ROS-Mediated Fragmentation Alters the Effects of Hyaluronan on Corneal Epithelial Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Fragmentation of HMWHA to Generate oxHA
2.2. HA Molecular Weight by Agarose Gel Electrophoresis
2.3. HA Molecular Weight Measurement by Gel Filtration High-Pressure Liquid Chromatography
2.4. Structural Analysis by NMR
2.5. Corneal Epithelial Cell Culture Conditions
2.6. Cell Viability Assay
2.7. Cell Proliferation Assay
2.8. In Vitro Cell Scratch Assay
2.9. In Vivo Wound Healing and Eye Drop Application
2.10. Statistical Analysis
3. Results
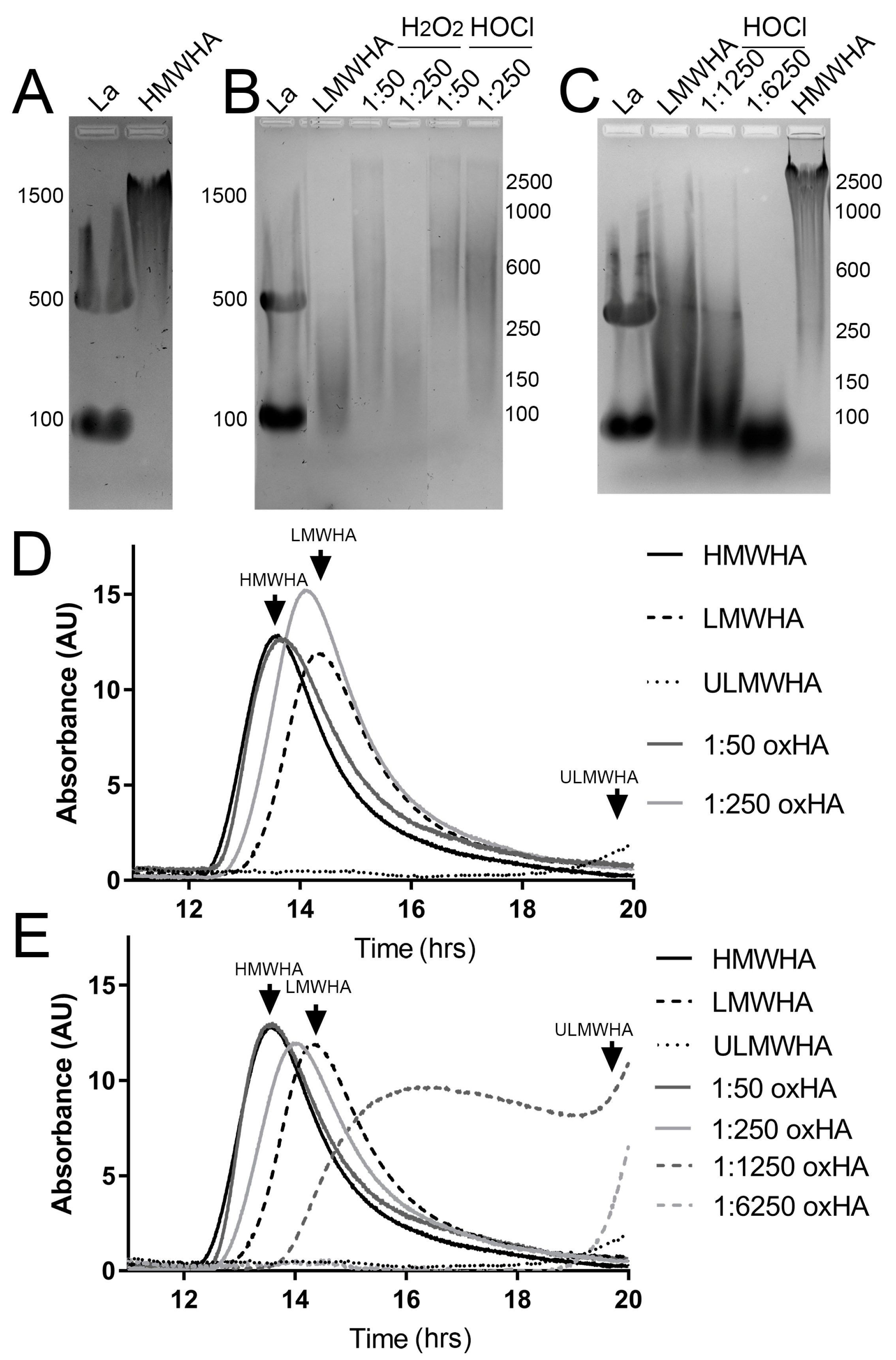
3.1. HA Fragmentation by H2O2 and HOCl
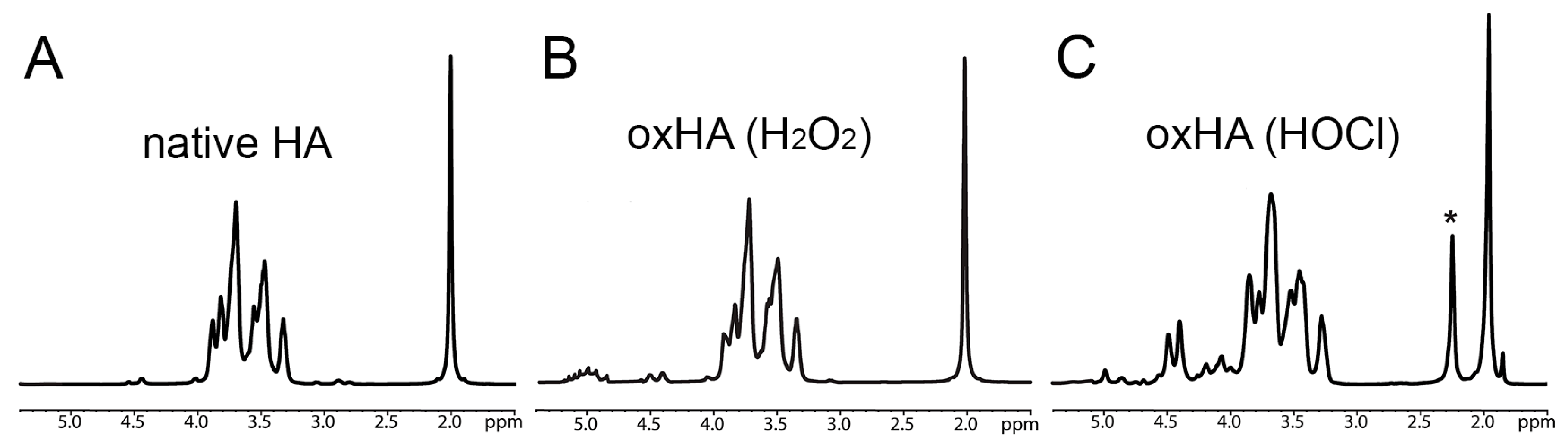
3.2. Structural Characterization of HA following Oxidation by H2O2 and HOCl
3.3. Effect of oxHA on the Viability of Corneal Epithelial Cells
3.4. Effect of oxHA on the Proliferation of Corneal Epithelial Cells
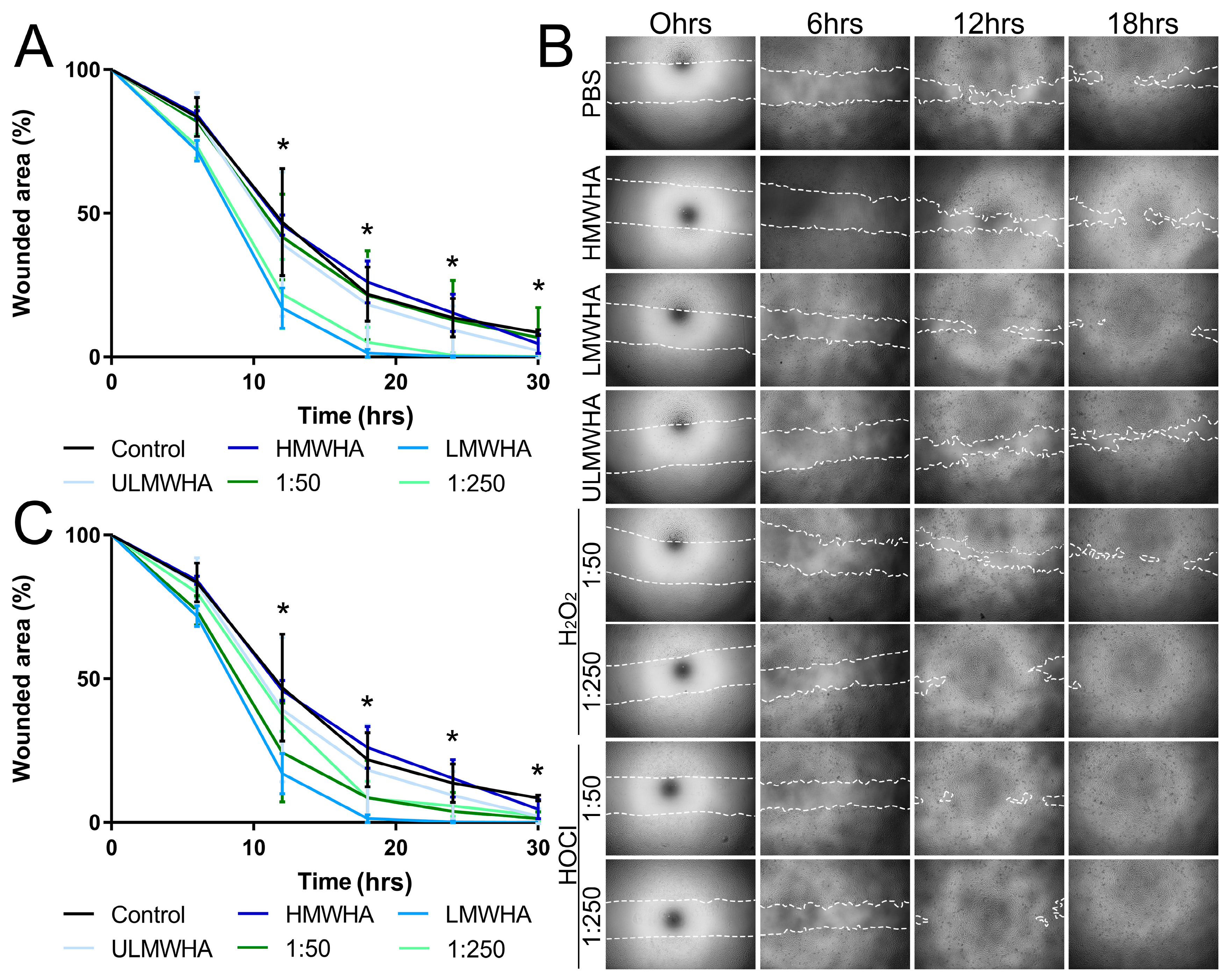
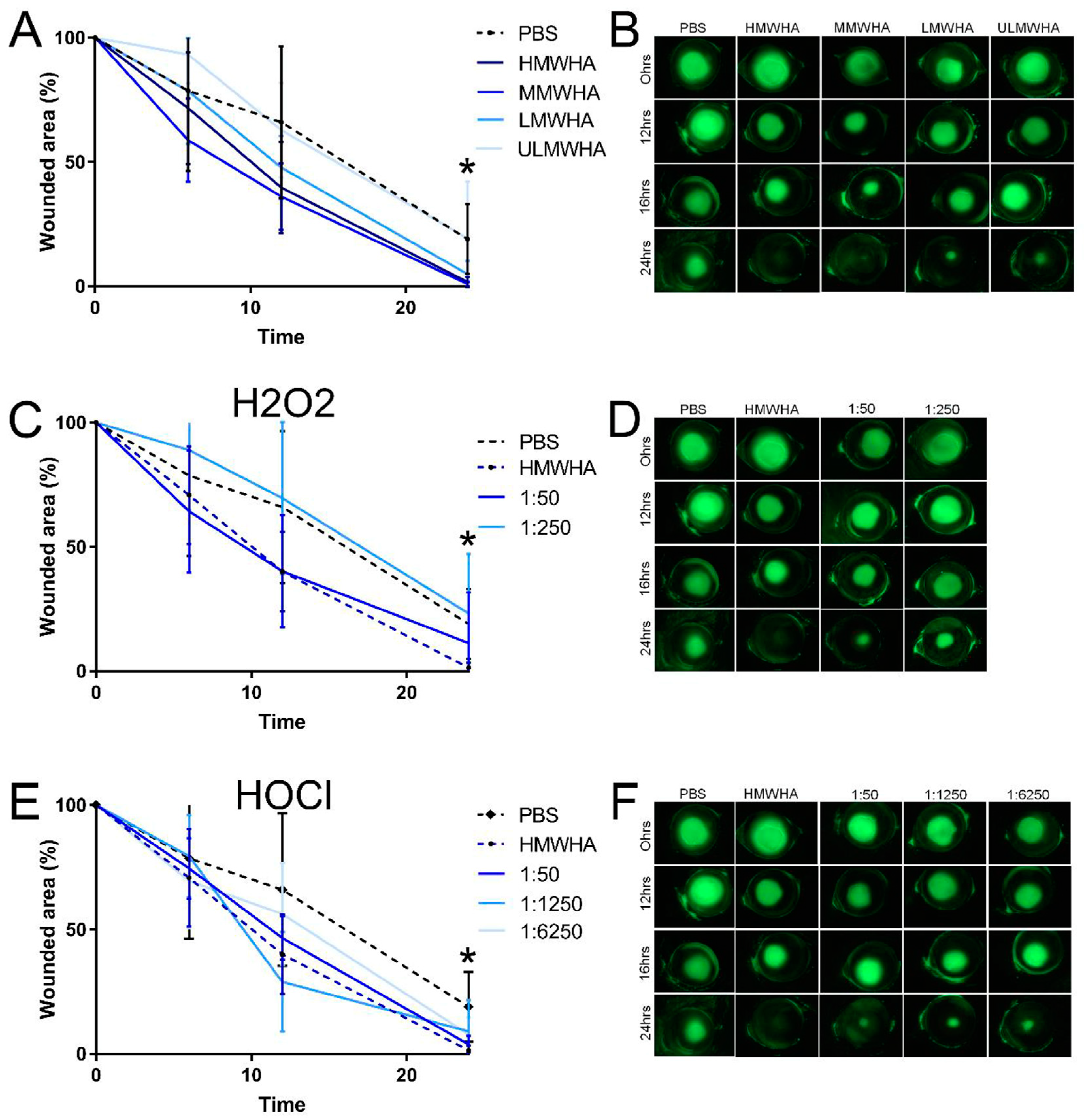
3.5. Effect of oxHA on the Migration of Corneal Epithelial Cells
3.6. Effect of oxHA on Corneal Epithelial Wound Healing In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liou, G.-Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Niethammer, P.; Grabher, C.; Look, A.T.; Mitchison, T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009, 459, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Bose, B.; Najwa, A.R.; Shenoy, P.S. Oxidative Damages to Eye Stem Cells, in Response to, Bright and Ultraviolet Light, Their Associated Mechanisms, and Salvage Pathways. Mol. Biotechnol. 2019, 61, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Duan-Arnold, Y.; Gyurdieva, A.; Johnson, A.; Jacobstein, D.A.; Danilkovitch, A. Soluble Factors Released by Endogenous Viable Cells Enhance the Antioxidant and Chemoattractive Activities of Cryopreserved Amniotic Membrane. Adv. Wound Care 2015, 4, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Von Zglinicki, T. Role of Oxidative Stress in Telomere Length Regulation and Replicative Senescence. Ann. N. Y. Acad. Sci. 2000, 908, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Williams, E.; Cadenas, E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem. J. 2001, 353, 411–416. [Google Scholar] [CrossRef]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef]
