Effect of Flavonols of Aronia melanocarpa Fruits on Morphofunctional State of Immunocompetent Organs of Rats under Cyclophosphamide-Induced Immunosuppression
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.1.1. Extract Preparation
2.1.2. Chemicals and Reagents
2.2. Ex Vivo Experiments
2.2.1. Histological Analysis of Thymus and Spleen
2.2.2. Thymus Tissue Analysis
2.2.3. Spleen Tissue Analysis
2.2.4. Effect of the Aronia Flavonol Fraction on Spleen and Thymus Indices
2.2.5. Determination of the Level of Lipid Peroxidation in the Blood of Rats
2.3. Statistical Analysis
3. Results
3.1. Effect of Flavonols on Immune Organ Mass Indices
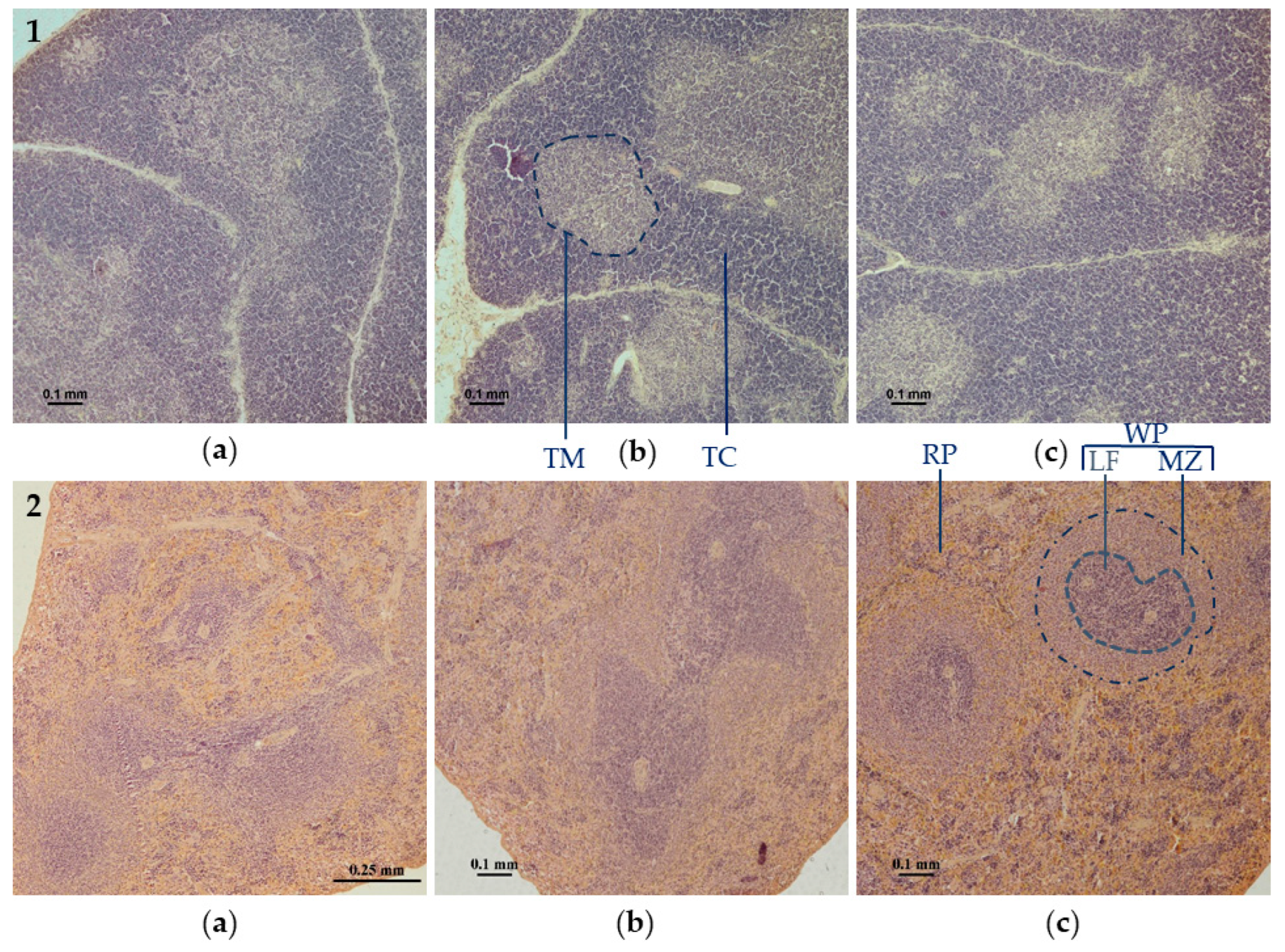
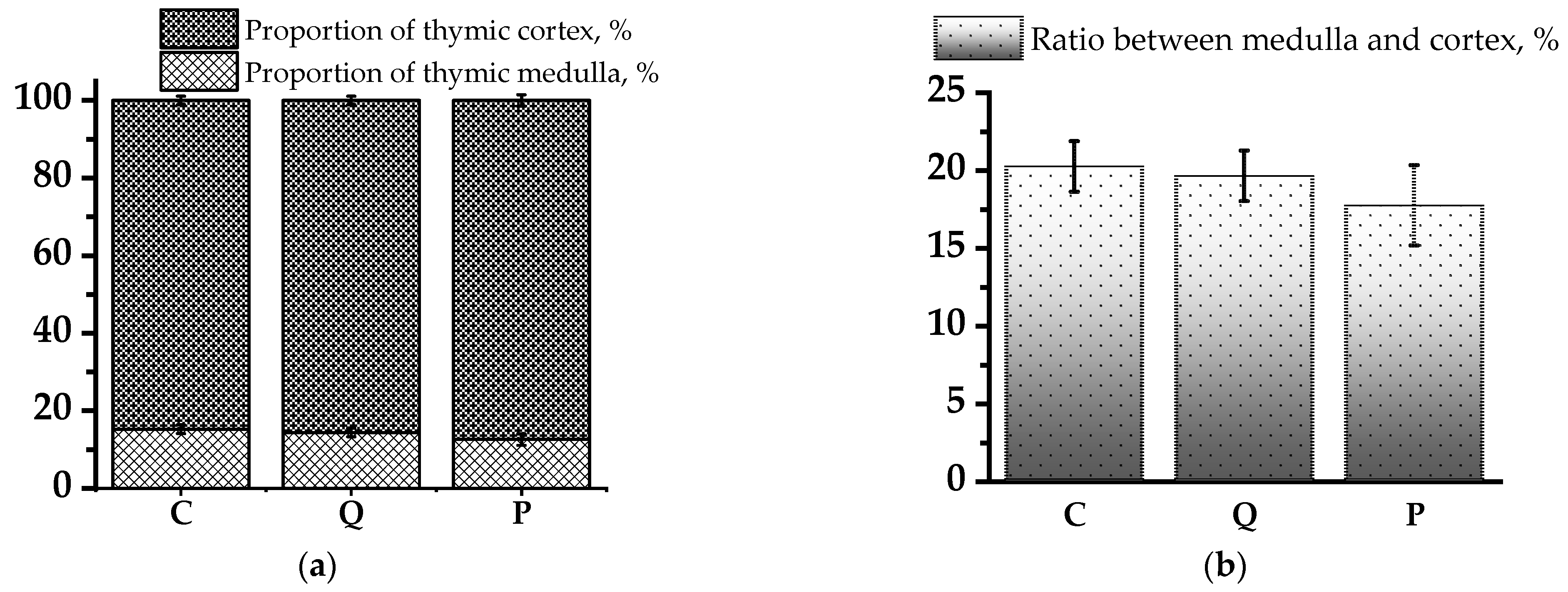
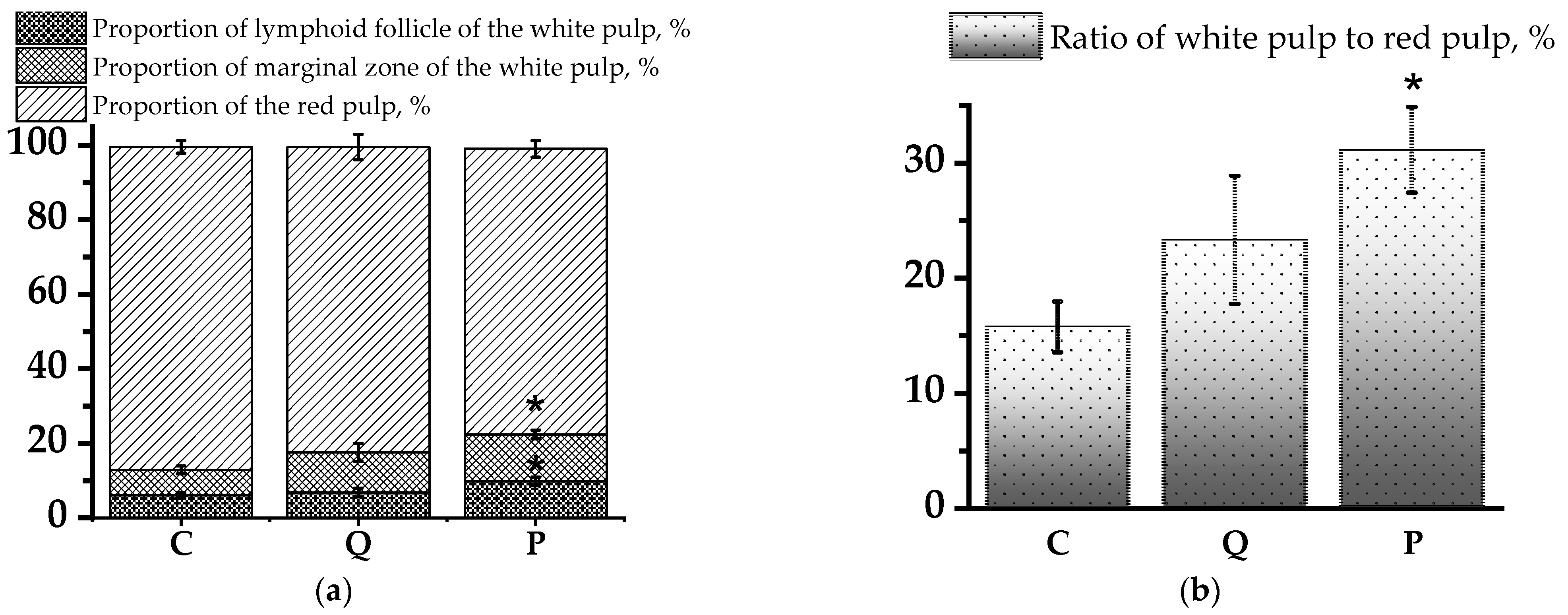
3.2. Evaluation of Morphological Changes in Rat Thymus and Spleen Tissues Using Light Microscopy
3.3. Effect of Flavonols on Lipid Peroxidation Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raj, S.; Gothandam, K. Immunomodulatory activity of methanolic extract of Amorphophallus commutatus var. wayanadensis under normal and cyclophosphamide induced immunosuppressive conditions in mice models. Food Chem. Toxicol. 2015, 81, 151–159. [Google Scholar] [CrossRef]
- Ding, Y.; Yan, Y.; Chen, D.; Ran, L.; Mi, J.; Lu, L.; Jing, B.; Li, X.; Zeng, X.; Cao, Y. Modulating effects of polysaccharides from the fruits of Lycium barbarum on the immune response and gut microbiota in cyclophosphamide-treated mice. Food Funct. 2019, 10, 3671–3683. [Google Scholar] [CrossRef]
- Tang, C.; Sun, J.; Zhou, B.; Jin, C.; Liu, J.; Kan, J.; Qian, C.; Zhang, N. Effects of polysaccharides from purple sweet potatoes on immune response and gut microbiota composition in normal and cyclophosphamide treated mice. Food Funct. 2018, 9, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Caglayan, C.; Kandemir, F.M.; Darendelioğlu, E.; Yıldırım, S.; Kucukler, S.; Dortbudak, M.B. Rutin ameliorates mercuric chloride-induced hepatotoxicity in rats via interfering with oxidative stress, inflammation and apoptosis. J. Trace Elem. Med. Biol. 2019, 56, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.A. Immunopathology in Toxicology and Drug Development; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Rezzani, R.; Nardo, L.; Favero, G.; Peroni, M.; Rodella, L.F. Thymus and aging: Morphological, radiological, and functional overview. Age 2014, 36, 313–351. [Google Scholar] [CrossRef]
