Bioactivation, Mutagenicity, DNA Damage, and Oxidative Stress Induced by 3,4-Dimethylaniline
Abstract
1. Background
2. Materials and Methods
2.1. Chinese Hamster Ovary (CHO) Cell Culture
2.2. Construction and Characterization of UV5/CHO Cell Lines
2.3. N-Acetyltransferase Assays In Vitro
2.4. N-Acetylation In Situ
2.5. Identification and Separation of Substrates and Their N-Acetylated Products
2.6. ROS
2.7. γH2AX In-Cell Western Staining
2.8. Colony Formation and HPRT Mutations
2.9. Statistical Analyses
3. Results
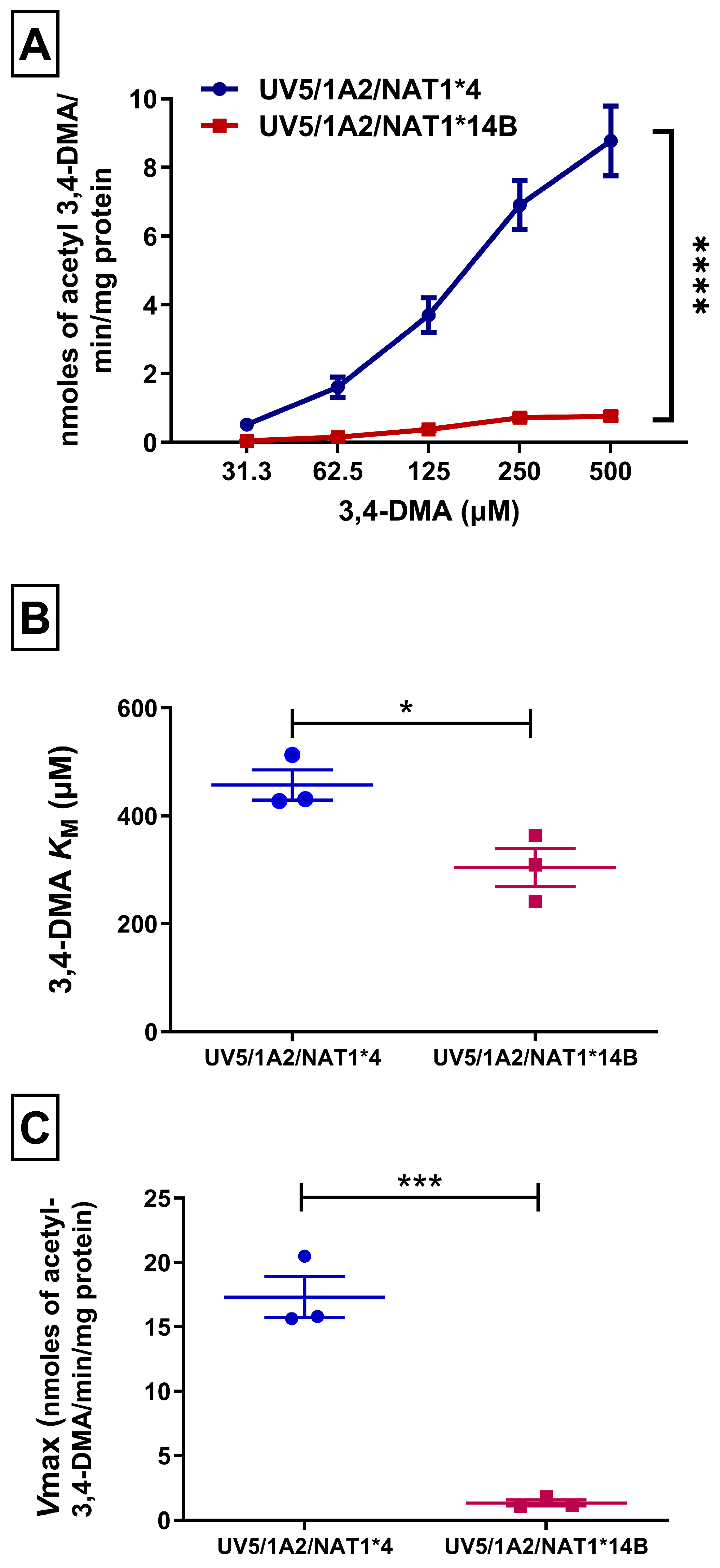
3.1. In Vitro N-Acetylation
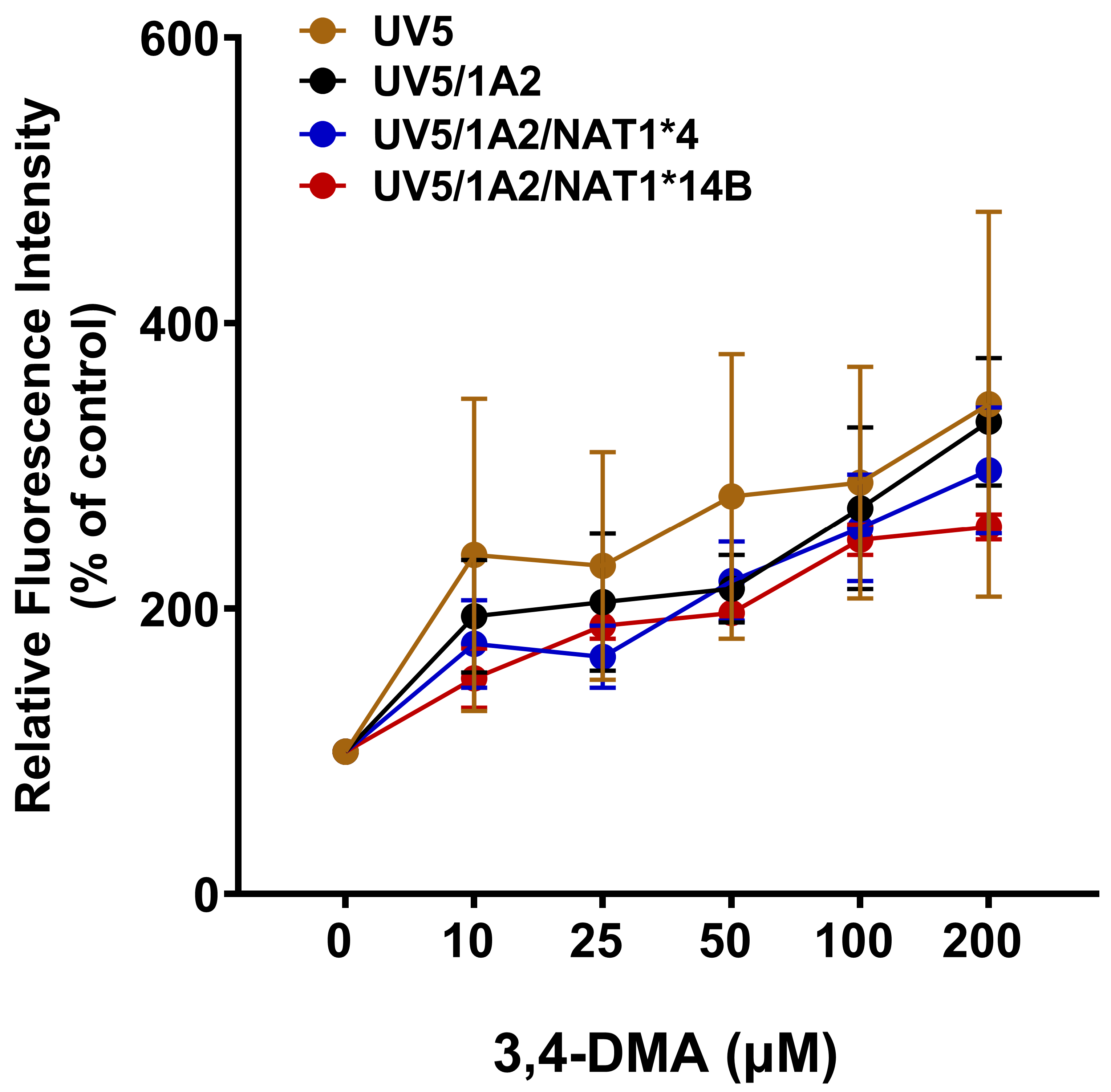
3.2. In Situ N-Acetylation
3.3. ROS
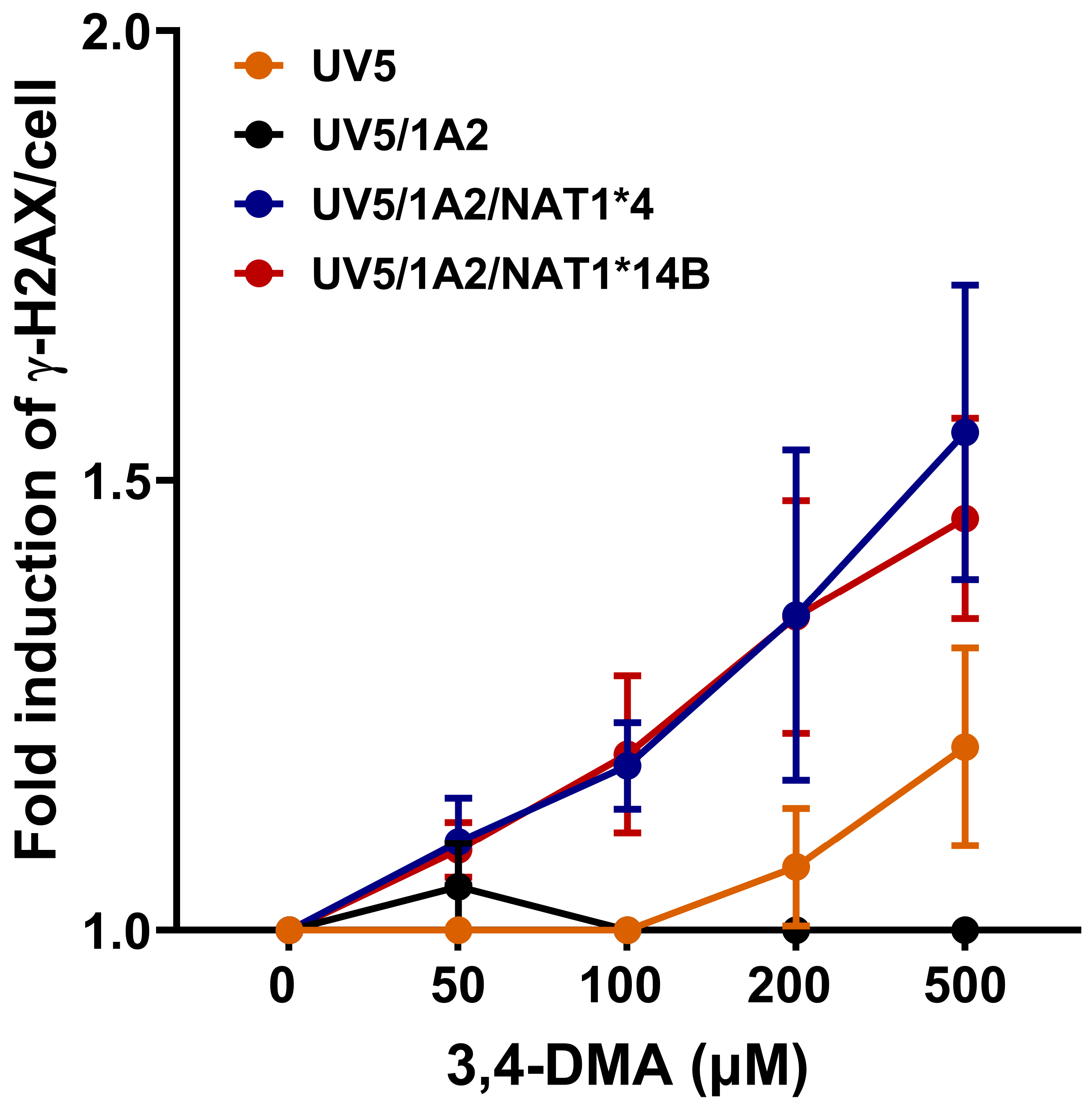
3.4. γH2AX Signal
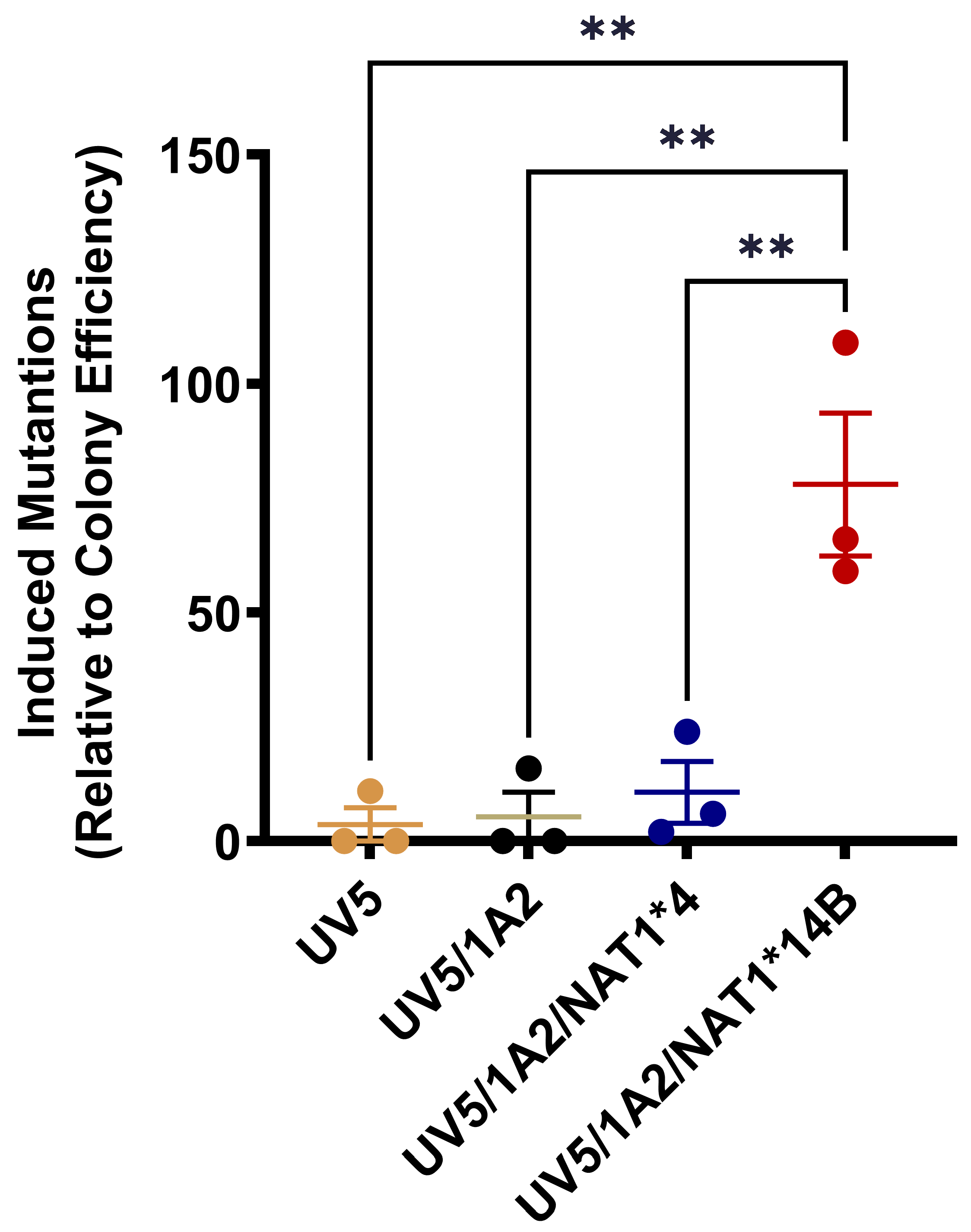
3.5. HPRT Mutations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skipper, P.L.; Kim, M.Y.; Sun, H.L.; Wogan, G.N.; Tannenbaum, S.R. Monocyclic aromatic amines as potential human carcinogens: Old is new again. Carcinogenesis 2010, 31, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, M.; Tang, J.; Zhang, Q.; Jiang, Y.; Li, H. A novel method Hybrid Photo-electrocatalytic Oxidation for the treatment of 3,4-dimethyaniline wastewater: Degradation mechanism and synergistic effect. J. Water Proc. Eng. 2020, 38, 101619. [Google Scholar] [CrossRef]
- Wang, S.; Hanna, D.; Sugamori, K.S.; Grant, D.M. Primary aromatic amines and cancer: Novel mechanistic insights using 4-aminobiphenyl as a model carcinogen. Pharmacol. Ther. 2019, 200, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.W.; Kim, M.Y.; Ye, W.; Ge, J.; Trudel, L.J.; Belanger, C.L.; Skipper, P.L.; Engelward, B.P.; Tannenbaum, S.R.; Wogan, G.N. Genotoxicity of 2,6- and 3,5-dimethylaniline in cultured mammalian cells: The role of reactive oxygen species. Toxicol. Sci. 2012, 130, 48–59. [Google Scholar] [CrossRef]
