Urate Levels as a Predictor of the Prevalence and Level of Cardiovascular Risk Factors: An Identificación de La PoBlación Española de Riesgo Cardiovascular y Renal Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
2.3. Recorded Variables
2.4. Statistical Analysis
3. Results
3.1. General Characteristics of the Sample
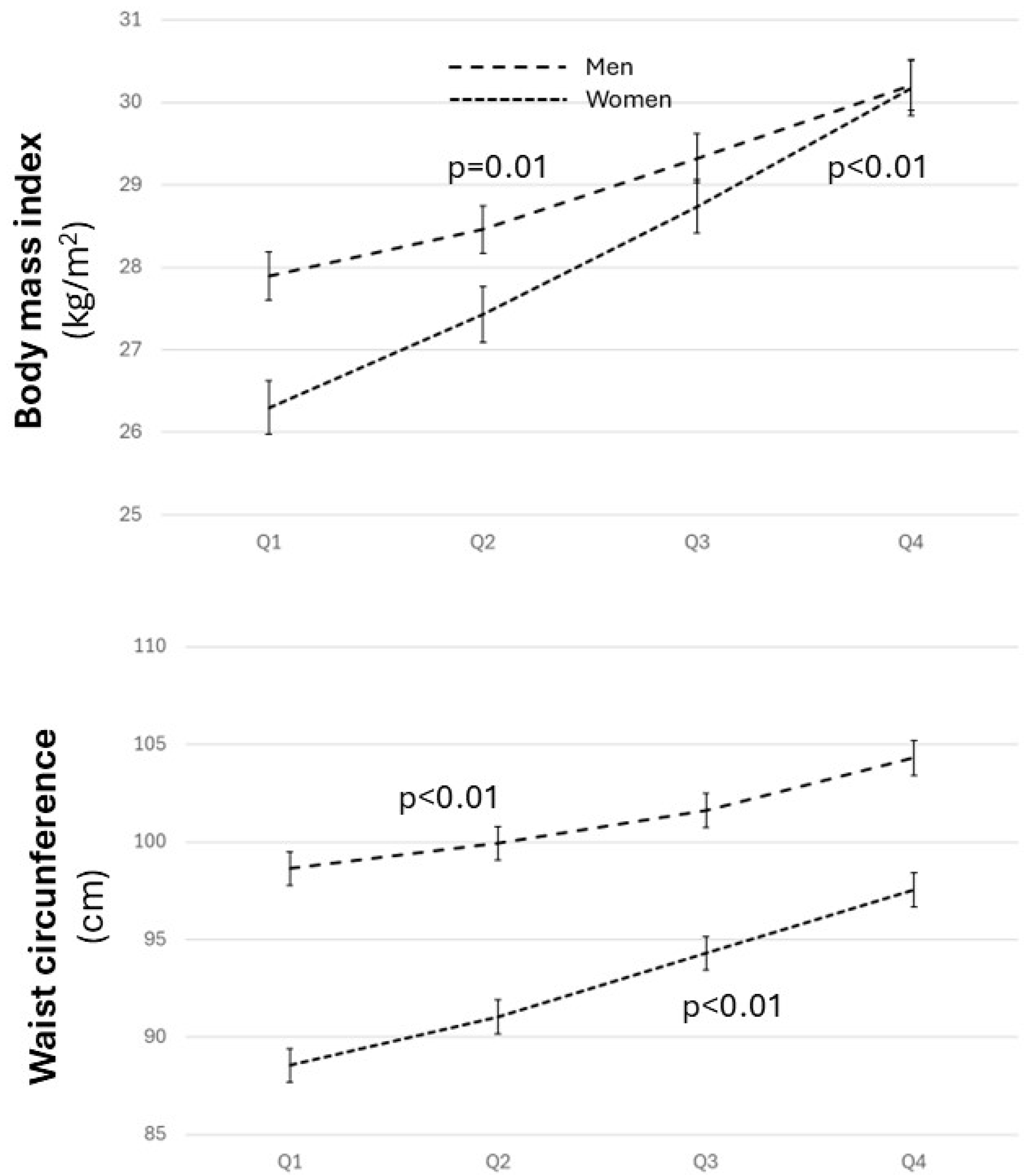
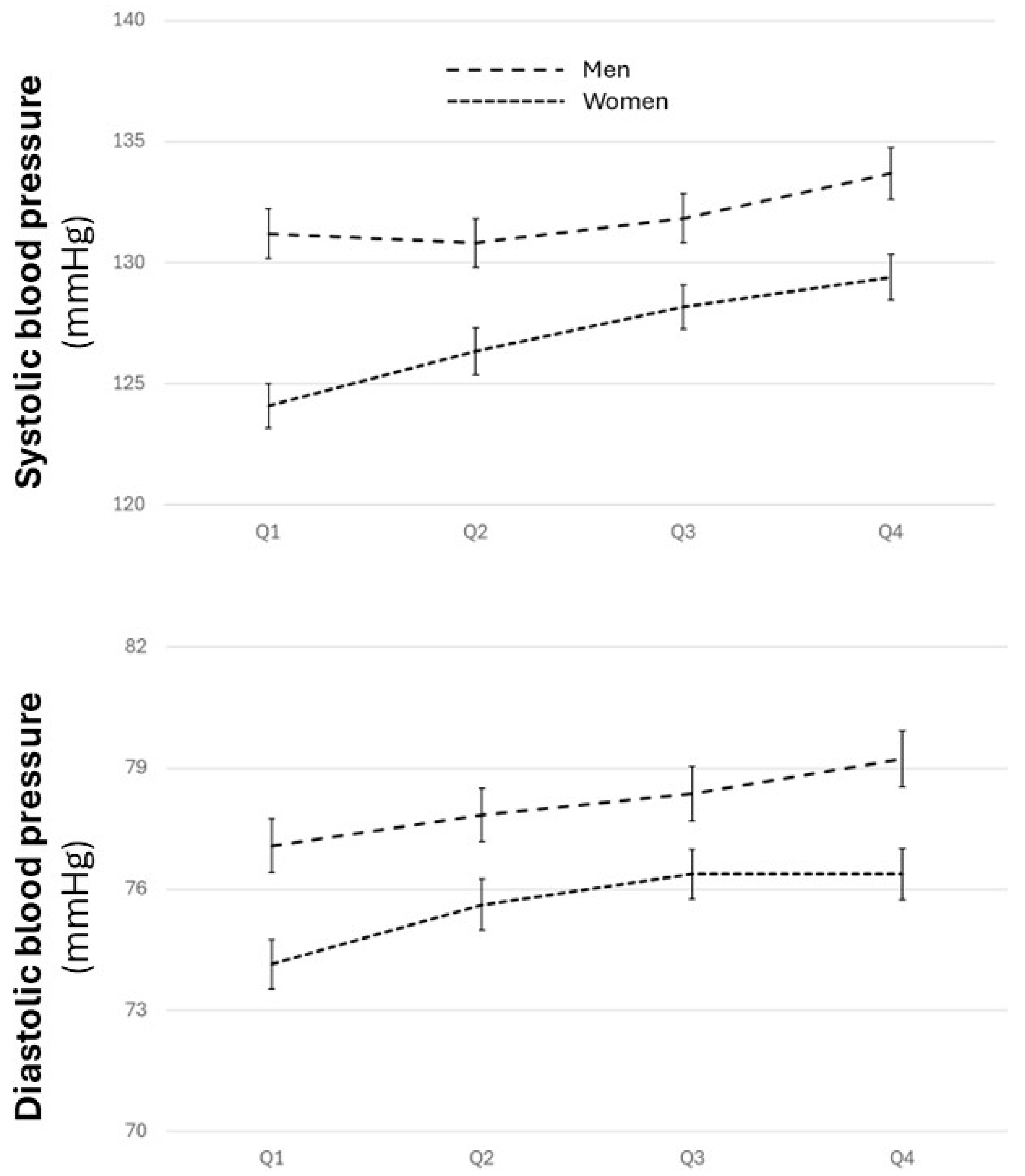
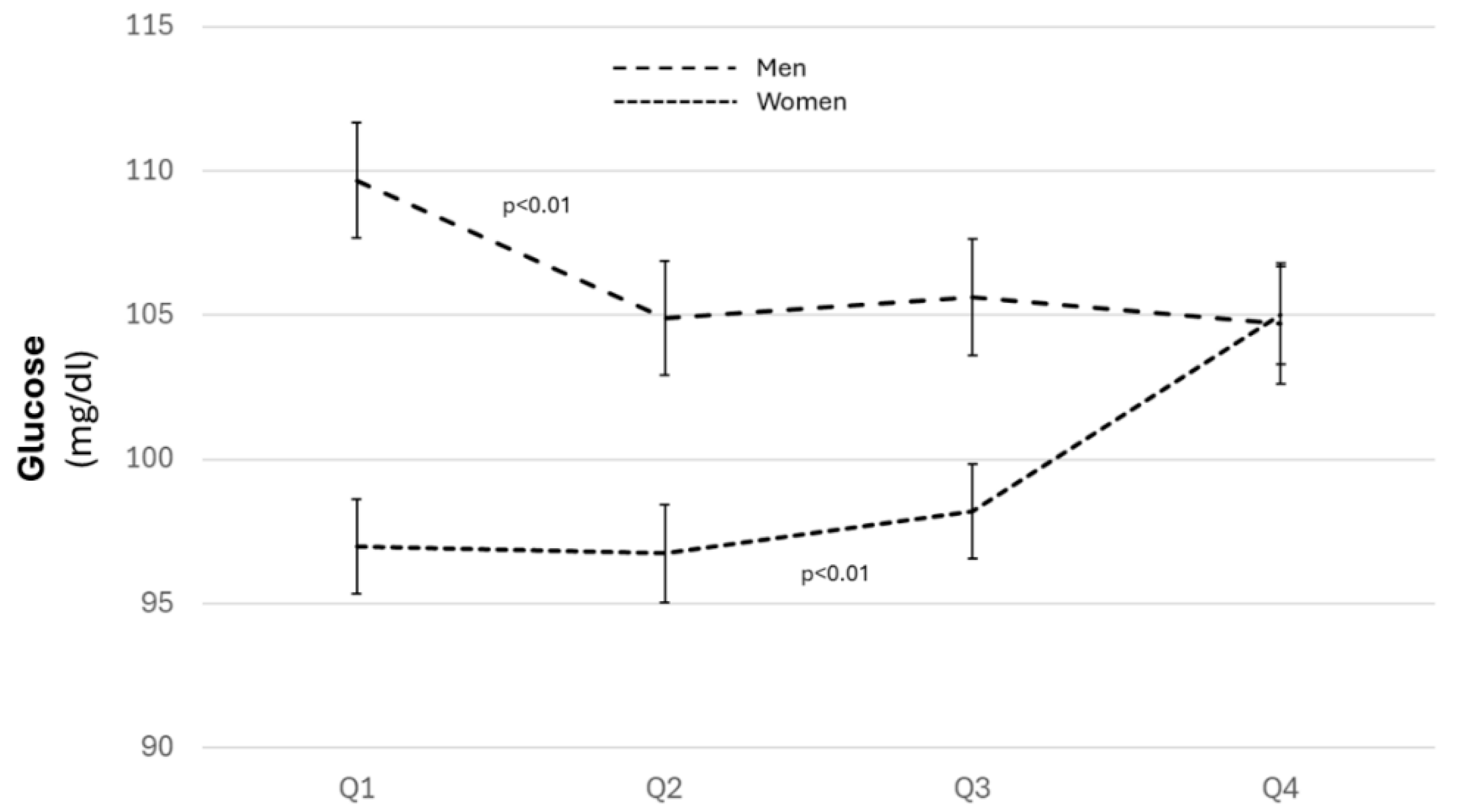
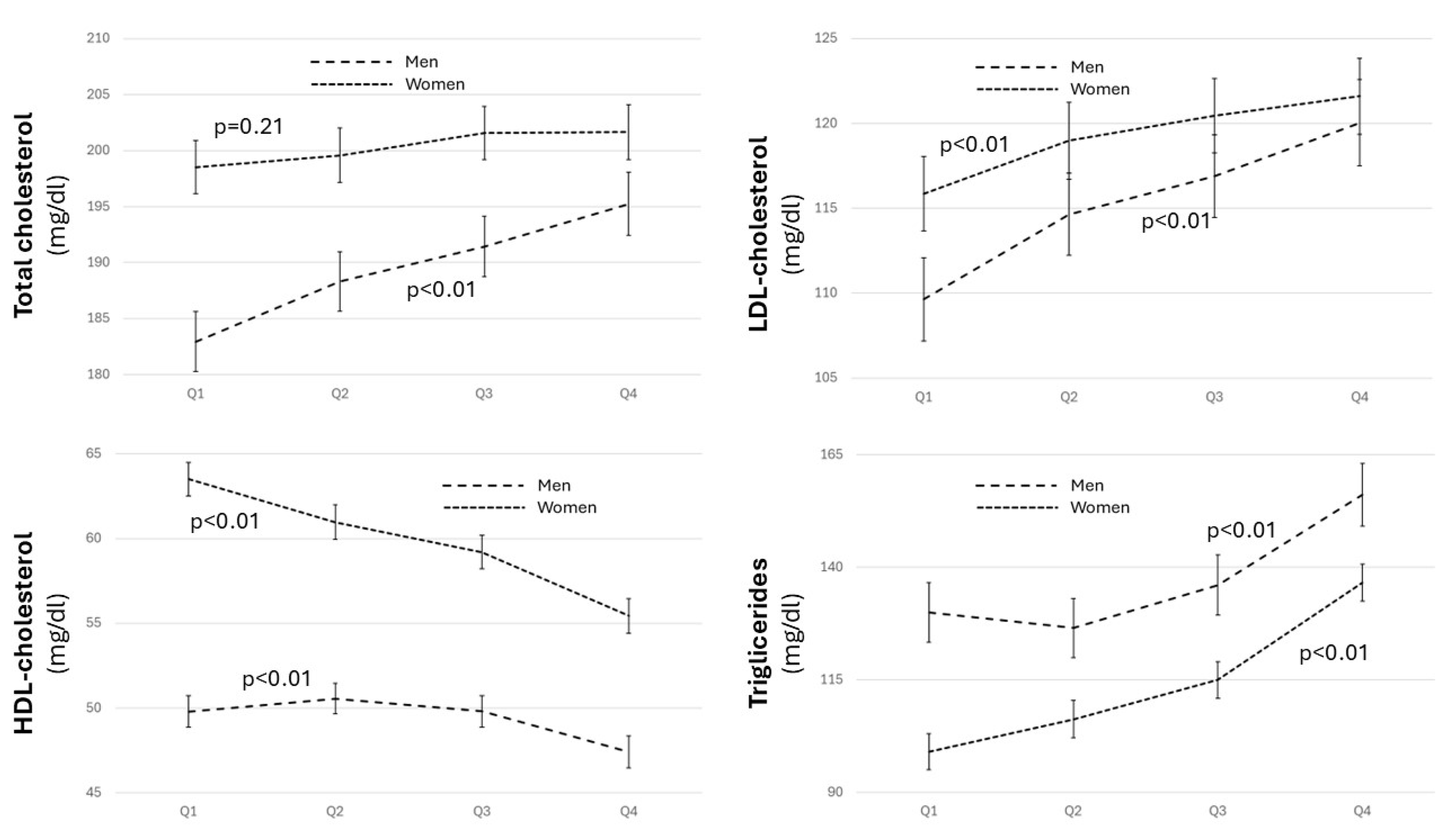
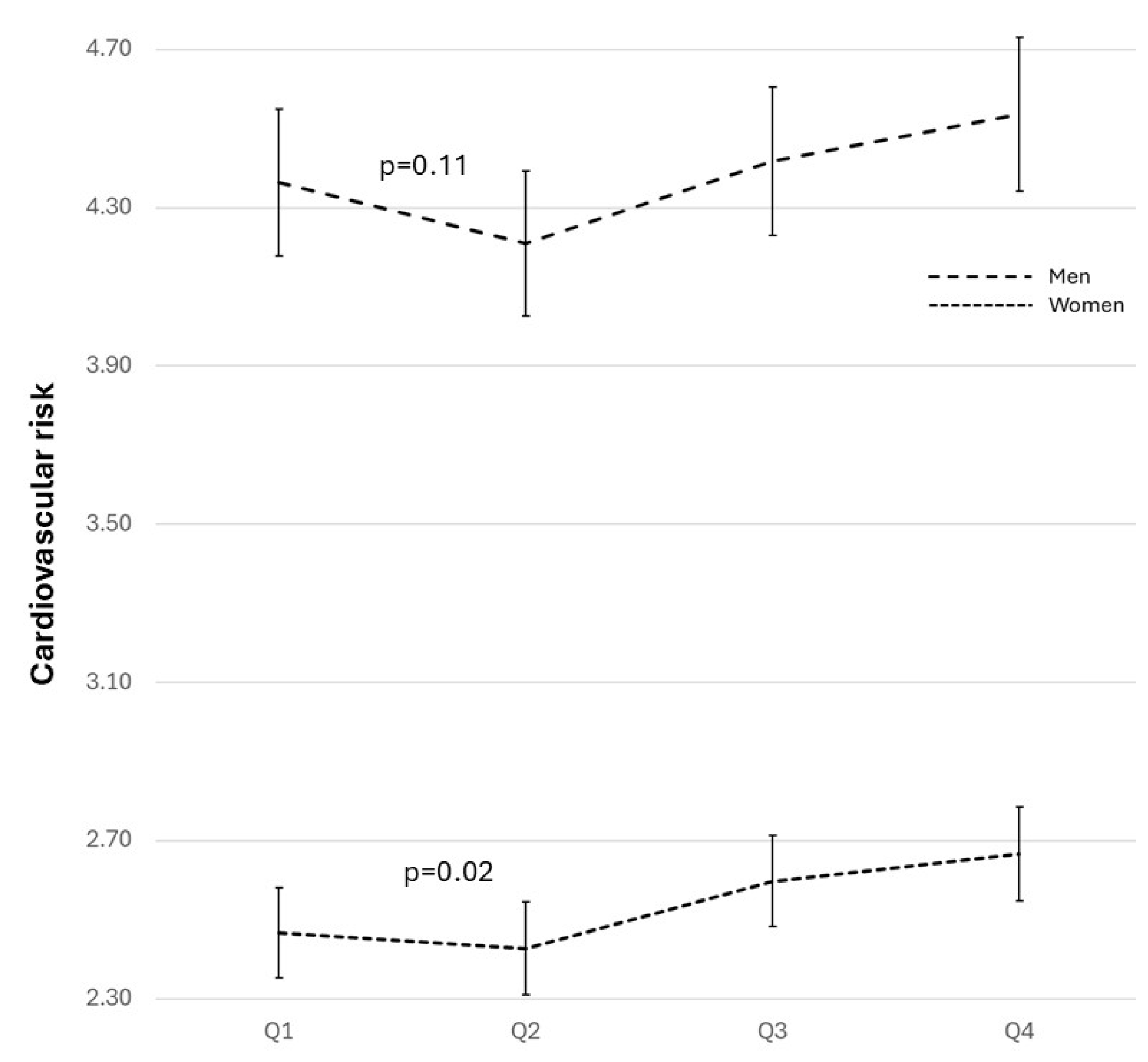
3.2. Distribution of Cardiovascular Risk Factors and Estimated Cardiovascular Risk
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and Atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Copur, S.; Demiray, A.; Kanbay, M. Uric Acid in Metabolic Syndrome: Does Uric Acid Have a Definitive Role? Eur. J. Intern. Med. 2022, 103, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Tanaka, A.; Node, K.; Kobayashi, Y. Uric Acid and Cardiovascular Disease: A Clinical Review. J. Cardiol. 2021, 78, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Corry, D.B.; Tuck, M.L. Uric Acid and the Vasculature. Curr. Hypertens. Rep. 2006, 8, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Sautin, Y.Y.; Johnson, R.J. Uric Acid: The Oxidant-Antioxidant Paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef]
- Patterson, R.A.; Horsley, E.T.M.; Leake, D.S. Prooxidant and Antioxidant Properties of Human Serum Ultrafiltrates toward LDL: Important Role of Uric Acid. J. Lipid Res. 2003, 44, 512–521. [Google Scholar] [CrossRef]
- Kutzing, M.K.; Firestein, B.L. Altered Uric Acid Levels and Disease States. J. Pharmacol. Exp. Ther. 2008, 324, 1–7. [Google Scholar] [CrossRef]
- Crawley, W.T.; Jungels, C.G.; Stenmark, K.R.; Fini, M.A. U-Shaped Association of Uric Acid to Overall-Cause Mortality and Its Impact on Clinical Management of Hyperuricemia. Redox Biol. 2022, 51, 102271. [Google Scholar] [CrossRef]
- Gherghina, M.E.; Peride, I.; Tiglis, M.; Neagu, T.P.; Niculae, A.; Checherita, I.A. Uric Acid and Oxidative Stress-Relationship with Cardiovascular, Metabolic, and Renal Impairment. Int. J. Mol. Sci. 2022, 23, 3188. [Google Scholar] [CrossRef]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the Management of Elevated Blood Pressure and Hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef]