- Ferrando-Martínez, S.; Ruiz-Mateos, E.; Dudakov, J.A.; Velardi, E.; Grillari, J.; Kreil, D.P.; Muñoz-Fernandez, M.Á.; van den Brink, M.R.; Leal, M. WNT signaling suppression in the senescent human thymus. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2015, 70, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Ni, X.; Song, X.; Wen, B.; Zhou, Y.; Zou, F.; Yang, M.; Peng, Z.; Zhu, H.; Zeng, Y. Fermented Yupingfeng polysaccharides enhance immunity by improving the foregut microflora and intestinal barrier in weaning rex rabbits. Appl. Microbiol. Biotechnol. 2016, 100, 8105–8120. [Google Scholar] [CrossRef]
- Steiniger, B.S. Human spleen microanatomy: Why mice do not suffice. Immunology 2015, 145, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Devi, H.P.; Mazumder, P.B. Methanolic extract of Curcuma caesia Roxb. prevents the toxicity caused by cyclophosphamide to bone marrow cells, liver and kidney of mice. Pharmacogn. Res. 2016, 8, 43. [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Njoku, R.-C.; Lawrence, C.J.; Charles, I.A.; Ikewuchi, J.C. Rutin ameliorates oxidative stress and preserves hepatic and renal functions following exposure to cadmium and ethanol. Pharm. Biol. 2017, 55, 2161–2169. [Google Scholar] [CrossRef]
- Zhai, J.; Zhang, F.; Gao, S.; Chen, L.; Feng, G.; Yin, J.; Chen, W. Schisandra chinensis extract decreases chloroacetaldehyde production in rats and attenuates cyclophosphamide toxicity in liver, kidney and brain. J. Ethnopharmacol. 2018, 210, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, X.; Li, S.; Wang, H.; Yu, L.; Wang, P. The effects of l-carnitine against cyclophosphamide-induced injuries in mouse testis. Basic Clin. Pharmacol. Toxicol. 2017, 120, 152–158. [Google Scholar] [CrossRef]
