Visfatin Enhances RANKL-Induced Osteoclastogenesis In Vitro: Synergistic Interactions and Its Role as a Mediator in Osteoclast Differentiation and Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies and Reagents
2.2. Visfatin Expression Analysis in Gene Expression Omnibus (GEO) Datasets
2.3. Mouse Bone Marrow-Derived Macrophage Preparation
2.4. Osteoclast Differentiation and Tartrate-Resistant Acid Phosphatase Staining
2.5. Resorption Pit Formation Assay
2.6. Western Blot Analysis
2.7. Enzyme-Linked Immunosorbent Assay
2.8. Immunocytochemistry
2.9. Reverse-Transcription Quantitative Real-Time Polymerase Chain Reaction
2.10. Statistical Analysis
3. Results
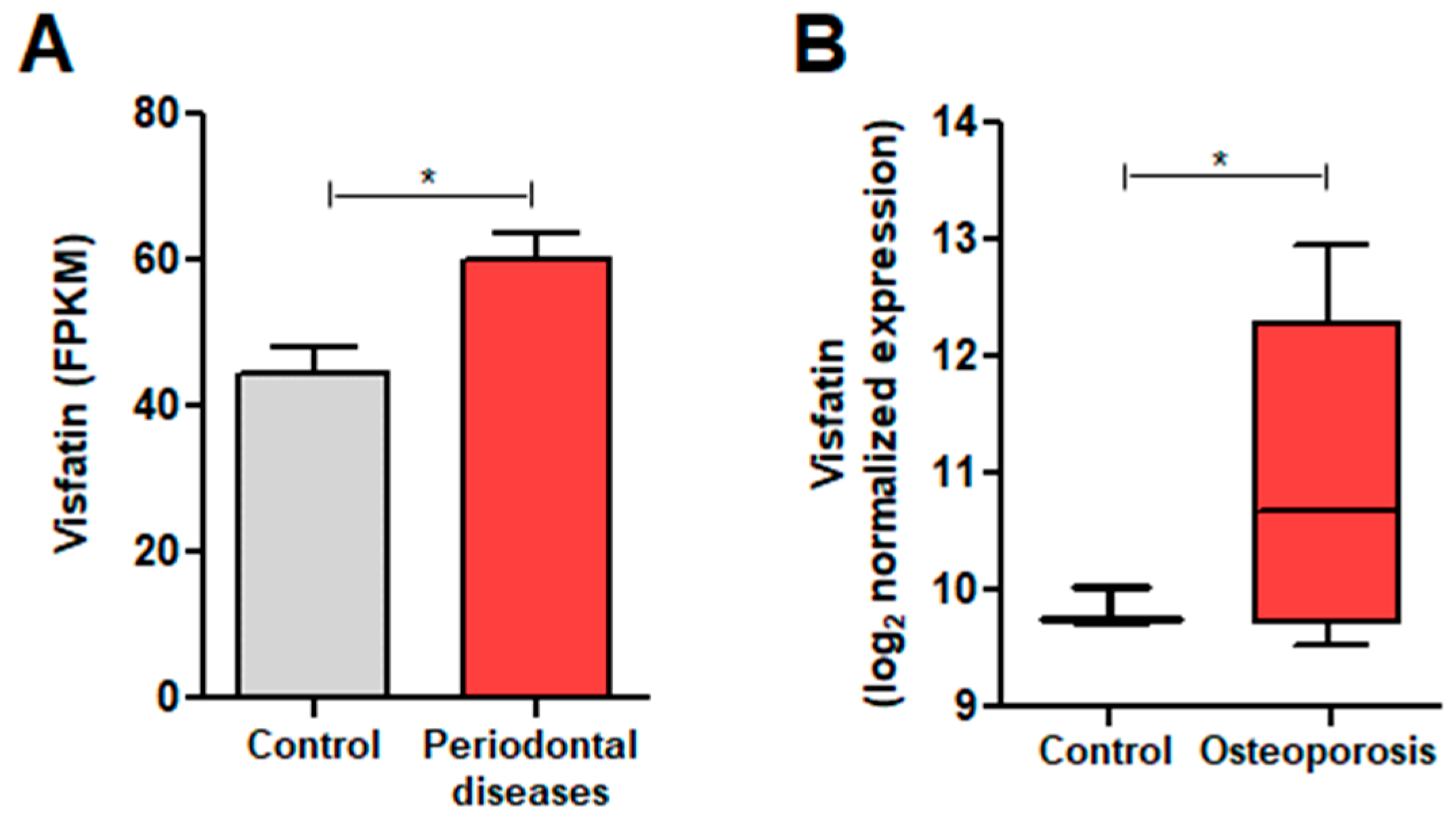
3.1. Visfatin Expression in Conditions with Elevated Osteoclast Activity
3.2. Visfatin Increased NFATc1 Protein Levels
3.3. Visfatin-Induced Osteoclast Differentiation Was RANKL-Dependent
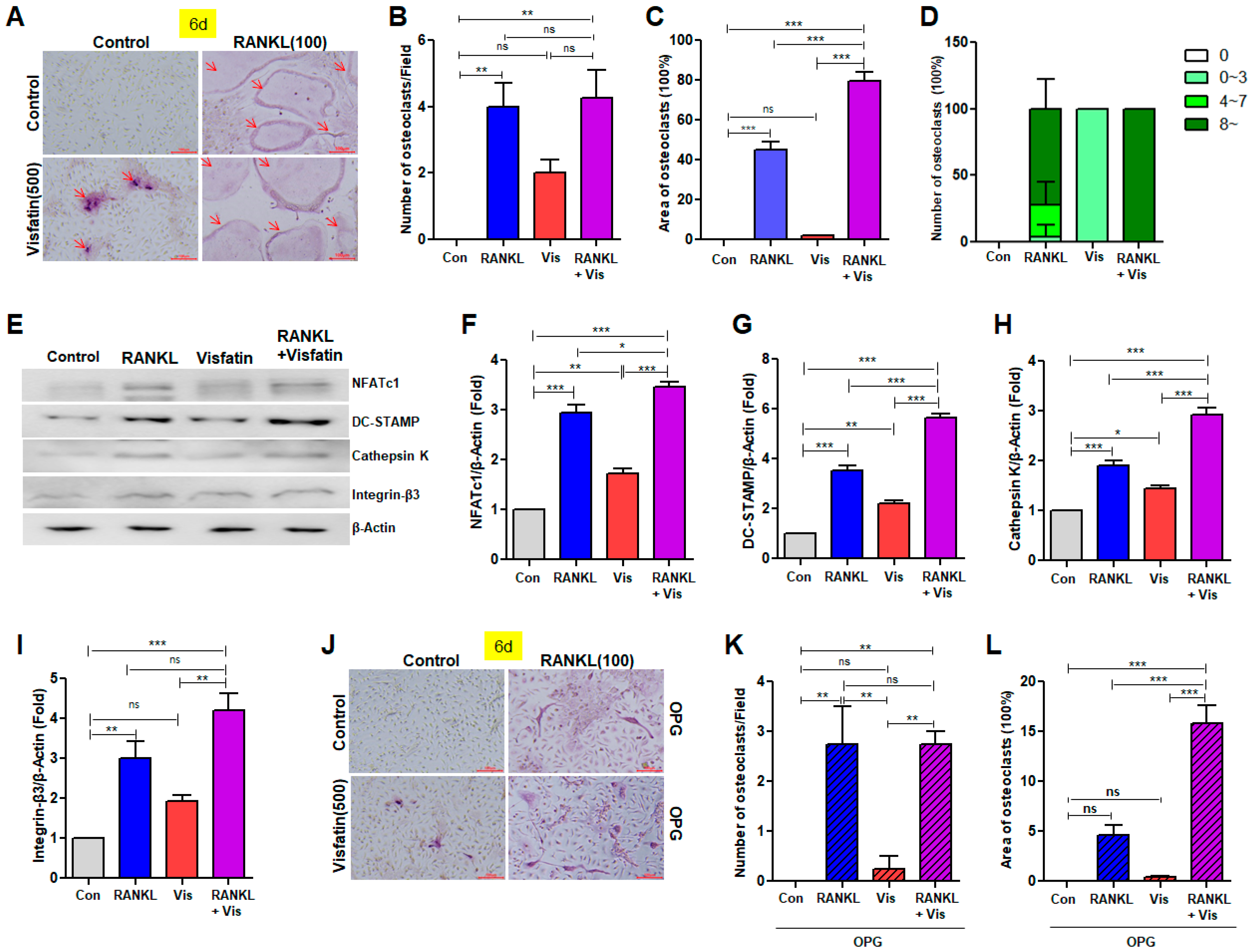
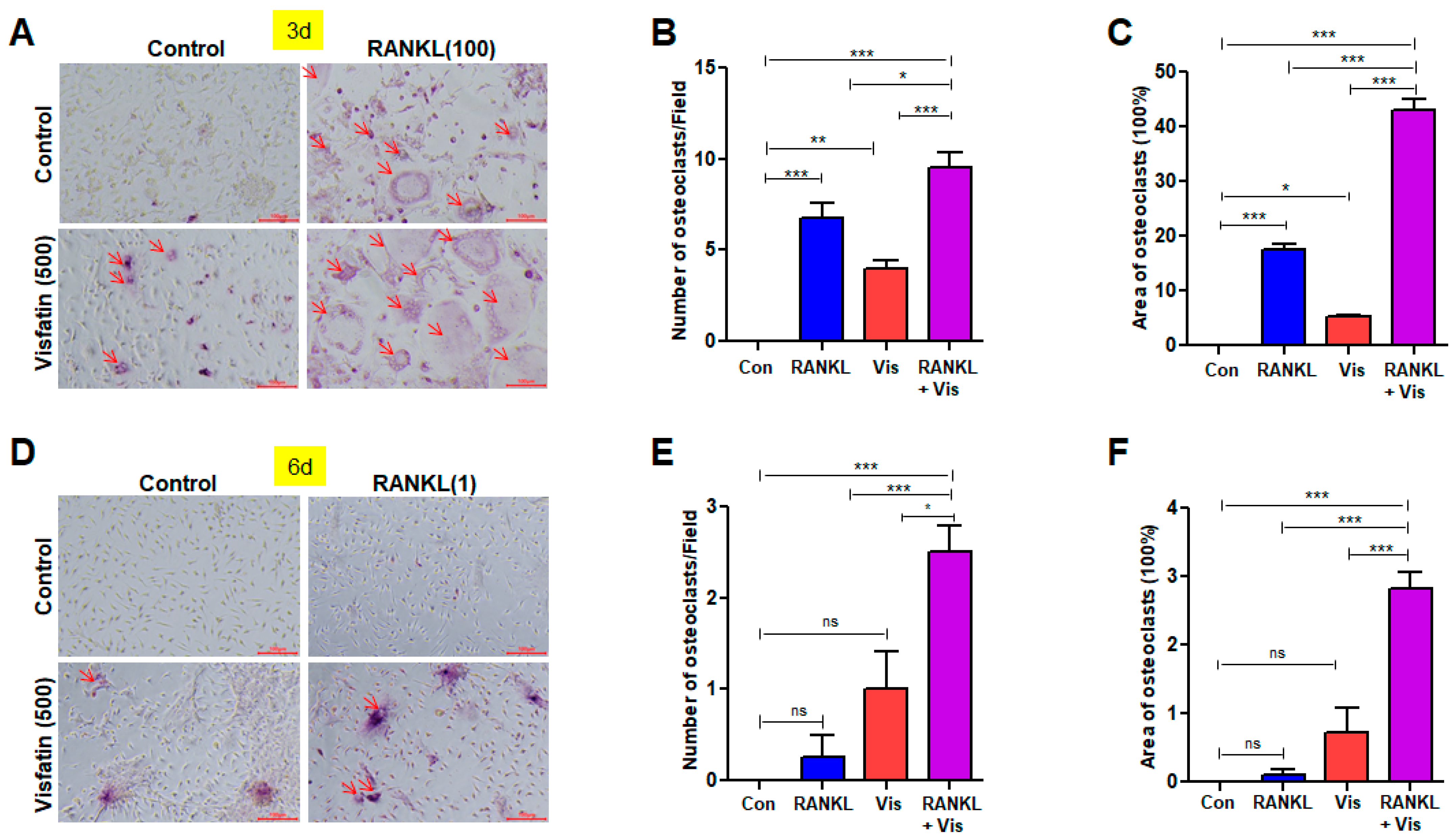
3.4. Visfatin Enhanced RANKL-Induced Osteoclast Differentiation
3.5. Visfatin Blockade Attenuated RANKL-Induced Osteoclast Differentiation
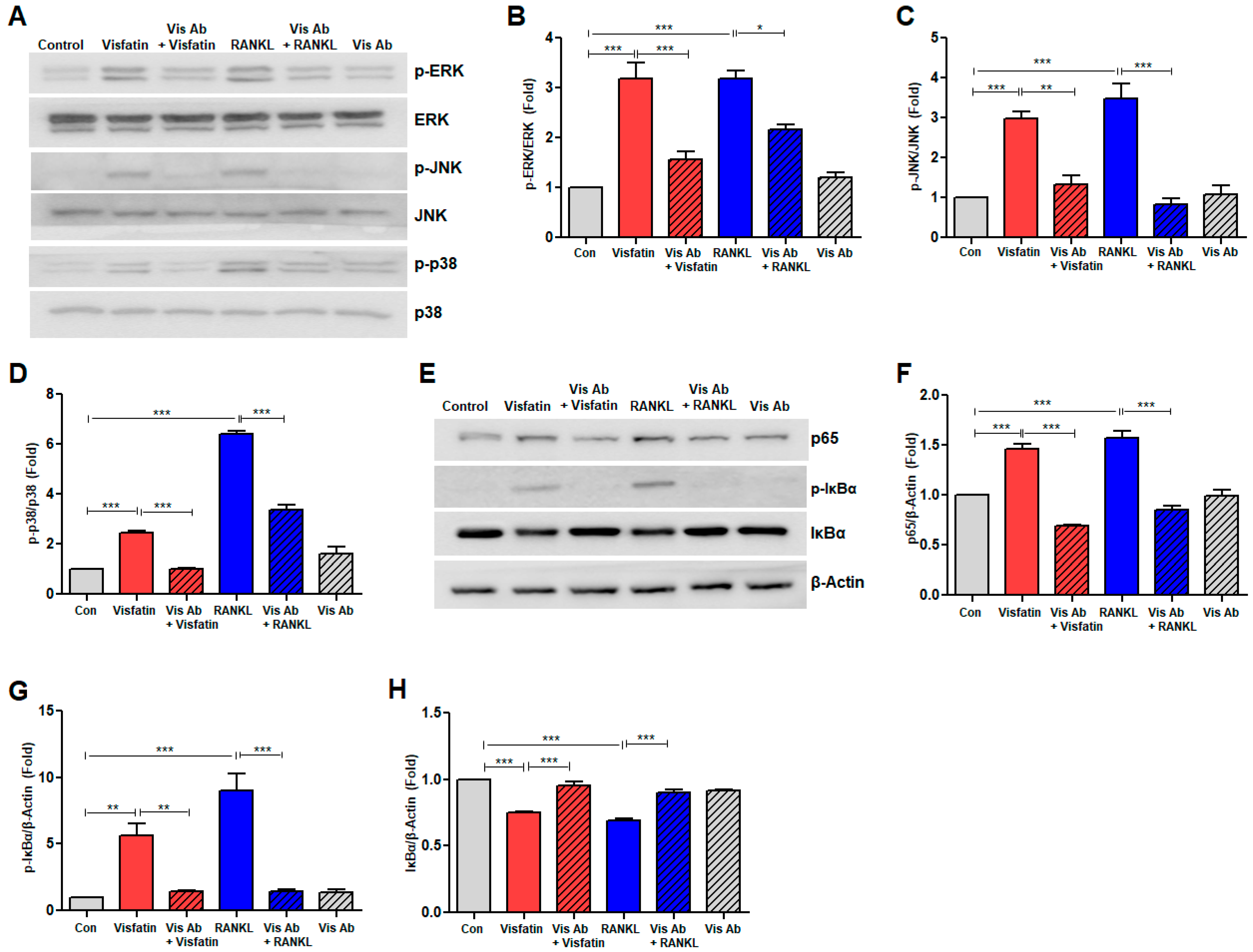
3.6. Visfatin Blockade Inhibited RANKL-Induced Activation of Mitogen-Activated Protein Kinase and NF-kB Signaling Pathways
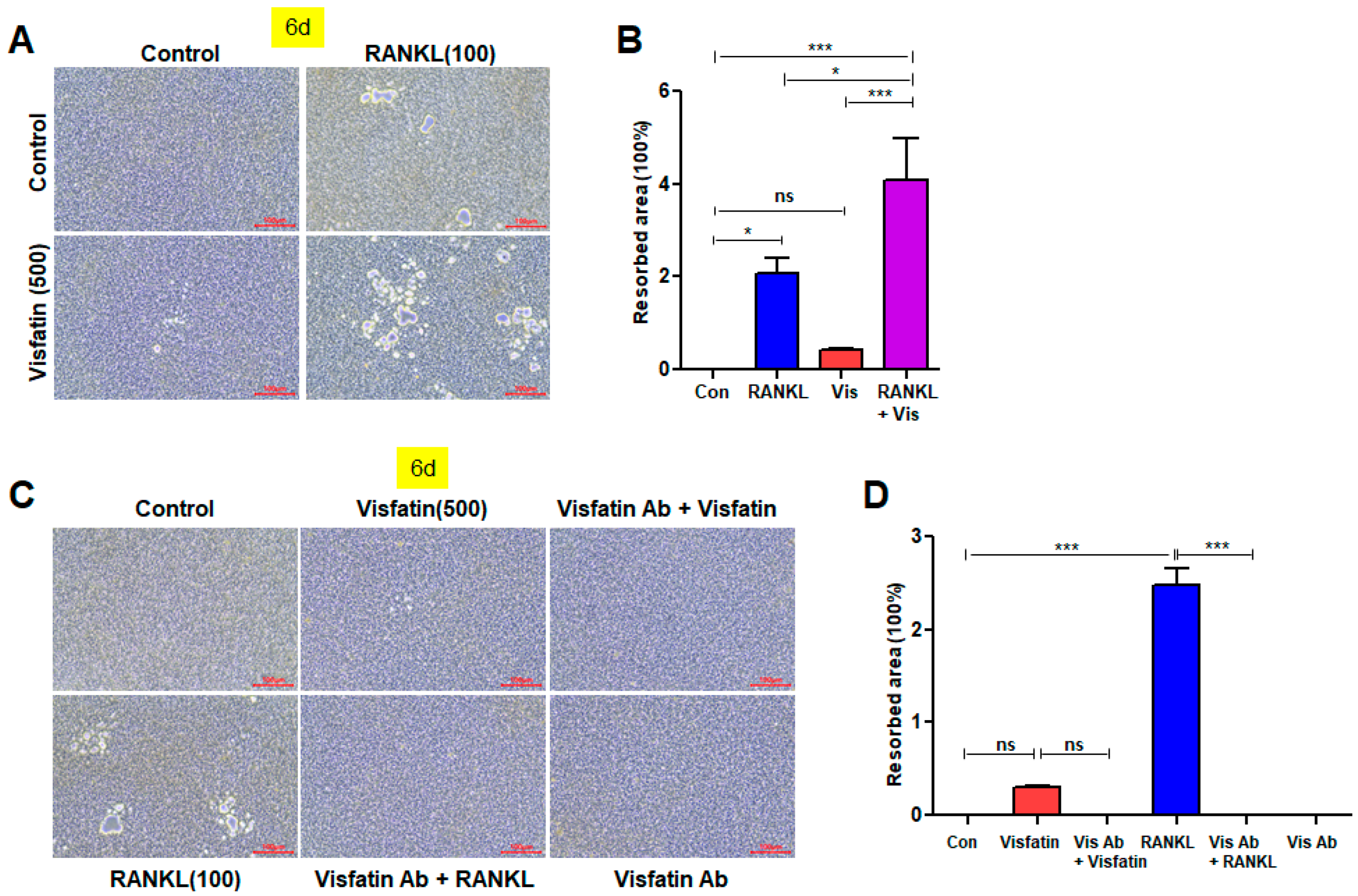
3.7. Visfatin Enhances the Resorptive Activity of RANKL-Induced Osteoclasts, While Visfatin Blockade Attenuates This Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zaidi, M. Skeletal remodeling in health and disease. Nat. Med. 2007, 13, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yamauchi, K.; Mitsunaga, T. A review on osteoclast diseases and osteoclastogenesis inhibitors recently developed from natural resources. Fitoterapia 2020, 142, 104482. [Google Scholar] [CrossRef] [PubMed]
- Omi, M.; Mishina, Y. Roles of osteoclasts in alveolar bone remodeling. Genesis 2022, 60, e23490. [Google Scholar] [CrossRef]