- Culleton, B.F.; Larson, M.G.; Kannel, W.B.; Levy, D. Serum Uric Acid and Risk for Cardiovascular Disease and Death: The Framingham Heart Study. Ann. Intern. Med. 1999, 131, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Maloberti, A.; Giannattasio, C.; Bombelli, M.; Desideri, G.; Cicero, A.F.G.; Muiesan, M.L.; Rosei, E.A.; Salvetti, M.; Ungar, A.; Rivasi, G.; et al. Hyperuricemia and Risk of Cardiovascular Outcomes: The Experience of the URRAH (Uric Acid Right for Heart Health) Project. High Blood Press. Cardiovasc. Prev. 2020, 27, 121–128. [Google Scholar] [CrossRef]
- Liu, L.; Gu, Y.; Li, C.; Zhang, Q.; Meng, G.; Wu, H.; Du, H.; Shi, H.; Xia, Y.; Guo, X.; et al. Serum Uric Acid Is an Independent Predictor for Developing Prehypertension: A Population-Based Prospective Cohort Study. J. Hum. Hypertens. 2017, 31, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Hayashi, T.; Tsumura, K.; Endo, G.; Fujii, S.; Okada, K. Serum Uric Acid and the Risk for Hyper-tension and Type 2 Diabetes in Japanese Men: The Osaka Health Survey. J. Hypertens. 2001, 19, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Qin, P.; Wang, C.; Ma, J.; Peng, X.; Xu, S.; Chen, H.; Zhao, D.; Wang, L.; Liu, D.; et al. Sex-Specific Associa-tion of Serum Uric Acid Level and Change in Hyperuricemia Status with Risk of Type 2 Diabetes Mellitus: A Large Cohort Study in China. J. Diabetes Res. 2020, 2020, 9637365. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zheng, T.; Zhang, D.; Yang, J.; Hu, X.; Yin, C.; Ren, X.; Li, J.; Shi, D.; Li, N.; et al. High-Level Uric Acid in Asymptomatic Hyperuricemia Could Be an Isolated Risk Factor of Cardio-Cerebrovascular Diseases: A Prospec-tive Cohort Study. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 3415–3425. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hou, Y.; Wang, X.; Li, Z.; Wang, X.; Li, H.; Shang, L.; Zhou, J.; Zhang, Y.; Ren, M.; et al. Relationship between Serum Uric Acid Levels and Different Types of Atrial Fibrillation: An Updated Meta-Analysis. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2756–2765. [Google Scholar] [CrossRef]
