Particulate Matter in Human Elderly: Higher Susceptibility to Cognitive Decline and Age-Related Diseases
Abstract
:1. Significance: Why This Review?
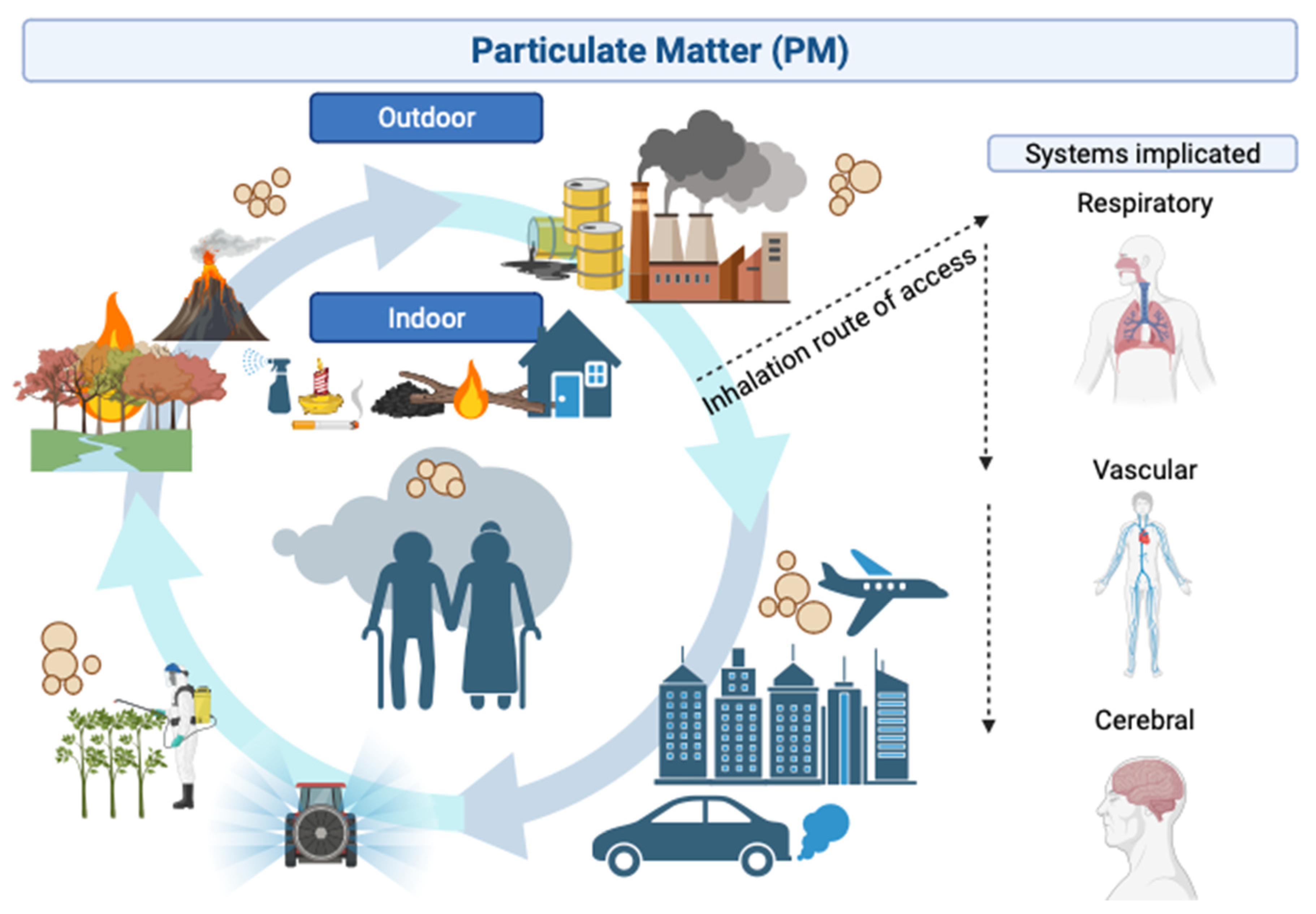
2. Etiology and Route of Access of Particulate Matter to the Human Body
2.1. Etiology of Particulate Matter
2.2. Route of Access and Biological Systems of Particulate Matter
| PM by Diameter | 1 Main Etiology | Deposited in: | References |
|---|---|---|---|
| PM0.1 or UFPM ≤100 nm | Smoke from wildfire | Cerebral cortex and cerebellum secondary to transport via the olfactory nerves | [11,28] |
| PM2.5 ≤2.5 μm | Traffic-related activities, industrial sites, factories, and agriculture | 2 Lungs and is also subject to olfactory transport and deposition in the olfactory cortex and other brain regions | [16,18,19,30] |
| PM10 ≤10 μm | Pollen, mold, and dust particles | Filtered out by the nose and upper airways | [16,18,19,29,30] |
3. Role of Particulate Matter in Cognitive Functions in Human Elderly
3.1. Cognitive Functions Affected
3.2. Cognitive Reserve Concept
| Reference | Author’s Name/Year | Country | PM Exposure | Cognitive Function Affected | Sex-Dependant? |
|---|---|---|---|---|---|
| [39] | Weuve et al., 2012 | USA | PM2.5–10, PM2.5 | GCF, verbal memory, digit span, and verbal fluency scores | ↓ in women |
