Organ-on-a-Chip Models—New Possibilities in Experimental Science and Disease Modeling
Abstract
1. Introduction
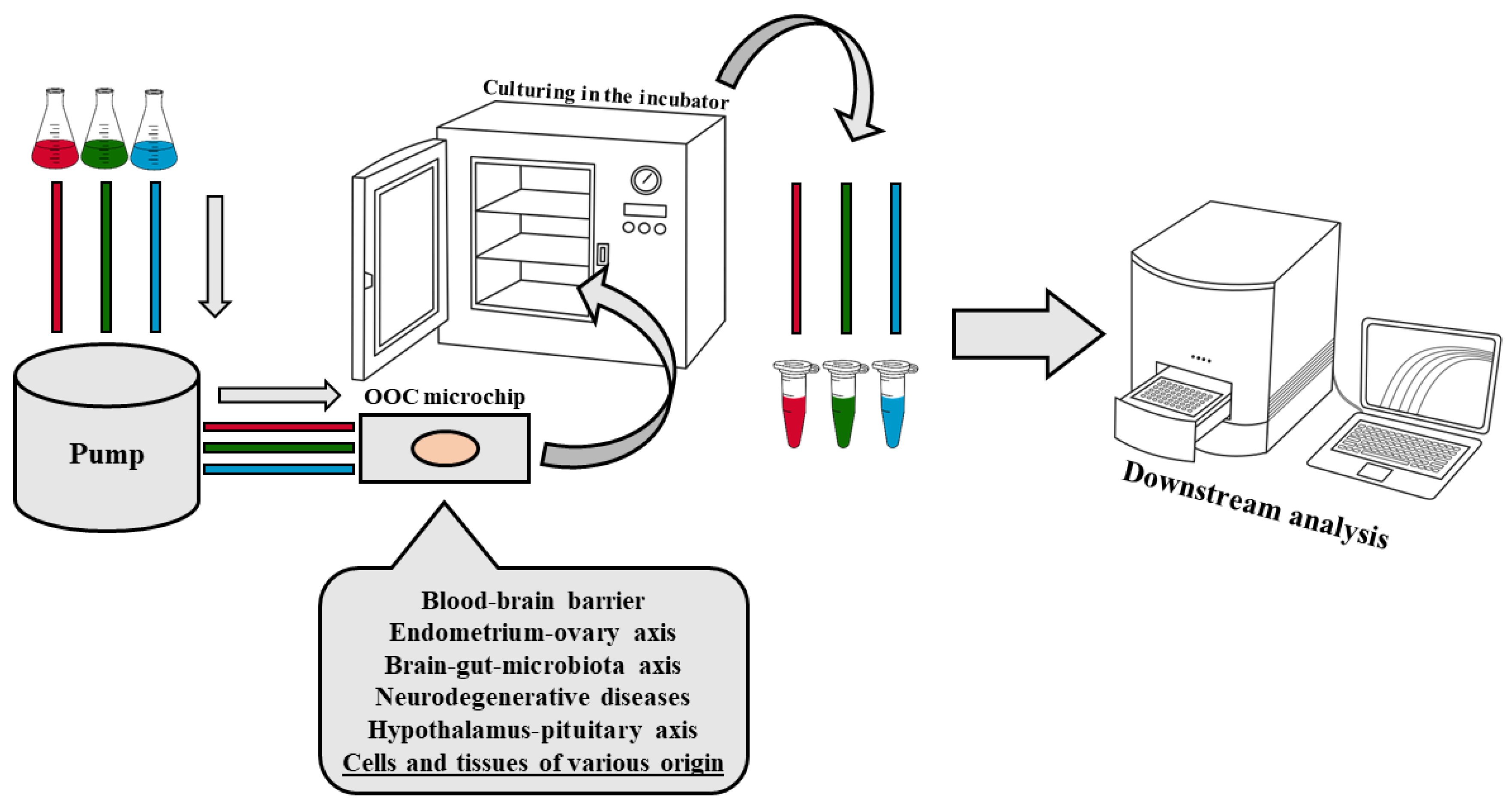
2. Organ-on-a-Chip Technology
2.1. Technical Aspects of the OOC Models’ Design
2.2. Biological Aspects of OOC Model Design
3. OOC Models in Neurosciences
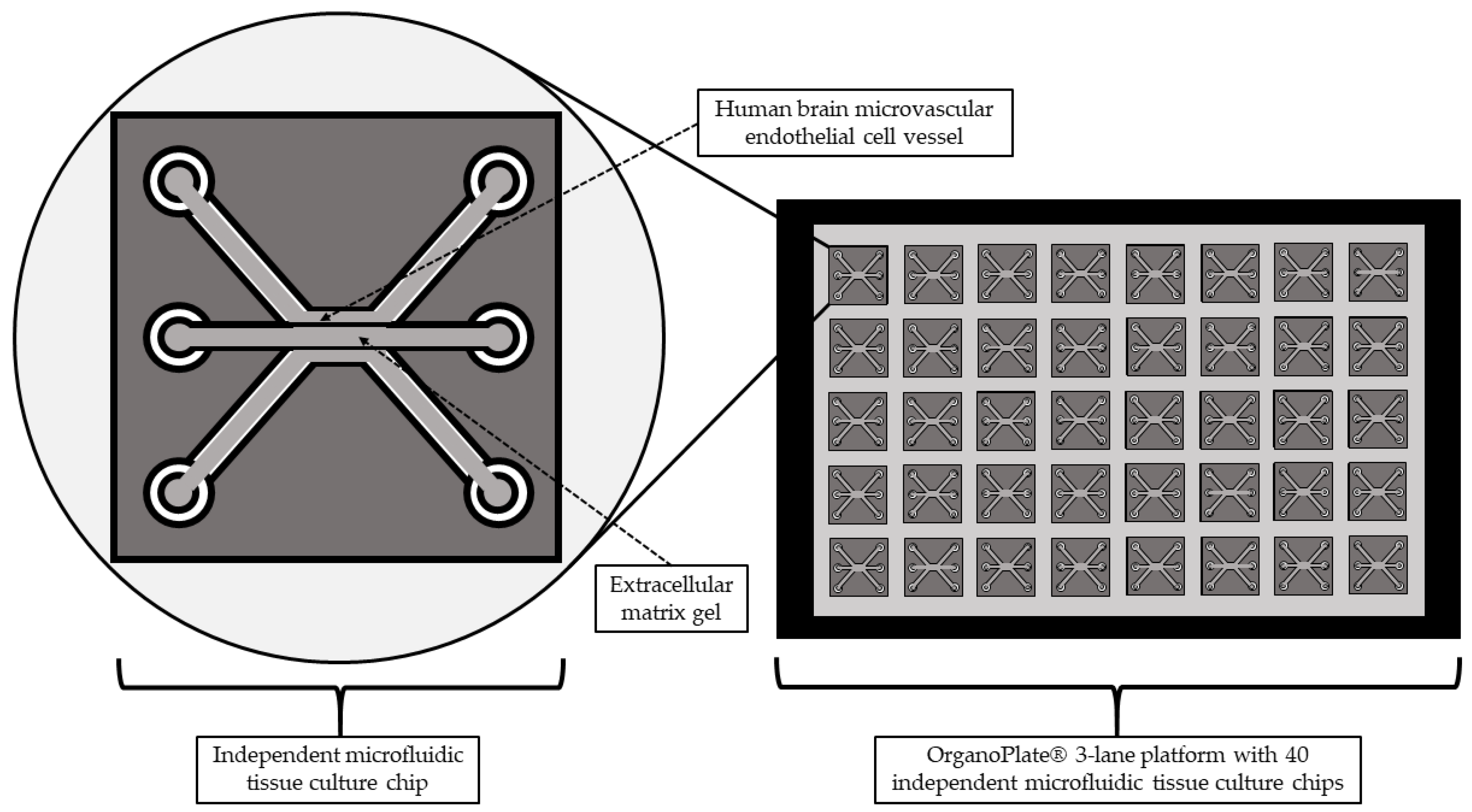
3.1. Blood–-Brain Barrier-on-a-Chip
3.2. Brain–Gut–Microbiota Axis-on-a-Chip
3.3. Amyotrophic Lateral Sclerosis-on-a-Chip
3.4. Parkinson’s Disease-on-a-Chip
4. The Introduction of the OOC Technology in Experimental Neuroendocrinology
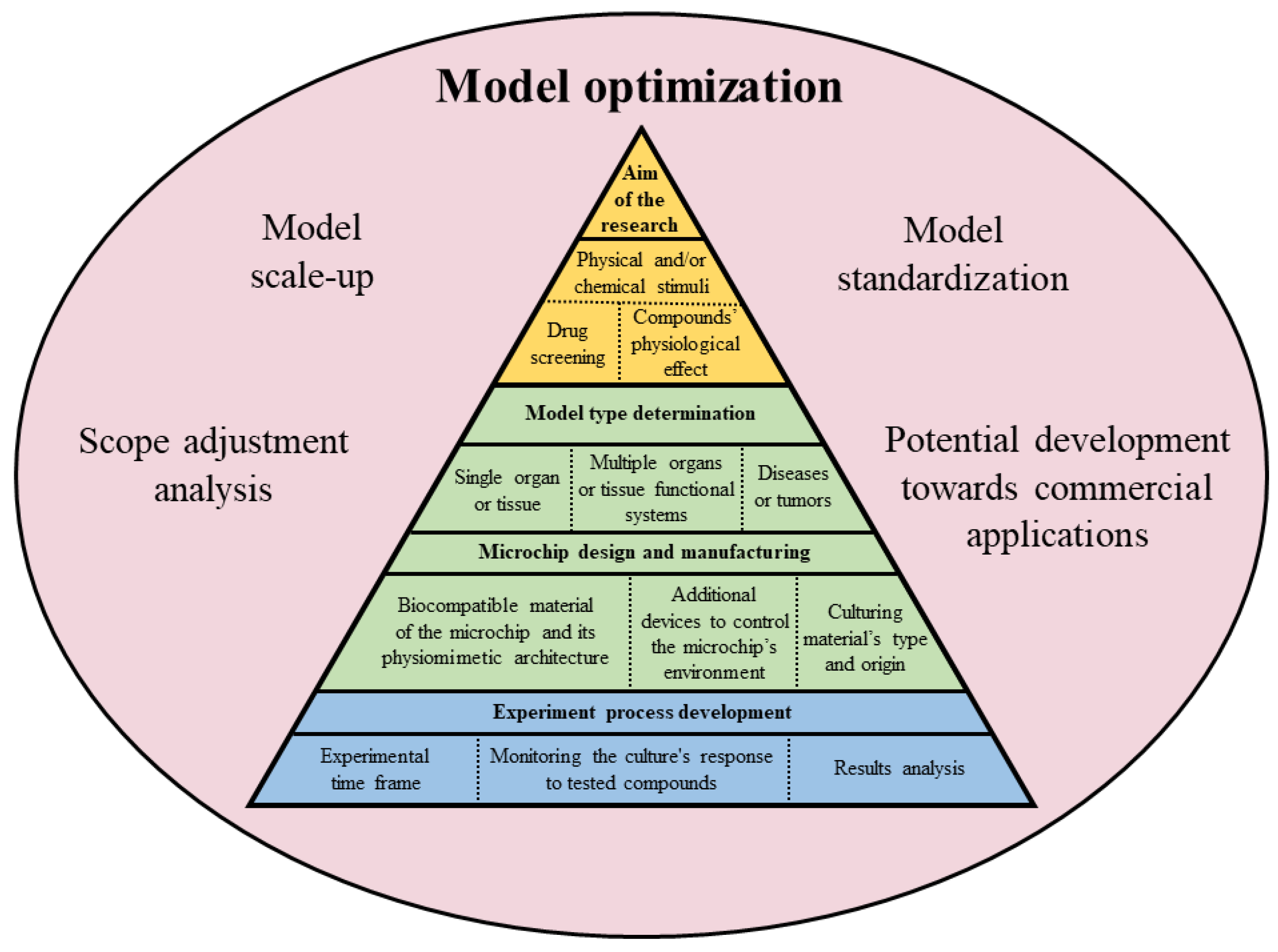
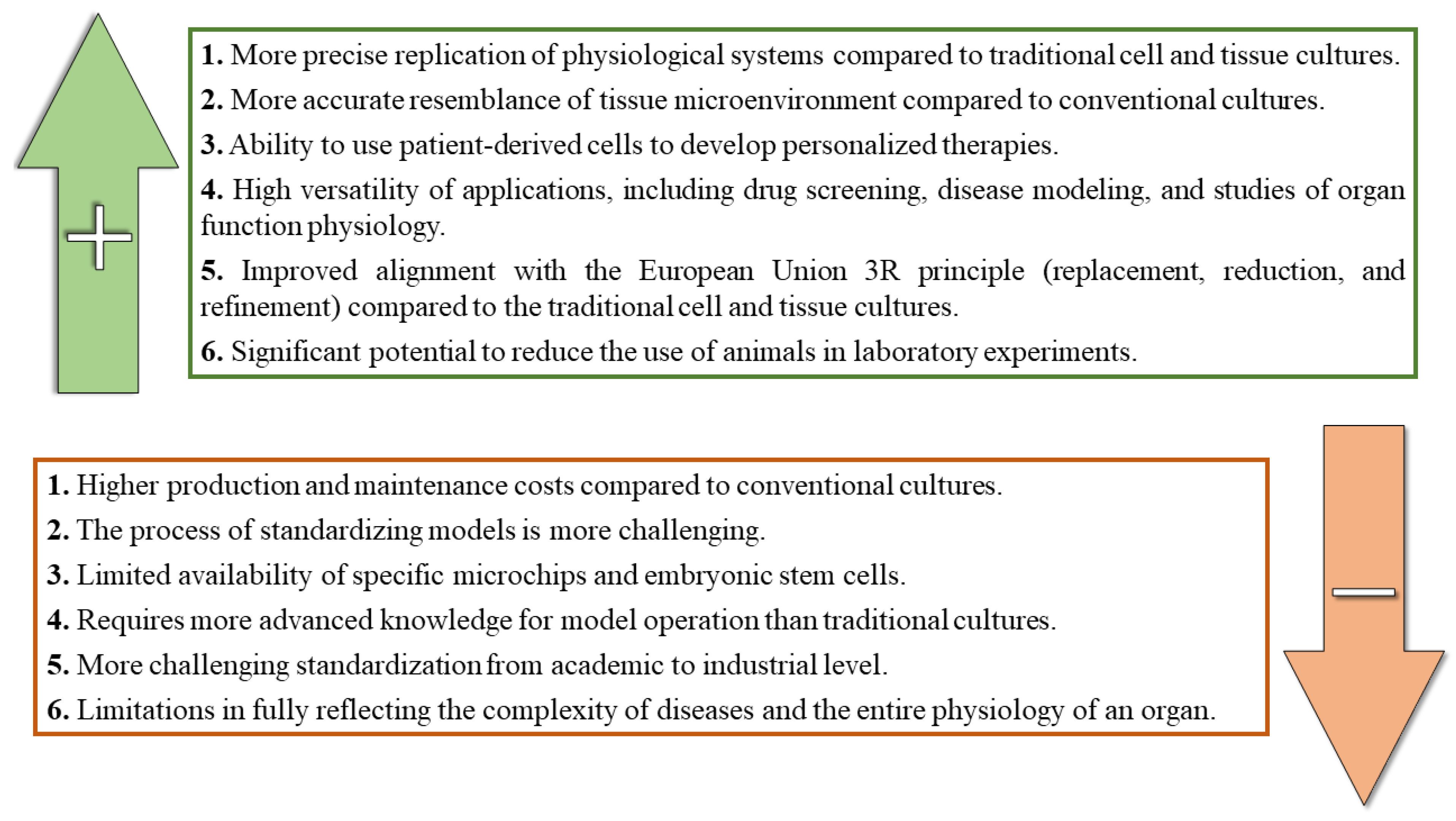
5. Benefits and Limitations of the OOC Technology
6. Future Direction of the OOC Technology in Neurosciences
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int. J. Mol. Sci. 2021, 22, 12200. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Białkowska, K.; Komorowski, P.; Bryszewska, M.; Miłowska, K. Spheroids as a Type of Three-Dimensional Cell Cultures—Examples of Methods of Preparation and the Most Important Application. Int. J. Mol. Sci. 2020, 21, 6225. [Google Scholar] [CrossRef] [PubMed]
- Jubelin, C.; Muñoz-Garcia, J.; Griscom, L.; Cochonneau, D.; Ollivier, E.; Heymann, M.-F.; Vallette, F.M.; Oliver, L.; Heymann, D. Three-Dimensional in Vitro Culture Models in Oncology Research. Cell Biosci. 2022, 12, 155. [Google Scholar] [CrossRef]
- Liu, X.; Su, Q.; Zhang, X.; Yang, W.; Ning, J.; Jia, K.; Xin, J.; Li, H.; Yu, L.; Liao, Y.; et al. Recent Advances of Organ-on-a-Chip in Cancer Modeling Research. Biosensors 2022, 12, 1045. [Google Scholar] [CrossRef]
- Rennane, S.; Baker, L.; Mulcahy, A. Estimating the Cost of Industry Investment in Drug Research and Development: A Review of Methods and Results. Inquiry 2021, 58, 4695802110597. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Peng, Y.; Li, H.; Chen, W. Organ-on-a-Chip: A New Paradigm for Drug Development. Trends Pharmacol. Sci. 2021, 42, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid Prototyping of Microfluidic Systems in Poly(Dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef]
- Andrus, J.; Bond, W.L. Photoengraving in Transistor Fabrication. In Transistor Technology; Bondi, F.J., Ed.; 1958; Volume III, pp. 151–162. [Google Scholar]
- Del Barrio, J.; Sánchez-Somolinos, C. Light to Shape the Future: From Photolithography to 4D Printing. Adv. Opt. Mater. 2019, 7, 1900598. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft Lithography. Angew. Chem. Int. Ed. 1998, 37, 550–575. [Google Scholar] [CrossRef]
- Yata, V.K. Microfluidics for Assisted Reproduction in Animals; Springer: Singapore, 2021; pp. 2–4. [Google Scholar] [CrossRef]
- Fanizza, F.; Campanile, M.; Forloni, G.; Giordano, C.; Albani, D. Induced Pluripotent Stem Cell-Based Organ-on-a-Chip as Personalized Drug Screening Tools: A Focus on Neurodegenerative Disorders. J. Tissue Eng. 2022, 13, 204173142210953. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ-on-a-Chip: Recent Breakthroughs and Future Prospects. BioMed Eng. OnLine 2020, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Human Organs-on-Chips for Disease Modelling, Drug Development and Personalized Medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef]
- Haring, A.P.; Sontheimer, H.; Johnson, B.N. Microphysiological Human Brain and Neural Systems-on-a-Chip: Potential Alternatives to Small Animal Models and Emerging Platforms for Drug Discovery and Personalized Medicine. Stem Cell Rev. Rep. 2017, 13, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Monteduro, A.G.; Rizzato, S.; Caragnano, G.; Trapani, A.; Giannelli, G.; Maruccio, G. Organs-on-Chips Technologies–A Guide from Disease Models to Opportunities for Drug Development. Biosens. Bioelectron. 2023, 231, 115271. [Google Scholar] [CrossRef]