- Moller, A.M.J.; Delaisse, J.; Olesen, J.B.; Madsen, J.S.; Canto, L.M.; Bechmann, T.; Rogatto, S.R.; Soe, K. Aging and menopause reprogram osteoclast precursors for aggressive bone resorption. Bone Res. 2020, 8, 27. [Google Scholar] [CrossRef]
- Kodama, J.; Kaito, T. Osteoclast Multinucleation: Review of Current Literature. Int. J. Mol. Sci. 2020, 21, 5685. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Kurotaki, D.; Yoshida, H.; Tamura, T. Epigenetic and transcriptional regulation of osteoclast differentiation. Bone 2020, 138, 115471. [Google Scholar] [CrossRef]
- Takayanagi, H. RANKL as the master regulator of osteoclast differentiation. J. Bone Miner. Metab. 2021, 39, 13–18. [Google Scholar] [CrossRef]
- Takeshita, S.; Fumoto, T.; Naoe, Y.; Ikeda, K. Age-related marrow adipogenesis is linked to increased expression of RANKL. J. Biol. Chem. 2014, 289, 16699–16710. [Google Scholar] [CrossRef]
- Yasuda, H. Discovery of the RANKL/RANK/OPG system. J. Bone Miner. Metab. 2021, 39, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zheng, T.; Zhao, B. Cytokine-mediated immunomodulation of osteoclastogenesis. Bone 2022, 164, 116540. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Guo, J.; Li, M. RANKL-independent modulation of osteoclastogenesis. J. Oral Biosci. 2019, 61, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; He, C.; He, W.; Yang, M.; Luo, X.; Li, C. Obesity and Bone Health: A Complex Link. Front. Cell Dev. Biol. 2020, 8, 600181. [Google Scholar] [CrossRef]
- Muruganandan, S.; Ionescu, A.M.; Sinal, C.J. At the Crossroads of the Adipocyte and Osteoclast Differentiation Programs: Future Therapeutic Perspectives. Int. J. Mol. Sci. 2020, 21, 2277. [Google Scholar] [CrossRef]
- Patil, J.D.; Fredericks, S. The role of adipokines in osteoporosis management: A mini review. Front. Endocrinol. 2024, 15, 1336543. [Google Scholar] [CrossRef]
- Fujita, Y.; Watanabe, K.; Maki, K. Serum leptin levels negatively correlate with trabecular bone mineral density in high-fat diet-induced obesity mice. J. Musculoskelet. Neuronal Interact. 2012, 12, 84–94. [Google Scholar]