- Moori, M.; Ghafoori, H.; Sariri, R. Nonenzymatic antioxidants in saliva of patients with systemic lupus erythematosus. Lupus 2016, 25, 265–271. [Google Scholar] [CrossRef]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- van Golen, R.F.; van Gulik, T.M.; Heger, M. Mechanistic overview of reactive species-induced degradation of the endothelial glycocalyx during hepatic ischemia/reperfusion injury. Free Radic. Biol. Med. 2012, 52, 1382–1402. [Google Scholar] [CrossRef]
- Shetty, R.; Sharma, A.; Pahuja, N.; Chevour, P.; Padmajan, N.; Dhamodaran, K.; Jayadev, C.; Nuijts, R.M.M.A.; Ghosh, A.; Nallathambi, J. Oxidative stress induces dysregulated autophagy in corneal epithelium of keratoconus patients. PLoS ONE 2017, 12, e0184628. [Google Scholar] [CrossRef]
- Yari, D.; Saravani, R.; Saravani, S.; Ebrahimian, K.; Galavi, H.R. Genetic Polymorphisms of Catalase and Glutathione Peroxidase-1 in Keratoconus. Iran. J. Public. Health 2018, 47, 1567–1574. [Google Scholar] [PubMed]
- Huo, Y.; Qiu, W.Y.; Pan, Q.; Yao, Y.F.; Xing, K.; Lou, M.F. Reactive oxygen species (ROS) are essential mediators in epidermal growth factor (EGF)-stimulated corneal epithelial cell proliferation, adhesion, migration, and wound healing. Exp. Eye Res. 2009, 89, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Buddi, R.; Lin, B.; Atilano, S.R.; Zorapapel, N.C.; Kenney, M.C.; Brown, D.J. Evidence of Oxidative Stress in Human Corneal Diseases. J. Histochem. Cytochem. 2002, 50, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Deepashree, S.; Niveditha, S.; Shivanandappa, T.; Ramesh, S.R. Oxidative stress resistance as a factor in aging: Evidence from an extended longevity phenotype of Drosophila melanogaster. Biogerontology 2019, 20, 497–513. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Atilano, S.R.; Chwa, M.; Kim, D.W.; Jordan, N.; Udar, N.; Coskun, P.; Jester, J.V.; Wallace, D.C.; Kenney, M.C. Hydrogen peroxide causes mitochondrial DNA damage in corneal epithelial cells. Cornea 2009, 28, 426–433. [Google Scholar] [CrossRef]
- Zhang, N.; Li, G.; Li, S.; Cai, C.; Zhang, F.; Linhardt, R.J.; Yu, G. Mass spectrometric evidence for the mechanism of free-radical depolymerization of various types of glycosaminoglycans. Carbohydr. Polym. 2020, 233, 115847. [Google Scholar] [CrossRef]
- Rees, M.D.; Hawkins, C.L.; Davies, M.J. Hypochlorite-mediated fragmentation of hyaluronan, chondroitin sulfates, and related N-acetyl glycosamines: Evidence for chloramide intermediates, free radical transfer reactions, and site-specific fragmentation. J. Am. Chem. Soc. 2003, 125, 13719–13733. [Google Scholar] [CrossRef] [PubMed]
