Phytochrome-Interacting Proteins
Abstract
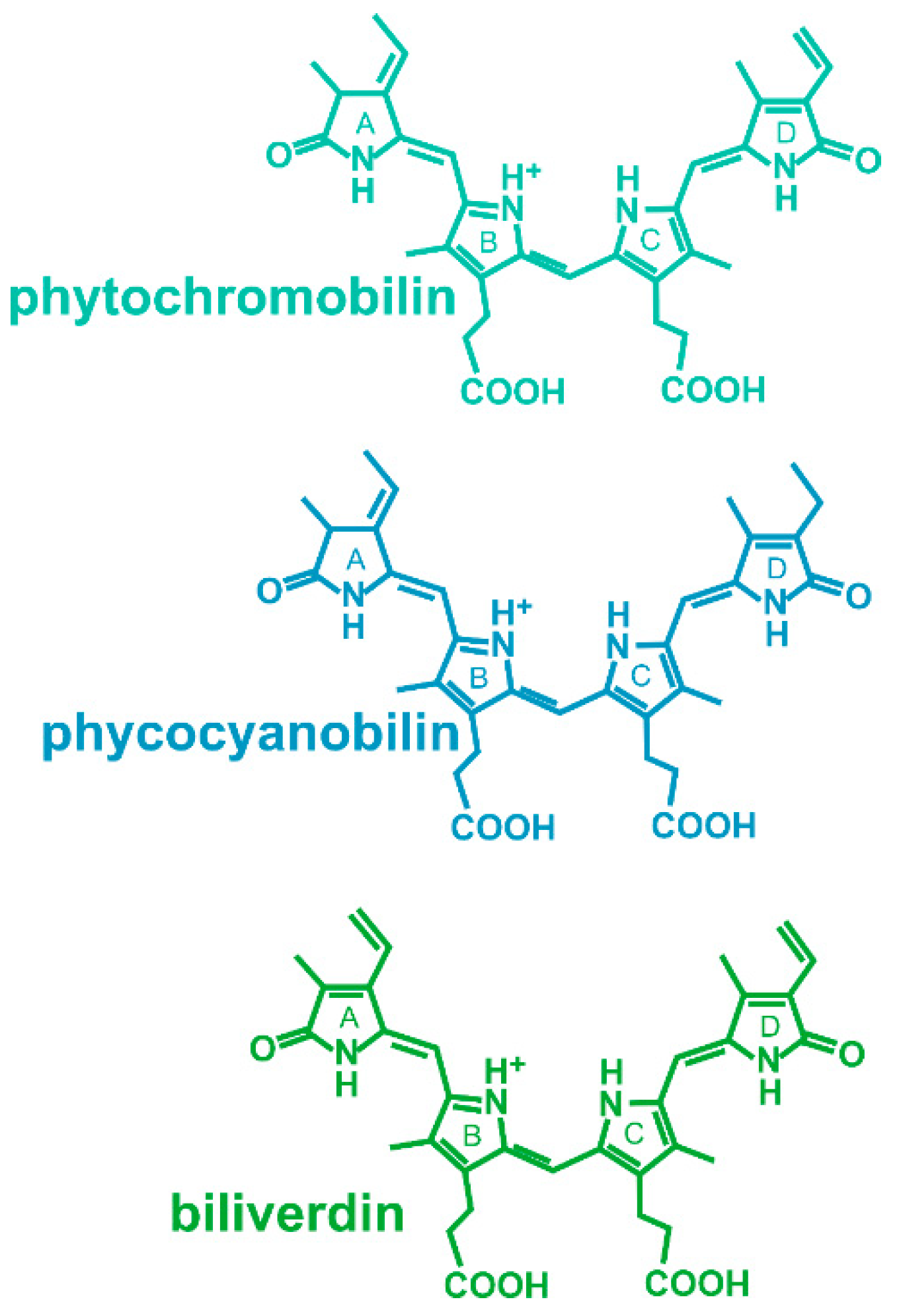
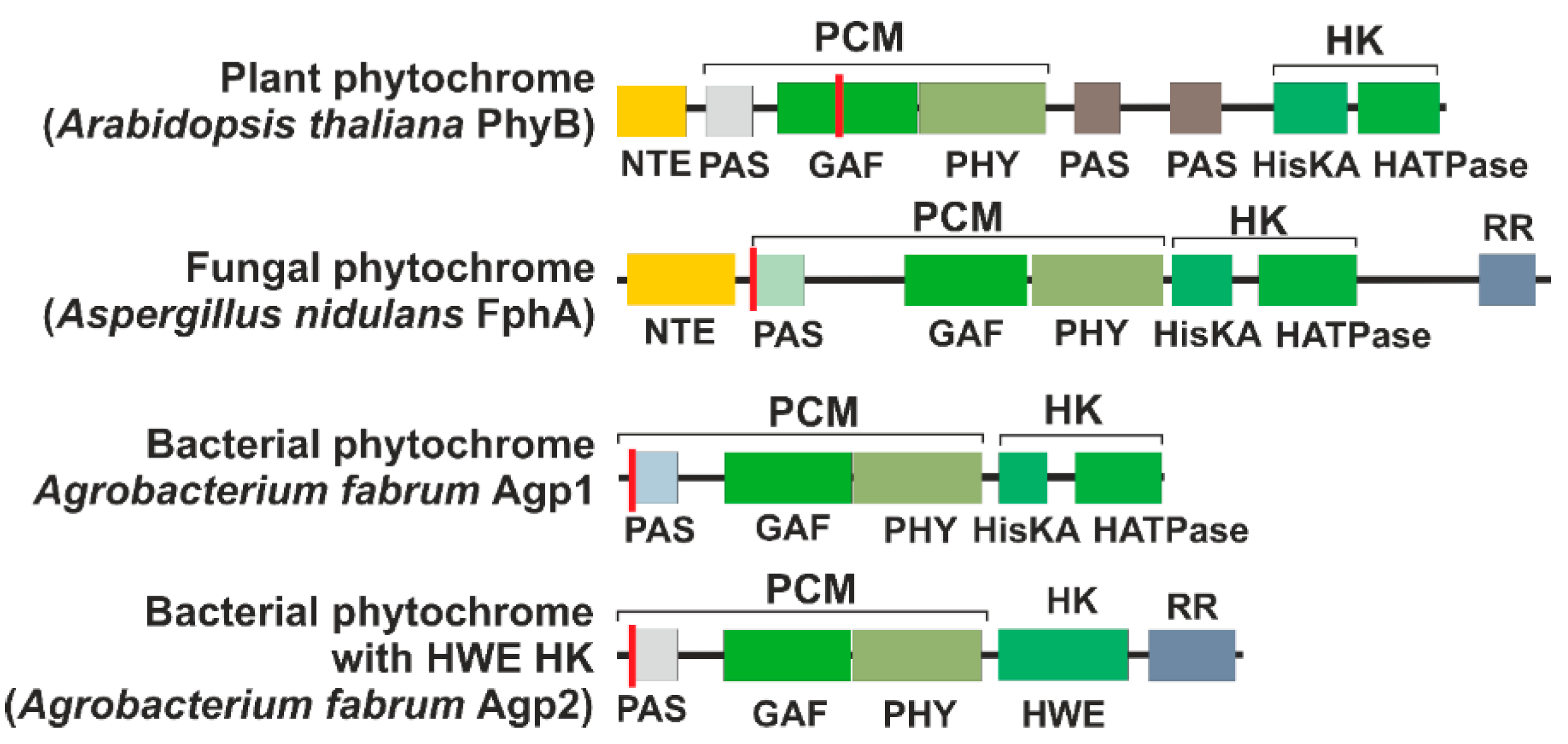
1. Introduction
2. Interaction Partners of Plant Phytochromes
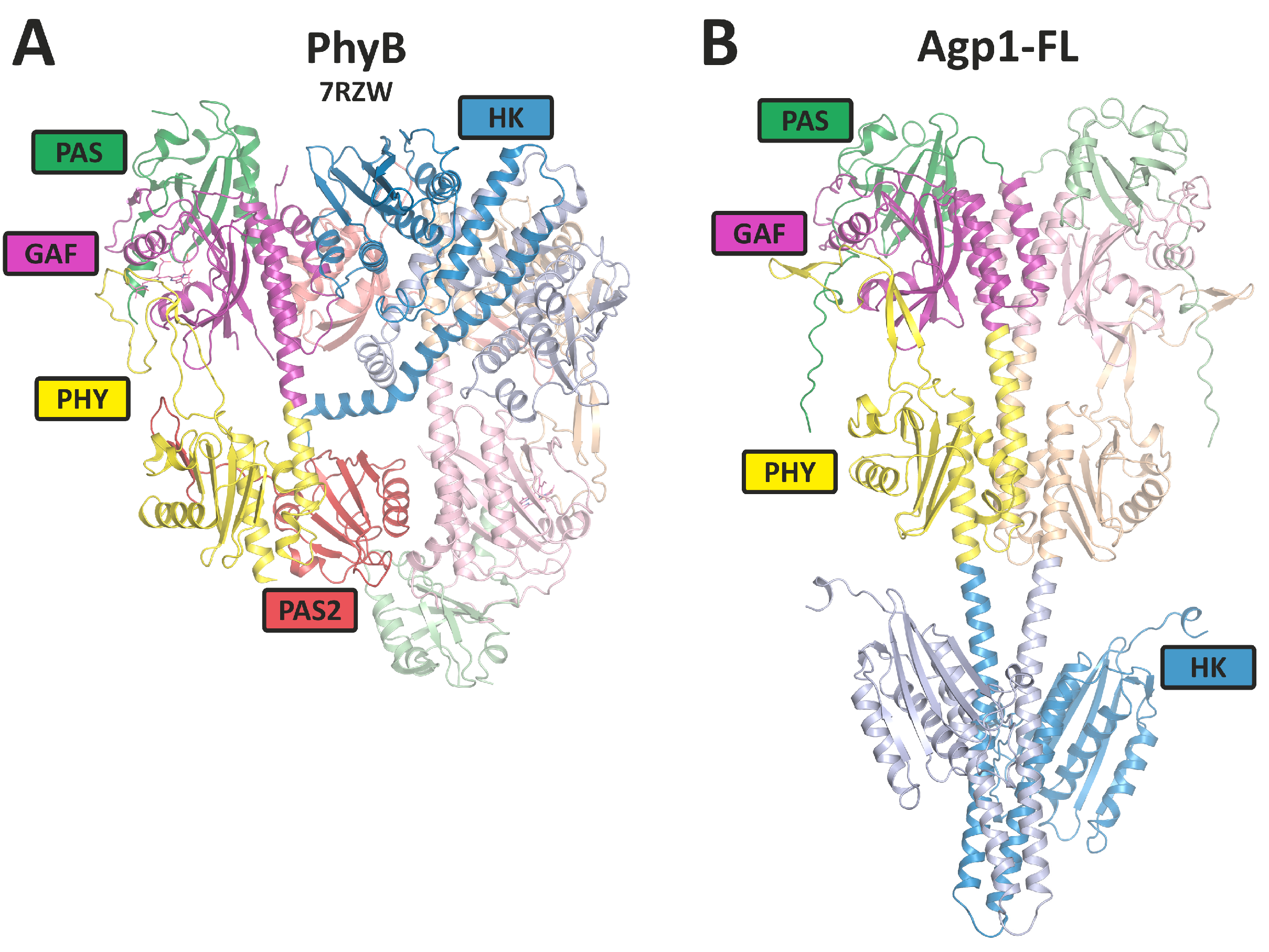
3. Homodimer and Heterodimer Formation
4. Interaction with PIF3 and Other PIF Proteins
5. Interaction with Cryptochrome (Cry)
6. Interaction with ARR4
7. Interaction with Phototropin
8. Interaction with COP1
9. Interaction with NDPK2
10. Interaction with PKS1
11. Interaction with SPA1
12. Other Plant Proteins Involved in Phy Signal Transduction
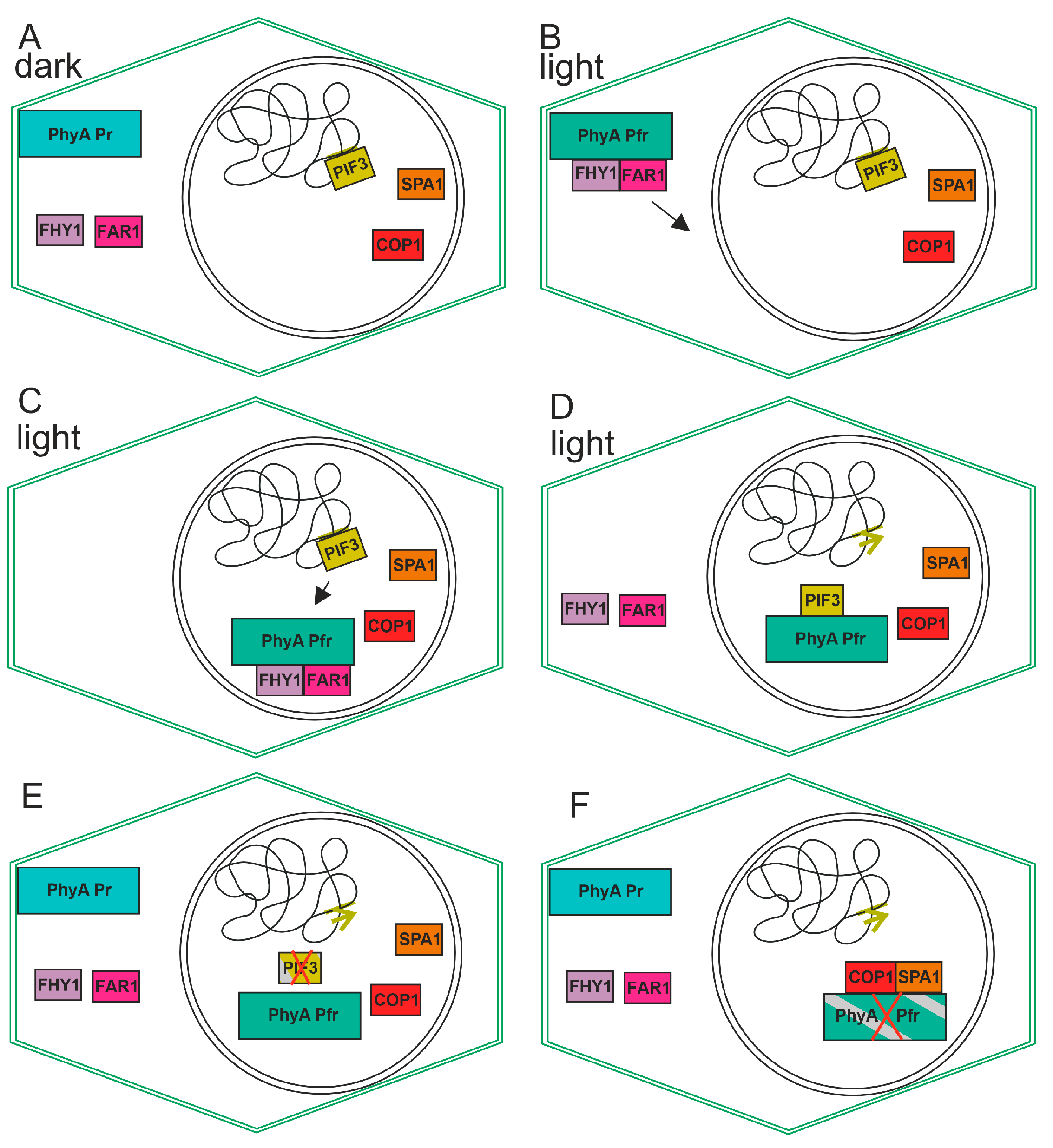
13. Interaction with FHY1 and FAR1
14. Fungi
15. Bacteria
16. Interactions of Bacterial Phytochromes with Response Regulators
17. The Transcription Factor PpsR
18. Interaction between Agrobacterium fabrum Phytochromes Agp1 and Agp2
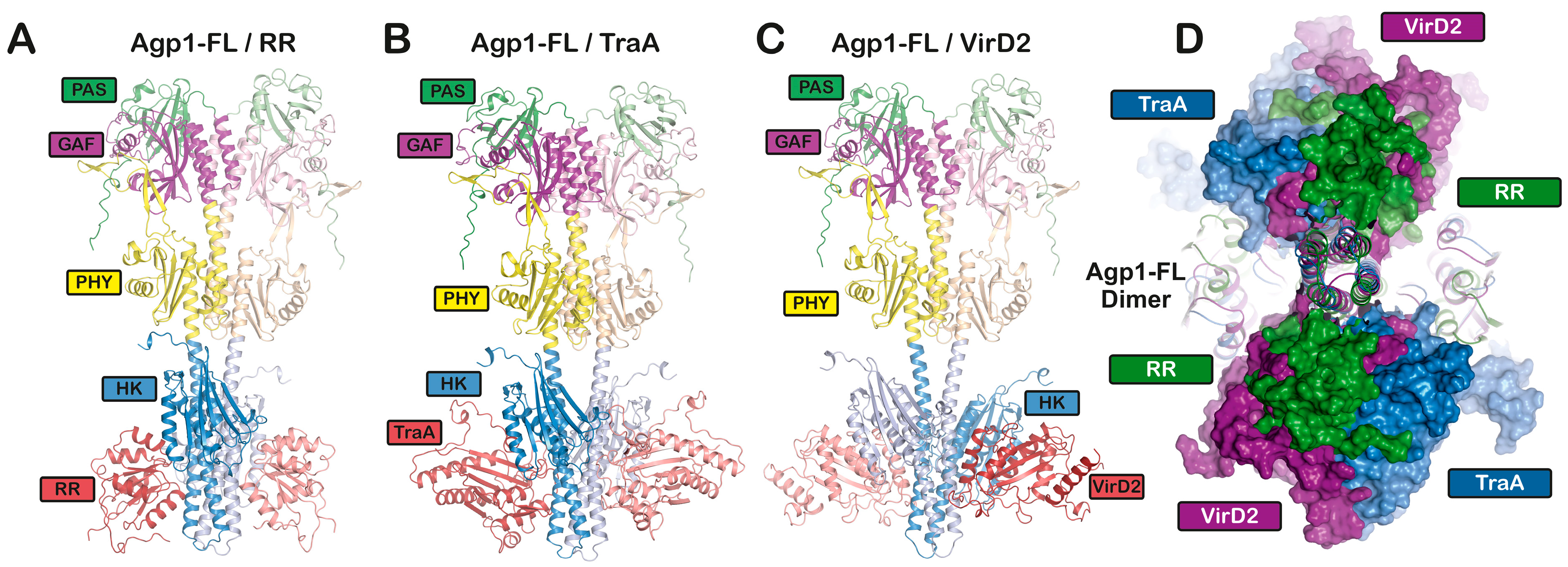
19. Possible Interaction of Agp1 or Agp2 with TraA and VirD2
20. Modeling the Interaction
21. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Huq, E.; Quail, P.H. Phytochrome signaling. In Handbook of Photosensory Receptors; Wiley: Hoboken, NJ, USA, 2005; pp. 151–170. [Google Scholar]
- Tu, S.-L.; Lagarias, J.C. The phytochromes. In Handbook of Photosensory Receptors; Wiley: Hoboken, NJ, USA, 2005; pp. 121–149. [Google Scholar]
- Vierstra, R.D.; Karniol, B. Phytochromes in microorganisms. In Handbook of Photosensory Receptors; Wiley: Hoboken, NJ, USA, 2005; pp. 171–195. [Google Scholar]
- Rockwell, N.C.; Duanmu, D.; Martin, S.S.; Bachy, C.; Price, D.C.; Bhattacharya, D.; Worden, A.Z.; Lagarias, J.C. Eukaryotic algal phytochromes span the visible spectrum. Proc. Natl. Acad. Sci. USA 2024, 111, 3871–3876. [Google Scholar] [CrossRef] [PubMed]
- Giraud, E.; Fardoux, J.; Fourrier, N.; Hannibal, L.; Genty, B.; Bouyer, P.; Dreyfus, B.; Vermeglio, A. Bacteriophytochrome controls photosystem synthesis in anoxygenic bacteria. Nature 2002, 417, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Rottwinkel, G.; Oberpichler, I.; Lamparter, T. Bathy phytochromes in rhizobial soil bacteria. J. Bacteriol. 2010, 192, 5124–5133. [Google Scholar] [CrossRef] [PubMed]
- Lamparter, T.; Carrascal, M.; Michael, N.; Martinez, E.; Rottwinkel, G.; Abian, J. The biliverdin chromophore binds covalently to a conserved cysteine residue in the n-terminus of Agrobacterium phytochrome Agp1. Biochemistry 2004, 43, 3659–3669. [Google Scholar] [CrossRef]
- Giraud, E.; Vuillet, L.; Hannibal, L.; Fardoux, J.; Zappa, S.; Adriano, J.M.; Berthomieu, C.; Bouyer, P.; Pignol, D.; Verméglio, A. A new type of bacteriophytochrome acts in tandem with a classical bacteriophytochrome to control the antennae synthesis in Rhodopseudomonas palustris. J. Biol. Chem. 2005, 280, 32389–32397. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, T.; Shimada, T.; Okajima, K.; Yoshihara, S.; Ochiai, Y.; Katayama, M.; Ikeuchi, M. Characterization of cyanobacteriochrome TePixJ from a thermophilic cyanobacterium Thermosynechococcus elongatus strain bp-1. Plant Cell Physiol. 2006, 47, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Ponting, C.P.; Aravind, L. PAS: A multifunctional domain family comes to light. Curr. Biol. 1997, 7, R674–R677. [Google Scholar] [CrossRef] [PubMed]