- Thommesen, L.; Stunes, A.K.; Monjo, M.; Grøsvik, K.; Tamburstuen, M.V.; Kjøbli, E.; Lyngstadaas, S.P.; Reseland, J.E.; Syversen, U. Expression and regulation of resistin in osteoblasts and osteoclasts indicate a role in bone metabolism. J. Cell. Biochem. 2006, 99, 824–834. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, H.; Li, Y.; Wang, Y.; Xing, R.; Mi, F.; Xiang, C.; Fu, R. Adiponectin inhibits the differentiation and maturation of osteoclasts via the mTOR pathway in multiple myeloma. Int. J. Mol. Med. 2020, 45, 1112–1120. [Google Scholar] [CrossRef]
- Chen, G.; Huang, L.; Wu, X.; Liu, X.; Xu, Q.; Li, F.; Dai, M.; Zhang, B. Adiponectin inhibits osteoclastogenesis by suppressing NF-κB and p38 signaling pathways. Biochem. Biophys. Res. Commun. 2018, 503, 2075–2082. [Google Scholar] [CrossRef]
- Lewis, J.W.; Edwards, J.R.; Naylor, A.J.; Mcgettrick, H.M. Adiponectin signalling in bone homeostasis, with age and in disease. Bone Res. 2021, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Sonoli, S.S.; Shivprasad, S.; Prasad, C.V.B.; Patil, A.B.; Desai, P.B.; Somannavar, M.S. Visfatin--a review. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 9–14. [Google Scholar] [PubMed]
- Dahl, T.B.; Holm, S.; Aukrust, P.; Halvorsen, B. Visfatin/NAMPT: A Multifaceted Molecule with Diverse Roles in Physiology and Pathophysiology. Annu. Rev. Nutr. 2012, 32, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Moschen, A.R.; Gerner, R.R.; Tilg, H. Pre-B Cell Colony Enhancing Factor/NAMPT/Visfatin in Inflammation and Obesity-Related Disorders. Curr. Pharm. Des. 2010, 16, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Tang, S.Y.; Luo, X.H.; Huang, J.; Cui, R.R.; Yuan, L.Q.; Zhou, H.D.; Wu, X.P.; Liao, E.Y. Insulin-like effects of visfatin on human osteoblasts. Calcif. Tissue Int. 2007, 80, 201–210. [Google Scholar] [CrossRef]