- Rees, M.D.; Hawkins, C.L.; Davies, M.J. Hypochlorite and superoxide radicals can act synergistically to induce fragmentation of hyaluronan and chondroitin sulphates. Biochem. J. 2004, 381, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Panasenko, O.M.; Gorudko, I.V.; Sokolov, A.V. Hypochlorous acid as a precursor of free radicals in living systems. Biochemistry 2013, 78, 1466–1489. [Google Scholar] [CrossRef] [PubMed]
- Rychlý, J.; Šoltés, L.; Stankovská, M.; Janigová, I.; Csomorová, K.; Sasinková, V.; Kogan, G.; Gemeiner, P. Unexplored capabilities of chemiluminescence and thermoanalytical methods in characterization of intact and degraded hyaluronans. Polym. Degrad. Stab. 2006, 91, 3174–3184. [Google Scholar] [CrossRef]
- Moseley, R.; Waddington, R.J.; Embery, G. Degradation of glycosaminoglycans by reactive oxygen species derived from stimulated polymorphonuclear leukocytes. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 1997, 1362, 221–231. [Google Scholar] [CrossRef]
- Laurent, T.C.; Fraser, J.R. The properties and turnover of hyaluronan. Ciba Found. Symp. 1986, 124, 9–29. [Google Scholar] [CrossRef]
- Itano, N.; Sawai, T.; Yoshida, M.; Lenas, P.; Yamada, Y.; Imagawa, M.; Shinomura, T.; Hamaguchi, M.; Yoshida, Y.; Ohnuki, Y.; et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J. Biol. Chem. 1999, 274, 25085–25092. [Google Scholar] [CrossRef]
- Temple-Wong, M.M.; Ren, S.; Quach, P.; Hansen, B.C.; Chen, A.C.; Hasegawa, A.; D’Lima, D.D.; Koziol, J.; Masuda, K.; Lotz, M.K.; et al. Hyaluronan concentration and size distribution in human knee synovial fluid: Variations with age and cartilage degeneration. Arthritis Res. Ther. 2016, 18, 18. [Google Scholar] [CrossRef]
- Puri, S.; Coulson-Thomas, Y.M.; Gesteira, T.F.; Coulson-Thomas, V.J. Distribution and Function of Glycosaminoglycans and Proteoglycans in the Development, Homeostasis and Pathology of the Ocular Surface. Front. Cell Dev. Biol. 2020, 8, 731. [Google Scholar] [CrossRef]
- Gesteira, T.F.; Sun, M.; Coulson-Thomas, Y.M.; Yamaguchi, Y.; Yeh, L.K.; Hascall, V.; Coulson-Thomas, V.J. Hyaluronan Rich Microenvironment in the Limbal Stem Cell Niche Regulates Limbal Stem Cell Differentiation. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4407–4421. [Google Scholar] [CrossRef]