- Leung, C.M.; De Haan, P.; Ronaldson-Bouchard, K.; Kim, G.-A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S.; et al. A Guide to the Organ-on-a-Chip. Nat. Rev. Methods Primers 2022, 2, 33. [Google Scholar] [CrossRef]
- Koyilot, M.C.; Natarajan, P.; Hunt, C.R.; Sivarajkumar, S.; Roy, R.; Joglekar, S.; Pandita, S.; Tong, C.W.; Marakkar, S.; Subramanian, L.; et al. Breakthroughs and Applications of Organ-on-a-Chip Technology. Cells 2022, 11, 1828. [Google Scholar] [CrossRef]
- Hadavi, D.; Tosheva, I.; Siegel, T.P.; Cuypers, E.; Honing, M. Technological Advances for Analyzing the Content of Organ-on-a-Chip by Mass Spectrometry. Front. Bioeng. Biotechnol. 2023, 11, 1197760. [Google Scholar] [CrossRef]
- Sances, S.; Ho, R.; Vatine, G.; West, D.; Laperle, A.; Meyer, A.; Godoy, M.; Kay, P.S.; Mandefro, B.; Hatata, S.; et al. Human iPSC-Derived Endothelial Cells and Microengineered Organ-Chip Enhance Neuronal Development. Stem Cell Rep. 2018, 10, 1222–1236. [Google Scholar] [CrossRef]
- Zhao, Q.; Cole, T.; Zhang, Y.; Tang, S.-Y. Mechanical Strain-Enabled Reconstitution of Dynamic Environment in Organ-on-a-Chip Platforms: A Review. Micromachines 2021, 12, 765. [Google Scholar] [CrossRef] [PubMed]
- Frimat, J.-P.; Luttge, R. The Need for Physiological Micro-Nanofluidic Systems of the Brain. Front. Bioeng. Biotechnol. 2019, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Van Noort, D.; Sung, J.H.; Park, S. Organ-on-a-Chip for Studying Gut-Brain Interaction Mediated by Extracellular Vesicles in the Gut Microenvironment. Int. J. Mol. Sci. 2021, 22, 13513. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.L.; Groenendijk, L.; Overdevest, R.; Fowke, T.M.; Annida, R.; Mocellin, O.; De Vries, H.E.; Wevers, N.R. Human BBB-on-a-Chip Reveals Barrier Disruption, Endothelial Inflammation, and T Cell Migration under Neuroinflammatory Conditions. Front. Mol. Neurosci. 2023, 16, 1250123. [Google Scholar] [CrossRef]
- Osaki, T.; Shin, Y.; Sivathanu, V.; Campisi, M.; Kamm, R.D. In Vitro Microfluidic Models for Neurodegenerative Disorders. Adv. Healthc. Mater. 2018, 7, 1700489. [Google Scholar] [CrossRef]
- Balestri, W.; Sharma, R.; Da Silva, V.A.; Bobotis, B.C.; Curle, A.J.; Kothakota, V.; Kalantarnia, F.; Hangad, M.V.; Hoorfar, M.; Jones, J.L.; et al. Modeling the Neuroimmune System in Alzheimer’s and Parkinson’s Diseases. J. Neuroinflamm 2024, 21, 32. [Google Scholar] [CrossRef]
- Imparato, G.; Urciuolo, F.; Netti, P.A. Organ on Chip Technology to Model Cancer Growth and Metastasis. Bioengineering 2022, 9, 28. [Google Scholar] [CrossRef]
- Yoon, J.; Kim, J.; Shah, Z.; Awasthi, A.; Mahajan, A.; Kim, Y. Advanced Human BBB-on-a-Chip: A New Platform for Alzheimer’s Disease Studies. Adv. Healthc. Mater. 2021, 10, 2002285. [Google Scholar] [CrossRef]
- Csöbönyeiová, M.; Polák, Š.; Danišovič, L. Toxicity Testing and Drug Screening Using iPSC-Derived Hepatocytes, Cardiomyocytes, and Neural Cells. Can. J. Physiol. Pharmacol. 2016, 94, 687–694. [Google Scholar] [CrossRef]
- Roy, N.; Kashyap, J.; Verma, D.; Tyagi, R.K.; Prabhakar, A. Prototype of a Smart Microfluidic Platform for the Evaluation of SARS-Cov-2 Pathogenesis, Along with Estimation of the Effectiveness of Potential Drug Candidates and Antigen–Antibody Interactions in Convalescent Plasma Therapy. Trans. Indian. Natl. Acad. Eng. 2020, 5, 241–250. [Google Scholar] [CrossRef]
- Kaarj, Y. Methods of Delivering Mechanical Stimuli to Organ-on-a-Chip. Micromachines 2019, 10, 700. [Google Scholar] [CrossRef]
- Thompson, C.L.; Fu, S.; Heywood, H.K.; Knight, M.M.; Thorpe, S.D. Mechanical Stimulation: A Crucial Element of Organ-on-Chip Models. Front. Bioeng. Biotechnol. 2020, 8, 602646. [Google Scholar] [CrossRef]
- Yan, J.; Li, Z.; Guo, J.; Liu, S.; Guo, J. Organ-on-a-Chip: A New Tool for In Vitro Research. Biosens. Bioelectron. 2022, 216, 114626. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Sun, H.; Lyu, T.; Ma, X.; Guo, J.; Tian, Y. Multi-Functional Nano-Doped Hollow Fiber from Microfluidics for Sensors and Micromotors. Biosensors 2024, 14, 186. [Google Scholar] [CrossRef]