- Managò, A.; Audrito, V.; Mazzola, F.; Sorci, L.; Gaudino, F.; Gizzi, K.; Vitale, N.; Incarnate, D.; Minazzato, G.; Ianniello, A.; et al. Extracellular nicotinate phosphoribosyltransferase binds Toll like receptor 4 and mediates inflammation. Nat. Commun. 2019, 10, 4116. [Google Scholar] [CrossRef]
- Camp, S.M.; Ceco, E.; Evenoski, C.L.; Danilov, S.M.; Zhou, T.; Chiang, E.T.; Moreno-Vinasco, L.; Mapes, B.; Zhao, J.; Gursoy, G.; et al. Unique Toll-Like Receptor 4 Activation by NAMPT/PBEF Induces NFκB Signaling and Inflammatory Lung Injury. Sci. Rep. 2015, 5, 13135. [Google Scholar] [CrossRef]
- Meier, F.M.P.; Frommer, K.W.; Peters, M.A.; Brentano, F.; Lefèvre, S.; Schröder, D.; Kyburz, D.; Steinmeyer, J.; Rehart, S.; Gay, S.; et al. Visfatin/Pre-B-cell Colony-enhancing Factor (PBEF), a Proinflammatory and Cell Motility-changing Factor in Rheumatoid Arthritis. J. Biol. Chem. 2012, 287, 28378–28385. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, J.H.; Lee, Y.J. The Role of Adipokines in Tumor Progression and Its Association with Obesity. Biomedicines 2024, 12, 97. [Google Scholar] [CrossRef]
- Dakroub, A.; Nasser, S.A.; Kobeissy, F.; Yassine, H.M.; Orekhov, A.; Sharifi-rad, J.; Iratni, R.; El-yazbi, A.F.; Eid, A.H. Visfatin: An emerging adipocytokine bridging the gap in the evolution of cardiovascular diseases. J. Cell. Physiol. 2021, 236, 6282–6296. [Google Scholar] [CrossRef]
- Ok, C.Y.; Park, S.; Jang, H.; Takata, T.; Bae, M.; Kim, Y.; Ryu, M.H.; Bae, S. Visfatin Induces Senescence of Human Dental Pulp Cells. Cells 2020, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Ok, C.Y.; Park, S.; Jang, H.; Bae, M.; Bae, S. Involvement of the visfatin/toll-like receptor 4 signaling axis in human dental pulp cell senescence: Protection via toll-like receptor 4 blockade. J. Dent. Sci. 2023, 18, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, S.; Shi, J.; Ning, N.; Wei, Y.; Zhang, Y. Periodontitis is associated with the increased levels of visfatin: A meta-analysis. BMC Oral Health 2023, 23, 799. [Google Scholar] [CrossRef] [PubMed]