- Aravind, L.; Ponting, C.P. The GAF domain: An evolutionary link between diverse phototransducing proteins. Trends Biochem. Sci. 1997, 22, 458–459. [Google Scholar] [CrossRef]
- Oka, Y.; Matsushita, T.; Mochizuki, N.; Suzuki, T.; Tokutomi, S.; Nagatani, A. Functional analysis of a 450-amino acid n-terminal fragment of phytochrome b in Arabidopsis. Plant Cell 2004, 16, 2104–2116. [Google Scholar] [CrossRef]
- Sharrock, R.A.; Clack, T. Heterodimerization of type ii phytochromes in Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 11500–11505. [Google Scholar] [CrossRef]
- Armstrong, D.R.; Berrisford, J.M.; Conroy, M.J.; Gutmanas, A.; Anyango, S.; Choudhary, P.; Clark, A.R.; Dana, J.M.; Deshpande, M.; Dunlop, R.; et al. Pdbe: Improved findability of macromolecular structure data in the PDB. Nucleic Acids Res. 2020, 48, D335–D343. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with alphafold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Nagano, S.; Scheerer, P.; Zubow, K.; Michael, N.; Inomata, K.; Lamparter, T.; Krauss, N. The crystal structures of the n-terminal photosensory core module of Agrobacterium phytochrome Agp1 as parallel and anti-parallel dimers. J. Biol. Chem. 2016, 291, 20674–20691. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.W.; Hendricks, S.B.; Borthwick, H.A.; Scully, N.J. Action spectrum for the photoperiodic control of floral initiation in biloxi soybean. Science 1945, 102, 152–155. [Google Scholar] [CrossRef]
- Flint, L.H.; McAlister, E.D. Wavelengths of radiation in the visible spectrum promoting the germination of light-sensitive lettuce seed. Smithson. Misc. Coll. 1937, 96, 1–8. [Google Scholar]
- Virgin, H.I. Action spectrum for elimination of lag phase in chlorophyll formation in previously dark grown leaves of wheat. Physiol. Plant. 1961, 14, 439–452. [Google Scholar] [CrossRef]
- Butler, W.L.; Norris, K.H.; Siegelman, H.W.; Hendricks, S.B. Detection, assay, and preliminary purification of the pigment controlling photoresponsive development of plants. Proc. Natl. Acad. Sci. USA 1959, 45, 1703–1708. [Google Scholar] [CrossRef]
- Kendrick, R.E.; Kronenberg, G.H.M. Photomorphogenesis in Plants, 2nd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994. [Google Scholar]
- Kehoe, D.M.; Grossman, A.R. Similarity of a chromatic adaptation sensor to phytochrome and ethylene receptors. Science 1996, 273, 1409–1412. [Google Scholar] [CrossRef]
- Ng, W.O.; Grossman, A.R.; Bhaya, D. Multiple light inputs control phototaxis in synechocystis sp. Strain pcc6803. J. Bacteriol. 2003, 185, 1599–1607. [Google Scholar] [CrossRef]
- Lamparter, T.; Babian, J.; Frohlich, K.; Mielke, M.; Weber, N.; Wunsch, N.; Zais, F.; Schulz, K.; Aschmann, V.; Spohrer, N.; et al. The involvement of type iv pili and the phytochrome Cpha in gliding motility, lateral motility and photophobotaxis of the cyanobacterium Phormidium lacuna. PLoS ONE 2022, 17, e0249509. [Google Scholar] [CrossRef]
- Lamparter, T.; Krauß, N.; Scheerer, P. Phytochromes from Agrobacterium fabrum. Photochem. Photobiol. 2017, 93, 642–655. [Google Scholar] [CrossRef] [PubMed]