- Kocahan, S.; Dogan, Z.; Erdemli, E.; Taskin, E. Protective effect of quercetin against oxidative stress-induced toxicity associated with doxorubicin and cyclophosphamide in rat kidney and liver tissue. Iran. J. Kidney Dis. 2017, 11, 124. [Google Scholar]
- Attia, A.A.; El-Twessy, M.Y.; Ghoneam, H.E.; Hammed, N.S. Histopathological and immunohistochemical studies on the role of α-Lipoic acid on cyclophosphamide-induced immunosuppressive effect in mice. Delta J. Sci. 2013, 36, 85–105. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Guo, B.; Ye, M.-J.; Liao, R.-F.; Li, S.-L. Protective effect of rutin against H2O2-induced oxidative stress and apoptosis in human lens epithelial cells. Curr. Eye Res. 2016, 41, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Zargar, S.; Siddiqi, N.J.; Al Daihan, S.K.; Wani, T.A. Protective effects of quercetin on cadmium fluoride induced oxidative stress at different intervals of time in mouse liver. Acta Biochim. Pol. 2015, 62, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Ebokaiwe, A.P.; Obasi, D.O.; Njoku, R.C.C.; Osawe, S.; Olusanya, O.; Kalu, W.O. Cyclophosphamide instigated hepatic-renal oxidative/inflammatory stress aggravates immunosuppressive indoleamine 2,3-dioxygenase in male rats: Abatement by quercetin. Toxicology 2021, 464, 153027. [Google Scholar] [CrossRef] [PubMed]
- Andreyeva, V.Y.; Sheykin, V.V.; Kalinkina, G.I.; Razina, T.G.; Zuyeva, Y.P.; Rybalkina, O.Y.; Ulrikh, A.V. Development of A Drug Based on the Fruits of Chokeberry (Aronia Melanocarpa (Michx.) Elliot) Improving the Efficiency of Cancer Chemotherapy. Khimija Rastit. Syrja 2020, 4, 219–226. [Google Scholar]
- Bushmeleva, K.; Vyshtakalyuk, A.; Terenzhev, D.; Belov, T.; Nikitin, E.; Zobov, V. Antioxidative and Immunomodulating Properties of Aronia melanocarpa Extract Rich in Anthocyanins. Plants 2022, 11, 3333. [Google Scholar] [CrossRef]
- Bushmeleva, K.; Vyshtakalyuk, A.; Terenzhev, D.; Belov, T.; Nikitin, E.; Zobov, V. Aronia melanocarpa Flavonol Extract—Antiradical and Immunomodulating Activities Analysis. Plants 2023, 12, 2976. [Google Scholar] [CrossRef]
- Slimestad, R.; Torskangerpoll, K.; Nateland, H.S.; Johannessen, T.; Giske, N.H. Flavonoids from black chokeberries, Aronia melanocarpa. J. Food Compos. Anal. 2005, 18, 61–68. [Google Scholar] [CrossRef]
