.Y.; Chang, S.C.; Kim, Y.M.; Lee, K.Y.; Lee, B.M.; Kim, H.S. Potential repositioning of GV1001 as a therapeutic agent for testosterone-induced benign prostatic hyperplasia. Int. J. Mol. Med. 2018, 42, 2260–2268. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, Y.J.; Kim, S.; Momeni, M.; Lee, A.; Treanor, A.; Kim, S.; Kim, R.H.; Park, N.H. GV1001 Inhibits the Severity of the Ligature-Induced Periodontitis and the Vascular Lipid Deposition Associated with the Periodontitis in Mice. Int. J. Mol. Sci. 2023, 24, 12566. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Kim, Y.; Kim, H.; Jeon, J.; Kim, T.W.; Park, J.H.; Hwnag, Y.I.; Lee, W.J.; Kang, J.S. The anti-fibrotic effect of GV1001 combined with gemcitabine on treatment of pancreatic ductal adenocarcinoma. Oncotarget 2016, 7, 75081–75093. [Google Scholar] [CrossRef]


| Gene | Primer Sequence (5′→3′) |
|---|---|
| Alp | (F) 5′-GGA CAT GCA GTA CGA GCT GA-3′ |
| (R) 5′-GCA GTG AAG GGC TTC TTG TC-3′ | |
| Bsp | (F) 5′-GCG AAG CAG AAG TGG ATG AAA -3′ |
| (R) 5′-TGC CTC TGT GCT GTT GGT ACT G -3′ | |
| Col1α1 | (F) 5′-CTG ACC TTC CTG CGC CTG ATG TCC-3′ |
| (R) 5′-GTC TGG GGC ACC AAC GTC CAA GGG-3′ | |
| Runx2 | (F) 5′-AGC AAC AGC AAC AGC AG-3′ |
| (R) 5′-GTA ATC TGA CTC TGT CCT TG-3′ | |
| Gapdh | (F) 5′-ACC ACA GTC CAT GCC ATC A-3′ |
| (R) 5′-TCC ACC ACC CTG TTG CTG T--3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piao, M.; Lee, S.H.; Hwang, J.W.; Kim, H.S.; Han, Y.H.; Lee, K.Y. The Cell-Penetrating Peptide GV1001 Enhances Bone Formation via Pin1-Mediated Augmentation of Runx2 and Osterix Stability. Biomolecules 2024, 14, 812. https://doi.org/10.3390/biom14070812
Piao M, Lee SH, Hwang JW, Kim HS, Han YH, Lee KY. The Cell-Penetrating Peptide GV1001 Enhances Bone Formation via Pin1-Mediated Augmentation of Runx2 and Osterix Stability. Biomolecules. 2024; 14(7):812. https://doi.org/10.3390/biom14070812
Chicago/Turabian StylePiao, Meiyu, Sung Ho Lee, Jin Wook Hwang, Hyung Sik Kim, Youn Ho Han, and Kwang Youl Lee. 2024. "The Cell-Penetrating Peptide GV1001 Enhances Bone Formation via Pin1-Mediated Augmentation of Runx2 and Osterix Stability" Biomolecules 14, no. 7: 812. https://doi.org/10.3390/biom14070812
APA StylePiao, M., Lee, S. H., Hwang, J. W., Kim, H. S., Han, Y. H., & Lee, K. Y. (2024). The Cell-Penetrating Peptide GV1001 Enhances Bone Formation via Pin1-Mediated Augmentation of Runx2 and Osterix Stability. Biomolecules, 14(7), 812. https://doi.org/10.3390/biom14070812