| [40] | Younan et al., 2020 | USA | PM2.5 | Episodic memory | - |
| [44] | Yao et al., 2022 | China | PM1, PM2.5, PM10 | Episodic memory, mental status | ↓ in women |
| [45] | Tonne et al., 2014 | UK | PM2.5, PM10 | Reasoning and memory | - |
| [46] | Wurth et al., 2018 | Puerto Rican in USA | PM2.5 | Recognition | No |
| [47] | Wang et al., 2020 | China | PM2.5 | MMSE | ↓ in men |
| [48] | Mo et al., 2023 | China | PM2.5 | GCF | ↓ in women |
| [50] | Ogurtsova et al., 2023 | Germany | PM10, PM2.5 | Immediate verbal memory | No |
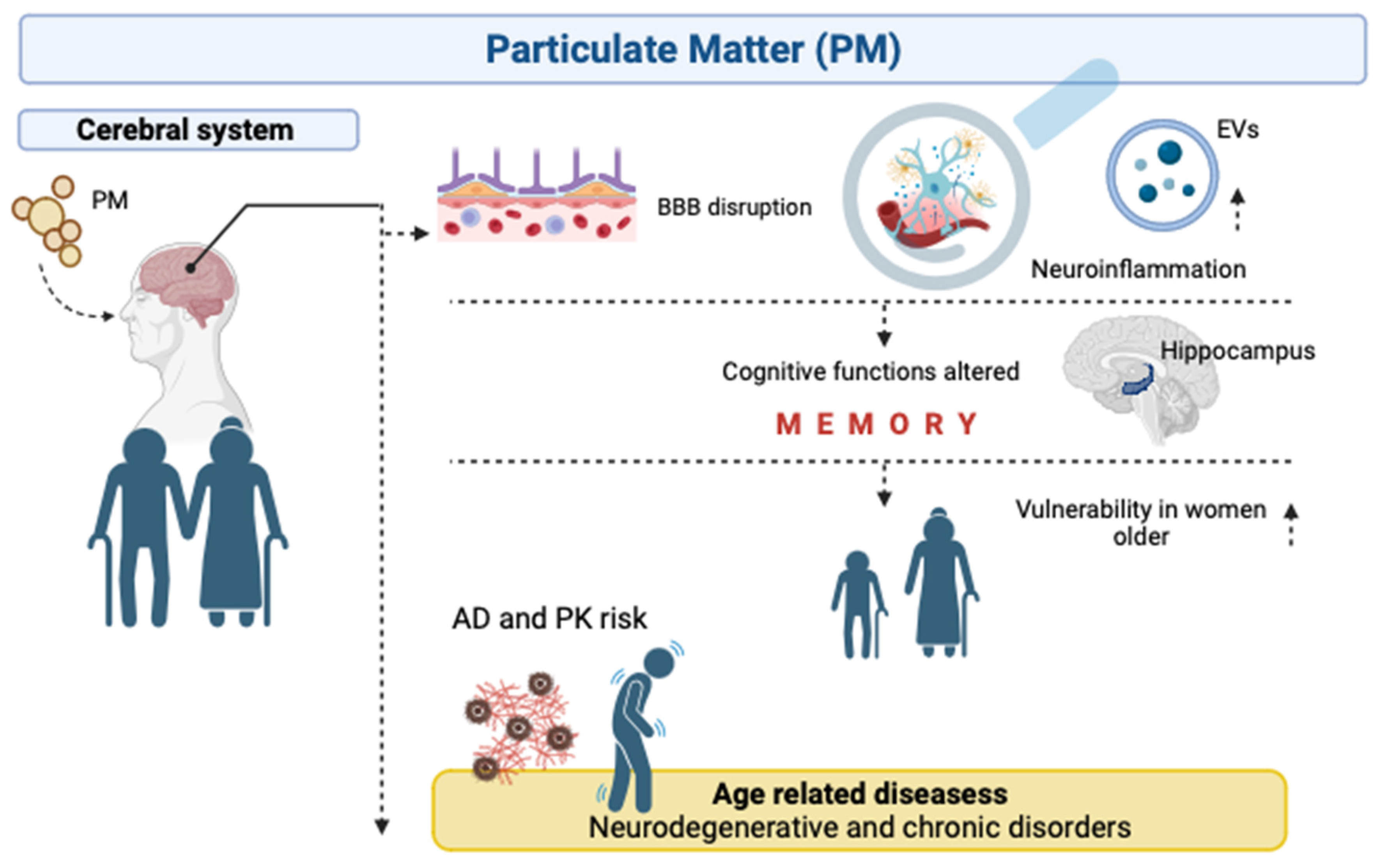
4. Particulate Matter and Neurodegenerative Diseases in Human Elderly
4.1. Inflammatory Processes
4.2. Mitochondrial Function
4.3. Changes in Gene Expressions
4.4. Neurodegenerative Diseases
Other Neurodegenerative Diseases
5. Particulate Matter, Extracellular Vesicles and Human Aging
5.1. Extracellular Vesicles, Particulate Matter and Age-Related Diseases
5.2. Extracellular Vesicles, Particulate Matter, and Neurological Diseases
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ltussey United States Environmental Protection Agency Air Quality Guide for Particle Pollution 0–50. Available online: https://www3.epa.gov/region1/airquality/pdfs/airqualityguideparticles.pdf (accessed on 10 October 2023).