- Mironov, A. A Guide to Preclinical Drug Research; Grif and K: Moscow, Russia, 2012; pp. 17–26. [Google Scholar]
- Commission, E. Commission recommendation of 18 June 2007 on guidelines for the accommodation and care of animals used for experimental and other scientific purposes. Off. J. Eur. Union 2007, 197, 1–89. [Google Scholar]
- Valcheva-Kuzmanova, S.; Borisova, P.; Galunska, B.; Krasnaliev, I.; Belcheva, A. Hepatoprotective effect of the natural fruit juice from Aronia melanocarpa on carbon tetrachloride-induced acute liver damage in rats. Exp. Toxicol. Pathol. 2004, 56, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Ku, C.S.; Pham, T.X.; Park, Y.; Martin, D.A.; Xie, L.; Taheri, R.; Lee, J.; Bolling, B.W. Aronia melanocarpa (chokeberry) polyphenol–rich extract improves antioxidant function and reduces total plasma cholesterol in apolipoprotein E knockout mice. Nutr. Res. 2013, 33, 406–413. [Google Scholar] [CrossRef]
- Borowska, S.; Brzóska, M.M. Chokeberries (Aronia melanocarpa) and their products as a possible means for the prevention and treatment of noncommunicable diseases and unfavorable health effects due to exposure to xenobiotics. Compr. Rev. Food Sci. Food Saf. 2016, 15, 982–1017. [Google Scholar] [CrossRef] [PubMed]
- Bushmeleva, K.; Vyshtakalyuk, A.; Terenzhev, D.; Belov, T.; Parfenov, A.; Sharonova, N.; Nikitin, E.; Zobov, V. Radical scavenging actions and immunomodulatory activity of Aronia melanocarpa propylene glycol extracts. Plants 2021, 10, 2458. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1978; Volume 52, pp. 302–310. [Google Scholar]
- Fu, Y.-P.; Feng, B.; Zhu, Z.-K.; Feng, X.; Chen, S.-F.; Li, L.-X.; Yin, Z.-Q.; Huang, C.; Chen, X.-F.; Zhang, B.-Z. The polysaccharides from Codonopsis pilosula modulates the immunity and intestinal microbiota of cyclophosphamide-treated immunosuppressed mice. Molecules 2018, 23, 1801. [Google Scholar] [CrossRef] [PubMed]
- Shirani, K.; Hassani, F.V.; Razavi-Azarkhiavi, K.; Heidari, S.; Zanjani, B.R.; Karimi, G. Phytotrapy of cyclophosphamide-induced immunosuppression. Environ. Toxicol. Pharmacol. 2015, 39, 1262–1275. [Google Scholar] [CrossRef]
