Functional Interaction between Adenosine A2A and mGlu5 Receptors Mediates STEP Phosphatase Activation and Promotes STEP/mGlu5R Binding in Mouse Hippocampus and Neuroblastoma Cell Line
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs
2.2. Animals
2.3. Preparation of Mouse Hippocampal Slices and Treatment
2.4. SH-SY5Y Cell Culture and Treatment
2.5. cAMP Measurements in SH-SY5Y Cell Culture
2.6. STEP Activity Assay on Immunoprecipitates
2.7. Co-Immunoprecipitation (Co-IP) Assay and Western Blot (WB) Analysis
2.8. Statistical Analysis
3. Results
3.1. Metabotropic mGlu5 and Adenosine A2A Receptors in the SH-SY5Y Neuroblastoma Cell Line
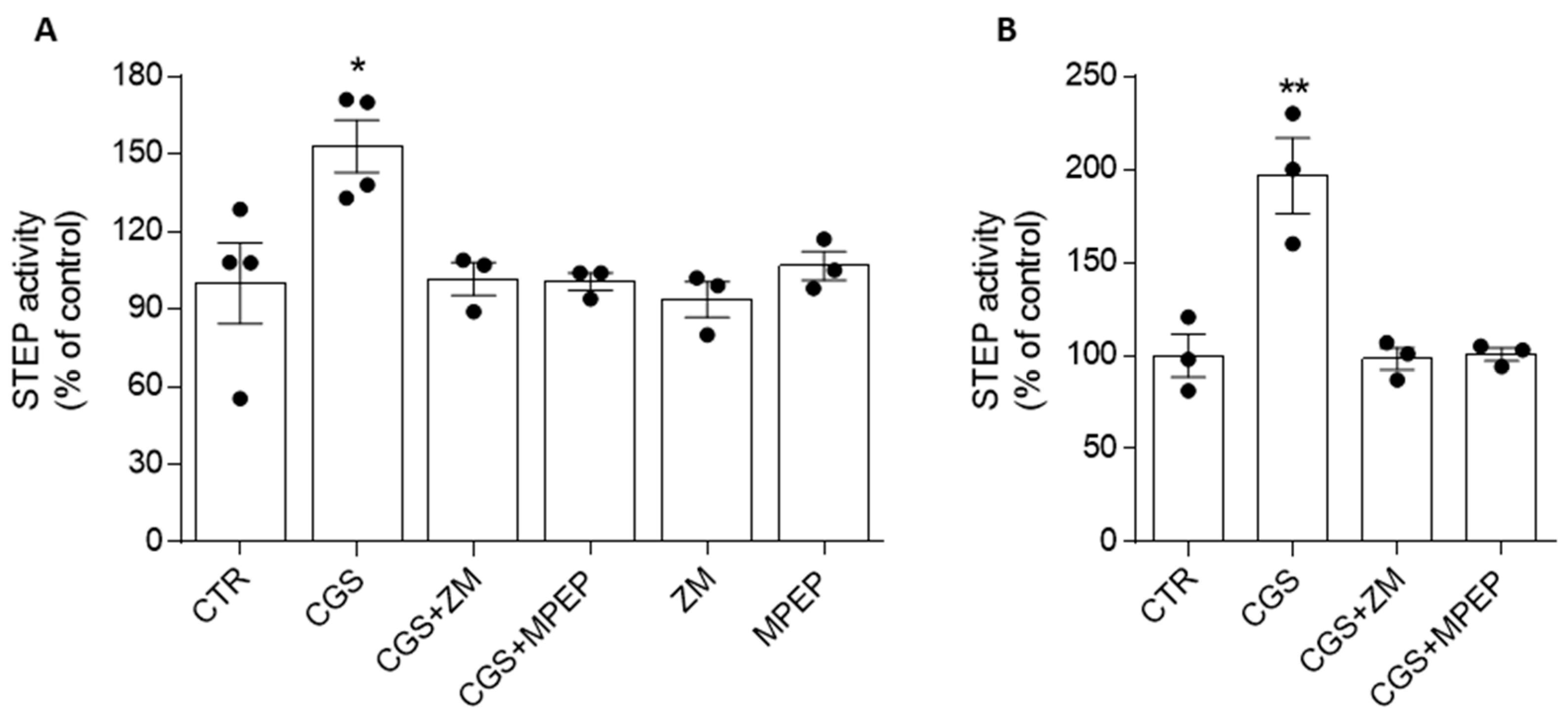
3.2. Adenosine A2ARs Modulate STEP Activity through the Involvement of mGlu5Rs
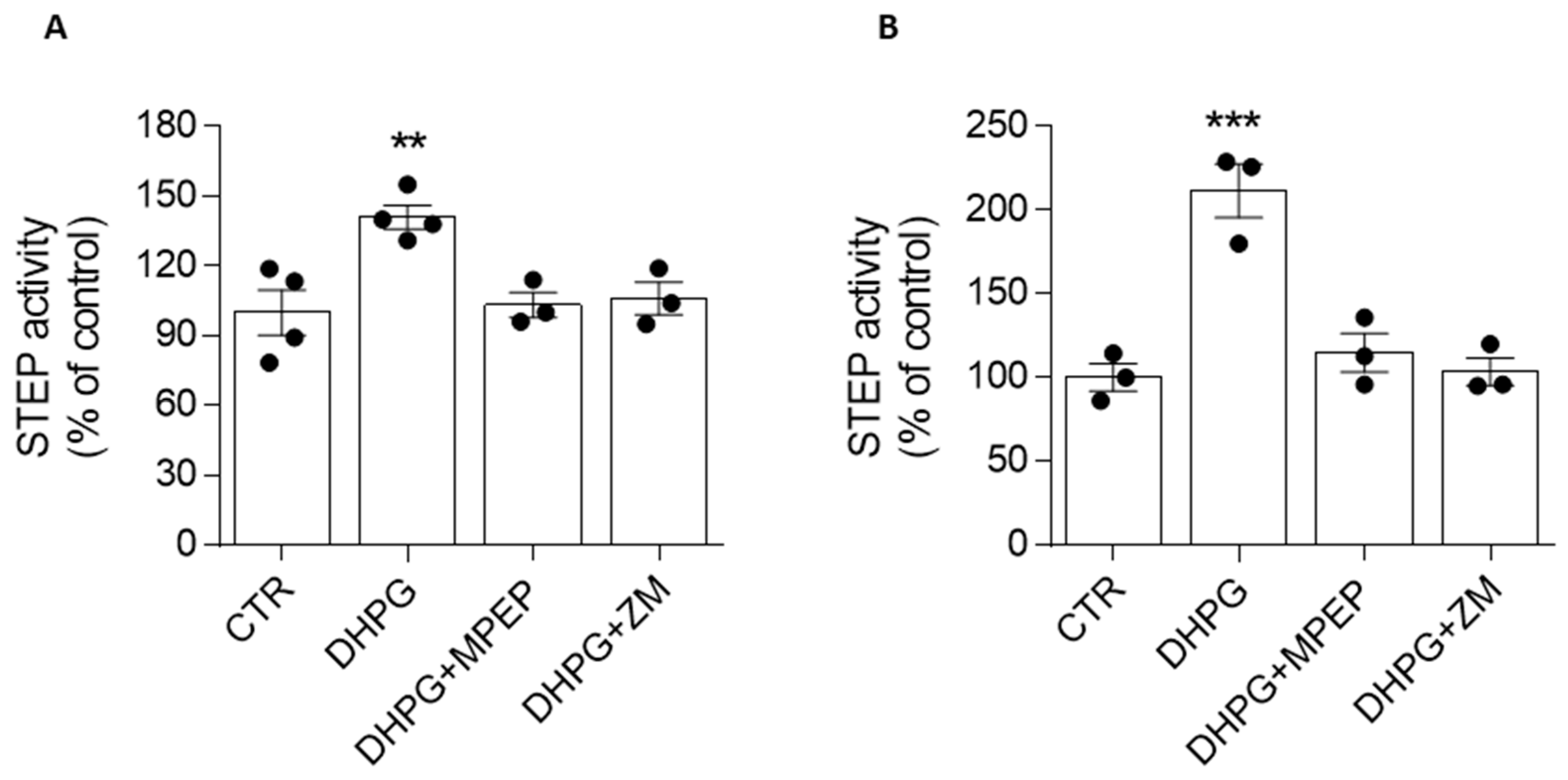
3.3. mGlu5R-Induced Increase in STEP Activity Is Prevented by the A2AR Antagonist
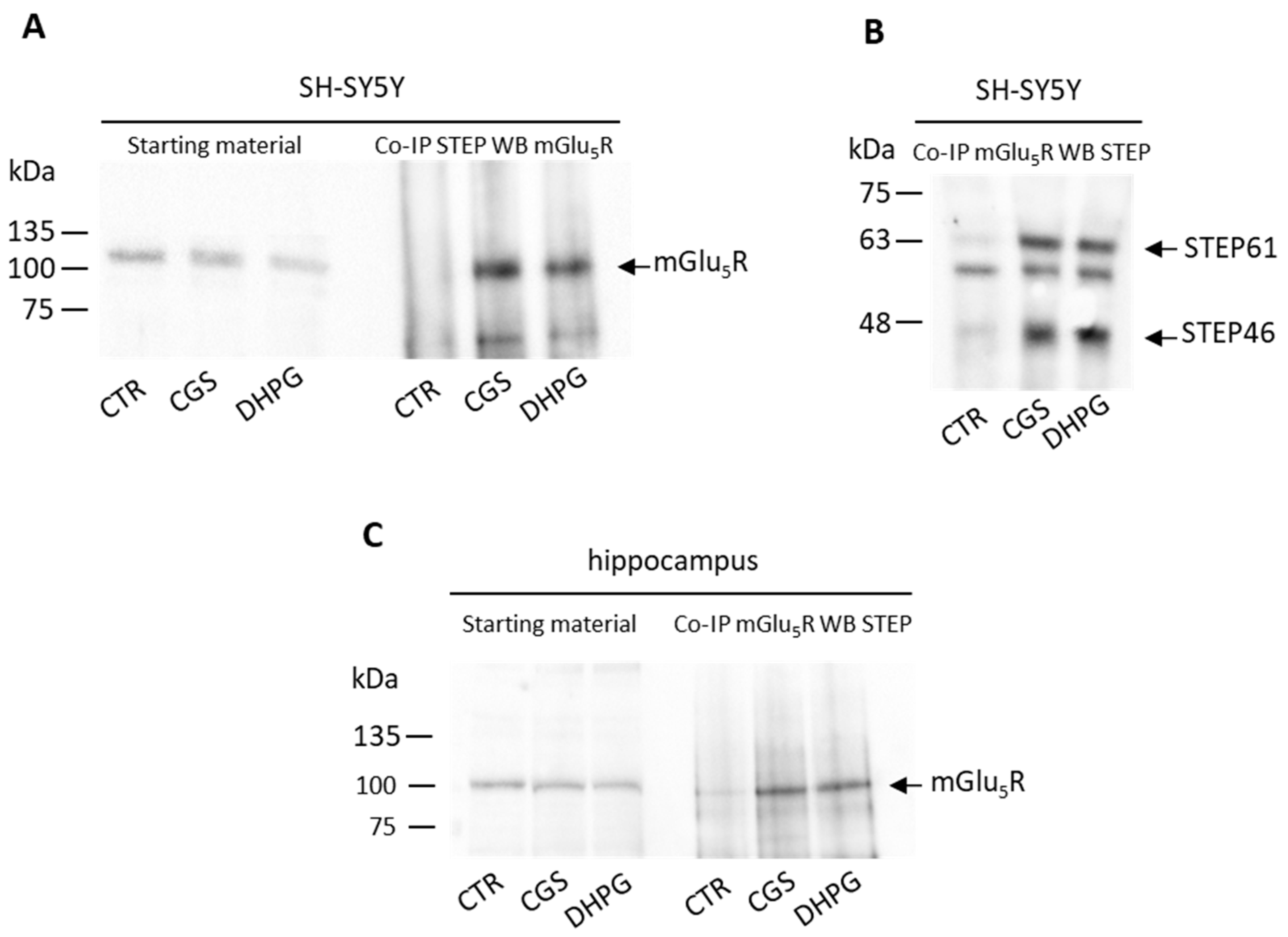
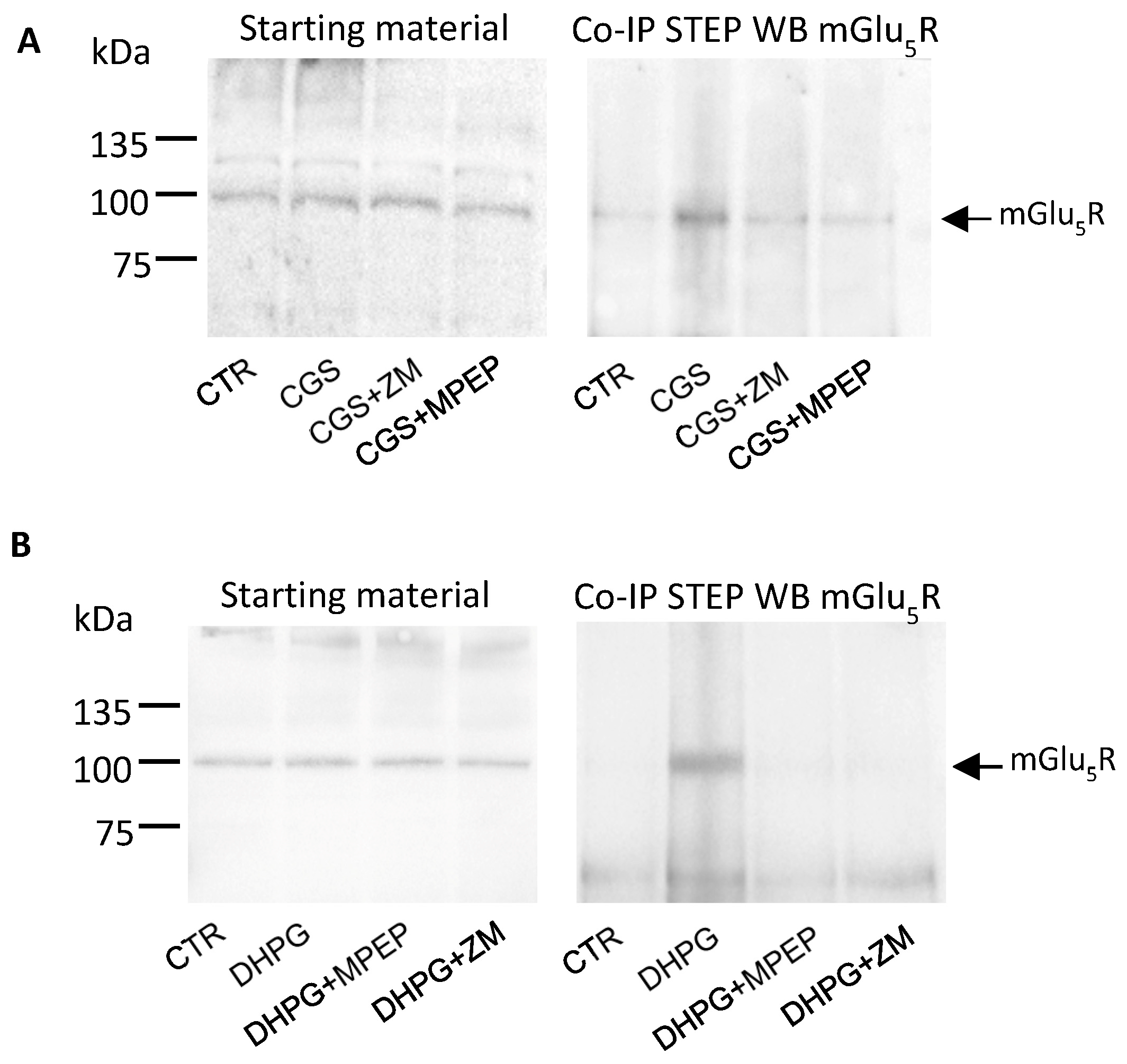
3.4. Stimulation of A2AR and mGlu5R Induces STEP and mGlu5R to Physically Interact
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef]
- Morin, N.; Di Paolo, T. Chapter Seven—Interaction of Adenosine Receptors with Other Receptors from Therapeutic Perspective in Parkinson’s Disease. Int. Rev. Neurobiol. 2014, 119, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Cabello, N.; Gandía, J.; Bertarelli, D.C.G.; Watanabe, M.; Lluís, C.; Franco, R.; Ferré, S.; Luján, R.; Ciruela, F. Metabotropic glutamate type 5, dopamine D2 and adenosine A2a receptors form higher-order oligomers in living cells. J. Neurochem. 2009, 109, 1497–1507. [Google Scholar] [CrossRef]