- Sun, M.; Puri, S.; Parfitt, G.J.; Mutoji, N.; Coulson-Thomas, V.J. Hyaluronan Regulates Eyelid and Meibomian Gland Morphogenesis. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3713–3727. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Puri, S.; Mutoji, K.N.; Coulson-Thomas, Y.M.; Hascall, V.C.; Jackson, D.G.; Gesteira, T.F.; Coulson-Thomas, V.J. Hyaluronan Derived from the Limbus is a Key Regulator of Corneal Lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- Mutoji, K.N.; Sun, M.; Elliott, G.; Moreno, I.Y.; Hughes, C.; Gesteira, T.F.; Coulson-Thomas, V.J. Extracellular Matrix Deposition and Remodeling after Corneal Alkali Burn in Mice. Int. J. Mol. Sci. 2021, 22, 5708. [Google Scholar] [CrossRef] [PubMed]
- Ooki, T.; Murata-Kamiya, N.; Takahashi-Kanemitsu, A.; Wu, W.; Hatakeyama, M. High-Molecular-Weight Hyaluronan Is a Hippo Pathway Ligand Directing Cell Density-Dependent Growth Inhibition via PAR1b. Dev. Cell 2019, 49, 590–604.e599. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Mekonnen, T.; Verma, S.; Zevallos-Delgado, C.; Singh, M.; Aglyamov, S.R.; Gesteira, T.F.; Larin, K.V.; Coulson-Thomas, V.J. Hyaluronan Modulates the Biomechanical Properties of the Cornea. Investig. Ophthalmol. Vis. Sci. 2022, 63, 6. [Google Scholar] [CrossRef]
- Cyphert, J.M.; Trempus, C.S.; Garantziotis, S. Size Matters: Molecular Weight Specificity of Hyaluronan Effects in Cell Biology. Int. J. Cell Biol. 2015, 2015, 563818. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, A.; Mahadevan, A.; Lauer, M.E.; Narvaez, R.J.; Ramesh, S.; Demler, C.M.; Souchet, N.R.; Hascall, V.C.; Midura, R.J.; Garantziotis, S.; et al. Midgut Laterality Is Driven by Hyaluronan on the Right. Dev. Cell 2018, 46, 533–551.e535. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef]
- Rayahin, J.E.; Buhrman, J.S.; Zhang, Y.; Koh, T.J.; Gemeinhart, R.A. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater. Sci. Eng. 2015, 1, 481–493. [Google Scholar] [CrossRef]
- Moseley, R.; Walker, M.; Waddington, R.J.; Chen, W.Y. Comparison of the antioxidant properties of wound dressing materials--carboxymethylcellulose, hyaluronan benzyl ester and hyaluronan, towards polymorphonuclear leukocyte-derived reactive oxygen species. Biomaterials 2003, 24, 1549–1557. [Google Scholar] [CrossRef]
- Ke, C.; Sun, L.; Qiao, D.; Wang, D.; Zeng, X. Antioxidant acitivity of low molecular weight hyaluronic acid. Food Chem. Toxicol. 2011, 49, 2670–2675. [Google Scholar] [CrossRef] [PubMed]
- Pauloin, T.; Dutot, M.; Warnet, J.M.; Rat, P. In vitro modulation of preservative toxicity: High molecular weight hyaluronan decreases apoptosis and oxidative stress induced by benzalkonium chloride. Eur. J. Pharm. Sci. 2008, 34, 263–273. [Google Scholar] [CrossRef]