- World Health Organization. WHO Global Air Quality Guidelines; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Clifford, A.; Lang, L.; Chen, R.; Anstey, K.J.; Seaton, A. Exposure to Air Pollution and Cognitive Functioning across the Life Course—A Systematic Literature Review. Environ. Res. 2016, 147, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The Global Prevalence of Dementia: A Systematic Review and Metaanalysis. Alzheimer’s Dement. 2013, 9, 63–75.e2. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, S.; Talwar, P.; Anthony, A.; Gupta, M.; Bala, K.; Agarwal, R.; Sharma, V.; Kukreti, R. Clinical Spectrum, Risk Factors, and Behavioral Abnormalities among Dementia Subtypes in a North Indian Population: A Hospital-Based Study. Dement. Geriatr. Cogn. Dis. Extra 2017, 7, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Boyle, P.A.; Wilson, R.S.; Yu, L.; Barr, A.M.; Honer, W.G.; Schneider, J.A.; Bennett, D.A. Much of Late Life Cognitive Decline Is Not Due to Common Neurodegenerative Pathologies. Ann. Neurol. 2013, 74, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Dimakakou, E.; Johnston, H.J.; Streftaris, G.; Cherrie, J.W. Exposure to Environmental and Occupational Particulate Air Pollution as a Potential Contributor to Neurodegeneration and Diabetes: A Systematic Review of Epidemiological Research. Int. J. Environ. Res. Public Health 2018, 15, 1704. [Google Scholar] [CrossRef] [PubMed]
- Sacks, J.D.; Stanek, L.W.; Luben, T.J.; Johns, D.O.; Buckley, B.J.; Brown, J.S.; Ross, M. Particulate Matter-Induced Health Effects: Who Is Susceptible? Environ. Health Perspect. 2011, 119, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Rodulfo-Cárdenas, R.; Ruiz-Sobremazas, D.; Biosca-Brull, J.; Cabré, M.; Blanco, J.; López-Granero, C.; Sánchez-Santed, F.; Colomina, M.T. The Influence of Environmental Particulate Matter Exposure during Late Gestation and Early Life on the Risk of Neurodevelopmental Disorders: A Systematic Review of Experimental Evidences. Environ. Res. 2023, 236, 116792. [Google Scholar] [CrossRef]
- Ruiz-Sobremazas, D.; Rodulfo-Cárdenas, R.; Ruiz-Coca, M.; Morales-Navas, M.; Teresa Colomina, M.; López-Granero, C.; Sánchez-Santed, F.; Perez-Fernandez, C. Uncovering the Link between Air Pollution and Neurodevelopmental Alterations during Pregnancy and Early Life Exposure: A Systematic Review. Neurosci. Biobehav. Rev. 2023, 152, 105314. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Ayala, A. Air Pollution, Ultrafine Particles, and Your Brain: Are Combustion Nanoparticle Emissions and Engineered Nanoparticles Causing Preventable Fatal Neurodegenerative Diseases and Common Neuropsychiatric Outcomes? Environ. Sci. Technol. 2022, 56, 6847–6856. [Google Scholar] [CrossRef]
- Costa, L.G.; Cole, T.B.; Dao, K.; Chang, Y.; Coburn, J. Effects of Air Pollution on the Nervous System and Its Possible Role in Neurodevelopmental and Neurodegenerative Disorders. Pharmacol. Ther. 2020, 210, 107523. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Reed, W.; Maronpot, R.R.; Henríquez-Roldán, C.; Delgado-Chavez, R.; Calderón-Garcidueñas, A.; Dragustinovis, I.; Franco-Lira, M.; Aragón-Flores, M.; Solt, A.C.; et al. Brain Inflammation and Alzheimer’s-like Pathology in Individuals Exposed to Severe Air Pollution. Toxicol. Pathol. 2004, 32, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Brugha, R.; Grigg, J. Urban Air Pollution and Respiratory Infections. Paediatr. Respir. Rev. 2014, 15, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the Air: A Review of the Effects of Particulate Matter Air Pollution on Human Health. J. Med. Toxicol. 2012, 8, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Cole, T.B.; Dao, K.; Chang, Y.C.; Garrick, J.M. Developmental Impact of Air Pollution on Brain Function. Neurochem. Int. 2019, 131, 104580. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, P.; Park, D.; Lee, Y.C. Recent Insights into Particulate Matter (PM2.5)-Mediated Toxicity in Humans: An Overview. Int. J. Environ. Res. Public Health 2022, 19, 7511. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Kabir, S. A Review on the Human Health Impact of Airborne Particulate Matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef]
- Robert, D.; Brook, S.R. Air Pollution and Cardiovascular Events. N. Engl. J. Med. 2007, 356, 2104–2106. [Google Scholar] [CrossRef]
- Hayes, R.B.; Lim, C.; Zhang, Y.; Cromar, K.; Shao, Y.; Reynolds, H.R.; Silverman, D.T.; Jones, R.R.; Park, Y.; Jerrett, M.; et al. PM2.5 Air Pollution and Cause-Specific Cardiovascular Disease Mortality. Int. J. Epidemiol. 2020, 49, 25–35. [Google Scholar] [CrossRef]
- Thurston, G.D.; Ahn, J.; Cromar, K.R.; Shao, Y.; Reynolds, H.R.; Jerrett, M.; Lim, C.C.; Shanley, R.; Park, Y.; Hayes, R.B. Ambient Particulate Matter Air Pollution Exposure and Mortality in the NIH-AARP Diet and Health Cohort. Environ. Health Perspect. 2016, 124, 484–490. [Google Scholar] [CrossRef]
- Kim, S.; Bae, H.; Lim, Y. Estimating the Health Burden of Ambient Fine Particulate Matter in Korea. In Proceedings of the ISEE 2022: 34th Annual Conference of the International Society of Environmental Epidemiology, Megaron, Athens, 18–21 September 2022; Volume 2022. [Google Scholar] [CrossRef]