- Lamparter, T.; Xue, P.; Elkurdi, A.; Kaeser, G.; Sauthof, L.; Scheerer, P.; Krauss, N. Phytochromes in Agrobacterium fabrum. Front. Plant Sci. 2021, 12, 642801. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Bai, Y.; Rottwinkel, G.; Averbukh, E.; Ma, Y.; Roeder, T.; Scheerer, P.; Krauss, N.; Lamparter, T. Phytochrome mediated responses in Agrobacterium fabrum: Growth, motility and plant infection. Curr. Microbiol. 2021, 78, 2708–2719. [Google Scholar] [CrossRef]
- Grombein, S.; Rüdiger, W.; Zimmermann, H. The structures of the phytochrome chromophore in both photoreversible forms. Hoppe Seylers Z. Physiol. Chem. 1975, 356, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Rüdiger, W.; Thümmler, F. Phytochrome, the visual pigment of plants. Angew. Chem.-Int. Ed. Engl. 1991, 30, 1216–1228. [Google Scholar] [CrossRef]
- Schmidt, A.; Sauthof, L.; Szczepek, M.; Lopez, M.F.; Escobar, F.V.; Qureshi, B.M.; Michael, N.; Buhrke, D.; Stevens, T.; Kwiatkowski, D.; et al. Structural snapshot of a bacterial phytochrome in its functional intermediate state. Nat. Commun. 2018, 9, 4912. [Google Scholar] [CrossRef]
- Essen, L.O.; Mailliet, J.; Hughes, J. The structure of a complete phytochrome sensory module in the Pr ground state. Proc. Natl. Acad. Sci. USA 2008, 105, 14709–14714. [Google Scholar] [CrossRef]
- Gourinchas, G.; Vide, U.; Winkler, A. Influence of the n-terminal segment and the phy-tongue element on light-regulation in bacteriophytochromes. J. Biol. Chem. 2019, 294, 4498–4510. [Google Scholar] [CrossRef]
- Otero, L.H.; Klinke, S.; Rinaldi, J.; Velazquez-Escobar, F.; Mroginski, M.A.; Lopez, M.F.; Malamud, F.; Vojnov, A.A.; Hildebrandt, P.; Goldbaum, F.A.; et al. Structure of the full-length bacteriophytochrome from the plant pathogen Xanthomonas campestris provides clues to its long-range signaling mechanism. J. Mol. Biol. 2016, 428, 3702–3720. [Google Scholar] [CrossRef]
- Burgie, E.S.; Li, H.; Gannam, Z.T.K.; McLoughlin, K.E.; Vierstra, R.D.; Li, H. The structure of Arabidopsis phytochrome a reveals topological and functional diversification among the plant photoreceptor isoforms. Nat. Plants 2023, 9, 1116–1129. [Google Scholar] [CrossRef]
- Li, H.; Burgie, E.S.; Gannam, Z.T.K.; Li, H.; Vierstra, R.D. Plant phytochrome b is an asymmetric dimer with unique signalling potential. Nature 2022, 604, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, X.; Ma, C.; Zhao, J.; Shang, X.; Wang, Z.; Xu, B.; Gao, N.; Deng, X.W.; Wang, J. Structural insights into plant phytochrome a as a highly sensitized photoreceptor. Cell Res. 2023, 33, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Wahlgren, W.Y.; Claesson, E.; Tuure, I.; Trillo-Muyo, S.; Bódizs, S.; Ihalainen, J.A.; Takala, H.; Westenhoff, S. Structural mechanism of signal transduction in a phytochrome histidine kinase. Nat. Commun. 2022, 13, 7673. [Google Scholar] [CrossRef] [PubMed]
- Gourinchas, G.; Etzl, S.; Göbl, C.; Vide, U.; Madl, T.; Winkler, A. Long-range allosteric signaling in red light-regulated diguanylyl cyclases. Sci. Adv. 2017, 3, e1602498. [Google Scholar] [CrossRef] [PubMed]