- Kumari, B.; Yadav, U.C.S. Adipokine visfatin’s role in pathogenesis of diabesity and related metabolic derangements. Curr. Mol. Med. 2018, 18, 116–125. [Google Scholar] [CrossRef]
- Franco-Trepat, E.; Guillán-Fresco, M.; Alonso-Pérez, A.; Jorge-Mora, A.; Francisco, V.; Gualillo, O.; Gómez, R. Visfatin Connection: Present and Future in Osteoarthritis and Osteoporosis. J. Clin. Med. 2019, 8, 1178. [Google Scholar] [CrossRef]
- Li, X.; Islam, S.; Xiong, M.; Nsumu, N.N.; Lee, M.W.; Zhang, L.Q.; Ueki, Y.; Heruth, D.P.; Lei, G.; Ye, S.Q. Epigenetic regulation of NfatC1 transcription and osteoclastogenesis by nicotinamide phosphoribosyl transferase in the pathogenesis of arthritis. Cell Death Discovery 2019, 5, 62. [Google Scholar] [CrossRef]
- Baek, J.M.; Ahn, S.; Cheon, Y.; Lee, M.S.; Oh, J.; Kim, J. Nicotinamide phosphoribosyltransferase inhibits receptor activator of nuclear factor-κB ligand-induced osteoclast differentiation in vitro. Mol. Med. Rep. 2017, 15, 784–792. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, H.J.; Park, C.K.; Kim, Y.; Lee, H.; Kim, J.; Kim, H. MicroRNA-124 regulates osteoclast differentiation. Bone 2013, 56, 383–389. [Google Scholar] [CrossRef]
- Cho, E.; Chen, Z.; Lee, J.; Lee, S.; Lee, T. PSTP-3,5-Me Inhibits Osteoclast Differentiation and Bone Resorption. Molecules 2019, 24, 3346. [Google Scholar] [CrossRef]
- Kim, S.; Bae, Y.; Bae, S.; Choi, K.; Yoon, K.; Koo, T.H.; Jang, H.; Yun, I.; Kim, K.; Kwon, Y.; et al. Visfatin enhances ICAM-1 and VCAM-1 expression through ROS-dependent NF-κB activation in endothelial cells. Biochim. Biophys. Acta 2008, 1783, 886–895. [Google Scholar] [CrossRef]
- Park, H.; Kim, S.S.; Wee, H.; Bae, M.; Ryu, M.H.; Bae, S. Visfatin promotes cell and tumor growth by upregulating Notch1 in breast cancer. Oncotarget 2014, 5, 5087–5099. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Bae, M.; Kim, S.; Lee, J.H.; Wee, H.; Bae, S. Upregulation of fibroblast growth factor-2 by visfatin that promotes endothelial angiogenesis. Biochem. Biophys. Res. Commun. 