- Zhang, Q.; Cong, R.; Hu, M.; Zhu, Y.; Yang, X. Immunoenhancement of edible fungal polysaccharides (lentinan, tremellan, and pachymaran) on cyclophosphamide-induced immunosuppression in mouse model. Evid.-Based Complement. Altern. Med. 2017, 2017, 9459156. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Park, Y.M.; Kim, J.; Oh, H.G.; Kim, K.S.; Kang, H.J.; Kim, R.R.; Kim, M.J.; Kim, S.H.; Yang, H.J. Orostachys japonicus A. Berger extracts induce immunity-enhancing effects on cyclophosphamide-treated immunosuppressed rats. BioMed Res. Int. 2019, 2019, 9461960. [Google Scholar] [CrossRef]
- Lee, S.I.; Kang, K.S. Omega-3 fatty acids modulate cyclophosphamide induced markers of immunosuppression and oxidative stress in pigs. Sci. Rep. 2019, 9, 2684. [Google Scholar] [CrossRef]
- Dong, A.; Yu, J.; Chen, X.; Wang, L.-S. Potential of dietary supplementation with berries to enhance immunity in humans. J. Food Bioact. 2021, 16, 19–24. [Google Scholar] [CrossRef]
- Akram, M.; Tahir, I.M.; Shah, S.M.A.; Mahmood, Z.; Altaf, A.; Ahmad, K.; Munir, N.; Daniyal, M.; Nasir, S.; Mehboob, H. Antiviral potential of medicinal plants against HIV, HSV, influenza, hepatitis, and coxsackievirus: A systematic review. Phytother. Res. 2018, 32, 811–822. [Google Scholar] [CrossRef]
- Ho, G.T.; Bräunlich, M.; Austarheim, I.; Wangensteen, H.; Malterud, K.E.; Slimestad, R.; Barsett, H. Immunomodulating activity of Aronia melanocarpa polyphenols. Int. J. Mol. Sci. 2014, 15, 11626–11636. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, N.; Azizullah, H.; Malterud, K.E.; Inngjerdingen, K.T.; Wangensteen, H. Immunomodulating polyphenols from Sideritis scardica. J. Funct. Foods 2022, 96, 105197. [Google Scholar] [CrossRef]
- Shukla, M.K.; Singh, S.K.; Pandey, S.; Gupta, P.K.; Choudhary, A.; Jindal, D.K.; Dua, K.; Kumar, D. Potential immunomodulatory activities of plant products. S. Afr. J. Bot. 2022, 149, 937–943. [Google Scholar] [CrossRef]