- Oughtred, R.; Stark, C.; Breitkreutz, B.J.; Rust, J.; Boucher, L.; Chang, C.; Kolas, N.; O’Donnell, L.; Leung, G.; McAdam, R.; et al. The biogrid interaction database: 2019 update. Nucleic Acids Res. 2019, 47, D529–D541. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.E.; Pratt, L.H. Partial characterization of undegraded oat phytochrome. Biochemistry 1980, 19, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Lagarias, J.C.; Mercurio, F.M. Structure function studies on phytochrome. Identification of light- induced conformational changes in 124-kda avena phytochrome in vitro. J. Biol. Chem. 1985, 260, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- Edgerton, M.D.; Jones, A.M. Subunit interactions in the carboxy-terminal domain of phytochrome. Biochemistry 1993, 32, 8239–8245. [Google Scholar] [CrossRef]
- Cheng, M.C.; Kathare, P.K.; Paik, I.; Huq, E. Phytochrome signaling networks. Annu. Rev. Plant Biol. 2021, 72, 217–244. [Google Scholar] [CrossRef]
- Castillon, A.; Shen, H.; Huq, E. Phytochrome interacting factors: Central players in phytochrome-mediated light signaling networks. Trends Plant Sci. 2007, 12, 514–521. [Google Scholar] [CrossRef]
- Duek, P.D.; Fankhauser, C. Bhlh class transcription factors take centre stage in phytochrome signalling. Trends Plant Sci. 2005, 10, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Samtani, H.; Sahu, K.; Sharma, A.K.; Khurana, J.P.; Khurana, P. Functions of phytochrome-interacting factors (pifs) in the regulation of plant growth and development: A comprehensive review. Int. J. Biol. Macromol. 2023, 244, 125234. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Tepperman, J.M.; Quail, P.H. Pif3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell 1998, 95, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Quail, P.H.; Boylan, M.T.; Parks, B.M.; Short, T.W.; Xu, Y.; Wagner, D. Phytochromes: Photosensory perception and signal transduction. Science 1995, 268, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Quail, P.H. Phytochrome-interacting factors. Semin. Cell Dev. Biol. 2000, 11, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Tepperman, J.M.; Quail, P.H. Binding of phytochrome b to its nuclear signaling partner pif3 is reversibly induced by light. Nature 1999, 400, 781–784. [Google Scholar] [CrossRef]
- Matsushita, T.; Mochizuki, N.; Nagatani, A. Dimers of the n-terminal domain of phytochrome b are functional in the nucleus. Nature 2003, 424, 571–574. [Google Scholar] [CrossRef]
- Kikis, E.A.; Oka, Y.; Hudson, M.E.; Nagatani, A.; Quail, P.H. Residues clustered in the light-sensing knot of phytochrome b are necessary for conformer-specific binding to signaling partner pif3. PLoS Genet. 2009, 5, e1000352. [Google Scholar] [CrossRef]
- Al Sady, B.; Ni, W.; Kircher, S.; Schäfer, E.; Quail, P.H. Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol. Cell 2006, 23, 439–446. [Google Scholar] [CrossRef]
- Bauer, D.; Viczian, A.; Kircher, S.; Nobis, T.; Nitschke, R.; Kunkel, T.; Panigrahi, K.C.; Adam, E.; Fejes, E.; Schäfer, E.; et al. Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell 2004, 16, 1433–1445. [Google Scholar] [CrossRef]