- Tebano, M.T.; Martire, A.; Popoli, P. Adenosine A(2A)-cannabinoid CB(1) receptor interaction: An integrative mechanism in striatal glutamatergic neurotransmission. Brain Res. 2012, 1476, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Chiarlone, A.; Medrano, M.; Puigdellivol, M.; Bibic, L.; Howell, L.A.; Resel, E.; Puente, N.; Casarejos, M.J.; Perucho, J.; et al. Singular Location and Signaling Profile of Adenosine A(2A)-Cannabinoid CB(1) Receptor Heteromers in the Dorsal Striatum. Neuropsychopharmacology 2018, 43, 964–977. [Google Scholar] [CrossRef]
- Ferré, S.; Ciruela, F. Functional and Neuroprotective Role of Striatal Adenosine A(2A) Receptor Heterotetramers. J. Caffeine Adenosine Res. 2019, 9, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Tebano, M.T.; Martire, A.; Rebola, N.; Pepponi, R.; Domenici, M.R.; Gro, M.C.; Schwarzschild, M.A.; Chen, J.F.; Cunha, R.A.; Popoli, P. Adenosine A2A receptors and metabotropic glutamate 5 receptors are co-localized and functionally interact in the hippocampus: A possible key mechanism in the modulation of N-methyl-D-aspartate effects. J. Neurochem. 2005, 95, 1188–1200. [Google Scholar] [CrossRef]
- Tebano, M.T.; Martire, A.; Pepponi, R.; Domenici, M.R.; Popoli, P. Is the functional interaction between adenosine A(2A) receptors and metabotropic glutamate 5 receptors a general mechanism in the brain? Differences and similarities between the striatum and the hippocampus. Purinergic Signal. 2006, 2, 619–625. [Google Scholar] [CrossRef][Green Version]
- Sarantis, K.; Tsiamaki, E.; Kouvaros, S.; Papatheodoropoulos, C.; Angelatou, F. Adenosine A(2)A receptors permit mGluR5-evoked tyrosine phosphorylation of NR2B (Tyr1472) in rat hippocampus: A possible key mechanism in NMDA receptor modulation. J. Neurochem. 2015, 135, 714–726. [Google Scholar] [CrossRef]