- Mantovani, A.; Targher, G.; Temporelli, P.L.; Lucci, D.; Gonzini, L.; Nicolosi, G.L.; Marchioli, R.; Tognoni, G.; Latini, R.; Cosmi, F.; et al. Prognostic Impact of Elevated Serum Uric Acid Levels on Long-Term Outcomes in Pa-tients with Chronic Heart Failure: A Post-Hoc Analysis of the GISSI-HF (Gruppo Italiano per Lo Studio Della So-pravvivenza Nella Insufficienza Cardiaca-Heart Failure) Trial. Metabolism 2018, 83, 205–215. [Google Scholar] [CrossRef]
- Lim, S.S.; Yang, Y.L.; Chen, S.C.; Wu, C.H.; Huang, S.S.; Chan, W.L.; Lin, S.J.; Chen, J.W.; Chou, C.Y.; Pan, J.P.; et al. Association of Variability in Uric Acid and Future Clinical Outcomes of Patient with Coronary Artery Disease Undergoing Percutaneous Coronary Intervention. Atherosclerosis 2020, 297, 40–46. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Braun, S.; Haase, H.-U.; Schulz, S.; Ranftl, S.; Hadamitzky, M.; Mehilli, J.; Schömig, A.; Kastrati, A. Prognostic Value of Uric Acid in Patients With Acute Coronary Syndromes. Am. J. Cardiol. 2012, 109, 1260–1265. [Google Scholar] [CrossRef]
- Lin, Y.K.; Lin, Y.P.; Lee, J.T.; Lin, C.S.; Wu, T.J.; Tsai, K.Z.; Su, F.Y.; Kwon, Y.; Hoshide, S.; Lin, G.M. Sex-Specific Association of Hyperuricemia with Cardiometabolic Abnormalities in a Military Cohort: The CHIEF Study. Medicine 2020, 99, e19535. [Google Scholar] [CrossRef] [PubMed]
- Casiglia, E.; Tikhonoff, V.; Virdis, A.; Masi, S.; Barbagallo, C.M.; Bombelli, M.; Bruno, B.; Cicero, A.F.G.; Cirillo, M.; Cirillo, P.; et al. Serum Uric Acid and Fatal Myocardial Infarction: Detection of Prognostic Cut-off Values: The URRAH (Uric Acid Right for Heart Health) Study. J. Hypertens. 2020, 38, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Cinza Sanjurjo, S.; Llisterri Caro, J.L.; Barquilla García, A.; Polo García, J.; Velilla Zancada, S.; Rodríguez Roca, G.C.; Micó Pérez, R.M.; Martín Sánchez, V.; Prieto Díaz, M.Á. Description of the sample, design and methods of the study for the identification of the Spanish population at cardiovascular and renal risk (IBERICAN). Med. De Fam. SEMERGEN 2020, 46, 4–15. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee; ElSayed, N.A.; Aleppo, G.; Bannuru, R.R.; Bruemmer, D.; Collins, B.S.; Ekhlaspour, L.; Gaglia, J.L.; Hilliard, M.E.; Johnson, E.L.; et al. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47, S20–S42. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Conroy, R. Estimation of Ten-Year Risk of Fatal Cardiovascular Disease in Europe: The SCORE Project. Eur. Heart J. 2003, 24, 987–1003. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary Patterns: A Mediterranean Diet Score and Its Relation to Clinical and Biological Markers of Cardiovascular Disease Risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef]
- Antelo-Pais, P.; Prieto-Díaz, M.Á.; Micó-Pérez, R.M.; Pallarés-Carratalá, V.; Velilla-Zancada, S.; Polo-García, J.; Barquilla-García, A.; Ginel-Mendoza, L.; Segura-Fragoso, A.; Vitelli-Storelli, F.; et al. Prevalence of Hyperuricemia and Its Association with Cardiovascular Risk Factors and Subclinical Target Organ Damage. J. Clin. Med. 2022, 12, 50. [Google Scholar] [CrossRef]
- Lurbe, E.; Torro, M.I.; Alvarez-Pitti, J.; Redon, J.; Borghi, C.; Redon, P. Uric Acid Is Linked to Cardiometabolic Risk Factors in Overweight and Obese Youths. J. Hypertens. 2018, 36, 1840–1846. [Google Scholar] [CrossRef]
- Rocha, E.P.A.A.; Vogel, M.; Stanik, J.; Pietzner, D.; Willenberg, A.; Körner, A.; Kiess, W. Serum Uric Acid Levels as an Indicator for Metabolically Unhealthy Obesity in Children and Adolescents. Horm. Res. Paediatr. 2018, 90, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, M.; Kuwabara, R.; Niwa, K.; Hisatome, I.; Smits, G.; Roncal-Jimenez, C.; MacLean, P.; Yracheta, J.; Ohno, M.; Lanaspa, M.; et al. Different Risk for Hypertension, Diabetes, Dyslipidemia, and Hyperuricemia According to Level of Body Mass Index in Japanese and American Subjects. Nutrients 2018, 10, 1011. [Google Scholar] [CrossRef] [PubMed]
- Shirasawa, T.; Ochiai, H.; Yoshimoto, T.; Nagahama, S.; Watanabe, A.; Yoshida, R.; Kokaze, A. Correction to: Cross-Sectional Study of Associations between Normal Body Weight with Central Obesity and Hyperuricemia in Japan. BMC Endocr. Disord. 2020, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Jiang, Y.; Huang, Y.; Song, W.; Li, X.; Huang, Y.; Ou, J.; Wei, Q.; Gu, J. The Comparison of Dyslipidemia and Serum Uric Acid in Patients with Gout and Asymptomatic Hyperuricemia: A Cross-Sectional Study. Lipids Health Dis. 2020, 19, 31. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Kao, T.W.; Yang, H.F.; Chou, C.W.; Wu, C.J.; Lai, C.H.; Sun, Y.S.; Wang, C.C.; Chen, W.L. The Associa-tion of Uric Acid with the Risk of Metabolic Syndrome, Arterial Hypertension or Diabetes in Young Subjects—An Observational Study. Clin. Chim. Acta 2018, 478, 68–73. [Google Scholar] [CrossRef]
- Osgood, K.; Krakoff, J.; Thearle, M. Serum Uric Acid Predicts Both Current and Future Components of the Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2013, 11, 157–162. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, X.; Kang, S.; Wu, Y. Serum Uric Acid Levels and Incidence of Impaired Fasting Glucose and Type 2 Diabetes Mellitus: A Meta-Analysis of Cohort Studies. Diabetes Res. Clin. Pract. 2013, 101, 88–96. [Google Scholar] [CrossRef]
- Kim, S.C.; Liu, J.; Solomon, D.H. Risk of Incident Diabetes in Patients with Gout: A Cohort Study. Arthritis Rheumatol. 2015, 67, 273–280. [Google Scholar] [CrossRef]
- Maloberti, A.; Dell’Oro, R.; Bombelli, M.; Quarti-Trevano, F.; Facchetti, R.; Mancia, G.; Grassi, G. Long-Term In-crease in Serum Uric Acid and Its Predictors over a 25 Year Follow-up: Results of the PAMELA Study. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 223–229. [Google Scholar] [CrossRef]
- Fadaei, R.; Davies, S.S. Oxidative Modification of HDL by Lipid Aldehydes Impacts HDL Function. Arch. Biochem. Biophys. 2022, 730, 109397. [Google Scholar] [CrossRef]
- Bawazier, L.A.; Sja’bani, M.; Irijanto, F.; Zulaela, Z.; Widiatmoko, A.; Kholiq, A.; Tomino, Y. Association of Serum Uric Acid, Morning Home Blood Pressure and Cardiovascular Risk Factors in a Population with Previous Pre-hypertension: A Cross-Sectional Study. BMJ Open 2020, 10, e038046. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Girerd, N.; Machu, J.-L.; Bozec, E.; Duarte, K.; Boivin, J.-M.; Wagner, S.; Ferreira, J.P.; Zannad, F.; Ros-signol, P. Impact of Uric Acid on Hypertension Occurrence and Target Organ Damage: Insights From the STAN-ISLAS Cohort With a 20-Year Follow-Up. Am. J. Hypertens. 2020, 33, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Agra, N.; Cruces-Sande, A.; Mendez-Alvarez, E.; Soto-Otero, R.; Cinza-Sanjurjo, S.; Lopez-Paz, J.-E.; Pose-Reino, A.; Hermida-Ameijeiras, A. Correlation between Blunted Nocturnal Decrease in Diastolic Blood Pressure and Oxidative Stress: An Observational Study. Antioxidants 2022, 11, 2430. [Google Scholar] [CrossRef] [PubMed]