- Barreca, D.; Trombetta, D.; Smeriglio, A.; Mandalari, G.; Romeo, O.; Felice, M.R.; Gattuso, G.; Nabavi, S.M. Food flavonols: Nutraceuticals with complex health benefits and functionalities. Trends Food Sci. Technol. 2021, 117, 194–204. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Black chokeberry Aronia melanocarpa L.—A qualitative composition, phenolic profile and antioxidant potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef] [PubMed]
- King, E.S.; Bolling, B.W. Composition, polyphenol bioavailability, and health benefits of aronia berry: A review. J. Food Bioact. 2020, 11, 13–30. [Google Scholar] [CrossRef]
- Kraus, M.D. Splenic histology and histopathology: An update. In Seminars in Diagnostic Pathology; Elsevier: Amsterdam, The Netherlands, 2003; Volume 20, pp. 84–93. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, Q.; Li, A.; Yang, M.; Huang, W.; Xu, H.; Zhao, Z.; Li, S. Immuno-enhancement effects of Yifei Tongluo Granules on cyclophosphamide-induced immunosuppression in Balb/c mice. J. Ethnopharmacol. 2016, 194, 72–82. [Google Scholar] [CrossRef]
- Liu, M.-W.; Su, M.-X.; Zhang, W.; Zhang, L.-M.; Wang, Y.-H.; Qian, C.-Y. Rhodiola rosea suppresses thymus T-lymphocyte apoptosis by downregulating tumor necrosis factor-α-induced protein 8-like-2 in septic rats. Int. J. Mol. Med. 2015, 36, 386–398. [Google Scholar] [CrossRef]
- Lim, S.-M.; Lee, H.S.; Jung, J.I.; Kim, S.M.; Kim, N.Y.; Seo, T.S.; Bae, J.-S.; Kim, E.J. Cyanidin-3-O-galactoside-enriched Aronia melanocarpa extract attenuates weight gain and adipogenic pathways in high-fat diet-induced obese C57BL/6 mice. Nutrients 2019, 11, 1190. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Anderson, R.A. An extract of chokeberry attenuates weight gain and modulates insulin, adipogenic and inflammatory signalling pathways in epididymal adipose tissue of rats fed a fructose-rich diet. Br. J. Nutr. 2012, 108, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Duchnowicz, P.; Ziobro, A.; Rapacka, E.; Koter-Michalak, M.; Bukowska, B. Changes in cholinesterase activity in blood of adolescent with metabolic syndrome after supplementation with extract from Aronia melanocarpa. BioMed Res. Int. 2018, 2018, 5670145. [Google Scholar] [CrossRef] [PubMed]
