Cimetidine Does Not Inhibit 5-Aminolevulinic Acid Synthase or Heme Oxygenase Activity: Implications for Treatment of Acute Intermittent Porphyria and Erythropoietic Protoporphyria
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Studies
2.2. Quantitation of Plasma ALA and PBG
2.3. RNA Extraction and Quantitative PCR for mRNA Quantification
2.4. ALAS Enzyme Activity Assay
2.5. Heme Oxygenase (HO) Activity Assay
2.6. Prokaryotic Expression and Purification of Human ALAS2
2.7. Statistical Analyses
3. Results
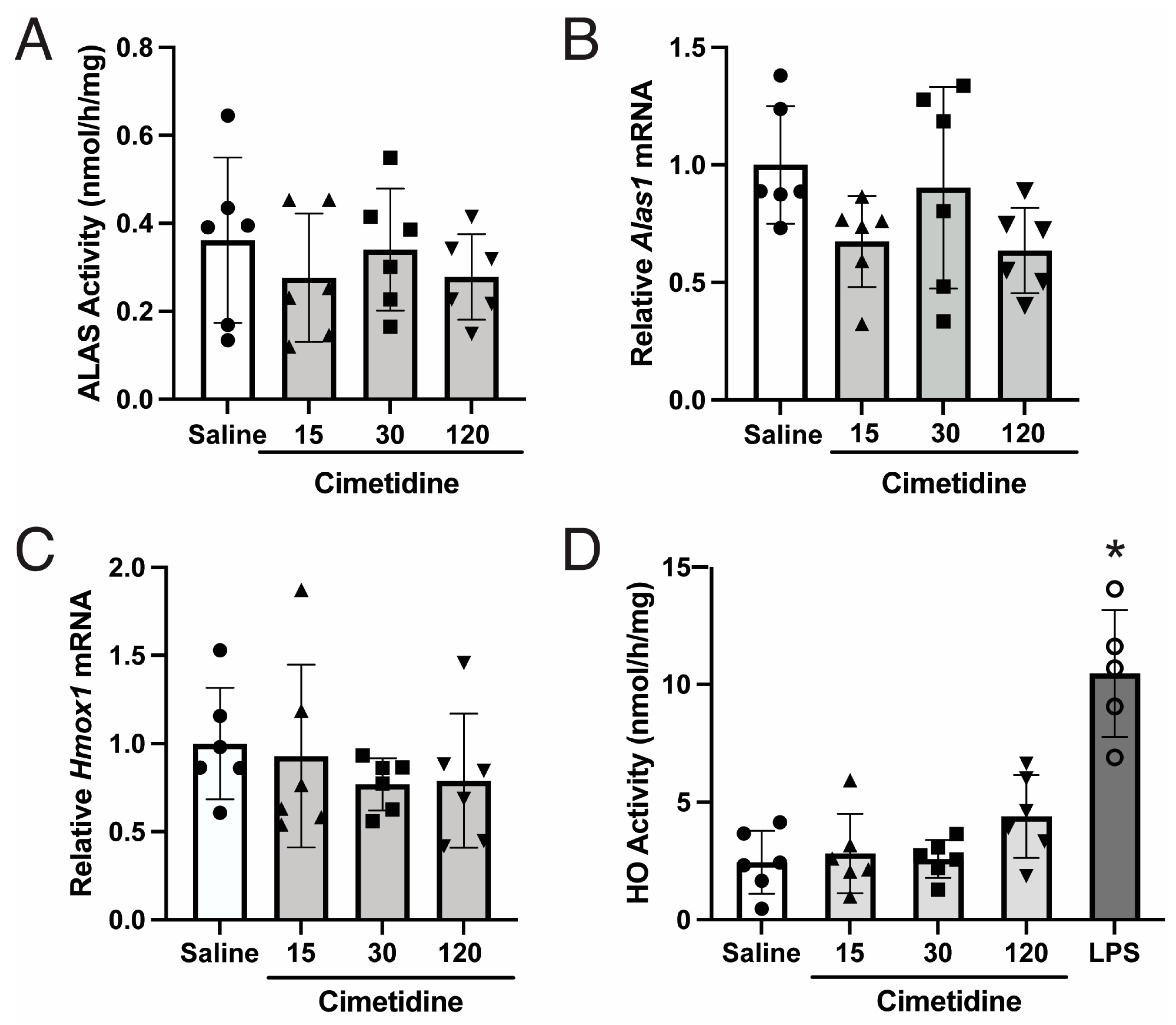
3.1. Cimetidine Administration Does Not Lead to Statistically Significant Decreases in Endogenous ALAS or HO Activity in Murine Livers
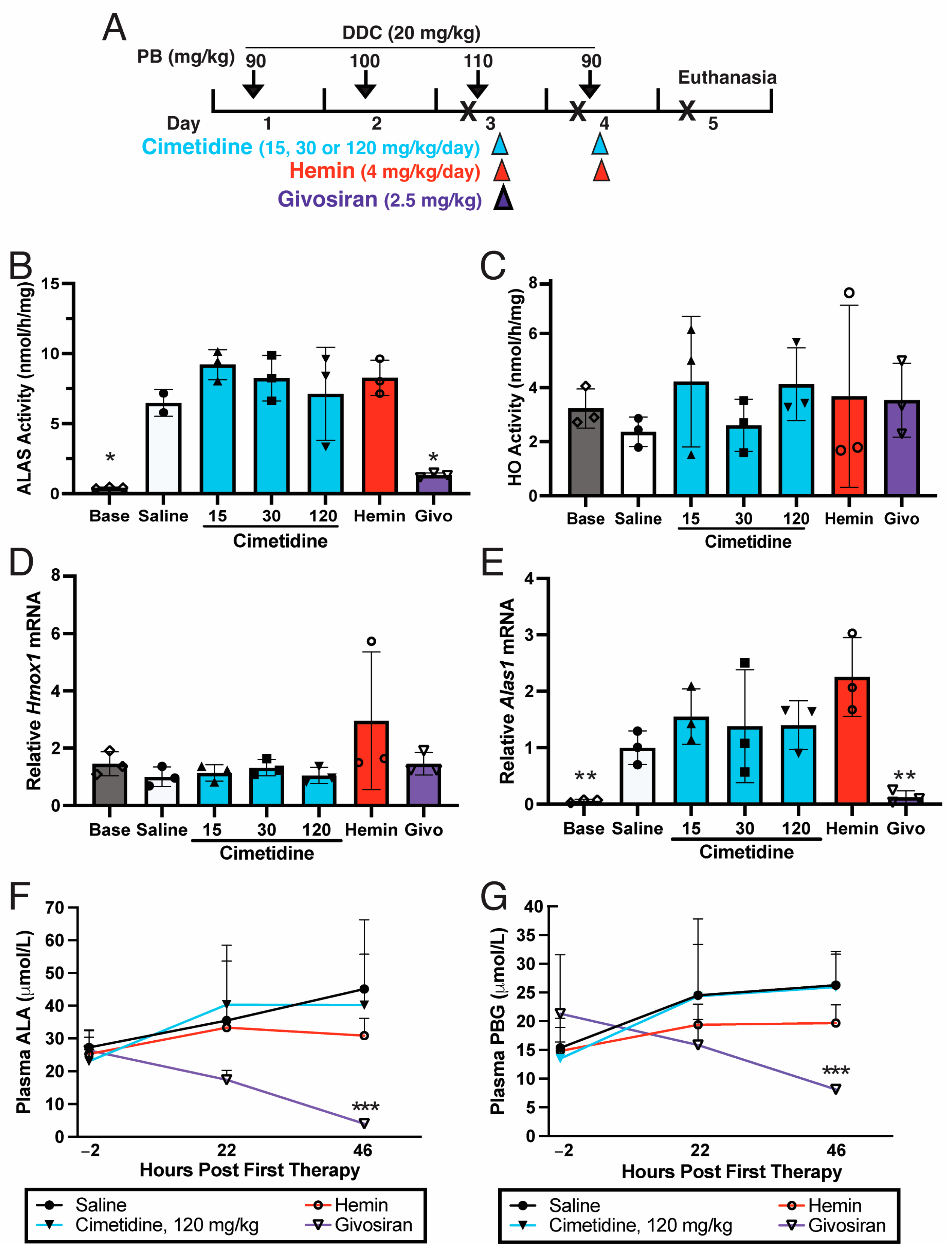
3.2. Cimetidine Does Not Decrease Hepatic ALAS Activity That Is Markedly Elevated during a Biochemical Acute Attack in AIP Mice
3.3. Cimetidine Does Not Inhibit HO Activity in PB/DDC-Induced AIP Mouse Livers
3.4. Cimetidine Does Not Decrease Plasma ALA and PBG Levels That Are Markedly Elevated during a Biochemical Acute Attack in AIP Mice
3.5. Cimetidine Does Not Alter Activity or Expression of ALAS or HO in Wildtype Murine Bone Marrow Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, K.E.; Sassa, S.; Bishop, D.F.; Desnick, R.J. Disorders of heme biosynthesis: X-linked sideroblastic anemia and the porphyrias. In The Metabolib and Molecular Bases of Inherited Disease; Scriver, C., Beaudet, A., Sly, W., Valle, D., Eds.; McGraw-Hill: New York, NY, USA, 2001; pp. 2961–3062. [Google Scholar]
- Balwani, M.; Desnick, R.J. The porphyrias: Advances in diagnosis and treatment. Blood 2012, 120, 4496–4504. [Google Scholar] [CrossRef] [PubMed]
- Bissell, D.M.; Anderson, K.E.; Bonkovsky, H.L. Porphyria. N. Engl. J. Med. 2017, 377, 2101. [Google Scholar] [CrossRef] [PubMed]
- Handschin, C.; Lin, J.; Rhee, J.; Peyer, A.K.; Chin, S.; Wu, P.H.; Meyer, U.A.; Spiegelman, B.M. Nutritional regulation of hepatic heme biosynthesis and porphyria through PGC-1α. Cell 2005, 122, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.J.; Podvinec, M.; Kaufmann, M.R.; Meyer, U.A. Drugs mediate the transcriptional activation of the 5-aminolevulinic acid synthase (ALAS1) gene via the chicken xenobiotic-sensing nuclear receptor (CXR). J. Biol. Chem. 2002, 277, 34717–34726. [Google Scholar] [CrossRef] [PubMed]