- Keshtiban, M.M.; Zand, M.M.; Ebadi, A.; Azizi, Z. PDMS-Based Porous Membrane for Medical Applications: Design, Development, and Fabrication. Biomed. Mater. 2023, 18, 045012. [Google Scholar] [CrossRef]
- Hassan, S.; Heinrich, M.; Cecen, B.; Prakash, J.; Zhang, Y.S. Biomaterials for On-Chip Organ Systems. In Biomaterials for Organ and Tissue Regeneration; Elsevier: Amsterdam, The Netherlands, 2020; pp. 669–707. [Google Scholar] [CrossRef]
- Osório, L.A.; Silva, E.; Mackay, R.E. A Review of Biomaterials and Scaffold Fabrication for Organ-on-a-Chip (OOAC) Systems. Bioengineering 2021, 8, 113. [Google Scholar] [CrossRef]
- Cao, U.M.N.; Zhang, Y.; Chen, J.; Sayson, D.; Pillai, S.; Tran, S.D. Microfluidic Organ-on-a-Chip: A Guide to Biomaterial Choice and Fabrication. Int. J. Mol. Sci. 2023, 24, 3232. [Google Scholar] [CrossRef]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and Rheological Properties of Methacrylamide Modified Gelatin Hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Campbell, S.B.; Wu, Q.; Yazbeck, J.; Liu, C.; Okhovatian, S.; Radisic, M. Beyond Polydimethylsiloxane: Alternative Materials for Fabrication of Organ-on-a-Chip Devices and Microphysiological Systems. ACS Biomater. Sci. Eng. 2021, 7, 2880–2899. [Google Scholar] [CrossRef]
- Qi, Z.; Xu, L.; Xu, Y.; Zhong, J.; Abedini, A.; Cheng, X.; Sinton, D. Disposable Silicon-Glass Microfluidic Devices: Precise, Robust and Cheap. Lab Chip 2018, 18, 3872–3880. [Google Scholar] [CrossRef]
- Oleaga, C.; Bernabini, C.; Smith, A.S.T.; Srinivasan, B.; Jackson, M.; McLamb, W.; Platt, V.; Bridges, R.; Cai, Y.; Santhanam, N.; et al. Multi-Organ Toxicity Demonstration in a Functional Human in Vitro System Composed of Four Organs. Sci. Rep. 2016, 6, 20030. [Google Scholar] [CrossRef]
- Alsharhan, A.T.; Acevedo, R.; Warren, R.; Sochol, R.D. 3D Microfluidics via Cyclic Olefin Polymer-Based in Situ Direct Laser Writing. Lab Chip 2019, 19, 2799–2810. [Google Scholar] [CrossRef]
- Esch, M.B.; Ueno, H.; Applegate, D.R.; Shuler, M.L. Modular, Pumpless Body-on-a-Chip Platform for the Co-Culture of GI Tract Epithelium and 3D Primary Liver Tissue. Lab Chip 2016, 16, 2719–2729. [Google Scholar] [CrossRef]
- Ayuso, J.M.; Monge, R.; Llamazares, G.A.; Moreno, M.; Agirregabiria, M.; Berganzo, J.; Doblaré, M.; Ochoa, I.; Fernández, L.J. SU-8 Based Microdevices to Study Self-Induced Chemotaxis in 3D Microenvironments. Front. Mater. 2015, 2, 37. [Google Scholar] [CrossRef]
- Li, R.; Lv, X.; Deng, Y. NOA 81 fabricated microfluidic chip for SH-SY5Y cell culture. In Proceeding of the 2015 IEEE International Conference on Mechatronics and Automation (ICMA), Beijing, China, 2–5 August 2015; pp. 994–998. [Google Scholar] [CrossRef]
- Li, R.; Lv, X.; Hasan, M.; Xu, J.; Xu, Y.; Zhang, X.; Qin, K.; Wang, J.; Zhou, D.; Deng, Y. A Rapidly Fabricated Microfluidic Chip for Cell Culture. J. Chromatogr. Sci. 2016, 54, 523–530. [Google Scholar] [CrossRef]
- Young, E.W.K.; Berthier, E.; Beebe, D.J. Assessment of Enhanced Autofluorescence and Impact on Cell Microscopy for Microfabricated Thermoplastic Devices. Anal. Chem. 2013, 85, 44–49. [Google Scholar] [CrossRef]
- Neiman, J.A.S.; Raman, R.; Chan, V.; Rhoads, M.G.; Raredon, M.S.B.; Velazquez, J.J.; Dyer, R.L.; Bashir, R.; Hammond, P.T.; Griffith, L.G. Photopatterning of Hydrogel Scaffolds Coupled to Filter Materials Using Stereolithography for Perfused 3D Culture of Hepatocytes. Biotechnol. Bioeng. 2015, 112, 777–787. [Google Scholar] [CrossRef]
- Domansky, K.; Leslie, D.C.; McKinney, J.; Fraser, J.P.; Sliz, J.D.; Hamkins-Indik, T.; Hamilton, G.A.; Bahinski, A.; Ingber, D.E. Clear Castable Polyurethane Elastomer for Fabrication of Microfluidic Devices. Lab Chip 2013, 13, 3956. [Google Scholar] [CrossRef]