- Pauloin, T.; Dutot, M.; Joly, F.; Warnet, J.M.; Rat, P. High molecular weight hyaluronan decreases UVB-induced apoptosis and inflammation in human epithelial corneal cells. Mol. Vis. 2009, 15, 577–583. [Google Scholar]
- Song, W.; Lee, B.H.; Tan, L.P.; Li, H. Cardiovascular engineering materials in translational medicine. In Biomaterials in Translational Medicine; Yang, L., Bhaduri, S.B., Webster, T.J., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 57–91. [Google Scholar]
- Calabro, A.; Benavides, M.; Tammi, M.; Hascall, V.C.; Midura, R.J. Microanalysis of enzyme digests of hyaluronan and chondroitin/dermatan sulfate by fluorophore-assisted carbohydrate electrophoresis (FACE). Glycobiology 2000, 10, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Andley, U.P.; Chakrabarti, B. Role of singlet oxygen in the degradation of hyaluronic acid. Biochem. Biophys. Res. Commun. 1983, 115, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Osterholt, H.C.; Dannevig, I.; Wyckoff, M.H.; Liao, J.; Akgul, Y.; Ramgopal, M.; Mija, D.S.; Cheong, N.; Longoria, C.; Mahendroo, M.; et al. Antioxidant protects against increases in low molecular weight hyaluronan and inflammation in asphyxiated newborn pigs resuscitated with 100% oxygen. PLoS ONE 2012, 7, e38839. [Google Scholar] [CrossRef]
- Frati, E.; Khatib, A.M.; Front, P.; Panasyuk, A.; Aprile, F.; Mitrovic, D.R. Degradation of hyaluronic acid by photosensitized riboflavin in vitro. Modulation of the effect by transition metals, radical quenchers, and metal chelators. Free Radic. Biol. Med. 1997, 22, 1139–1144. [Google Scholar] [CrossRef]
- Ruhela, D.; Riviere, K.; Szoka, F.C. Efficient Synthesis of an Aldehyde Functionalized Hyaluronic Acid and Its Application in the Preparation of Hyaluronan−Lipid Conjugates. Bioconjugate Chem. 2006, 17, 1360–1363. [Google Scholar] [CrossRef]
- Soltes, L.; Brezova, V.; Stankovska, M.; Kogan, G.; Gemeiner, P. Degradation of high-molecular-weight hyaluronan by hydrogen peroxide in the presence of cupric ions. Carbohydr. Res. 2006, 341, 639–644. [Google Scholar] [CrossRef]
- Channa, R.; Zafar, S.N.; Canner, J.K.; Haring, R.S.; Schneider, E.B.; Friedman, D.S. Epidemiology of Eye-Related Emergency Department Visits. JAMA Ophthalmol. 2016, 134, 312–319. [Google Scholar] [CrossRef]