- Miller, K.A.; Siscovick, D.S.; Sheppard, L.; Shepherd, K.; Sullivan, J.H.; Anderson, G.L.; Kaufman, J.D. Long-Term Exposure to Air Pollution and Incidence of Cardiovascular Events in Women. N. Engl. J. Med. 2007, 356, 447–458. [Google Scholar] [CrossRef]
- Seaton, A.; Godden, D.; MacNee, W.; Donaldson, K. Particulate Air Pollution and Acute Health Effects. Lancet 1995, 345, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Jalali, S.; Karbakhsh, M.; Momeni, M.; Taheri, M.; Amini, S.; Mansourian, M.; Sarrafzadegan, N. Long-Term Exposure to PM2.5 and Cardiovascular Disease Incidence and Mortality in an Eastern Mediterranean Country: Findings Based on a 15-Year Cohort Study. Environ. Health 2021, 20, 112. [Google Scholar] [CrossRef] [PubMed]
- Alexeeff, S.E.; Liao, N.S.; Liu, X.; Van Den Eeden, S.K.; Sidney, S. Long-Term PM2.5 Exposure and Risks of Ischemic Heart Disease and Stroke Events: Review and Meta-Analysis. J. Am. Heart Assoc. 2021, 10, e016890. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of Inhaled Ultrafine Particles to the Brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Ajmani, G.S.; Suh, H.H.; Pinto, J.M. Effects of Ambient Air Pollution Exposure on Olfaction: A Review. Environ. Health Perspect. 2016, 124, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Finch, C.E. A Critical Review of Assays for Hazardous Components of Air Pollution. Free Radic. Biol. Med. 2018, 117, 202–217. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Calderón-Garcidueñas, L. Air Pollution: Mechanisms of Neuroinflammation and CNS Disease. Trends Neurosci. 2009, 32, 506–516. [Google Scholar] [CrossRef]
- Fann, N.; Risley, D. The Public Health Context for PM2.5 and Ozone Air Quality Trends. Air Qual. Atmos. Health 2013, 6, 1–11. [Google Scholar] [CrossRef]
- Rivas, I.; Basagaña, X.; Cirach, M.; López-Vicente, M.; Suades-González, E.; Garcia-Esteban, R.; Álvarez-Pedrerol, M.; Dadvand, P.; Sunyer, J. Association between Early Life Exposure to Air Pollution and Working Memory and Attention. Environ. Health Perspect. 2019, 127, 057002. [Google Scholar] [CrossRef]
- Aghaei, M.; Janjani, H.; Yousefian, F.; Jamal, A.; Yunesian, M. Association between Ambient Gaseous and Particulate Air Pollutants and Attention Deficit Hyperactivity Disorder (ADHD) in Children; a Systematic Review. Environ. Res. 2019, 173, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Mora-Tiscareño, A.; Ontiveros, E.; Gómez-Garza, G.; Barragán-Mejía, G.; Broadway, J.; Chapman, S.; Valencia-Salazar, G.; Jewells, V.; Maronpot, R.R.; et al. Air Pollution, Cognitive Deficits and Brain Abnormalities: A Pilot Study with Children and Dogs. Brain Cogn. 2008, 68, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-H.M.; Hsu, H.-H.L.; Coull, B.A.; Bellinger, D.C.; Kloog, I.; Schwartz, J.; Wright, R.O.; Wright, R.J. Prenatal Particulate Air Pollution and Neurodevelopment in Urban Children: Examining Sensitive Windows and Sex-Specific Associations. Environ. Int. 2016, 87, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Schikowski, T.; Altuğ, H. The Role of Air Pollution in Cognitive Impairment and Decline. Neurochem. Int. 2020, 136, 104708. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Kwong, J.C.; Copes, R.; Tu, K.; Villeneuve, P.J.; van Donkelaar, A.; Hystad, P.; Martin, R.V.; Murray, B.J.; Jessiman, B.; et al. Living near Major Roads and the Incidence of Dementia, Parkinson’s Disease, and Multiple Sclerosis: A Population-Based Cohort Study. Lancet 2017, 389, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Weuve, J.; Puett, R.C.; Schwartz, J.; Yanosky, J.D.; Laden, F.; Grodstein, F. Exposure to Particulate Air Pollution and Cognitive Decline in Older Women. Arch. Intern. Med. 2012, 172, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Younan, D.; Petkus, A.J.; Widaman, K.F.; Wang, X.; Casanova, R.; Espeland, M.A.; Gatz, M.; Henderson, V.W.; Manson, J.E.; Rapp, S.R.; et al. Particulate Matter and Episodic Memory Decline Mediated by Early Neuroanatomic Biomarkers of Alzheimer’s Disease. Brain 2020, 143, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Heusinkveld, H.J.; Wahle, T.; Campbell, A.; Westerink, R.H.S.; Tran, L.; Johnston, H.; Stone, V.; Cassee, F.R.; Schins, R.P.F. Neurodegenerative and Neurological Disorders by Small Inhaled Particles. Neurotoxicology 2016, 56, 94–106. [Google Scholar] [CrossRef]
- Power, M.C.; Weisskopf, M.G.; Alexeeff, S.E.; Coull, B.A.; Avron, S.; Schwartz, J. Traffic-Related Air Pollution and Cognitive Function in a Cohort of Older Men. Environ. Health Perspect. 2011, 119, 682–687. [Google Scholar] [CrossRef]
- Zeng, Y.; Gu, D.; Purser, J.; Hoenig, H.; Christakis, N. Associations of Environmental Factors with Elderly Health and Mortality in China. Am. J. Public Health 2010, 100, 298–305. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, K.; Xiang, H. Association between Cognitive Function and Ambient Particulate Matters in Middle-Aged and Elderly Chinese Adults: Evidence from the China Health and Retirement Longitudinal Study (CHARLS). Sci. Total Environ. 2022, 828, 154297. [Google Scholar] [CrossRef] [PubMed]