- Temido-Ferreira, M.; Ferreira, D.G.; Batalha, V.L.; Marques-Morgado, I.; Coelho, J.E.; Pereira, P.; Gomes, R.; Pinto, A.; Carvalho, S.; Canas, P.M.; et al. Age-related shift in LTD is dependent on neuronal adenosine A2A receptors interplay with mGluR5 and NMDA receptors. Mol. Psychiatry 2020, 25, 1876–1900. [Google Scholar] [CrossRef]
- Nicoletti, F.; Di Menna, L.; Iacovelli, L.; Orlando, R.; Zuena, A.R.; Conn, P.J.; Dogra, S.; Joffe, M.E. GPCR interactions involving metabotropic glutamate receptors and their relevance to the pathophysiology and treatment of CNS disorders. Neuropharmacology 2023, 235, 109569. [Google Scholar] [CrossRef] [PubMed]
- Goebel-Goody, S.M.; Baum, M.; Paspalas, C.D.; Fernandez, S.M.; Carty, N.C.; Kurup, P.; Lombroso, P.J. Therapeutic implications for striatal-enriched protein tyrosine phosphatase (STEP) in neuropsychiatric disorders. Pharmacol. Rev. 2012, 64, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, T.; Lombroso, P.J. Disruption of striatal-enriched protein tyrosine phosphatase (STEP) function in neuropsychiatric disorders. Neurosci. Res. 2014, 89, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Won, S.; Roche, K.W. Regulation of glutamate receptors by striatal-enriched tyrosine phosphatase 61 (STEP61). J. Physiol. 2021, 599, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Bagwe, P.V.; Deshpande, R.D.; Juhasz, G.; Sathaye, S.; Joshi, S.V. Uncovering the Significance of STEP61 in Alzheimer’s Disease: Structure, Substrates, and Interactome. Cell. Mol. Neurobiol. 2023, 1–15, epub ahead of print. [Google Scholar] [CrossRef]