- Dzau, V.J.; Antman, E.M.; Black, H.R.; Hayes, D.L.; Manson, J.E.; Plutzky, J.; Popma, J.J.; Stevenson, W. The Cardiovascular Disease Continuum Validated: Clinical Evidence of Improved Patient Outcomes. Circulation 2006, 114, 2850–2870. [Google Scholar] [CrossRef] [PubMed]
- Maloberti, A.; Qualliu, E.; Occhi, L.; Sun, J.; Grasso, E.; Tognola, C.; Tavecchia, G.; Cartella, I.; Milani, M.; Vallerio, P.; et al. Hyperuricemia Prevalence in Healthy Subjects and Its Relationship with Cardiovascular Target Organ Damage. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 178–185. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Tian, W.; Shi, L.; Chen, L.; Li, J.; Zhao, S.; Qi, G. Association between Serum Uric Acid Levels and Coronary Artery Disease in Different Age and Gender: A Cross-Sectional Study. Aging Clin. Exp. Res. 2019, 31, 1783–1790. [Google Scholar] [CrossRef]
- Cinza-Sanjurjo, S.; Micó-Pérez, R.M.; Velilla-Zancada, S.; Prieto-Díaz, M.A.; Rodríguez-Roca, G.C.; Barquilla García, A.; Polo García, J.; Martín Sánchez, V.; Llisterri Caro, J.L. Factores Asociados al Riesgo Cardiovascular y Enfermedad Cardiovascular y Renal En El Estudio IBERICAN (Identificación de La PoBlación Española de RIesgo CArdiovascular y ReNal): Resultados Definitivos. Med. De Fam. SEMERGEN 2020, 46, 368–378. [Google Scholar] [CrossRef]
- Comité Científico Estudio—IBERICAN. Estudio IBERICAN: ¿el Framingham Español? Semer. Rev. Española De Med. De Fam. 2015, 41, 1–2. [Google Scholar] [CrossRef]
- Mena-Sánchez, G.; Babio, N.; Becerra-Tomás, N.; Martínez-González, M.Á.; Díaz-López, A.; Corella, D.; Zomeño, M.D.; Romaguera, D.; Vioque, J.; Alonso-Gómez, Á.M.; et al. Association between Dairy Product Consumption and Hyperuricemia in an Elderly Population with Metabolic Syndrome. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 214–222. [Google Scholar] [CrossRef]
- Stack, A.G.; Hanley, A.; Casserly, L.F.; Cronin, C.J.; Abdalla, A.A.; Kiernan, T.J.; Murthy, B.V.R.; Hegarty, A.; Han-nigan, A.; Nguyen, H.T. Independent and Conjoint Associations of Gout and Hyperuricaemia with Total and Cardiovascular Mortality. QJM Int. J. Med. 2013, 106, 647–658. [Google Scholar] [CrossRef]