- Monte, E.; Tepperman, J.M.; Al Sady, B.; Kaczorowski, K.A.; Alonso, J.M.; Ecker, J.R.; Li, X.; Zhang, Y.; Quail, P.H. The phytochrome-interacting transcription factor, PIF3, acts early, selectively, and positively in light-induced chloroplast development. Proc. Natl. Acad. Sci. USA 2004, 101, 16091–16098. [Google Scholar] [CrossRef] [PubMed]
- Phee, B.K.; Kim, J.I.; Shin, D.H.; Yoo, J.; Park, K.J.; Han, Y.J.; Kwon, Y.K.; Cho, M.H.; Jeon, J.S.; Bhoo, S.H.; et al. A novel protein phosphatase indirectly regulates phytochrome-interacting factor 3 via phytochrome. Biochem. J. 2008, 415, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Bernula, P.; Pettkó-Szandtner, A.; Hajdu, A.; Kozma-Bognár, L.; Josse, E.M.; Adám, E.; Nagy, F.; Viczián, A. Sumoylation of phytochrome interacting factor 3 promotes photomorphogenesis in Arabidopsis thaliana. New Phytol. 2021, 229, 2050–2061. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.M.; Xu, S.L.; Tepperman, J.M.; Stanley, D.J.; Maltby, D.A.; Gross, J.D.; Burlingame, A.L.; Wang, Z.Y.; Quail, P.H. A mutually assured destruction mechanism attenuates light signaling in Arabidopsis. Science 2014, 344, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Leivar, P.; Tepperman, J.M.; Monte, E.; Calderon, R.H.; Liu, T.L.; Quail, P.H. Definition of early transcriptional circuitry involved in light-induced reversal of pif-imposed repression of photomorphogenesis in young Arabidopsis seedlings. Plant Cell 2009, 21, 3535–3553. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhu, Z.Q. Pif4 and pif4-interacting proteins: At the nexus of plant light, temperature and hormone signal integrations. Int. J. Mol. Sci. 2021, 22, 10304. [Google Scholar] [CrossRef] [PubMed]
- Tavridou, E.; Pireyre, M.; Ulm, R. Degradation of the transcription factors pif4 and pif5 under UV-b promotes UVR8-mediated inhibition of hypocotyl growth in Arabidopsis. Plant J. 2020, 101, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; Huq, E.; Kikis, E.A.; Al Sady, B.; Lanzatella, C.; Quail, P.H. A novel molecular recognition motif necessary for targeting photoactivated phytochrome signaling to specific basic helix-loop-helix transcription factors. Plant Cell 2004, 16, 3033–3044. [Google Scholar] [CrossRef]
- Zhou, Y.; Park, S.H.; Soh, M.Y.; Chua, N.H. Ubiquitin-specific proteases ubp12 and ubp13 promote shade avoidance response by enhancing pif7 stability. Proc. Natl. Acad. Sci. USA 2021, 118, e2103633118. [Google Scholar] [CrossRef]
- Xu, T.F.; Hiltbrunner, A. Phytochrome interacting factors from physcomitrella patens are active in Arabidopsis and complement the pif quadruple mutant. Plant Signal. Behav. 2017, 12, e1388975. [Google Scholar] [CrossRef]
- Wareing, P.F.; Black, M. Similar effects of blue and infra-red radiation on light-sensitive seeds. Nature 1958, 181, 1420–1421. [Google Scholar] [CrossRef]
- Sugai, M.; Furuya, M. Photomorphogenesis in pteris vittata I. Phytochrome-mediated spore germination and blue light interaction. Plant Cell Physiol. 1967, 8, 737–748. [Google Scholar]