- Johnson, B.N.; Lancaster, K.Z.; Hogue, I.B.; Meng, F.; Kong, Y.L.; Enquist, L.W.; McAlpine, M.C. 3D Printed Nervous System on a Chip. Lab Chip 2016, 16, 1393–1400. [Google Scholar] [CrossRef]
- Park, J.Y.; Ryu, H.; Lee, B.; Ha, D.-H.; Ahn, M.; Kim, S.; Kim, J.Y.; Jeon, N.L.; Cho, D.-W. Development of a Functional Airway-on-a-Chip by 3D Cell Printing. Biofabrication 2018, 11, 15002. [Google Scholar] [CrossRef]
- Park, S.-R.; Kook, M.G.; Kim, S.-R.; Lee, J.W.; Yu, Y.S.; Park, C.H.; Lim, S.; Oh, B.-C.; Jung, Y.; Hong, I.-S. A Microscale 3D Organ on a Chip for Recapitulating Reciprocal Neuroendocrine Crosstalk between the Hypothalamus and the Pituitary Gland. Biofabrication 2024, 16, 25011. [Google Scholar] [CrossRef]
- Boghdeh, N.A.; Risner, K.H.; Barrera, M.D.; Britt, C.M.; Schaffer, D.K.; Alem, F.; Brown, J.A.; Wikswo, J.P.; Narayanan, A. Application of a Human Blood Brain Barrier Organ-on-a-Chip Model to Evaluate Small Molecule Effectiveness against Venezuelan Equine Encephalitis Virus. Viruses 2022, 14, 2799. [Google Scholar] [CrossRef]
- Ronaldson-Bouchard, K.; Vunjak-Novakovic, G. Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development. Cell Stem Cell 2018, 22, 310–324. [Google Scholar] [CrossRef]
- Weiskirchen, S.; Schröder, S.K.; Buhl, E.M.; Weiskirchen, R. A Beginner’s Guide to Cell Culture: Practical Advice for Preventing Needless Problems. Cells 2023, 12, 682. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Palasantzas, V.E.J.M.; Tamargo-Rubio, I.; Le, K.; Slager, J.; Wijmenga, C.; Jonkers, I.H.; Kumar, V.; Fu, J.; Withoff, S. iPSC-Derived Organ-on-a-Chip Models for Personalized Human Genetics and Pharmacogenomics Studies. Trends Genet. 2023, 39, 268–284. [Google Scholar] [CrossRef]
- Osaki, T.; Uzel, S.G.M.; Kamm, R.D. Microphysiological 3D Model of Amyotrophic Lateral Sclerosis (ALS) from Human iPS-Derived Muscle Cells and Optogenetic Motor Neurons. Sci. Adv. 2018, 4, eaat5847. [Google Scholar] [CrossRef]
- Pediaditakis, I.; Kodella, K.R.; Manatakis, D.V.; Le, C.Y.; Hinojosa, C.D.; Tien-Street, W.; Manolakos, E.S.; Vekrellis, K.; Hamilton, G.A.; Ewart, L.; et al. Modeling Alpha-Synuclein Pathology in a Human Brain-Chip to Assess Blood-Brain Barrier Disruption. Nat. Commun. 2021, 12, 5907. [Google Scholar] [CrossRef]
- Wnorowski, A.; Yang, H.; Wu, J.C. Progress, Obstacles, and Limitations in the Use of Stem Cells in Organ-on-a-Chip Models. Adv. Drug Deliv. Rev. 2019, 140, 3–11. [Google Scholar] [CrossRef]
- Wang, X.; Miao, Y.-H.; Zhao, X.-M.; Liu, X.; Hu, Y.-W.; Deng, D.-W. Perspectives on Organ-on-a-Chip Technology for Natural Products Evaluation. Food Med. Homol. 2024, 1, 9420013. [Google Scholar] [CrossRef]
- Teli, P.; Kale, V.; Vaidya, A. Beyond Animal Models: Revolutionizing Neurodegenerative Disease Modeling Using 3D in Vitro Organoids, Microfluidic Chips, and Bioprinting. Cell Tissue Res. 2023, 394, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Praça, C.; Rosa, S.C.; Sevin, E.; Cecchelli, R.; Dehouck, M.-P.; Ferreira, L.S. Derivation of Brain Capillary-like Endothelial Cells from Human Pluripotent Stem Cell-Derived Endothelial Progenitor Cells. Stem Cell Rep. 2019, 13, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Su, S.-H.; Song, Y.; Stephens, A.; Situ, M.; McCloskey, M.C.; McGrath, J.L.; Andjelkovic, A.V.; Singer, B.H.; Kurabayashi, K. A Tissue Chip with Integrated Digital Immunosensors: In Situ Brain Endothelial Barrier Cytokine Secretion Monitoring. Biosens. Bioelectron. 2023, 224, 115030. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Lee, H.Y.; Choi, Y.Y.; Mo, S.J.; Jeon, S.; Ha, J.H.; Park, S.D.; Shim, J.-J.; Lee, J.; Chung, B.G. Effect of Gut Microbiota-Derived Metabolites and Extracellular Vesicles on Neurodegenerative Disease in a Gut-Brain Axis Chip. Nano Converg. 2024, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Richardson, L.S.; Kim, S.; Han, A.; Menon, R. Modeling Ascending Infection with a Feto-Maternal Interface Organ-on-Chip. Lab Chip 2020, 20, 4486–4501. [Google Scholar] [CrossRef]