- Mallozzi, C.; Pepponi, R.; Visentin, S.; Chiodi, V.; Lombroso, P.J.; Bader, M.; Popoli, P.; Domenici, M.R. The activity of the Striatal-enriched protein tyrosine phosphatase in neuronal cells is modulated by adenosine A(2A) receptor. J. Neurochem. 2020, 152, 284–298. [Google Scholar] [CrossRef]
- Giralt, A.; Saavedra, A.; Carreton, O.; Xifro, X.; Alberch, J.; Perez-Navarro, E. Increased PKA signaling disrupts recognition memory and spatial memory: Role in Huntington’s disease. Hum. Mol. Genet. 2011, 20, 4232–4247. [Google Scholar] [CrossRef]
- Domenici, M.R.; Mallozzi, C.; Pepponi, R.; Casella, I.; Chiodi, V.; Ferrante, A.; Popoli, P. Insight into the Role of the STriatal-Enriched Protein Tyrosine Phosphatase (STEP) in A(2A) Receptor-Mediated Effects in the Central Nervous System. Front. Pharmacol. 2021, 12, 647742. [Google Scholar] [CrossRef]
- Won, S.; Incontro, S.; Li, Y.; Nicoll, R.A.; Roche, K.W. The STEP 61 interactome reveals subunit-specific AMPA receptor binding and synaptic regulation. Proc. Natl. Acad. Sci. USA 2019, 116, 8028. [Google Scholar] [CrossRef]
- Ferre, S.; Karcz-Kubicha, M.; Hope, B.T.; Popoli, P.; Burgueno, J.; Gutierrez, M.A.; Casado, V.; Fuxe, K.; Goldberg, S.R.; Lluis, C.; et al. Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: Implications for striatal neuronal function. Proc. Natl. Acad. Sci. USA 2002, 99, 11940–11945. [Google Scholar] [CrossRef]
- Domenici, M.R.; Pepponi, R.; Martire, A.; Tebano, M.T.; Potenza, R.L.; Popoli, P. Permissive role of adenosine A2A receptors on metabotropic glutamate receptor 5 (mGluR5)-mediated effects in the striatum. J. Neurochem. 2004, 90, 1276–1279. [Google Scholar] [CrossRef] [PubMed]