- Podvinec, M.; Handschin, C.; Looser, R.; Meyer, U.A. Identification of the xenosensors regulating human 5-aminolevulinate synthase. Proc. Natl. Acad. Sci. USA 2004, 101, 9127–9132. [Google Scholar] [CrossRef] [PubMed]
- Murray, M. Microsomal cytochrome P450-dependent steroid metabolism in male sheep liver. Quantitative importance of 6β-hydroxylation and evidence for the involvement of a P450 from the IIIA subfamily in the pathway. J. Steroid Biochem. Mol. Biol. 1991, 38, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Murray, M. Participation of a cytochrome P450 enzyme from the 2C subfamily in progesterone 21-hydroxylation in sheep liver. J. Steroid Biochem. Mol. Biol. 1992, 43, 591–593. [Google Scholar] [CrossRef]
- Cheng, Z.; Guo, S.; Copps, K.; Dong, X.; Kollipara, R.; Rodgers, J.T.; Depinho, R.A.; Puigserver, P.; White, M.F. Foxo1 integrates insulin signaling with mitochondrial function in the liver. Nat. Med. 2009, 15, 1307–1311. [Google Scholar] [CrossRef]
- Solares, I.; Izquierdo-Sanchez, L.; Morales-Conejo, M.; Jerico, D.; Castelbon, F.J.; Cordoba, K.M.; Sampedro, A.; Lumbreras, C.; Moreno-Aliaga, M.J.; Enriquez de Salamanca, R.; et al. High Prevalence of Insulin Resistance in Asymptomatic Patients with Acute Intermittent Porphyria and Liver-Targeted Insulin as a Novel Therapeutic Approach. Biomedicines 2021, 9, 255. [Google Scholar] [CrossRef]
- Sonin, N.V.; Garcia-Pagan, J.C.; Nakanishi, K.; Zhang, J.X.; Clemens, M.G. Patterns of vasoregulatory gene expression in the liver response to ischemia/reperfusion and endotoxemia. Shock 1999, 11, 175–179. [Google Scholar] [CrossRef]
- Suzuki, T.; Takahashi, T.; Yamasaki, A.; Fujiwara, T.; Hirakawa, M.; Akagi, R. Tissue-specific gene expression of heme oxygenase-1 (HO-1) and non-specific δ-aminolevulinate synthase (ALAS-N) in a rat model of septic multiple organ dysfunction syndrome. Biochem. Pharmacol. 2000, 60, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, S.; Nakamura, T.; Kataoka, T.; Taketani, S. Egr-1 regulates the transcriptional repression of mouse δ-aminolevulinic acid synthase 1 by heme. Gene 2011, 472, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Kolluri, S.; Sadlon, T.J.; May, B.K.; Bonkovsky, H.L. Haem repression of the housekeeping 5-aminolaevulinic acid