- Tantengco, O.A.G.; Richardson, L.S.; Radnaa, E.; Kammala, A.K.; Kim, S.; Medina, P.M.B.; Han, A.; Menon, R. Modeling Ascending Ureaplasma Parvum Infection through the Female Reproductive Tract Using Vagina-cervix-decidua-organ-on-a-chip and Feto-maternal Interface-organ-on-a-chip. FASEB J. 2022, 36, e22551. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a0204122015. [Google Scholar] [CrossRef]
- Alahmari, A. Blood-Brain Barrier Overview: Structural and Functional Correlation. Neural Plast. 2021, 2021, 6564585. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood–Brain Barrier Overview on Structure, Function, Impairment, and Biomarkers of Integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Marino, A.; Tricinci, O.; Battaglini, M.; Filippeschi, C.; Mattoli, V.; Sinibaldi, E.; Ciofani, G. A 3D Real-Scale, Biomimetic, and Biohybrid Model of the Blood-Brain Barrier Fabricated through Two-Photon Lithography. Small 2018, 14, 1702959. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.I.; Sei, Y.J.; Park, H.-J.; Kim, J.; Ryu, Y.; Choi, J.J.; Sung, H.-J.; MacDonald, T.J.; Levey, A.I.; Kim, Y. Microengineered Human Blood–Brain Barrier Platform for Understanding Nanoparticle Transport Mechanisms. Nat. Commun. 2020, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Pensabene, V.; Markov, D.A.; Allwardt, V.; Neely, M.D.; Shi, M.; Britt, C.M.; Hoilett, O.S.; Yang, Q.; Brewer, B.M.; et al. Recreating Blood-Brain Barrier Physiology and Structure on Chip: A Novel Neurovascular Microfluidic Bioreactor. Biomicrofluidics 2015, 9, 054124. [Google Scholar] [CrossRef] [PubMed]
- Motallebnejad, P.; Thomas, A.; Swisher, S.L.; Azarin, S.M. An Isogenic hiPSC-Derived BBB-on-a-Chip. Biomicrofluidics 2019, 13, 64119. [Google Scholar] [CrossRef] [PubMed]
- Chang, L. Brain–Gut–Microbiota Axis in Depression: A Historical Overview and Future Directions. Brain Res. Bull. 2022, 182, 44–56. [Google Scholar] [CrossRef]
- Góralczyk-Bińkowska, A.; Szmajda-Krygier, D.; Kozłowska, E. The Microbiota–Gut–Brain Axis in Psychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 11245. [Google Scholar] [CrossRef]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The Role of Microbiota-Gut-Brain Axis in Neuropsychiatric and Neurological Disorders. Pharm. Res. 2021, 172, 105840. [Google Scholar] [CrossRef]
- Szlis, M.; Wójcik-Gładysz, A.; Przybył, B.J. Central Obestatin Administration Affect the LH and FSH Secretory Activity in Peripubertal Sheep. Theriogenology 2020, 145, 10–17. [Google Scholar] [CrossRef]
- Wójcik-Gładysz, A.; Wańkowska, M.; Gajewska, A.; Misztal, T.; Zielińska-Górska, M.; Szlis, M.; Polkowska, J. Effects of Intracerebroventricular Infusions of Ghrelin on Secretion of Follicle-Stimulating Hormone in Peripubertal Female Sheep. Reprod. Fertil. Dev. 2016, 28, 2065. [Google Scholar] [CrossRef]
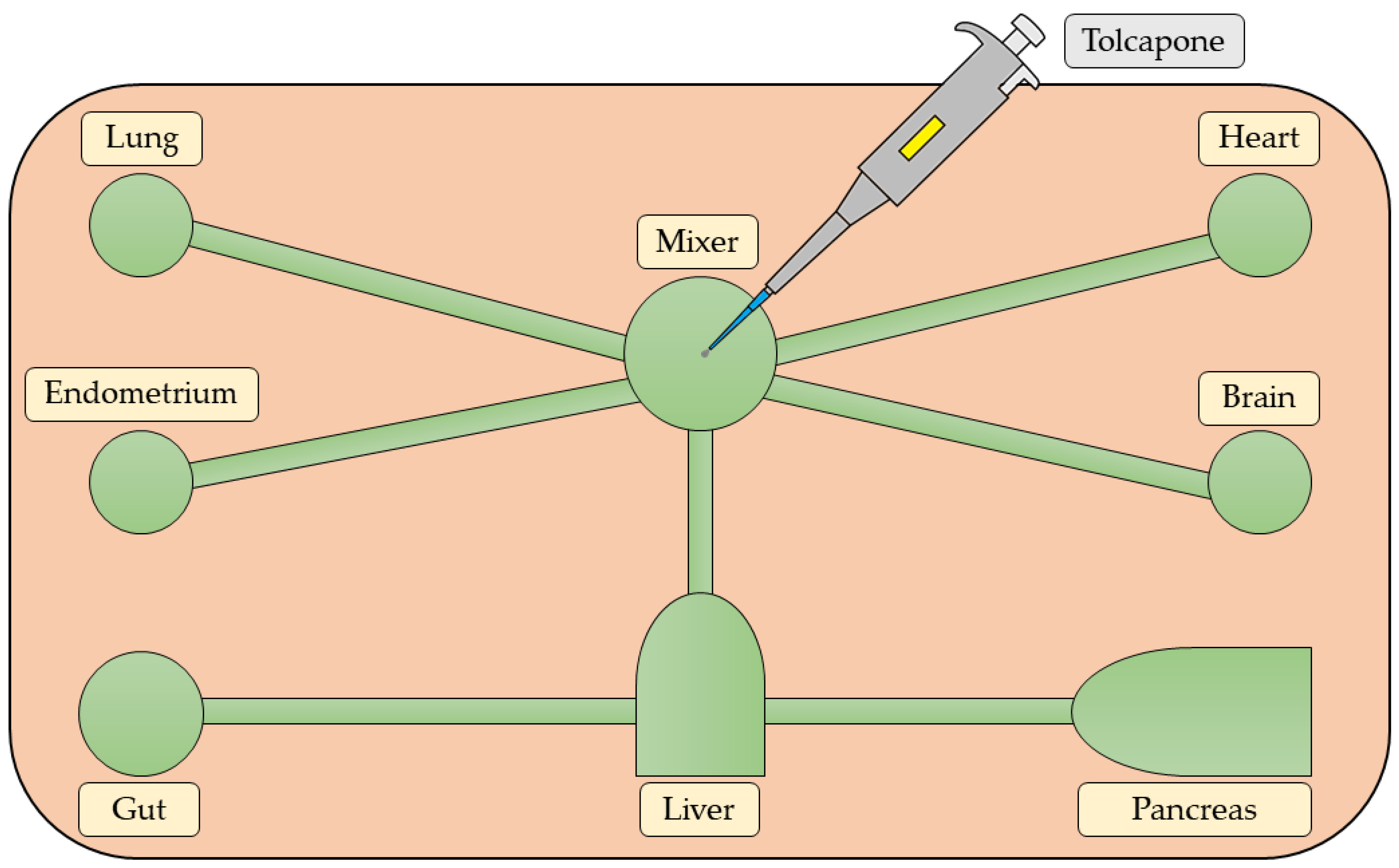
- Trapecar, M.; Wogram, E.; Svoboda, D.; Communal, C.; Omer, A.; Lungjangwa, T.; Sphabmixay, P.; Velazquez, J.; Schneider, K.; Wright, C.W.; et al. Human Physiomimetic Model Integrating Microphysiological Systems of the Gut, Liver, and Brain for Studies of Neurodegenerative Diseases. Sci. Adv. 2021, 7, eabd1707. [Google Scholar] [CrossRef]
- Wang, X.; Cirit, M.; Wishnok, J.S.; Griffith, L.G.; Tannenbaum, S.R. Analysis of an Integrated Human Multiorgan Microphysiological System for Combined Tolcapone Metabolism and Brain Metabolomics. Anal. Chem. 2019, 91, 8667–8675. [Google Scholar] [CrossRef] [PubMed]
- Arjmand, B.; Larijani, B. Organ on a Chip: A Novel in Vitro Biomimetic Strategy in Amyotrophic Lateral Sclerosis (ALS) Modeling. Front. Neurol. 2022, 12, 788462. [Google Scholar] [CrossRef] [PubMed]
- De Jongh, R.; Spijkers, X.M.; Pasteuning-Vuhman, S.; Vulto, P.; Pasterkamp, R.J. Neuromuscular Junction-on-a-chip: ALS Disease Modeling and Read-out Development in Microfluidic Devices. J. Neurochem. 2021, 157, 393–412. [Google Scholar] [CrossRef] [PubMed]