- Krania, P.; Dimou, E.; Bantouna, M.; Kouvaros, S.; Tsiamaki, E.; Papatheodoropoulos, C.; Sarantis, K.; Angelatou, F. Adenosine A(2A) receptors are required for glutamate mGluR5- and dopamine D1 receptor-evoked ERK1/2 phosphorylation in rat hippocampus: Involvement of NMDA receptor. J. Neurochem. 2018, 145, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Moult, P.R.; Schnabel, R.; Kilpatrick, I.C.; Bashir, Z.I.; Collingridge, G.L. Tyrosine dephosphorylation underlies DHPG-induced LTD. Neuropharmacology 2002, 43, 175–180. [Google Scholar] [CrossRef]
- Zhang, Y.; Venkitaramani, D.V.; Gladding, C.M.; Zhang, Y.; Kurup, P.; Molnar, E.; Collingridge, G.L.; Lombroso, P.J. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J. Neurosci. 2008, 28, 10561–10566. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lin, R.; Chang, L.; Xu, S.; Wei, X.; Zhang, J.; Wang, C.; Anwyl, R.; Wang, Q. Enhancement of long-term depression by soluble amyloid beta protein in rat hippocampus is mediated by metabotropic glutamate receptor and involves activation of p38MAPK, STEP and caspase-3. Neuroscience 2013, 253, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Conn, P.J.; Pin, J.P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 205–237. [Google Scholar] [CrossRef]
- Landucci, E.; Berlinguer-Palmini, R.; Baccini, G.; Boscia, F.; Gerace, E.; Mannaioni, G.; Pellegrini-Giampietro, D.E. The Neuroprotective Effects of mGlu1 Receptor Antagonists Are Mediated by an Enhancement of GABAergic Synaptic Transmission via a Presynaptic CB1 Receptor Mechanism. Cells 2022, 11, 3015. [Google Scholar] [CrossRef]
- Vezzi, V.; Ambrosio, C.; Grò, M.C.; Molinari, P.; Süral, G.; Costa, T.; Onaran, H.O.; Cotecchia, S. Vasopressin receptor 2 mutations in the nephrogenic syndrome of inappropriate antidiuresis show different mechanisms of constitutive activation for G protein coupled receptors. Sci. Rep. 2020, 10, 9111. [Google Scholar] [CrossRef]