synthase gene in the hepatoma cell line LMH. Biochem. J. 2005, 392, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Nomura, K.; Katoh, Y.; Yamashita, R.; Kaneko, K.; Furuyama, K. Novel Mechanisms for Heme-dependent Degradation of ALAS1 Protein as a Component of Negative Feedback Regulation of Heme Biosynthesis. J. Biol. Chem. 2016, 291, 20516–20529. [Google Scholar] [CrossRef] [PubMed]
- Lathrop, J.T.; Timko, M.P. Regulation by heme of mitochondrial protein transport through a conserved amino acid motif. Science 1993, 259, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Munakata, H.; Sun, J.Y.; Yoshida, K.; Nakatani, T.; Honda, E.; Hayakawa, S.; Furuyama, K.; Hayashi, N. Role of the heme regulatory motif in the heme-mediated inhibition of mitochondrial import of 5-aminolevulinate synthase. J. Biochem. 2004, 136, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Dowman, J.K.; Gunson, B.K.; Bramhall, S.; Badminton, M.N.; Newsome, P.N. Liver transplantation from donors with acute intermittent porphyria. Ann. Intern. Med. 2011, 154, 571–572. [Google Scholar] [CrossRef]
- Wang, B. The acute hepatic porphyrias. Transl. Gastroenterol. Hepatol. 2021, 6, 24. [Google Scholar] [CrossRef]
- Kazamel, M.; Pischik, E.; Desnick, R.J. Pain in acute hepatic porphyrias: Updates on pathophysiology and management. Front. Neurol. 2022, 13, 1004125. [Google Scholar] [CrossRef]
- Bonkovsky, H.L.; Healey, J.F.; Lourie, A.N.; Gerron, G.G. Intravenous heme-albumin in acute intermittent porphyria: Evidence for repletion of hepatic hemoproteins and regulatory heme pools. Am. J. Gastroenterol. 1991, 86, 1050–1056. [Google Scholar]
- Bonkowsky, H.L.; Tschudy, D.P.; Collins, A.; Doherty, J.; Bossenmaier, I.; Cardinal, R.; Watson, C.J. Repression of the overproduction of porphyrin precursors in acute intermittent porphyria by intravenous infusions of hematin. Proc. Natl. Acad. Sci. USA 1971, 68, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Balwani, M.; Sardh, E.; Ventura, P.; Peiro, P.A.; Rees, D.C.; Stolzel, U.; Bissell, D.M.; Bonkovsky, H.L.; Windyga, J.; Anderson, K.E.; et al. Phase 3 Trial of RNAi Therapeutic Givosiran for Acute Intermittent Porphyria. N. Engl. J. Med. 2020, 382, 2289–2301. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Liebow, A.; Yasuda, M.; Gan, L.; Racie, T.; Maier, M.; Kuchimanchi, S.; Foster, D.; Milstein, S.; Charisse, K.; et al. Preclinical Development of a Subcutaneous ALAS1 RNAi Therapeutic for Treatment of Hepatic Porphyrias Using Circulating RNA Quantification. Mol. Ther. Nucleic Acids 2015, 4, e263. [Google Scholar] [CrossRef] [PubMed]