- Santoso, J.W.; McCain, M.L. Neuromuscular Disease Modeling on a Chip. Dis. Model. Mech. 2020, 13, dmm044867. [Google Scholar] [CrossRef]
- Chennampally, P.; Sayed-Zahid, A.; Soundararajan, P.; Sharp, J.; Cox, G.A.; Collins, S.D.; Smith, R.L. A Microfluidic Approach to Rescue ALS Motor Neuron Degeneration Using Rapamycin. Sci. Rep. 2021, 11, 18168. [Google Scholar] [CrossRef]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the Diagnosis of Parkinson’s Disease. Lancet Neurol. 2022, 20, 385–397. [Google Scholar] [CrossRef]
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef]
- Fernandes, J.T.S.; Chutna, O.; Chu, V.; Conde, J.P.; Outeiro, T.F. A Novel Microfluidic Cell Co-Culture Platform for the Study of the Molecular Mechanisms of Parkinson’s Disease and Other Synucleinopathies. Front. Neurosci. 2016, 10, 511. [Google Scholar] [CrossRef]
- De Rus Jacquet, A.; Alpaugh, M.; Denis, H.L.; Tancredi, J.L.; Boutin, M.; Decaestecker, J.; Beauparlant, C.; Herrmann, L.; Saint-Pierre, M.; Parent, M.; et al. The Contribution of Inflammatory Astrocytes to BBB Impairments in a Brain-Chip Model of Parkinson’s Disease. Nat. Commun. 2023, 14, 3651. [Google Scholar] [CrossRef]
- Demers, C.J.; Soundararajan, P.; Chennampally, P.; Cox, G.A.; Briscoe, J.; Collins, S.D.; Smith, R.L. Development-on-Chip: In Vitro Neural Tube Patterning with a Microfluidic Device. Development 2016, 143, 1884–1892. [Google Scholar] [CrossRef]
- Chakrabarti, J.; Pandey, R.; Churko, J.M.; Eschbacher, J.; Mallick, S.; Chen, Y.; Hermes, B.; Mallick, P.; Stansfield, B.N.; Pond, K.W.; et al. Development of Human Pituitary Neuroendocrine Tumor Organoids to Facilitate Effective Targeted Treatments of Cushing’s Disease. Cells 2022, 11, 3344. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-K.; Wong, S.Z.H.; Pather, S.R.; Nguyen, P.T.T.; Zhang, F.; Zhang, D.Y.; Zhang, Z.; Lu, L.; Fang, W.; Chen, L.; et al. Generation of Hypothalamic Arcuate Organoids from Human Induced Pluripotent Stem Cells. Cell Stem Cell 2021, 28, 1657–1670.e10. [Google Scholar] [CrossRef]
- Przybył, B.; Wójcik-Gładysz, A.; Gajewska, A.; Szlis, M. Brain-Derived Neurotrophic Factor (BDNF) Affects Somatotrophic Axis Activity in Sheep. J. Anim. Feed. Sci. 2021, 30, 329–339. [Google Scholar] [CrossRef]
- Wójcik-Gładysz, A.; Szlis, M.; Przybył, B.J.; Polkowska, J. Obestatin May Affect the GnRH/KNDy Gene Network in Sheep Hypothalamus. Res. Vet. Sci. 2019, 123, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-R.; Kim, S.-R.; Lee, J.W.; Park, C.H.; Yu, W.-J.; Lee, S.-J.; Chon, S.J.; Lee, D.H.; Hong, I.-S. Development of a Novel Dual Reproductive Organ on a Chip: Recapitulating Bidirectional Endocrine Crosstalk between the Uterine Endometrium and the Ovary. Biofabrication 2021, 13, 15001. [Google Scholar] [CrossRef]
- Boeri, L.; Izzo, L.; Sardelli, L.; Tunesi, M.; Albani, D.; Giordano, C. Advanced Organ-on-a-Chip Devices to Investigate Liver Multi-Organ Communication: Focus on Gut, Microbiota and Brain. Bioengineering 2019, 6, 91. [Google Scholar] [CrossRef]
- Marzagalli, M.; Pelizzoni, G.; Fedi, A.; Vitale, C.; Fontana, F.; Bruno, S.; Poggi, A.; Dondero, A.; Aiello, M.; Castriconi, R.; et al. A Multi-Organ-on-Chip to Recapitulate the Infiltration and the Cytotoxic Activity of Circulating NK Cells in 3D Matrix-Based Tumor Model. Front. Bioeng. Biotechnol. 2022, 10, 945149. [Google Scholar] [CrossRef]
- Schwerdtfeger, L.A.; Tobet, S.A. From Organotypic Culture to Body-on-a-chip: A Neuroendocrine Perspective. J. Neuroendocrinol. 2019, 31, e126502019. [Google Scholar] [CrossRef]
- Ong, L.J.Y.; Fan, X.; Rujia Sun, A.; Mei, L.; Toh, Y.-C.; Prasadam, I. Controlling Microenvironments with Organs-on-Chips for Osteoarthritis Modelling. Cells 2023, 12, 579. [Google Scholar] [CrossRef]