- Ahmad, M.; Cashmore, A.R. Hy4 gene of a. Thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 1993, 366, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Huala, E.; Oeller, P.W.; Liscum, E.; Han, I.S.; Larsen, E.; Briggs, W.R. Arabidopsis nph1: A protein kinase with a putative redox-sensing domain. Science 1997, 278, 2120–2123. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Jarillo, J.A.; Smirnova, O.; Cashmore, A.R. The Cry1 blue light photoreceptor of Arabidopsis interacts with phytochrome a in vitro. Mol. Cell 1998, 1, 939–948. [Google Scholar] [CrossRef]
- Neff, M.M.; Chory, J. Genetic interactions between phytochrome a, phytochrome b, and cryptochrome 1 during Arabidopsis development. Plant Physiol. 1998, 118, 27–36. [Google Scholar] [CrossRef] [PubMed]
- de Wit, M.; Keuskamp, D.H.; Bongers, F.J.; Hornitschek, P.; Gommers, C.M.M.; Reinen, E.; Martinez-Ceron, C.; Fankhauser, C.; Pierik, R. Integration of phytochrome and cryptochrome signals determines plant growth during competition for light. Curr. Biol. 2016, 26, 3320–3326. [Google Scholar] [CrossRef]
- Brandstatter, I.; Kieber, J.J. Two genes with similarity to bacterial response regulators are rapidly and specifically induced by cytokinin in Arabidopsis. Plant Cell 1998, 10, 1009–1019. [Google Scholar] [CrossRef]
- Sweere, U.; Eichenberg, K.; Lohrmann, J.; Mira-Rodado, V.; Baurle, I.; Kudla, J.; Nagy, F.; Schäfer, E.; Harter, K. Interaction of the response regulator arr4 with phytochrome b in modulating red light signaling. Science 2001, 294, 1108–1111. [Google Scholar] [CrossRef]
- Jaedicke, K.; Lichtenthaler, A.L.; Meyberg, R.; Zeidler, M.; Hughes, J. A phytochrome-phototropin light signaling complex at the plasma membrane. Proc. Natl. Acad. Sci. USA 2012, 109, 12231–12236. [Google Scholar] [CrossRef]
- Jang, I.C.; Henriques, R.; Seo, H.S.; Nagatani, A.; Chua, N.H. Arabidopsis phytochrome interacting factor proteins promote phytochrome b polyubiquitination by cop1 e3 ligase in the nucleus. Plant Cell 2010, 22, 2370–2383. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Yi, H.; Lee, J.; Kwon, Y.-K.; Soh, M.S.; Shin, B.; Luka, Z.; Hahn, T.-R.; Song, P.-S. Phytochrome signalling is mediated through nucleoside diphosphate kinase 2. Nature 1999, 401, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Kim, J.I.; Song, P.S. Ndpk2 as a signal transducer in the phytochrome-mediated light signaling. J. Biol. Chem. 2005, 280, 5740–5749. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Kim, J.I.; Song, P.S. Autophosphorylation of Arabidopsis nucleoside diphosphate kinase 2 occurs only on its active histidine residue. Biochemistry 2006, 45, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Hetmann, A.; Kowalczyk, S. Nucleoside diphosphate kinase isoforms regulated by phytochrome a isolated from oat coleoptiles. Acta Biochim. Pol. 2009, 56, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Z.; Tohge, T.; Lalor, P.; Dockery, P.; Devaney, N.; Esteves-Ferreira, A.A.; Fernie, A.R.; Sulpice, R. Phytochrome a and b regulate primary metabolism in Arabidopsis leaves in response to light. Front. Plant Sci. 2017, 8, 1394. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, C.; Yeh, K.C.; Lagarias, J.C.; Zhang, H.; Elich, T.D.; Chory, J. Pks1, a substrate phosphorylated by phytochrome that modulates light signaling in Arabidopsis. Science 1999, 284, 1539–1541. [Google Scholar] [CrossRef]