- Tonne, C.; Elbaz, A.; Beevers, S.; Singh-Manoux, A. Traffic-Related Air Pollution in Relation to Cognitive Function in Older Adults. Epidemiology 2014, 25, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Wurth, R.; Kioumourtzoglou, M.A.; Tucker, K.L.; Griffith, J.; Manjourides, J.; Suh, H. Fine Particle Sources and Cognitive Function in an Older Puerto Rican Cohort in Greater Boston. Environ. Epidemiol. 2018, 2, e022. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, T.; Lv, Y.; Kraus, V.B.; Zhang, Y.; Mao, C.; Yin, Z.; Shi, W.; Zhou, J.; Zheng, T.; et al. Fine Particulate Matter and Poor Cognitive Function among Chinese Older Adults: Evidence from a Community-Based, 12-Year Prospective Cohort Study. Environ. Health Perspect. 2020, 128, 067013. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.; Wang, Y.; Peng, M.; Wang, Q.; Zheng, H.; Zhan, Y.; Ma, Z.; Yang, Z.; Liu, L.; Hu, K.; et al. Sex Disparity in Cognitive Aging Related to Later-Life Exposure to Ambient Air Pollution. Sci. Total Environ. 2023, 886, 163980. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Hsieh, P.I.; Chen, J.K.; Kuo, E.; Yu, H.L.; Chiou, J.M.; Chen, J.H. Effect of Indoor Air Quality on the Association of Long-Term Exposure to Low-Level Air Pollutants with Cognition in Older Adults. Environ. Res. 2023, 233, 115483. [Google Scholar] [CrossRef]
- Ogurtsova, K.; Soppa, V.J.; Weimar, C.; Jöckel, K.H.; Jokisch, M.; Hoffmann, B. Association of Long-Term Air Pollution and Ambient Noise with Cognitive Decline in the Heinz Nixdorf Recall Study. Environ. Pollut. 2023, 331, 121898. [Google Scholar] [CrossRef]
- Gallagher, M.; Koh, M.T. Episodic Memory on the Path to Alzheimer’s Disease. Curr. Opin. Neurobiol. 2011, 21, 929–934. [Google Scholar] [CrossRef]
- Katzman, R.; Terry, R.; DeTeresa, R.; Brown, T.; Davies, P.; Fuld, P.; Renbing, X.; Peck, A. Clinical, Pathological, and Neurochemical Changes in Dementia: A Subgroup with Preserved Mental Status and Numerous Neocortical Plaques. Ann. Neurol. 1988, 23, 138–144. [Google Scholar] [CrossRef]
- Bigio, E.H.; Hynan, L.S.; Sontag, E.; Satumtira, S.; White, C.L. Synapse Loss Is Greater in Presenile than Senile Onset Alzheimer Disease: Implications for the Cognitive Reserve Hypothesis. Neuropathol. Appl. Neurobiol. 2002, 28, 218–227. [Google Scholar] [CrossRef]
- Staff, R.T.; Murray, A.D.; Deary, I.J.; Whalley, L.J. What Provides Cerebral Reserve? Brain 2004, 127, 1191–1199. [Google Scholar] [CrossRef]
- Opdebeeck, C.; Martyr, A.; Clare, L. Cognitive Reserve and Cognitive Function in Healthy Older People: A Meta-Analysis. Aging Neuropsychol. Cogn. 2016, 23, 40–60. [Google Scholar] [CrossRef]
- Barulli, D.; Stern, Y. Efficiency, Capacity, Compensation, Maintenance, Plasticity: Emerging Concepts in Cognitive Reserve. Trends Cogn. Sci. 2013, 17, 502–509. [Google Scholar] [CrossRef]
- León, I.; García-García, J.; Roldán-Tapia, L. Estimating Cognitive Reserve in Healthy Adults Using the Cognitive Reserve Scale. PLoS ONE 2014, 9, e102632. [Google Scholar] [CrossRef]
- Stern, Y. Cognitive Reserve: Implications for Assessment and Intervention. Folia Phoniatr. Logop. 2013, 65, 49–54. [Google Scholar] [CrossRef]
- Stern, Y. Cognitive Reserve☆. Neuropsychologia 2009, 47, 2015–2028. [Google Scholar] [CrossRef]
- Sass, V.; Kravitz-Wirtz, N.; Karceski, S.M.; Hajat, A.; Crowder, K.; Takeuchi, D. The Effects of Air Pollution on Individual Psychological Distress. Health Place 2017, 48, 72–79. [Google Scholar] [CrossRef]
- Lim, Y.H.; Kim, H.; Kim, J.H.; Bae, S.; Park, H.Y.; Hong, Y.C. Air Pollution and Symptoms of Depression in Elderly Adults. Environ. Health Perspect. 2012, 120, 1023–1028. [Google Scholar] [CrossRef]
- Power, M.C.; Kioumourtzoglou, M.A.; Hart, J.E.; Okereke, O.I.; Laden, F.; Weisskopf, M.G. The Relation between Past Exposure to Fine Particulate Air Pollution and Prevalent Anxiety: Observational Cohort Study. BMJ 2015, 350, h1111. [Google Scholar] [CrossRef]
- Chen, J.-C.; Wang, X.; Serre, M.; Cen, S.; Franklin, M.; Espeland, M. Particulate Air Pollutants, Brain Structure, and Neurocognitive Disorders in Older Women; 2004. [Google Scholar]
- Chen, C.; Hayden, K.M.; Kaufman, J.D.; Espeland, M.A.; Whitsel, E.A.; Serre, M.L.; Vizuete, W.; Orchard, T.S.; Wang, X.; Chui, H.C.; et al. Adherence to a MIND-Like Dietary Pattern, Long-Term Exposure to Fine Particulate Matter Air Pollution, and MRI-Based Measures of Brain Volume: The Women’s Health Initiative Memory Study-MRI. Environ. Health Perspect. 2021, 129, 127008. [Google Scholar] [CrossRef]
- Wilker, E.H.; Preis, S.R.; Beiser, A.S.; Wolf, P.A.; Au, R.; Kloog, I.; Li, W.; Schwartz, J.; Koutrakis, P.; Decarli, C.; et al. Long-Term Exposure to Fine Particulate Matter, Residential Proximity to Major Roads and Measures of Brain Structure. Stroke 2015, 46, 1161–1166. [Google Scholar] [CrossRef]
- CAMPBELL, A. Inflammation, Neurodegenerative Diseases, and Environmental Exposures. Ann. N. Y. Acad. Sci. 2004, 1035, 117–132. [Google Scholar] [CrossRef]
- Ejaz, S.; Anwar, K.; Ashraf, M. MRI and Neuropathological Validations of the Involvement of Air Pollutants in Cortical Selective Neuronal Loss. Environ. Sci. Pollut. Res. 2014, 21, 3351–3362. [Google Scholar] [CrossRef]
- Jankowska-Kieltyka, M.; Roman, A.; Nalepa, I. The Air We Breathe: Air Pollution as a Prevalent Proinflammatory Stimulus Contributing to Neurodegeneration. Front. Cell. Neurosci. 2021, 15, 647643. [Google Scholar] [CrossRef]