- Griffith, L.G.; Swartz, M.A. Capturing Complex 3D Tissue Physiology in Vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic Organs-on-Chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Foo, G.W.; Aggarwal, N.; Chang, M.W. Organ-on-Chip Technology: Opportunities and Challenges. Biotechnol. Notes 2024, 5, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Picollet-D’hahan, N.; Zuchowska, A.; Lemeunier, I.; Le Gac, S. Multiorgan-on-a-Chip: A Systemic Approach To Model and Decipher Inter-Organ Communication. Trends Biotechnol 2021, 39, 788–810. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, J.; Bai, H.; Wang, H.; Hao, S.; Ding, Y.; Peng, B.; Zhang, J.; Li, L.; Huang, W. An Overview of Organs-on-Chips Based on Deep Learning. Research 2022, 2022, 9869518. [Google Scholar] [CrossRef] [PubMed]
- Meneses, J.; Conceição, F.; Van Der Meer, A.D.; De Wit, S.; Moreira Teixeira, L. Guiding Organs-on-Chips towards Applications: A Balancing Act between Integration of Advanced Technologies and Standardization. Front. Lab Chip Technol. 2024, 3, 1376964. [Google Scholar] [CrossRef]
- Tronolone, J.J.; Mathur, T.; Chaftari, C.P.; Jain, A. Evaluation of the Morphological and Biological Functions of Vascularized Microphysiological Systems with Supervised Machine Learning. Ann. Biomed. Eng. 2023, 51, 1723–1737. [Google Scholar] [CrossRef]
- Dressler, O.J.; Howes, P.D.; Choo, J.; de Mello, A.J. Reinforcement Learning for Dynamic Microfluidic Control. ACS Omega 2018, 3, 10084–10091. [Google Scholar] [CrossRef]
- Galan, E.A.; Zhao, H.; Wang, X.; Dai, Q.; Huck, W.T.S.; Ma, S. Intelligent Microfluidics: The Convergence of Machine Learning and Microfluidics in Materials Science and Biomedicine. Matter 2020, 3, 1893–1922. [Google Scholar] [CrossRef]
- Jodat, Y.A.; Kang, M.G.; Kiaee, K.; Kim, G.J.; Martinez, A.F.H.; Rosenkranz, A.; Bae, H.; Shin, S.R. Human-Derived Organ-on-a-Chip for Personalized Drug Development. CPD 2019, 24, 5471–5486. [Google Scholar] [CrossRef]
- Deng, S.; Li, C.; Cao, J.; Cui, Z.; Du, J.; Fu, Z.; Yang, H.; Chen, P. Organ-on-a-Chip Meets Artificial Intelligence in Drug Evaluation. Theranostics 2023, 13, 4526–4558. [Google Scholar] [CrossRef]
- Kumar, S.; Dagli, N.; Haque, M. Human-on-Chip Technology: Current State and Future Challenges. Bangladesh J. Med. Sci. 2024, 23, 7–11. [Google Scholar] [CrossRef]

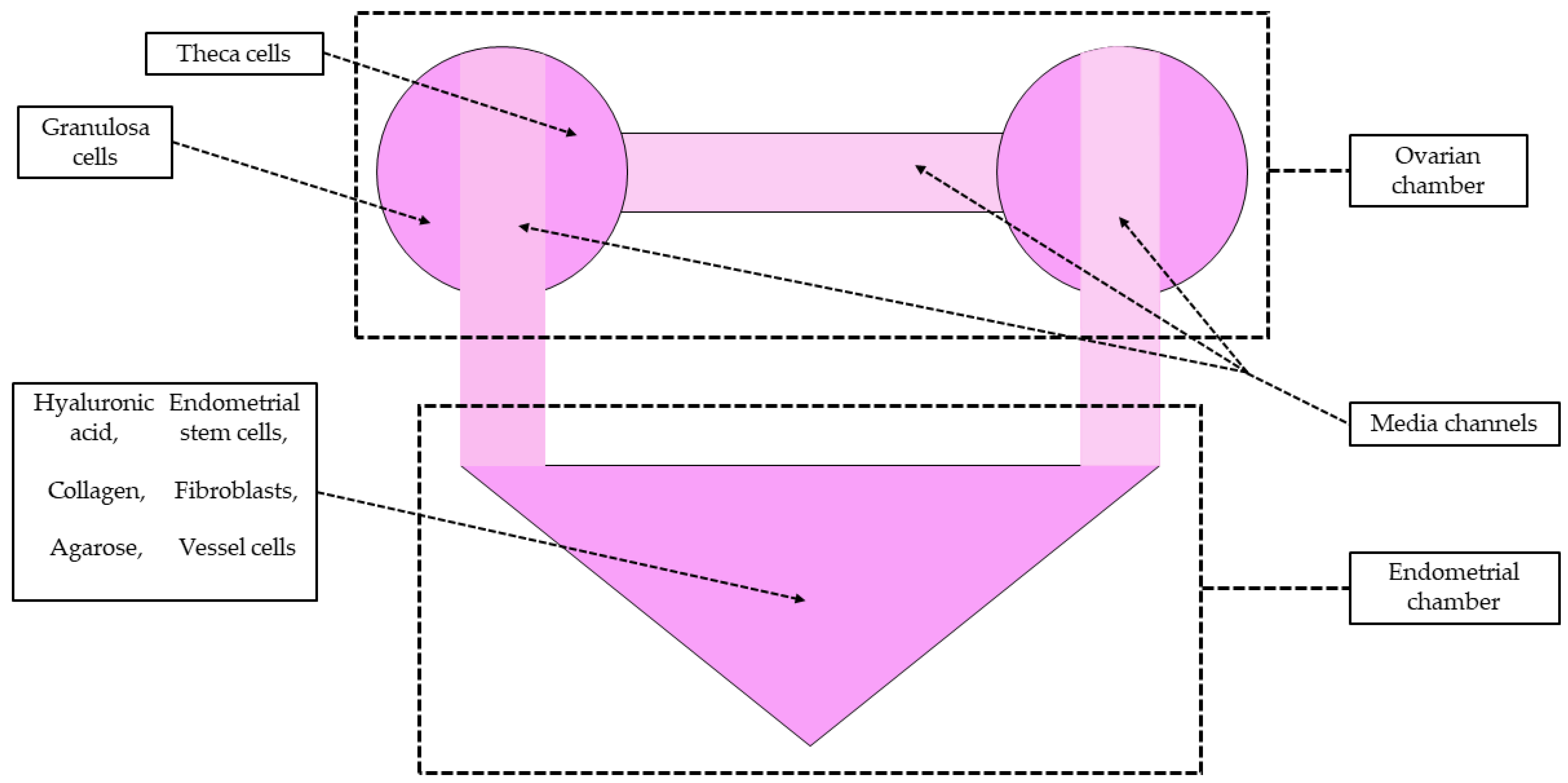
| Material | Advantages | Disadvantages | References |
|---|---|---|---|
| Poly(dimethylsiloxane) (PDMS) | Flexibility and cost-effectiveness, ease of fabrication, biocompatibility | Absorption of small molecules, leakage issues | [41] |
| Silicon glass | Precision and robustness, optical clarity, chemical compatibility | High fabrication cost, limited reusability, complex fabrication process | [42] |
| Thermoplastics | High optical transparency, solvent resistance, low gas permeability | Relatively high rigidity, complex manufacturing process, limited compatibility with certain photomaterials | [44,45] |
| Epoxy resins and adhesives | Advantageous mechanical properties, ability to integrate multiple sensors and actuators, hydrophilicity | Challenges with turning it hydrophobic, leakage issues | [46,47,48] |
| Thermoplastics combined with hydrogels or elastomers | Hydrogels: soft mechanical properties closely mimic native tissue, biocompatible microenvironment provides improves cell viability Polyurethane elastomers: optical transparency and biocompatibility, strong bonding capabilities | Hydrogels: swelling issues, challenging process of bonding with filters; Polyurethane elastomers: cytotoxic concerns, lower gas permeability than PDMS, processing challenges regarding degassing and molding | [49,50,51] |
| Organ/Structure Replicated | Disease/Condition Addressed | Cells/Tissues Used | Main Findings | Reference |
|---|---|---|---|---|