- Canals, M.; Angulo, E.; Casadó, V.; Canela, E.I.; Mallol, J.; Viñals, F.; Staines, W.; Tinner, B.; Hillion, J.; Agnati, L.; et al. Molecular mechanisms involved in the adenosine A and A receptor-induced neuronal differentiation in neuroblastoma cells and striatal primary cultures. J. Neurochem. 2005, 92, 337–348. [Google Scholar] [CrossRef]
- Gomez-Castro, F.; Zappettini, S.; Pressey, J.C.; Silva, C.G.; Russeau, M.; Gervasi, N.; Figueiredo, M.; Montmasson, C.; Renner, M.; Canas, P.M.; et al. Convergence of adenosine and GABA signaling for synapse stabilization during development. Science 2021, 374, eabk2055. [Google Scholar] [CrossRef]
- Paul, S.; Snyder, G.L.; Yokakura, H.; Picciotto, M.R.; Nairn, A.C.; Lombroso, P.J. The Dopamine/D1 receptor mediates the phosphorylation and inactivation of the protein tyrosine phosphatase STEP via a PKA-dependent pathway. J. Neurosci. 2000, 20, 5630–5638. [Google Scholar] [CrossRef] [PubMed]
- Valjent, E.; Pascoli, V.; Svenningsson, P.; Paul, S.; Enslen, H.; Corvol, J.; Stipanovich, A.; Caboche, J.; Lombroso, P.J.; Nairn, A.C.; et al. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc. Natl. Acad. Sci. USA 2005, 102, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Borroto-Escuela, D.O.; Ferraro, L.; Fuxe, K. Molecular Integration in Adenosine Heteroreceptor Complexes Through Allosteric and De-Phosphorylation (STEP) Mechanisms and its Role in Brain Disease. Front. Pharmacol. 2022, 12, 781381. [Google Scholar] [CrossRef]