- Sardh, E.; Harper, P.; Balwani, M.; Stein, P.; Rees, D.; Bissell, D.M.; Desnick, R.; Parker, C.; Phillips, J.; Bonkovsky, H.L.; et al. Phase 1 Trial of an RNA Interference Therapy for Acute Intermittent Porphyria. N. Engl. J. Med. 2019, 380, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Gan, L.; Chen, B.; Kadirvel, S.; Yu, C.; Phillips, J.D.; New, M.I.; Liebow, A.; Fitzgerald, K.; Querbes, W.; et al. RNAi-mediated silencing of hepatic Alas1 effectively prevents and treats the induced acute attacks in acute intermittent porphyria mice. Proc. Natl. Acad. Sci. USA 2014, 111, 7777–7782. [Google Scholar] [CrossRef] [PubMed]
- Baccino, E.; Lan Cheong Wah, L.S.; Bressollette, L.; Mottier, D. Cimetidine in the treatment of acute intermittent porphyria. JAMA 1989, 262, 3000. [Google Scholar] [CrossRef] [PubMed]
- Cherem, J.H.; Malagon, J.; Nellen, H. Cimetidine and acute intermittent porphyria. Ann. Intern. Med. 2005, 143, 694–695. [Google Scholar] [CrossRef]
- Horie, Y.; Norimoto, M.; Tajima, F.; Sasaki, H.; Nanba, E.; Kawasaki, H. Clinical usefulness of cimetidine treatment for acute relapse in intermittent porphyria. Clin. Chim. Acta 1995, 234, 171–175. [Google Scholar] [CrossRef]
- Horie, Y.; Udagawa, M.; Hirayama, C. Clinical usefulness of cimetidine for the treatment of acute intermittent porphyria--a preliminary report. Clin. Chim. Acta 1987, 167, 267–271. [Google Scholar] [CrossRef]
- Marcus, D.L.; Halbrecht, J.L.; Bourque, A.L.; Lew, G.; Nadel, H.; Freedman, M.L. Effect of cimetidine on δ-aminolevulinic acid synthase and microsomal heme oxygenase in rat liver. Biochem. Pharmacol. 1984, 33, 2005–2008. [Google Scholar] [CrossRef]
- Marcus, D.L.; Nadel, H.; Lew, G.; Freedman, M.L. Cimetidine suppresses chemically induced experimental hepatic porphyria. Am. J. Med. Sci. 1990, 300, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, R.L.; Martini, R.; Baumgartner, M.; Erne, B.; Borg, J.; Zielasek, J.; Ricker, K.; Steck, A.; Toyka, K.V.; Meyer, U.A. Motor neuropathy in porphobilinogen deaminase-deficient mice imitates the peripheral neuropathy of human acute porphyria. J. Clin. Investig. 