- de Carbonnel, M.; Davis, P.; Roelfsema, M.R.G.; Inoue, S.; Schepens, I.; Lariguet, P.; Geisler, M.; Shimazaki, K.; Hangarter, R.; Fankhauser, C. The Arabidopsis phytochrome kinase substrate2 protein is a phototropin signaling element that regulates leaf flattening and leaf positioning. Plant Physiol. 2010, 152, 1391–1405. [Google Scholar] [CrossRef]
- Sheerin, D.J.; Menon, C.; Oven-Krockhaus, S.Z.; Enderle, B.; Zhu, L.; Johnen, P.; Schleifenbaum, F.; Stierhof, Y.D.; Huq, E.; Hiltbrunner, A. Light-activated phytochrome a and b interact with members of the spa family to promote photomorphogenesis in Arabidopsis by reorganizing the cop1/spa complex. Plant Cell 2015, 27, 189–201. [Google Scholar] [CrossRef]
- Andersson, C.R.; Kay, S.A. Cop1 and hy5 interact to mediate light-induced gene expression. BioEssays 1998, 20, 445–448. [Google Scholar] [CrossRef]
- Liscum, E.; Hangarter, R.P. Photomorphogenic mutants of Arabidopsis thaliana reveal activities of multiple photosensory systems during light-stimulated apical-hook opening. Planta 1993, 191, 214–221. [Google Scholar] [CrossRef]
- Fairchild, C.D.; Schumaker, M.A.; Quail, P.H. Hfr1 encodes an atypical bhlh protein that acts in phytochrome a signal transduction. Genes Dev. 2000, 14, 2377–2391. [Google Scholar] [PubMed]
- Hiltbrunner, A.; Viczian, A.; Bury, E.; Tscheuschler, A.; Kircher, S.; Toth, R.; Honsberger, A.; Nagy, F.; Fankhauser, C.; Schäfer, E. Nuclear accumulation of the phytochrome a photoreceptor requires fhy1. Curr. Biol. 2005, 15, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Hudson, M.; Ringli, C.; Boylan, M.T.; Quail, P.H. The far1 locus encodes a novel nuclear-protein specific to phytochrome-a signaling. Genes Dev. 1999, 13, 2017–2027. [Google Scholar] [CrossRef]
- Rausenberger, J.; Tscheuschler, A.; Nordmeier, W.; Wust, F.; Timmer, J.; Schafer, E.; Fleck, C.; Hiltbrunner, A. Photoconversion and nuclear trafficking cycles determine phytochrome a’s response profile to far-red light. Cell 2011, 146, 813–825. [Google Scholar] [CrossRef]
- Blumenstein, A.; Vienken, K.; Tasler, R.; Purschwitz, J.; Veith, D.; Frankenberg-Dinkel, N.; Fischer, R. The aspergillus nidulans phytochrome fpha represses sexual development in red light. Curr. Biol. 2005, 15, 1833–1838. [Google Scholar] [CrossRef]
- Purschwitz, J.; Muller, S.; Fischer, R. Mapping the interaction sites of aspergillus nidulans phytochrome fpha with the global regulator vea and the white collar protein lreb. Mol. Genet. Genom. 2009, 281, 35–42. [Google Scholar] [CrossRef]
- Yeh, K.C.; Wu, S.H.; Murphy, J.T.; Lagarias, J.C. A cyanobacterial phytochrome two-component light sensory system. Science 1997, 277, 1505–1508. [Google Scholar] [CrossRef]
- Hughes, J.; Lamparter, T.; Mittmann, F.; Hartmann, E.; Gärtner, W.; Wilde, A.; Börner, T. A prokaryotic phytochrome. Nature 1997, 386, 663. [Google Scholar] [CrossRef]
- Bai, Y.; Rottwinkel, G.; Feng, J.; Liu, Y.; Lamparter, T. Bacteriophytochromes control conjugation in Agrobacterium fabrum. J. Photochem. Photobiol. B 2016, 161, 192–199. [Google Scholar] [CrossRef]
- Lamparter, T.; Michael, N.; Mittmann, F.; Esteban, B. Phytochrome from Agrobacterium tumefaciens has unusual spectral properties and reveals an n-terminal chromophore attachment site. Proc. Natl. Acad. Sci. USA 2002, 99, 11628–11633. [Google Scholar] [CrossRef] [PubMed]