- Kobets, T.; Duan, J.D.; Brunnemann, K.D.; Vock, E.; Deschl, U.; Williams, G.M. DNA-damaging activities of twenty-four structurally diverse unsubstituted and substituted cyclic compounds in embryo-fetal chicken livers. Mutat. Res. 2019, 844, 10–24. [Google Scholar] [CrossRef]
- Leggett, C.S.; Doll, M.A.; States, J.C.; Hein, D.W. Acetylation of putative arylamine and alkylaniline carcinogens in immortalized human fibroblasts transfected with rapid and slow acetylator N-acetyltransferase 2 haplotypes. Arch. Toxicol. 2021, 95, 311–319. [Google Scholar] [CrossRef]
- Millner, L.M.; Doll, M.A.; Cai, J.; States, J.C.; Hein, D.W. Phenotype of the most common “slow acetylator” arylamine N-acetyltransferase 1 genetic variant (NAT1*14B) is substrate-dependent. Drug Metab. Dispos. 2012, 40, 198–204. [Google Scholar] [CrossRef]
- Bouchardy, C.; Mitrunen, K.; Wikman, H.; Husgafvel-Pursiainen, K.; Dayer, P.; Benhamou, S.; Hirvonen, A. N-acetyltransferase NAT1 and NAT2 genotypes and lung cancer risk. Pharmacogenetics 1998, 8, 291–298. [Google Scholar] [CrossRef] [PubMed]
- El Kawak, M.; Dhaini, H.R.; Jabbour, M.E.; Moussa, M.A.; El Asmar, K.; Aoun, M. Slow N-acetylation as a possible contributor to bladder carcinogenesis. Mol. Carcinog. 2020, 59, 1017–1027. [Google Scholar] [CrossRef]
- Habil, M.R.; Hein, D.W. Effects of dose and human N-acetyltransferase 1 genetic polymorphism in benzidine metabolism and genotoxicity. Arch. Toxicol. 2023, 97, 1765–1772. [Google Scholar] [CrossRef]
- Thompson, L.H.; Rubin, J.S.; Cleaver, J.E.; Whitmore, G.F.; Brookman, K. A screening method for isolating DNA repair-deficient mutants of CHO cells. Somat. Cell Genet. 1980, 6, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef]