1999, 103, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, R.L.; Porcher, C.; Grandchamp, B.; Ledermann, B.; Burki, K.; Brandner, S.; Aguzzi, A.; Meyer, U.A. Porphobilinogen deaminase deficiency in mice causes a neuropathy resembling that of human hepatic porphyria. Nat. Genet. 1996, 12, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Bishop, D.F.; Fowkes, M.; Cheng, S.H.; Gan, L.; Desnick, R.J. AAV8-mediated gene therapy prevents induced biochemical attacks of acute intermittent porphyria and improves neuromotor function. Mol. Ther. 2010, 18, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Heerfordt, I.M.; Lerche, C.M.; Wulf, H.C. Cimetidine for erythropoietic protoporphyria. Photodiagn. Photodyn. Ther. 2022, 38, 102793. [Google Scholar] [CrossRef]
- Kiberd, J.; Finlayson, L. Delayed photosensitivity in a child with erythropoietic protoporphyria: A case report. SAGE Open Med. Case Rep. 2018, 6, 2050313X18772125. [Google Scholar] [CrossRef]
- Tu, J.H.; Sheu, S.L.; Teng, J.M. Novel Treatment Using Cimetidine for Erythropoietic Protoporphyria in Children. JAMA Dermatol. 2016, 152, 1258–1261. [Google Scholar] [CrossRef]
- Yasuda, M.; Gan, L.; Chen, B.; Yu, C.; Zhang, J.; Gama-Sosa, M.A.; Pollak, D.D.; Berger, S.; Phillips, J.D.; Edelmann, W.; et al. Homozygous hydroxymethylbilane synthase knockout mice provide pathogenic insights into the severe neurological impairments present in human homiozygous dominant acute intermittent porphyria. Hum. Mol. Genet. 2019, 28, 1755–1767. [Google Scholar] [CrossRef]
- Bergonia, H.A.; Franklin, M.R.; Kushner, J.P.; Phillips, J.D. A method for determining delta-aminolevulinic acid synthase activity in homogenized cells and tissues. Clin. Biochem. 2015, 48, 788–795. [Google Scholar] [CrossRef]
- Tenhunen, R.; Marver, H.S.; Schmid, R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc. Natl. Acad. Sci. USA 1968, 61, 748–755. [Google Scholar] [CrossRef]
- Converso, D.P.; Taille, C.; Carreras, M.C.; Jaitovich, A.; Poderoso, J.J.; Boczkowski, J. HO-1 is located in liver mitochondria and modulates mitochondrial heme content and metabolism. FASEB J. 2006, 20, 1236–1238. [Google Scholar] [CrossRef]