- Brunekreef, B.; Holgate, S.T. Air Pollution and Health. Lancet 2002, 360, 1233–1242. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging Roles of Oxidative Stress in Brain Aging and Alzheimer’s Disease. Neurobiol Aging 2021, 107, 86–95. [Google Scholar] [CrossRef]
- Marogianni, C.; Sokratous, M.; Dardiotis, E.; Hadjigeorgiou, G.M.; Bogdanos, D.; Xiromerisiou, G. Neurodegeneration and Inflammation—An Interesting Interplay in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 8421. [Google Scholar] [CrossRef]
- Arias-Pérez, R.D.; Taborda, N.A.; Gómez, D.M.; Narvaez, J.F.; Porras, J.; Hernandez, J.C. Inflammatory Effects of Particulate Matter Air Pollution. Environ. Sci. Pollut. Res. 2020, 27, 42390–42404. [Google Scholar] [CrossRef]
- Dionisio-Santos, D.A.; Olschowka, J.A.; O’Banion, M.K. Exploiting Microglial and Peripheral Immune Cell Crosstalk to Treat Alzheimer’s Disease. J. Neuroinflam. 2019, 16, 74. [Google Scholar] [CrossRef]
- Block, M.L.; Elder, A.; Auten, R.L.; Bilbo, S.D.; Chen, H.; Chen, J.-C.; Cory-Slechta, D.A.; Costa, D.; Diaz-Sanchez, D.; Dorman, D.C.; et al. The Outdoor Air Pollution and Brain Health Workshop. Neurotoxicology 2012, 33, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Brockmeyer, S.; D’Angiulli, A. How Air Pollution Alters Brain Development: The Role of Neuroinflammation. Transl. Neurosci. 2016, 7, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Kavanaugh, M.; Block, M.; D’Angiulli, A.; Delgado-Chávez, R.; Torres-Jardón, R.; González-Maciel, A.; Reynoso-Robles, R.; Osnaya, N.; Villarreal-Calderon, R.; et al. Neuroinflammation, Hyperphosphorylated Tau, Diffuse Amyloid Plaques, and Down-Regulation of the Cellular Prion Protein in Air Pollution Exposed Children and Young Adults. J. Alzheimer’s Dis. 2012, 28, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; Klocke, C.; Morris-Schaffer, K.; Conrad, K.; Sobolewski, M.; Cory-Slechta, D.A. Cognitive Effects of Air Pollution Exposures and Potential Mechanistic Underpinnings. Curr. Environ. Health Rep. 2017, 4, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Solt, A.C.; Henríquez-Roldán, C.; Torres-Jardón, R.; Nuse, B.; Herritt, L.; Villarreal-Calderón, R.; Osnaya, N.; Stone, I.; García, R.; et al. Long-Term Air Pollution Exposure Is Associated with Neuroinflammation, an Altered Innate Immune Response, Disruption of the Blood-Brain Barrier, Ultrafine Particulate Deposition, and Accumulation of Amyloid β-42 and α-Synuclein in Children and Young Adults. Toxicol. Pathol. 2008, 36, 289–310. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Dasgupta, S.; Mishra, P.K.; Chaudhury, K. Air Pollution-Induced Epigenetic Changes: Disease Development and a Possible Link with Hypersensitivity Pneumonitis. Environ. Sci. Pollut. Res. 2021, 28, 55981–56002. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Kuntic, M.; Hahad, O.; Delogu, L.G.; Rohrbach, S.; Di Lisa, F.; Schulz, R.; Münzel, T. Effects of Air Pollution Particles (Ultrafine and Fine Particulate Matter) on Mitochondrial Function and Oxidative Stress—Implications for Cardiovascular and Neurodegenerative Diseases. Arch. Biochem. Biophys. 2020, 696, 108662. [Google Scholar] [CrossRef]
- Angelopoulou, E.; Paudel, Y.N.; Papageorgiou, S.G.; Piperi, C. Environmental Impact on the Epigenetic Mechanisms Underlying Parkinson’s Disease Pathogenesis: A Narrative Review. Brain Sci. 2022, 12, 175. [Google Scholar] [CrossRef]
- Marques, S.C.F.; Oliveira, C.R.; Pereira, C.M.F.; Outeiro, T.F. Epigenetics in Neurodegeneration: A New Layer of Complexity. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 348–355. [Google Scholar] [CrossRef]
- Giambò, F.; Leone, G.M.; Gattuso, G.; Rizzo, R.; Cosentino, A.; Cinà, D.; Teodoro, M.; Costa, C.; Tsatsakis, A.; Fenga, C.; et al. Genetic and Epigenetic Alterations Induced by Pesticide Exposure: Integrated Analysis of Gene Expression, MicroRNA Expression, and DNA Methylation Datasets. Public Health 2021, 18, 8697. [Google Scholar] [CrossRef]
- Rasmi, Y.; Shokati, A.; Hassan, A.; Aziz, S.G.G.; Bastani, S.; Jalali, L.; Moradi, F.; Alipour, S. The Role of DNA Methylation in Progression of Neurological Disorders and Neurodegenerative Diseases as Well as the Prospect of Using DNA Methylation Inhibitors as Therapeutic Agents for Such Disorders. IBRO Neurosci. Rep. 2023, 14, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Solaimani, P.; Saffari, A.; Sioutas, C.; Bondy, S.C.; Campbell, A. Exposure to Ambient Ultrafine Particulate Matter Alters the Expression of Genes in Primary Human Neurons. Neurotoxicology 2017, 58, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Kilburn, K.H. Effects of Diesel Exhaust on Neurobehavioral and Pulmonary Functions. Arch. Environ. Health Int. J. 2000, 55, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Seagrave, J.C.; McDonald, J.D.; Bedrick, E.; Edgerton, E.S.; Gigliotti, A.P.; Jansen, J.J.; Ke, L.; Naeher, L.P.; Seilkop, S.K.; Zheng, M.; et al. Lung Toxicity of Ambient Particulate Matter from Southeastern U.S. Sites with Different Contributing Sources: Relationships between Composition and Effects. Environ. Health Perspect. 2006, 114, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.-R.; Lin, Y.-T.; Hwang, B.-F. Ozone, Particulate Matter, and Newly Diagnosed Alzheimer’s Disease: A Population-Based Cohort Study in Taiwan. J. Alzheimer’s Dis. 2015, 44, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Ailshire, J.A.; Clarke, P. Fine Particulate Matter Air Pollution and Cognitive Function among U.S. Older Adults. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2015, 70, 322–328. [Google Scholar] [CrossRef]