2008, 379, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Takeshita, S.; Barker, J.E.; Kanagawa, O.; Ross, F.P.; Teitelbaum, S.L. TNF-α induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J. Clin. Investig. 2000, 106, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Jules, J.; Zhang, P.; Ashley, J.W.; Wei, S.; Shi, Z.; Liu, J.; Michalek, S.M.; Feng, X. Molecular Basis of Requirement of Receptor Activator of Nuclear Factor κB Signaling for Interleukin 1-mediated Osteoclastogenesis. J. Biol. Chem. 2012, 287, 15728–15738. [Google Scholar] [CrossRef] [PubMed]
- Okabe, I.; Kikuchi, T.; Mogi, M.; Takeda, H.; Aino, M.; Kamiya, Y.; Fujimura, T.; Goto, H.; Okada, K.; Hasegawa, Y.; et al. IL-15 and RANKL Play a Synergistically Important Role in Osteoclastogenesis. J. Cell. Biochem. 2016, 118, 739–747. [Google Scholar] [CrossRef]
- Feng, W.; Liu, H.; Luo, T.; Liu, D.; Du, J.; Sun, J.; Wang, W.; Han, X.; Yang, K.; Guo, J.; et al. Combination of IL-6 and sIL-6R differentially regulate varying levels of RANKL-induced osteoclastogenesis through NF-κB, ERK and JNK signaling pathways. Sci. Rep. 2017, 7, 41411. [Google Scholar] [CrossRef]
- Hung, A.C.; Lo, S.; Hou, M.; Lee, Y.; Tsai, C.; Chen, Y.; Liu, W.; Su, Y.; Lo, Y.; Wang, C.; et al. Extracellular Visfatin-Promoted Malignant Behavior in Breast Cancer Is Mediated Through c-Abl and STAT3 Activation. Clin. Cancer Res. 2016, 22, 4478–4490. [Google Scholar] [CrossRef]
- Nokhbehsaim, M.; Keser, S.; Jäger, A.; Jepsen, S.; Deschner, J. Regulation of Regenerative Periodontal Healing by NAMPT. Mediat. Inflamm. 2013, 2013, 202530. [Google Scholar] [CrossRef]
- Li, Y.; Xin, C.; Xie, J.; Sun, X. Association between visfatin and periodontitis: A systematic review and meta-analysis. PeerJ 2024, 12, e17187. [Google Scholar] [CrossRef]
- Matsunaga, N.; Tsuchimori, N.; Matsumoto, T.; Ii, M. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol. Pharmacol. 2011, 79, 34–41. [Google Scholar] [CrossRef]