- Jeng, B.H. Abrasions, Planned Defects, and Persistent Epithelial Defects in Corneal Epithelial Wound Healing. JAMA Ophthalmol. 2016, 134, 1176–1177. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Wang, H.; Pan, X.; Zhang, C.; Zhang, K.; Chen, Z.; Dong, W.; Xie, A.; Qi, X. Dendritic Hydrogels with Robust Inherent Antibacterial Properties for Promoting Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 11144–11155. [Google Scholar] [CrossRef] [PubMed]
- Chanock, S.J.; el Benna, J.; Smith, R.M.; Babior, B.M. The respiratory burst oxidase. J. Biol. Chem. 1994, 269, 24519–24522. [Google Scholar] [CrossRef] [PubMed]
- Colavitti, R.; Pani, G.; Bedogni, B.; Anzevino, R.; Borrello, S.; Waltenberger, J.; Galeotti, T. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J. Biol. Chem. 2002, 277, 3101–3108. [Google Scholar] [CrossRef]
- Sundaresan, M.; Yu, Z.X.; Ferrans, V.J.; Irani, K.; Finkel, T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 1995, 270, 296–299. [Google Scholar] [CrossRef]
- Buffa, R.; Hermannová, M.; Sojka, M.; Svozil, V.; Šulc, P.; Halamková, P.; Pospíšilová, M.; Krejčí, H.; Velebný, V. Hyaluronic acid chloramide—Synthesis, chemical structure, stability and analysis of antimicrobials. Carbohydr. Polym. 2020, 250, 116928. [Google Scholar] [CrossRef]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Davies, M.J. Degradation of Hyaluronic Acid, Poly- and Mono-Saccharides, and Model Compounds by Hypochlorite: Evidence for Radical Intermediates and Fragmentation. Free Radic. Biol. Med. 1998, 24, 1396–1410. [Google Scholar] [CrossRef]
- Lee, E.; Miki, Y.; Katsura, H.; Kariya, K. Mechanism of inactivation of myeloperoxidase by propylthiouracil. Biochem. Pharmacol. 1990, 39, 1467–1471. [Google Scholar] [CrossRef]
- Speth, C.; Brodde, M.F.; Hagleitner, M.; Rambach, G.; Van Aken, H.; Dierich, M.; Kehrel, B.E. Neutrophils Turn Plasma Proteins into Weapons against HIV-1. PLoS ONE 2013, 8, e66073. [Google Scholar] [CrossRef]
- Cowman, M.K. Methods for Hyaluronan Molecular Mass Determination by Agarose Gel Electrophoresis. Methods Mol. Biol. 2019, 1952, 91–102. [Google Scholar] [CrossRef]
- Lee, H.G.; Cowman, M.K. An agarose gel electrophoretic method for analysis of hyaluronan molecular weight distribution. Anal. Biochem. 1994, 219, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Lin, X.; Verma, S.; Puri, S.; Hascall, V.; Gesteira, T.F.; Coulson-Thomas, V.J. Characterization of the molecular weight of hyaluronan in eye products using a novel method of size exclusion high-pressure liquid chromatography. Transl. Vis. Sci. Technol. 2023, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.M.; Li, L.; Fisher, S.; Pearce, V.P.; Shay, J.W.; Wright, W.E.; Cavanagh, H.D.; Jester, J.V. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Investig. Ophthalmol. Vis. Sci. 2005, 46, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, O.; Yuan, Y.; Coulson-Thomas, V.J.; Gesteira, T.F.; Call, M.K.; Zhang, Y.; Zhang, J.; Chang, S.-H.; Xie, C.; Liu, C.-Y.; et al. Lumican binds ALK5 to promote epithelium wound healing. PLoS ONE 2013, 8, e82730. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]
- Puri, S.; Sun, M.; Mutoji, K.N.; Gesteira, T.F.; Coulson-Thomas, V.J. Epithelial Cell Migration and Proliferation Patterns during Initial Wound Closure in Normal Mice and an Experimental Model of Limbal Stem Cell Deficiency. Investig. Ophthalmol. Vis. Sci. 2020, 61, 27. [Google Scholar] [CrossRef]
- Hawkins, L.; Davies, J. EPR studies on the selectivity of hydroxyl radical attack on amino acids and peptides. J. Chem. Soc. Perkin Trans. 1998, 2, 2617–2622. [Google Scholar] [CrossRef]
- Qu, C.; Rilla, K.; Tammi, R.; Tammi, M.; Kroger, H.; Lammi, M.J. Extensive CD44-dependent hyaluronan coats on human bone marrow-derived mesenchymal stem cells produced by hyaluronan synthases HAS1, HAS2 and HAS3. Int. J. Biochem. Cell Biol. 2014, 48, 45–54. [Google Scholar] [CrossRef]
- Coulson-Thomas, V.J.; Gesteira, T.F.; Hascall, V.; Kao, W. Umbilical cord mesenchymal stem cells suppress host rejection: The role of the glycocalyx. J. Biol. Chem. 2014, 289, 23465–23481. [Google Scholar] [CrossRef]
- Simoni, R.D.; Hill, R.L.; Vaughan, M.; Hascall, V. The Discovery of Hyaluronan by Karl Meyer. J. Biol. Chem. 2002, 277, e1–e2. [Google Scholar] [CrossRef]
- Zhou, J.; Ge, L.; Jia, C.; Zheng, X.; Cui, H.; Zong, R.; Bao, X.; Yin, Y.; Ma, J.X.; Li, W.; et al. ROS-mediated Different Homeostasis of Murine Corneal Epithelial Progenitor Cell Line under Oxidative Stress. Sci. Rep. 2016, 6, 36481. [Google Scholar] [CrossRef] [PubMed]
- Krasinski, R.; Tchorzewski, H.; Lewkowicz, P. Antioxidant effect of hyaluronan on polymorphonuclear leukocyte-derived reactive oxygen species is dependent on its molecular weight and concentration and mainly involves the extracellular space. Adv. Hyg. Exp. Med. 2009, 63, 205–212. [Google Scholar]
- Termeer, C.C.; Hennies, J.; Voith, U.; Ahrens, T.; Weiss, J.M.; Prehm, P.; Simon, J.C. Oligosaccharides of hyaluronan are potent activators of dendritic cells. J. Immunol. 2000, 165, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Williams, J.D.; Al-Assaf, S.; Phillips, G.O.; Phillips, A.O. Hyaluronan and proximal tubular cell migration. Kidney Int. 2004, 65, 823–833. [Google Scholar] [CrossRef][Green Version]