- Um, J.W.; Kaufman, A.C.; Kostylev, M.; Heiss, J.K.; Stagi, M.; Takahashi, H.; Kerrisk, M.E.; Vortmeyer, A.; Wisniewski, T.; Koleske, A.J.; et al. Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer abeta oligomer bound to cellular prion protein. Neuron 2013, 79, 887–902. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.; Vasefi, M.; Vander Tuin, C.; McQuaid, R.; Anisman, H.; Ferguson, S.G. Chronic Pharmacological mGluR5 Inhibition Prevents Cognitive Impairment and Reduces Pathogenesis in an Alzheimer Disease Mouse Model. Cell Rep. 2016, 15, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Launay, A.; Nebie, O.; Vijaya Shankara, J.; Lebouvier, T.; Buee, L.; Faivre, E.; Blum, D. The role of adenosine A(2A) receptors in Alzheimer’s disease and tauopathies. Neuropharmacology 2023, 226, 109379. [Google Scholar] [CrossRef]
- Ferrante, A.; Boussadia, Z.; Borreca, A.; Mallozzi, C.; Pedini, G.; Pacini, L.; Pezzola, A.; Armida, M.; Vincenzi, F.; Varani, K.; et al. Adenosine A(2A) receptor inhibition reduces synaptic and cognitive hippocampal alterations in Fmr1 KO mice. Transl. Psychiatry 2021, 11, 112–115. [Google Scholar] [CrossRef]
- Pop, A.S.; Gomez-Mancilla, B.; Neri, G.; Willemsen, R.; Gasparini, F. Fragile X syndrome: A preclinical review on metabotropic glutamate receptor 5 (mGluR5) antagonists and drug development. Psychopharmacology 2014, 231, 1217–1226. [Google Scholar] [CrossRef]
- Scharf, S.H.; Jaeschke, G.; Wettstein, J.G.; Lindemann, L. Metabotropic glutamate receptor 5 as drug target for Fragile X syndrome. Curr. Opin. Pharmacol. 2015, 20, 124–134. [Google Scholar] [CrossRef]
- Westmark, P.R.; Dekundy, A.; Gravius, A.; Danysz, W.; Westmark, C.J. Rescue of Fmr1(KO) phenotypes with mGluR(5) inhibitors: MRZ-8456 versus AFQ-056. Neurobiol. Dis. 2018, 119, 190–198. [Google Scholar] [CrossRef]