- Gan, J.; Skipper, P.L.; Gago-Dominguez, M.; Arakawa, K.; Ross, R.K.; Yu, M.C.; Tannenbaum, S.R. Alkylaniline-hemoglobin adducts and risk of non-smoking-related bladder cancer. J. Natl. Cancer Inst. 2004, 96, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, A.; Arand, M.; MAK Commission. Xylidine isomers (2, 3-xylidine, 2, 5-xylidine, 3, 4-xylidine, 3, 5-xylidine). MAK Collect. Occup. Health Saf. 2023, 8, Doc064. [Google Scholar]
- Hein, D.W. N-acetyltransferase SNPs: Emerging concepts serve as a paradigm for understanding complexities of personalized medicine. Expert Opin. Drug Metab. Toxicol. 2009, 5, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Von Vett, A.; Zhang, N.; Walters, K.J.; Wagner, C.R.; Hanna, P.E. Arylamine N-acetyltransferases: Characterization of the substrate specificities and molecular interactions of environmental arylamines with human NAT1 and NAT2. Chem. Res. Toxicol. 2007, 20, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Dombrovsky, L.; Tempel, W.; Martin, F.; Loppnau, P.; Goodfellow, G.H.; Grant, D.M.; Plotnikov, A.N. Structural basis of substrate-binding specificity of human arylamine N-acetyltransferases. J. Biol. Chem. 2007, 282, 30189–30197. [Google Scholar] [CrossRef]
- Walraven, J.M.; Trent, J.O.; Hein, D.W. Structure-function analyses of single nucleotide polymorphisms in human N-acetyltransferase 1. Drug Metab. Rev. 2008, 40, 169–184. [Google Scholar] [CrossRef][Green Version]
- Zhou, X.; Ma, Z.; Dong, D.; Wu, B. Arylamine N-acetyltransferases: A structural perspective. Br. J. Pharmacol. 2013, 169, 748–760. [Google Scholar] [CrossRef]