- Barkovits, K.; Schubert, B.; Heine, S.; Scheer, M.; Frankenberg-Dinkel, N. Function of the bacteriophytochrome bphp in the rpos/las quorum-sensing network of Pseudomonas aeruginosa. Microbiology 2011, 157, 1651–1664. [Google Scholar] [CrossRef] [PubMed]
- Stock, A.M.; Robinson, V.L.; Goudreau, P.N. Two-component signal transduction. Annu. Rev. Biochem. 2000, 69, 183–215. [Google Scholar] [CrossRef] [PubMed]
- Bhoo, S.H.; Davis, S.J.; Walker, J.; Karniol, B.; Vierstra, R.D. Bacteriophytochromes are photochromic histidine kinases using a biliverdin chromophore. Nature 2001, 414, 776–779. [Google Scholar] [CrossRef]
- Xue, P.; El Kurdi, A.; Kohler, A.; Ma, H.; Kaeser, G.; Ali, A.; Fischer, R.; Krauss, N.; Lamparter, T. Evidence for weak interaction between phytochromes agp1 and agp2 from Agrobacterium fabrum. FEBS Lett. 2019, 593, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Gomelsky, M.; Kaplan, S. Appa, a redox regulator of photosystem formation in rhodobacter sphaeroides 2.4.1, is a flavoprotein. Identification of a novel fad binding domain. J. Biol. Chem. 1998, 273, 35319–35325. [Google Scholar] [CrossRef] [PubMed]
- Jaubert, M.; Zappa, S.; Fardoux, J.; Adriano, J.M.; Hannibal, L.; Elsen, S.; Lavergne, J.; Vermeglio, A.; Giraud, E.; Pignol, D. Light and redox control of photosynthesis gene expression in bradyrhizobium: Dual roles of two ppsr. J. Biol. Chem. 2004, 279, 44407–44416. [Google Scholar] [CrossRef] [PubMed]
- Kaberniuk, A.A.; Shemetov, A.A.; Verkhusha, V.V. A bacterial phytochrome-based optogenetic system controllable with near-infrared light. Nat. Methods 2016, 13, 591–597. [Google Scholar] [CrossRef]
- Rottwinkel, G. Studien zu Verbreitung, Charakteristika und Funktionen der Bakteriophytochrome in Rhizobiales. Ph.D. Thesis, Karlsruhe Institute of Technology (KIT), Karlsruhe, Germany, 2011. [Google Scholar]
- Stanger, F.V.; de Beer, T.A.P.; Dranow, D.M.; Schirmer, T.; Phan, I.; Dehio, C. The bid domain of type iv secretion substrates forms a conserved four-helix bundle topped with a hook. Structure 2017, 25, 203–211. [Google Scholar] [CrossRef]
- Somers, D.E.; Kim, W.Y.; Geng, R. The f-box protein zeitlupe confers dosage-dependent control on the circadian clock, photomorphogenesis, and flowering time. Plant Cell 2004, 16, 769–782. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaeser, G.; Krauß, N.; Roughan, C.; Sauthof, L.; Scheerer, P.; Lamparter, T. Phytochrome-Interacting Proteins. Biomolecules 2024, 14, 9. https://doi.org/10.3390/biom14010009
Kaeser G, Krauß N, Roughan C, Sauthof L, Scheerer P, Lamparter T. Phytochrome-Interacting Proteins. Biomolecules. 2024; 14(1):9. https://doi.org/10.3390/biom14010009
Chicago/Turabian StyleKaeser, Gero, Norbert Krauß, Clare Roughan, Luisa Sauthof, Patrick Scheerer, and Tilman Lamparter. 2024. "Phytochrome-Interacting Proteins" Biomolecules 14, no. 1: 9. https://doi.org/10.3390/biom14010009
APA StyleKaeser, G., Krauß, N., Roughan, C., Sauthof, L., Scheerer, P., & Lamparter, T. (2024). Phytochrome-Interacting Proteins. Biomolecules, 14(1), 9. https://doi.org/10.3390/biom14010009