- Taylor, D.; Kneynsberg, A.; van Roijen, M.; Gotz, J. Tyrosine phosphatase STEP(61) in human dementia and in animal models with amyloid and tau pathology. Mol. Brain 2023, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R. A novel phosphatase inhibitor may be a STEP toward ameliorating cognitive dysfunction. PLoS Biol. 2014, 12, e1001924. [Google Scholar] [CrossRef] [PubMed][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallozzi, C.; Pepponi, R.; Gaddini, L.; Casella, I.; Chiodi, V.; Popoli, P.; Domenici, M.R. Functional Interaction between Adenosine A2A and mGlu5 Receptors Mediates STEP Phosphatase Activation and Promotes STEP/mGlu5R Binding in Mouse Hippocampus and Neuroblastoma Cell Line. Biomolecules 2023, 13, 1350. https://doi.org/10.3390/biom13091350
Mallozzi C, Pepponi R, Gaddini L, Casella I, Chiodi V, Popoli P, Domenici MR. Functional Interaction between Adenosine A2A and mGlu5 Receptors Mediates STEP Phosphatase Activation and Promotes STEP/mGlu5R Binding in Mouse Hippocampus and Neuroblastoma Cell Line. Biomolecules. 2023; 13(9):1350. https://doi.org/10.3390/biom13091350
Chicago/Turabian StyleMallozzi, Cinzia, Rita Pepponi, Lucia Gaddini, Ida Casella, Valentina Chiodi, Patrizia Popoli, and Maria Rosaria Domenici. 2023. "Functional Interaction between Adenosine A2A and mGlu5 Receptors Mediates STEP Phosphatase Activation and Promotes STEP/mGlu5R Binding in Mouse Hippocampus and Neuroblastoma Cell Line" Biomolecules 13, no. 9: 1350. https://doi.org/10.3390/biom13091350
APA StyleMallozzi, C., Pepponi, R., Gaddini, L., Casella, I., Chiodi, V., Popoli, P., & Domenici, M. R. (2023). Functional Interaction between Adenosine A2A and mGlu5 Receptors Mediates STEP Phosphatase Activation and Promotes STEP/mGlu5R Binding in Mouse Hippocampus and Neuroblastoma Cell Line. Biomolecules, 13(9), 1350. https://doi.org/10.3390/biom13091350