- Chen, P.-H.; Chen, Y.-W.; Liu, W.-J.; Hsu, S.-W.; Chen, C.-H.; Lee, C.-L. Approximate Mortality Risks between Hyperuricemia and Diabetes in the United States. J. Clin. Med. 2019, 8, 2127. [Google Scholar] [CrossRef] [PubMed]

| Q1 | Q2 | Q3 | Q4 | p | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1609 | 1821 | 1697 | 1800 | ||||||
| N | Mean [SD] | N | Mean [SD] | N | Mean [SD] | N | Mean [SD] | ||
| Age (years) | 1609 | 55.91 [14.94] | 1821 | 56.91 [15.06] | 1697 | 59.55 [14.14] | 1800 | 62.15 [13.55] | <0.001 |
| N | % | N | % | N | % | N | % | ||
| Women | 873 | 54.3% | 993 | 54.5% | 878 | 51.7% | 995 | 55.3% | 0.136 |
| Ethnicity | |||||||||
| White | 1545 | 96.0% | 1747 | 95.9% | 1641 | 96.7% | 1745 | 96.9% | 0.656 |
| Black | 4 | 0.2% | 7 | 0.4% | 7 | 0.4% | 12 | 0.7% | |
| Latin American | 49 | 3.0% | 55 | 3.0% | 41 | 2.4% | 34 | 1.9% | |
| Asian | 2 | 0.1% | 1 | 0.1% | 1 | 0.1% | 3 | 0.2% | |
| Berber | 9 | 0.6% | 11 | 0.6% | 7 | 0.4% | 6 | 0.3% | |
| Habitat | |||||||||
| Urban | 947 | 58.9% | 1056 | 58.0% | 999 | 58.9% | 1059 | 58.8% | 0.986 |
| Semi-urban | 342 | 21.3% | 398 | 21.9% | 353 | 20.8% | 381 | 21.2% | |
| Rural | 319 | 19.8% | 367 | 20.2% | 344 | 20.3% | 360 | 20.0% | |
| Education | |||||||||
| Unschooled | 116 | 7.2% | 126 | 6.9% | 160 | 9.4% | 225 | 12.5% | 0.124 |
| Primary education | 863 | 53.6% | 1014 | 55.7% | 931 | 54.9% | 1006 | 55.9% | |
| Higher education | 377 | 23.4% | 430 | 23.6% | 376 | 22.2% | 378 | 21.0% | |
| University education | 253 | 15.7% | 251 | 13.8% | 230 | 13.6% | 191 | 10.6% | |
| Employment | |||||||||
| Jobholder | 739 | 46.0% | 810 | 44.6% | 718 | 42.5% | 647 | 36.0% | 0.138 |
| Unemployed | 141 | 8.8% | 160 | 8.8% | 143 | 8.5% | 125 | 7.0% | |
| Retired | 493 | 30.7% | 609 | 33.6% | 617 | 36.5% | 766 | 42.7% | |
| Student | 23 | 1.4% | 29 | 1.6% | 15 | .9% | 9 | .5% | |
| Housekeeping | 209 | 13.0% | 207 | 11.4% | 197 | 11.7% | 248 | 13.8% | |
| Income | |||||||||
| Annual income lower than 18,000 EUR | 664 | 41.3% | 744 | 40.9% | 719 | 42.4% | 793 | 44.1% | 0.935 |