- ÇELİK, H.; Karabörk, Ş.; Şaylan, A.; Çetinkaya, A. Protective and histopathologic effects of Aronia melanocarpa extract against Cyclophosphamide-induced delayed toxicity on the bladder of female Wistar rats. Authorea Prepr. 2022. [Google Scholar] [CrossRef]
- Tasinov, O.; Dincheva, I.; Badjakov, I.; Grupcheva, C.; Galunska, B. Comparative phytochemical analysis of Aronia melanocarpa L. Fruit juices on bulgarian market. Plants 2022, 11, 1655. [Google Scholar] [CrossRef]

| Group C 1 | Group Q 2 | Group P 3 | |
|---|---|---|---|
| Day 1 | 362.5 ± 9.1 | 322.5 ± 7.2 ° | 326.3 ± 14.3 |
| Day 21 | 390.8 ± 8.4 * | 344.5 ± 6.7 *° | 352.5 ± 14.4 |
| Group C 1 | Group Q 2 | Group P 3 | |
|---|---|---|---|
| Day 1 | 31.66 ± 1.16 | 29.75 ± 0.77 | 26.35 ± 0.55 * |
| Day 21 | 37.53 ± 2.69 ° | 32.77 ± 0.81 ° | 24.95 ± 0.85 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bushmeleva, K.; Vyshtakalyuk, A.; Terenzhev, D.; Belov, T.; Nikitin, E.; Zobov, V. Effect of Flavonols of Aronia melanocarpa Fruits on Morphofunctional State of Immunocompetent Organs of Rats under Cyclophosphamide-Induced Immunosuppression. Biomolecules 2024, 14, 578. https://doi.org/10.3390/biom14050578
Bushmeleva K, Vyshtakalyuk A, Terenzhev D, Belov T, Nikitin E, Zobov V. Effect of Flavonols of Aronia melanocarpa Fruits on Morphofunctional State of Immunocompetent Organs of Rats under Cyclophosphamide-Induced Immunosuppression. Biomolecules. 2024; 14(5):578. https://doi.org/10.3390/biom14050578
Chicago/Turabian StyleBushmeleva, Kseniya, Alexandra Vyshtakalyuk, Dmitriy Terenzhev, Timur Belov, Evgeniy Nikitin, and Vladimir Zobov. 2024. "Effect of Flavonols of Aronia melanocarpa Fruits on Morphofunctional State of Immunocompetent Organs of Rats under Cyclophosphamide-Induced Immunosuppression" Biomolecules 14, no. 5: 578. https://doi.org/10.3390/biom14050578
APA StyleBushmeleva, K., Vyshtakalyuk, A., Terenzhev, D., Belov, T., Nikitin, E., & Zobov, V. (2024). Effect of Flavonols of Aronia melanocarpa Fruits on Morphofunctional State of Immunocompetent Organs of Rats under Cyclophosphamide-Induced Immunosuppression. Biomolecules, 14(5), 578. https://doi.org/10.3390/biom14050578


_Kwok.png)