- Galli, U.; Travelli, C.; Massarotti, A.; Fakhfouri, G.; Rahimian, R.; Tron, G.C.; Genazzani, A.A. Medicinal chemistry of nicotinamide phosphoribosyltransferase (NAMPT) inhibitors. J. Med. Chem. 2013, 56, 6279–6296. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, D.; Arezzini, B.; Pecorelli, A.; Valacchi, G.; Martorana, P.A.; Gardi, C. Reactivity of mouse alveolar macrophages to cigarette smoke is strain dependent. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2010, 298, L704–L713. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hou, L.; Yang, L.; Chang, N.; Zhao, X.; Zhou, X.; Dong, C.; Liu, F.; Yang, L.; Li, L. Macrophage Sphingosine1-Phosphate Receptor 2 Blockade Attenuates Liver Inflammation and Fibrogenesis Triggered by NLRP3 Inflammasome. Front. Immunol. 2020, 11, 1149. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Jiang, H.; Takashita, E.; Inoue, H.; Kadowaki, S.; Mori, K.J. Strain specific production of a negative regulator of IL-3 (NIL-3): Difference in the negative feedback mechanism of hemopoiesis among mouse strains. Cell Struct. Funct. 1997, 22, 407–411. [Google Scholar] [CrossRef][Green Version]
- Zhao, X.; Patil, S.; Xu, F.; Lin, X.; Qian, A. Role of Biomolecules in Osteoclasts and Their Therapeutic Potential for Osteoporosis. Biomolecules 2021, 11, 747. [Google Scholar] [CrossRef]
- Matsumoto, T.; Endo, I. RANKL as a target for the treatment of osteoporosis. J. Bone Miner. Metab. 2021, 39, 91–105. [Google Scholar] [CrossRef]
- Reid, I.R.; Billington, E.O. Drug therapy for osteoporosis in older adults. Lancet 2022, 399, 1080–1092. [Google Scholar] [CrossRef]



| Gene | Accession Number | Primer Sequence |
|---|---|---|
| Visfatin (756 bp) | NM_021524.2 | Forward: 5′- GGCCACAAATTCCAGAGAACAG-3′ |
| Reverse: 5′- CCAAATGAGCAGATGCCCCTA -3′ | ||
| NFATc1 (188 bp) | NM_001164110.1 | Forward: 5′- CCAGTATACCAGCTCTGCCA-3′ |
| Reverse: 5′- GTGGGAAGTCAGAAGTGGGT-3′ | ||
| RANK (181 bp) | NM_009399.5 | Forward: 5′- AGAAGACGGTGCTGGAGTCT-3′ |