- Whitby, F.G.; Phillips, J.D.; Kushner, J.P.; Hill, C.P. Crystal structure of human uroporphyrinogen decarboxylase. EMBO J. 1998, 17, 2463–2471. [Google Scholar] [CrossRef]
- Phillips, J.D.; Kushner, J.P.; Whitby, F.G.; Hill, C.P. Characterization and crystallization of human uroporphyrinogen decarboxylase. Protein Sci. 1997, 6, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Sheftel, A.D.; Kim, S.F.; Ponka, P. Non-heme induction of heme oxygenase-1 does not alter cellular iron metabolism. J. Biol. Chem. 2007, 282, 10480–10486. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fujita, Y.; Sato-Matsumura, K.C. Effective treatment for porphyria cutanea tarda with oral cimetidine. J. Dermatol. 2010, 37, 677–679. [Google Scholar] [CrossRef] [PubMed]
- Horie, Y.; Tanaka, K.; Okano, J.; Ohgi, N.; Kawasaki, H.; Yamamoto, S.; Kondo, M.; Sassa, S. Cimetidine in the treatment of porphyria cutanea tarda. Intern. Med. 1996, 35, 717–719. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Granata, F.; Duca, L.; Graziadei, G.; Brancaleoni, V.; Missineo, P.; De Luca, G.; Fustinoni, S.; Di Pierro, E. Inflammatory involvement into phototoxic reaction in erythropoietic protoporphyria (EPP) patients. Immunol. Res. 2019, 67, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.W. Mechanisms of phototoxicity in porphyria cutanea tarda and erythropoietic protoporphyria. Immunol. Ser. 1989, 46, 671–685. [Google Scholar]
- Tanaka, T.; Kochi, T.; Shirakami, Y.; Mori, T.; Kurata, A.; Watanabe, N.; Moriwaki, H.; Shimizu, M. Cimetidine and Clobenpropit Attenuate Inflammation-Associated Colorectal Carcinogenesis in Male ICR Mice. Cancers 2016, 8, 25. [Google Scholar] [CrossRef]
- Chiabrando, D.; Mercurio, S.; Tolosano, E. Heme and erythropoieis: More than a structural role. Haematologica 2014, 99, 973–983. [Google Scholar] [CrossRef]
- Kramer, M.F.; Gunaratne, P.; Ferreira, G.C. Transcriptional regulation of the murine erythroid-specific 5-aminolevulinate synthase gene. Gene 2000, 247, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.D. Heme biosynthesis and the porphyrias. Mol. Genet. Metab. 2019, 128, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Pawliuk, R.; Tighe, R.; Wise, R.J.; Mathews-Roth, M.M.; Leboulch, P. Prevention of murine erythropoietic protoporphyria-associated skin photosensitivity and liver disease by dermal and hepatic ferrochelatase. J. Investig. Dermatol. 2005, 124, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G.A.; Ferreira, G.C. An Extended C-Terminus, the Possible Culprit for Differential Regulation of 5-Aminolevulinate Synthase Isoforms. Front. Mol. Biosci. 2022, 9, 920668. [Google Scholar] [CrossRef]