- Tzircotis, G.; Thorne, R.F.; Isacke, C.M. Chemotaxis towards hyaluronan is dependent on CD44 expression and modulated by cell type variation in CD44-hyaluronan binding. J. Cell Sci. 2005, 118, 5119–5128. [Google Scholar] [CrossRef]
- Camillieri, G.; Bucolo, C.; Rossi, S.; Drago, F. Hyaluronan-induced stimulation of corneal wound healing is a pure pharmacological effect. J. Ocul. Pharmacol. Ther. 2004, 20, 548–553. [Google Scholar] [CrossRef]
- Sugiyama, T.; Miyauchi, S.; Machida, A.; Miyazaki, K.; Tokuyasu, K.; Nakazawa, K. The effect of sodium hyaluronate on the migration of rabbit corneal epithelium. II. The effect of topical administration. J. Ocul. Pharmacol. 1991, 7, 53–64. [Google Scholar] [CrossRef]
- Ling, K.; Bastion, M.C. Use of commercially available sodium hyaluronate 0.18% eye drops for corneal epithelial healing in diabetic patients. Int. Ophthalmol. 2019, 39, 2195–2203. [Google Scholar] [CrossRef]
- Nakamura, M.; Hikida, M.; Nakano, T. Concentration and molecular weight dependency of rabbit corneal epithelial wound healing on hyaluronan. Curr. Eye Res. 1992, 11, 981–986. [Google Scholar] [CrossRef]
- Scheuer, C.A.; Rah, M.J.; Reindel, W.T. Increased concentration of hyaluronan in tears after soaking contact lenses in Biotrue multipurpose solution. Clin. Ophthalmol. 2016, 10, 1945–1952. [Google Scholar] [CrossRef] [PubMed][Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Moreno, I.Y.; Nguyen, L.; Gesteira, T.F.; Coulson-Thomas, V.J. ROS-Mediated Fragmentation Alters the Effects of Hyaluronan on Corneal Epithelial Wound Healing. Biomolecules 2023, 13, 1385. https://doi.org/10.3390/biom13091385
Lin X, Moreno IY, Nguyen L, Gesteira TF, Coulson-Thomas VJ. ROS-Mediated Fragmentation Alters the Effects of Hyaluronan on Corneal Epithelial Wound Healing. Biomolecules. 2023; 13(9):1385. https://doi.org/10.3390/biom13091385
Chicago/Turabian StyleLin, Xiao, Isabel Y. Moreno, Lawrence Nguyen, Tarsis F. Gesteira, and Vivien J. Coulson-Thomas. 2023. "ROS-Mediated Fragmentation Alters the Effects of Hyaluronan on Corneal Epithelial Wound Healing" Biomolecules 13, no. 9: 1385. https://doi.org/10.3390/biom13091385
APA StyleLin, X., Moreno, I. Y., Nguyen, L., Gesteira, T. F., & Coulson-Thomas, V. J. (2023). ROS-Mediated Fragmentation Alters the Effects of Hyaluronan on Corneal Epithelial Wound Healing. Biomolecules, 13(9), 1385. https://doi.org/10.3390/biom13091385