- Butcher, N.J.; Ilett, K.F.; Minchin, R.F. Functional polymorphism of the human arylamine N-acetyltransferase type 1 gene caused by C190T and G560A mutations. Pharmacogenetics 1998, 8, 67–72. [Google Scholar] [CrossRef]
- Butcher, N.J.; Arulpragasam, A.; Minchin, R.F. Proteasomal degradation of N-acetyltransferase 1 is prevented by acetylation of the active site cysteine: A mechanism for the slow acetylator phenotype and substrate-dependent down-regulation. J. Biol. Chem. 2004, 279, 22131–22137. [Google Scholar] [CrossRef] [PubMed]
- Fretland, A.J.; Doll, M.A.; Leff, M.A.; Hein, D.W. Functional characterization of nucleotide polymorphisms in the coding region of N-acetyltransferase 1. Pharmacogenetics 2001, 11, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Fretland, A.J.; Doll, M.A.; Zhu, Y.; Smith, L.; Leff, M.A.; Hein, D.W. Effect of nucleotide substitutions in N-acetyltransferase-1 on N-acetylation (deactivation) and O-acetylation (activation) of arylamine carcinogens: Implications for cancer predisposition. Cancer Detect. Prev. 2002, 26, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Hughes, N.C.; Janezic, S.A.; McQueen, K.L.; Jewett, M.A.S.; Castranio, T.; Bell, D.A.; Grant, D.M. Identification and characterization of variant alleles of human acetyltransferase NAT1 with defective function using p-aminosalicylate as in vivo and in vitro probe. Pharmacogenetics 1998, 8, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Salazar-González, R.A.; Turiján-Espinoza, E.; Hein, D.W.; Niño-Moreno, P.C.; Romano-Moreno, S.; Milán-Segovia, R.C.; Portales-Pérez, D.P. Arylamine N-acetyltransferase 1 in situ N-acetylation on CD3+ peripheral blood mononuclear cells correlate with NATb mRNA and NAT1 haplotype. Arch. Toxicol. 2018, 92, 661–668. [Google Scholar] [CrossRef]