| Cimetidine Concentration (μmoles/L) | PLP Concentration (μmoles/L) | ALAS Activity (Mean ± SD, pmol/h) |
|---|---|---|
| 0 | 0 | 94 ± 6 |
| 0.06 | 0 | 102 ± 4 |
| 0.2 | 0 | 96 ± 5 |
| 0.6 | 0 | 101 ± 2 |
| 2 | 0 | 100 ± 10 |
| 6 | 0 | 104 ± 9 |
| 20 | 0 | 95 ± 5 |
| 0 | 20 | 170 ± 12 |
| 0.2 | 20 | 176 ± 15 |
| 0.6 | 20 | 170 ± 9 |
| 2 | 20 | 166 ± 3 |
| 6 | 20 | 164 ± 16 |
| 20 | 20 | 152 ± 18 |
| 60 | 20 | 154 ± 13 |
| 0 | 80 | 163 ± 8 |
| 0.8 | 80 | 155 ± 3 |
| 2.4 | 80 | 170 ± 5 |
| 8 | 80 | 165 ± 7 |
| 24 | 80 | 161 ± 0.3 |
| 80 | 80 | 158 ± 9 |
| 240 | 80 | 157 ± 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasuda, M.; Lee, S.; Gan, L.; Bergonia, H.A.; Desnick, R.J.; Phillips, J.D. Cimetidine Does Not Inhibit 5-Aminolevulinic Acid Synthase or Heme Oxygenase Activity: Implications for Treatment of Acute Intermittent Porphyria and Erythropoietic Protoporphyria. Biomolecules 2024, 14, 27. https://doi.org/10.3390/biom14010027
Yasuda M, Lee S, Gan L, Bergonia HA, Desnick RJ, Phillips JD. Cimetidine Does Not Inhibit 5-Aminolevulinic Acid Synthase or Heme Oxygenase Activity: Implications for Treatment of Acute Intermittent Porphyria and Erythropoietic Protoporphyria. Biomolecules. 2024; 14(1):27. https://doi.org/10.3390/biom14010027
Chicago/Turabian StyleYasuda, Makiko, Sangmi Lee, Lin Gan, Hector A. Bergonia, Robert J. Desnick, and John D. Phillips. 2024. "Cimetidine Does Not Inhibit 5-Aminolevulinic Acid Synthase or Heme Oxygenase Activity: Implications for Treatment of Acute Intermittent Porphyria and Erythropoietic Protoporphyria" Biomolecules 14, no. 1: 27. https://doi.org/10.3390/biom14010027
APA StyleYasuda, M., Lee, S., Gan, L., Bergonia, H. A., Desnick, R. J., & Phillips, J. D. (2024). Cimetidine Does Not Inhibit 5-Aminolevulinic Acid Synthase or Heme Oxygenase Activity: Implications for Treatment of Acute Intermittent Porphyria and Erythropoietic Protoporphyria. Biomolecules, 14(1), 27. https://doi.org/10.3390/biom14010027