| Blood–brain barrier (BBB) | Drug discovery related to the BBB | Brain capillary-like endothelial cells (BCLECs) derived from human-induced pluripotent stem cells (hiPSCs) | A two-step differentiation protocol to derive BCLECs from hiPSCs, demonstrating that the combination of VEGF, Wnt3a, and retinoic acid significantly improved the expression of endothelial markers and barrier properties, including transendothelial electrical resistance and paracellular permeability. The derived BCLECs exhibited moderate expression of P-glycoprotein and responded to inflammatory stimuli, indicating their potential for modeling BBB function in vitro | [65] |
| BBB using the tissue chip platform “DigiTACK” | Neuroinflammation and related neurological disorders, with a focus on cytokine secretion dynamics | Primary mouse brain microvascular endothelial cells (mBMECs) | The DigiTACK platform enabled longitudinal monitoring of cytokine secretion from the mBMECs barrier, revealing significant differences in cytokine profiles between luminal and abluminal sides and demonstrating the potential for high-throughput analysis in studying CNS disease mechanisms | [66] |
| A gut–brain axis chip that mimics the intestinal and neural environment | Alzheimer’s disease model to evaluate the effects of gut microbiota-derived metabolites and exosomes | hiPSCs differentiated into induced neural stem cells and Caco-2 cells | Metabolites and exosomes derived from gut microbiota influenced neural growth, maturation, and synaptic plasticity, suggesting their potential as therapeutic candidates for neurodevelopmental and neurodegenerative disorders | [67] |
| A feto-maternal interface organ-on-chip (FMi-OOC) model | Ascending infections and their associated inflammatory responses that are significant risk factors for spontaneous preterm birth (PTB) | Primary human cells from the fetal membranes, including decidual cells, chorion trophoblasts, amnion mesenchymal cells, and epithelial cells | The FMi-OOC successfully demonstrated the propagation of lipopolysaccharide-induced inflammation from the maternal to fetal compartments, revealing distinct inflammatory cytokine profiles and highlighting the immune imbalance that can contribute to PTB. The model maintained key physiological characteristics of the in vivo environment, validating its utility for studying the feto-maternal interface | [68] |
| A six-chamber vagina–cervix–decidua-organ-on-a-chip (VCD-OOC) model that mimics the female reproductive tract during pregnancy | Ascending Ureaplasma parvum infection and its association with PTB | Vaginal epithelial cells, cervical epithelial and stromal cells, and decidual cells, all derived from immortalized human cell lines | U. parvum infection did not cause significant cell death or massive inflammation in the VCD-OOC model. However, combined with lipopolysaccharides, it induced a substantial inflammatory response. In vivo studies showed that the vaginal inoculation of U. parvum alone resulted in low PTB rates, while intra-amniotic injection significantly increased PTB rates | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wysoczański, B.; Świątek, M.; Wójcik-Gładysz, A. Organ-on-a-Chip Models—New Possibilities in Experimental Science and Disease Modeling. Biomolecules 2024, 14, 1569. https://doi.org/10.3390/biom14121569
Wysoczański B, Świątek M, Wójcik-Gładysz A. Organ-on-a-Chip Models—New Possibilities in Experimental Science and Disease Modeling. Biomolecules. 2024; 14(12):1569. https://doi.org/10.3390/biom14121569
Chicago/Turabian StyleWysoczański, Bartłomiej, Marcin Świątek, and Anna Wójcik-Gładysz. 2024. "Organ-on-a-Chip Models—New Possibilities in Experimental Science and Disease Modeling" Biomolecules 14, no. 12: 1569. https://doi.org/10.3390/biom14121569
APA StyleWysoczański, B., Świątek, M., & Wójcik-Gładysz, A. (2024). Organ-on-a-Chip Models—New Possibilities in Experimental Science and Disease Modeling. Biomolecules, 14(12), 1569. https://doi.org/10.3390/biom14121569