| Reverse: 5′- TAGGAGCAGTGAACCAGTCG-3′ | ||
| TRAF6 (151 bp) | NM_009424.3 | Forward: 5′- GCCCAGGCTGTTCATAATGT-3′ |
| Reverse: 5′- TCGCCCACGTACATACTCTG-3′ | ||
| TRAP (190 bp) | NM_001102405.1 | Forward: 5′- CAGTTGGCAGCAGCCAAGGAG-3′ |
| Reverse: 5′- TCCGTGCTCGGCGATGGACC-3′ | ||
| DC-STAMP (264 bp) | NM_029422.4 | Forward: 5′- CTAGCTGGCTGGACTTCATCC-3′ |
| Reverse: 5′- TCATGCTGTCTAGGAGACCTC-3′ | ||
| CD36 (99 bp) | NM_001159558.2 | Forward: 5′- TCCTCTGACATTTGCAGGTCTA-3′ |
| Reverse: 5′- AAAGGCATTGGCTGGAAGAA-3′ | ||
| OSCAR (310 bp) | NM_175632 | Forward: 5′- CTGCTGGATACGGATCAGCTCCCCAGA-3′ |
| Reverse: 5′- CCAAGGAGCCAGAACCTTCGAAACT-3′ | ||
| GAPDH (139 bp) | NM_001289726.2 | Forward: 5′- GGAGAAACCTGCCAAGTATG-3′ |
| Reverse: 5′- GAAGAGTGGGAGTTGCTGTT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ok, C.Y.; Kwon, R.J.; Jang, H.-O.; Bae, M.-K.; Bae, S.-K. Visfatin Enhances RANKL-Induced Osteoclastogenesis In Vitro: Synergistic Interactions and Its Role as a Mediator in Osteoclast Differentiation and Activation. Biomolecules 2024, 14, 1500. https://doi.org/10.3390/biom14121500
Ok CY, Kwon RJ, Jang H-O, Bae M-K, Bae S-K. Visfatin Enhances RANKL-Induced Osteoclastogenesis In Vitro: Synergistic Interactions and Its Role as a Mediator in Osteoclast Differentiation and Activation. Biomolecules. 2024; 14(12):1500. https://doi.org/10.3390/biom14121500
Chicago/Turabian StyleOk, Chang Youp, Ryuk Jun Kwon, Hye-Ock Jang, Moon-Kyoung Bae, and Soo-Kyung Bae. 2024. "Visfatin Enhances RANKL-Induced Osteoclastogenesis In Vitro: Synergistic Interactions and Its Role as a Mediator in Osteoclast Differentiation and Activation" Biomolecules 14, no. 12: 1500. https://doi.org/10.3390/biom14121500
APA StyleOk, C. Y., Kwon, R. J., Jang, H.-O., Bae, M.-K., & Bae, S.-K. (2024). Visfatin Enhances RANKL-Induced Osteoclastogenesis In Vitro: Synergistic Interactions and Its Role as a Mediator in Osteoclast Differentiation and Activation. Biomolecules, 14(12), 1500. https://doi.org/10.3390/biom14121500