- Zhu, Y.; Hein, D.W. Functional effects of single nucleotide polymorphisms in the coding region of human N-acetyltransferase 1. Pharmacogenomics J. 2008, 8, 339–348. [Google Scholar] [CrossRef]
- Habil, M.R.; Doll, M.A.; Hein, D.W. Acetyl coenzyme A kinetic studies on N-acetylation of environmental carcinogens by human N-acetyltransferase 1 and its NAT1*14B variant. Front. Pharmacol. 2022, 13, 931323. [Google Scholar] [CrossRef]
- Doll, M.A.; Hein, D.W. 560G>A (rs4986782) (R187Q) single nucleotide polymorphism in arylamine N-acetyltransferase 1 increases affinity for the aromatic amine carcinogens 4-aminobiphenyl and N-hydroxy-4-aminobiphenyl: Implications for cancer risk assessment. Front. Pharmacol. 2022, 13, 820082. [Google Scholar] [CrossRef]
- Tuli, H.S.; Kaur, J.; Vashishth, K.; Sak, K.; Sharma, U.; Choudhary, R.; Behl, T.; Singh, T.; Sharma, S.; Saini, A.K.; et al. Molecular mechanisms behind ROS regulation in cancer: A balancing act between augmented tumorigenesis and cell apoptosis. Arch. Toxicol. 2023, 97, 103–120. [Google Scholar] [CrossRef]
- Goetz, M.E.; Luch, A. Reactive species: A cell damaging rout assisting to chemical carcinogens. Cancer Lett. 2008, 266, 73–83. [Google Scholar] [CrossRef]
- Kim, M.Y. Role of cytochrome P450 1A2 and N-acetyltransferase 2 in 2,6-dimethylaniline induced genotoxicity. Braz. J. Pharm. Sci. 2022, 58, e19221. [Google Scholar] [CrossRef]
- Qi, Y.; Toyooka, T.; Kashiwagi, H.; Yanagiba, Y.; Koda, S.; Ohta, H.; Wang, R.-S. 2,4-Dimethylaniline generates phosphorylated histone H2AX in human urothelial and hepatic cells through reactive oxygen species produced by cytochrome P450 2E1. Arch. Toxicol. 2018, 92, 3093–3101. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, D.J.; Sheil, M.L.; Streicker, M.A.; Johnson, G.E. A weight of evidence assessment of the genotoxicity of 2,6-xylidine based on existing and new data, with relevance to safety of lidocaine exposure. Reg. Toxicol. Pharmacol. 2021, 119, 104838. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.-W.; Kuo, H.-C.; Tong, S.-Y.; Yang, Y.-S.; Chuang, Y.-C.; Tseng, C.-Y. In vitro and In vivo analysis of the effects of 3,5-DMA and its metabolites in neural oxidative stress and neurodevelopmental toxicity. Toxicol. Sci. 2019, 168, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Rahmanian, N.; Shokrzadeh, M.; Eskandani, M. Recent advances in gammaH2AX biomarker-based genotoxicity assays: A marker of DNA damage and repair. DNA Repair 2021, 108, 103243. [Google Scholar] [CrossRef]
- Tronnet, S.; Oswald, E. Quantification of Colibactin-associated genotoxicity in HeLa cells by in cell Western (ICW) using γ-H2AX as a marker. Bio-Protoc. 2018, 8, e2771. [Google Scholar] [CrossRef]
- Kopp, B.; Khoury, L.; Audebert, M. Validation of the γH2AX biomarker for genotoxicity assessment: A review. Arch. Toxicol. 2019, 93, 2103–2114. [Google Scholar] [CrossRef]
- Kusakabe, H.; Yamakage, K.; Wakuri, S.; Sasaki, K.; Nakagawa, Y.; Watanabe, M.; Hayashi, M.; Sofuni, T.; Ono, H.; Tanaka, N. Relevance of chemical structure and cytotoxicity to the induction of chromosome aberrations based on the testing results of 98 high production volume industrial chemicals. Mutat. Res. 2002, 517, 187–198. [Google Scholar] [CrossRef]
- Toyooka, T.; Nie, J.; Ohta, H.; Koda, S.; Wang, R.S. Comparative gamma-H2AX analysis for assessment of the genotoxicity of six aromatic amines implicated in bladder cancer in human urothelial cell line. Toxicol. Vitr. 2020, 66, 104880. [Google Scholar]