- Ailshire, J.A.; Crimmins, E.M. Fine Particulate Matter Air Pollution and Cognitive Function Among Older US Adults. Am. J. Epidemiol. 2014, 180, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Parra, K.L.; Alexander, G.E.; Raichlen, D.A.; Klimentidis, Y.C.; Furlong, M.A. Exposure to Air Pollution and Risk of Incident Dementia in the UK Biobank. Environ. Res. 2022, 209, 112895. [Google Scholar] [CrossRef]
- Aström, D.O.; Adolfsson, R.; Segersson, D.; Forsberg, B.; Oudin, A. Local Contrasts in Concentration of Ambient Particulate Air Pollution (PM2.5) and Incidence of Alzheimer’s Disease and Dementia: Results from the Betula Cohort in Northern Sweden. J. Alzheimer’s Dis. 2021, 81, 83–85. [Google Scholar] [CrossRef]
- Oudin, A.; Andersson, J.; Sundström, A.; Nordin Adolfsson, A.; Oudin Åström, D.; Adolfsson, R.; Forsberg, B.; Nordin, M. Traffic-Related Air Pollution as a Risk Factor for Dementia: No Clear Modifying Effects of APOE ε4 in the Betula Cohort. J. Alzheimer’s Dis. 2019, 71, 733–740. [Google Scholar] [CrossRef]
- Oudin, A.; Bråbäck, L.; Åström, D.O.; Strömgren, M.; Forsberg, B. Association between Neighbourhood Air Pollution Concentrations and Dispensed Medication for Psychiatric Disorders in a Large Longitudinal Cohort of Swedish Children and Adolescents. BMJ Open 2016, 6, e010004. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, R.M.; Blanco, M.N.; Li, G.; Adar, S.D.; Carone, M.; Szpiro, A.A.; Kaufman, J.D.; Larson, T.V.; Larson, E.B.; Crane, P.K.; et al. Fine Particulate Matter and Dementia Incidence in the Adult Changes in Thought Study. Environ. Health Perspect. 2021, 129, 087001. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Mora-Tiscareño, A.; Franco-Lira, M.; Zhu, H.; Lu, Z.; Solorio, E.; Torres-Jardón, R.; D’Angiulli, A. Decreases in Short Term Memory, IQ, and Altered Brain Metabolic Ratios in Urban Apolipoprotein Ε4 Children Exposed to Air Pollution. J. Alzheimer’s Dis. 2015, 45, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Yan, H. Effect of Long-Term Particulate Matter Exposure on Parkinson’s Risk. Environ. Geochem. Health 2020, 42, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Kioumourtzoglou, M.A.; Schwartz, J.D.; Weisskopf, M.G.; Melly, S.J.; Wang, Y.; Dominici, F.; Zanobetti, A. Long-Term PM2.5 Exposure and Neurological Hospital Admissions in the Northeastern United States. Environ. Health Perspect. 2016, 124, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Palacios, N.; Fitzgerald, K.C.; Hart, J.E.; Weisskopf, M.; Schwarzschild, M.A.; Ascherio, A.; Laden, F. Air Pollution and Risk of Parkinson’s Disease in a Large Prospective Study of Men. Environ. Health Perspect. 2017, 125, 087011. [Google Scholar] [CrossRef]
- Palacios, N.; Fitzgerald, K.C.; Hart, J.E.; Weisskopf, M.G.; Schwarzschild, M.A.; Ascherio, A.; Laden, F. Particulate Matter and Risk of Parkinson Disease in a Large Prospective Study of Women. Environ. Health 2014, 13, 80. [Google Scholar] [CrossRef]
- Liu, R.; Young, M.T.; Chen, J.C.; Kaufman, J.D.; Chen, H. Ambient Air Pollution Exposures and Risk of Parkinson Disease. Environ. Health Perspect. 2016, 124, 1759–1765. [Google Scholar] [CrossRef]
- Chen, C.Y.; Hung, H.J.; Chang, K.H.; Hsu, C.Y.; Muo, C.H.; Tsai, C.H.; Wu, T.N. Long-Term Exposure to Air Pollution and the Incidence of Parkinson’s Disease: A Nested Case-Control Study. PLoS ONE 2017, 12, e0182834. [Google Scholar] [CrossRef]
- Bai, L.; Burnett, R.T.; Kwong, J.C.; Hystad, P.; van Donkelaar, A.; Brook, J.R.; Tu, K.; Copes, R.; Goldberg, M.S.; Martin, R.V.; et al. Long-Term Exposure to Air Pollution and the Incidence of Multiple Sclerosis: A Population-Based Cohort Study. Environ. Res. 2018, 166, 437–443. [Google Scholar] [CrossRef]
- Gregory, A.C.; Shendell, D.G.; Okosun, I.S.; Gieseker, K.E. Multiple Sclerosis Disease Distribution and Potential Impact of Environmental Air Pollutants in Georgia. Sci. Total Environ. 2008, 396, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Parks, R.M.; Nunez, Y.; Balalian, A.A.; Gibson, E.A.; Hansen, J.; Raaschou-Nielsen, O.; Ketzel, M.; Khan, J.; Brandt, J.; Vermeulen, R.; et al. Long-Term Traffic-Related Air Pollutant Exposure and Amyotrophic Lateral Sclerosis Diagnosis in Denmark: A Bayesian Hierarchical Analysis. Epidemiology 2022, 33, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Malek, A.M.; Arena, V.C.; Song, R.; Whitsel, E.A.; Rager, J.R.; Stewart, J.; Yanosky, J.D.; Liao, D.; Talbott, E.O. Long-Term Air Pollution and Risk of Amyotrophic Lateral Sclerosis Mortality in the Women’s Health Initiative Cohort. Environ. Res. 2023, 216, 114510. [Google Scholar] [CrossRef] [PubMed]
- Seelen, M.; Toro Campos, R.A.; Veldink, J.H.; Visser, A.E.; Hoek, G.; Brunekreef, B.; van der Kooi, A.J.; de Visser, M.; Raaphorst, J.; van den Berg, L.H.; et al. Long-Term Air Pollution Exposure and Amyotrophic Lateral Sclerosis in Netherlands: A Population-Based Case–Control Study. Environ. Health Perspect. 2017, 125, 097023. [Google Scholar] [CrossRef] [PubMed]