| Annual income between 18,000 EUR and 100,000 EUR | 927 | 57.6% | 1057 | 58.0% | 953 | 56.2% | 983 | 54.6% | |
| Annual income higher than 100,000 EUR | 18 | 1.1% | 20 | 1.1% | 25 | 1.5% | 24 | 1.3% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antelo-Pais, P.; Prieto-Díaz, M.Á.; Micó-Pérez, R.M.; Pallarés-Carratalá, V.; Velilla-Zancada, S.; Polo-García, J.; Barquilla-García, A.; Ginel-Mendoza, L.; Segura-Fragoso, A.; Vitelli-Storelli, F.; et al. Urate Levels as a Predictor of the Prevalence and Level of Cardiovascular Risk Factors: An Identificación de La PoBlación Española de Riesgo Cardiovascular y Renal Study. Biomolecules 2024, 14, 1530. https://doi.org/10.3390/biom14121530
Antelo-Pais P, Prieto-Díaz MÁ, Micó-Pérez RM, Pallarés-Carratalá V, Velilla-Zancada S, Polo-García J, Barquilla-García A, Ginel-Mendoza L, Segura-Fragoso A, Vitelli-Storelli F, et al. Urate Levels as a Predictor of the Prevalence and Level of Cardiovascular Risk Factors: An Identificación de La PoBlación Española de Riesgo Cardiovascular y Renal Study. Biomolecules. 2024; 14(12):1530. https://doi.org/10.3390/biom14121530
Chicago/Turabian StyleAntelo-Pais, Paula, Miguel Ángel Prieto-Díaz, Rafael M. Micó-Pérez, Vicente Pallarés-Carratalá, Sonsoles Velilla-Zancada, José Polo-García, Alfonso Barquilla-García, Leovigildo Ginel-Mendoza, Antonio Segura-Fragoso, Facundo Vitelli-Storelli, and et al. 2024. "Urate Levels as a Predictor of the Prevalence and Level of Cardiovascular Risk Factors: An Identificación de La PoBlación Española de Riesgo Cardiovascular y Renal Study" Biomolecules 14, no. 12: 1530. https://doi.org/10.3390/biom14121530
APA StyleAntelo-Pais, P., Prieto-Díaz, M. Á., Micó-Pérez, R. M., Pallarés-Carratalá, V., Velilla-Zancada, S., Polo-García, J., Barquilla-García, A., Ginel-Mendoza, L., Segura-Fragoso, A., Vitelli-Storelli, F., Martín-Sánchez, V., Hermida-Ameijerias, Á., Cinza-Sanjurjo, S., & on behalf of the Investigators of the IBERICAN Study and of the Spanish Society of Primary Care Physicians (SEMERGEN) Foundation. (2024). Urate Levels as a Predictor of the Prevalence and Level of Cardiovascular Risk Factors: An Identificación de La PoBlación Española de Riesgo Cardiovascular y Renal Study. Biomolecules, 14(12), 1530. https://doi.org/10.3390/biom14121530