- Zimmer, D.; Mazurek, J.; Petzold, G.; Bhuyan, B.K. Bacterial mutagenicity and mammalian cell DNA damage by several substituted anilines. Mutat. Res. 1980, 77, 317–326. [Google Scholar] [CrossRef]
- Kohara, A.; Matsumoto, M.; Hirose, A.; Hayashi, M.; Honma, M.; Suzuki, T. Mutagenic properties of dimethylaniline isomers in mice as evaluated by comet, micronucleus and transgenic mutation assays. Genes Environ. 2018, 40, 18. [Google Scholar] [CrossRef] [PubMed]
- Habil, M.R. Investigation of Human N-Acetyltransferases (NAT1 and NAT2) Genetic Polymorphisms in Susceptibility to Aromatic Amine and Alkylaniline Genotoxicity. Ph.D. Dissertation, University of Louisville, Louisville, KY, USA, December 2022. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habil, M.R.; Salazar-González, R.A.; Doll, M.A.; Hein, D.W. Bioactivation, Mutagenicity, DNA Damage, and Oxidative Stress Induced by 3,4-Dimethylaniline. Biomolecules 2024, 14, 1562. https://doi.org/10.3390/biom14121562
Habil MR, Salazar-González RA, Doll MA, Hein DW. Bioactivation, Mutagenicity, DNA Damage, and Oxidative Stress Induced by 3,4-Dimethylaniline. Biomolecules. 2024; 14(12):1562. https://doi.org/10.3390/biom14121562
Chicago/Turabian StyleHabil, Mariam R., Raúl A. Salazar-González, Mark A. Doll, and David W. Hein. 2024. "Bioactivation, Mutagenicity, DNA Damage, and Oxidative Stress Induced by 3,4-Dimethylaniline" Biomolecules 14, no. 12: 1562. https://doi.org/10.3390/biom14121562
APA StyleHabil, M. R., Salazar-González, R. A., Doll, M. A., & Hein, D. W. (2024). Bioactivation, Mutagenicity, DNA Damage, and Oxidative Stress Induced by 3,4-Dimethylaniline. Biomolecules, 14(12), 1562. https://doi.org/10.3390/biom14121562