- Myung, W.; Lee, H.; Kim, H. Short-Term Air Pollution Exposure and Emergency Department Visits for Amyotrophic Lateral Sclerosis: A Time-Stratified Case-Crossover Analysis. Environ. Int. 2019, 123, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Mas-Bargues, C.; Alique, M.; Barrús-Ortiz, M.T.; Borrás, C.; Rodrigues-Díez, R. Exploring New Kingdoms: The Role of Extracellular Vesicles in Oxi-Inflamm-Aging Related to Cardiorenal Syndrome. Antioxidants 2022, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Mariani, J.; Iodice, S.; Cantone, L.; Solazzo, G.; Marraccini, P.; Conforti, E.; Bulsara, P.A.; Lombardi, M.S.; Howlin, R.P.; Bollati, V.; et al. Particulate Matter Exposure and Allergic Rhinitis: The Role of Plasmatic Extracellular Vesicles and Bacterial Nasal Microbiome. Int. J. Environ. Res. Public Health 2021, 18, 689. [Google Scholar] [CrossRef]
- Bonzini, M.; Pergoli, L.; Cantone, L.; Hoxha, M.; Spinazzè, A.; Del Buono, L.; Favero, C.; Carugno, M.; Angelici, L.; Broggi, L.; et al. Short-Term Particulate Matter Exposure Induces Extracellular Vesicle Release in Overweight Subjects. Environ. Res. 2017, 155, 228–234. [Google Scholar] [CrossRef]
- Eckhardt, C.M.; Baccarelli, A.A.; Wu, H. Environmental Exposures and Extracellular Vesicles: Indicators of Systemic Effects and Human Disease. Curr. Environ. Health Rep. 2022, 9, 465–476. [Google Scholar] [CrossRef]
- Ferrari, L.; Borghi, F.; Iodice, S.; Catelan, D.; Rossi, S.; Giusti, I.; Grisotto, L.; Rovelli, S.; Spinazzè, A.; Alinovi, R.; et al. INSIDE Project: Individual Air Pollution Exposure, Extracellular Vesicles Signaling and Hypertensive Disorder Development in Pregnancy. Int. J. Environ. Res. Public Health 2020, 17, 9046. [Google Scholar] [CrossRef]
- Pope, C.A.; Bhatnagar, A.; McCracken, J.P.; Abplanalp, W.; Conklin, D.J.; O’Toole, T. Exposure to Fine Particulate Air Pollution Is Associated With Endothelial Injury and Systemic Inflammation. Circ. Res. 2016, 119, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, M.G.; Lee, S.H.; Abdelazim, A.M.; Saadeldin, I.M.; Abomughaid, M.M. Role of Extracellular Vesicles in Compromising Cellular Resilience to Environmental Stressors. Biomed. Res. Int. 2021, 2021, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Benedikter, B.J.; Wouters, E.F.M.; Savelkoul, P.H.M.; Rohde, G.G.U.; Stassen, F.R.M. Extracellular Vesicles Released in Response to Respiratory Exposures: Implications for Chronic Disease. J. Toxicol. Environ. Health Part B 2018, 21, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Harischandra, D.S.; Ghaisas, S.; Rokad, D.; Kanthasamy, A.G. Exosomes in Toxicology: Relevance to Chemical Exposure and Pathogenesis of Environmentally Linked Diseases. Toxicol. Sci. 2017, 158, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, S.; Baccarelli, A.; Prada, D. Role of Brain Extracellular Vesicles in Air Pollution-Related Cognitive Impairment and Neurodegeneration. Environ. Res. 2022, 204, 112316. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Hornung, S.; Taha, H.B.; Bitan, G. Biomarkers for Parkinsonian Disorders in CNS-Originating EVs: Promise and Challenges. Acta Neuropathol. 2023, 145, 515–540. [Google Scholar] [CrossRef] [PubMed]
- Schraufnagel, D.E.; Balmes, J.R.; de Matteis, S.; Hoffman, B.; Kim, W.J.; Perez-Padilla, R.; Rice, M.; Sood, A.; Vanker, A.; Wuebbles, D.J. Health Benefits of Air Pollution Reduction. Ann. Am. Thorac. Soc. 2019, 16, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Slovic, A.D.; de Oliveira, M.A.; Biehl, J.; Ribeiro, H. How Can Urban Policies Improve Air Quality and Help Mitigate Global Climate Change: A Systematic Mapping Review. J. Urban Health 2016, 93, 73–95. [Google Scholar] [CrossRef]
- Vedrenne, M.; Pérez, J.; Lumbreras, J.; Rodríguez, M.E. Life Cycle Assessment as a Policy-Support Tool: The Case of Taxis in the City of Madrid. Energy Policy 2014, 66, 185–197. [Google Scholar] [CrossRef]
- Brand, C.; Dekker, H.J.; Behrendt, F. Cycling, Climate Change and Air Pollution. Adv. Transp. Policy Plan. 2022, 10, 235–264. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Granero, C.; Polyanskaya, L.; Ruiz-Sobremazas, D.; Barrasa, A.; Aschner, M.; Alique, M. Particulate Matter in Human Elderly: Higher Susceptibility to Cognitive Decline and Age-Related Diseases. Biomolecules 2024, 14, 35. https://doi.org/10.3390/biom14010035
López-Granero C, Polyanskaya L, Ruiz-Sobremazas D, Barrasa A, Aschner M, Alique M. Particulate Matter in Human Elderly: Higher Susceptibility to Cognitive Decline and Age-Related Diseases. Biomolecules. 2024; 14(1):35. https://doi.org/10.3390/biom14010035
Chicago/Turabian StyleLópez-Granero, Caridad, Leona Polyanskaya, Diego Ruiz-Sobremazas, Angel Barrasa, Michael Aschner, and Matilde Alique. 2024. "Particulate Matter in Human Elderly: Higher Susceptibility to Cognitive Decline and Age-Related Diseases" Biomolecules 14, no. 1: 35. https://doi.org/10.3390/biom14010035
APA StyleLópez-Granero, C., Polyanskaya, L., Ruiz-Sobremazas, D., Barrasa, A., Aschner, M., & Alique, M. (2024). Particulate Matter in Human Elderly: Higher Susceptibility to Cognitive Decline and Age-Related Diseases. Biomolecules, 14(1), 35. https://doi.org/10.3390/biom14010035









