Diurnal Variation of L-Arginine and the Cardiovascular Risk Markers Asymmetric and Symmetric Dimethylarginine and Homoarginine in Rotating Night Shift Workers and Controls
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Protocol
2.3. Measurement of L-Arginine and Its Metabolites by Liquid Chromatography–Tandem Mass Spectrometry
2.4. Assessment of Plasma Melatonin and Cortisol
2.5. 24-h Blood Pressure Measurements
2.6. Statistical Analyses
3. Results
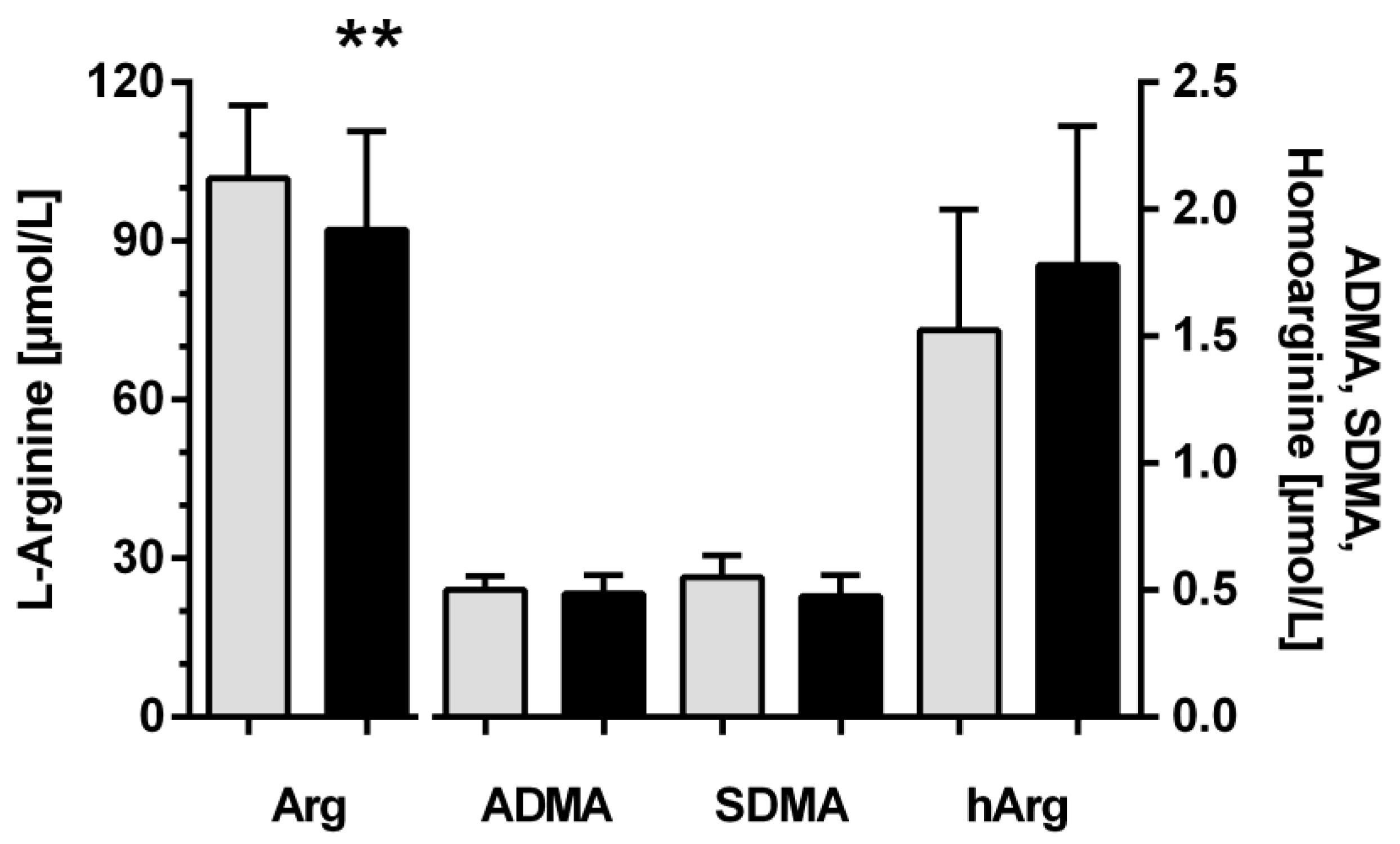
3.1. Baseline Characteristics
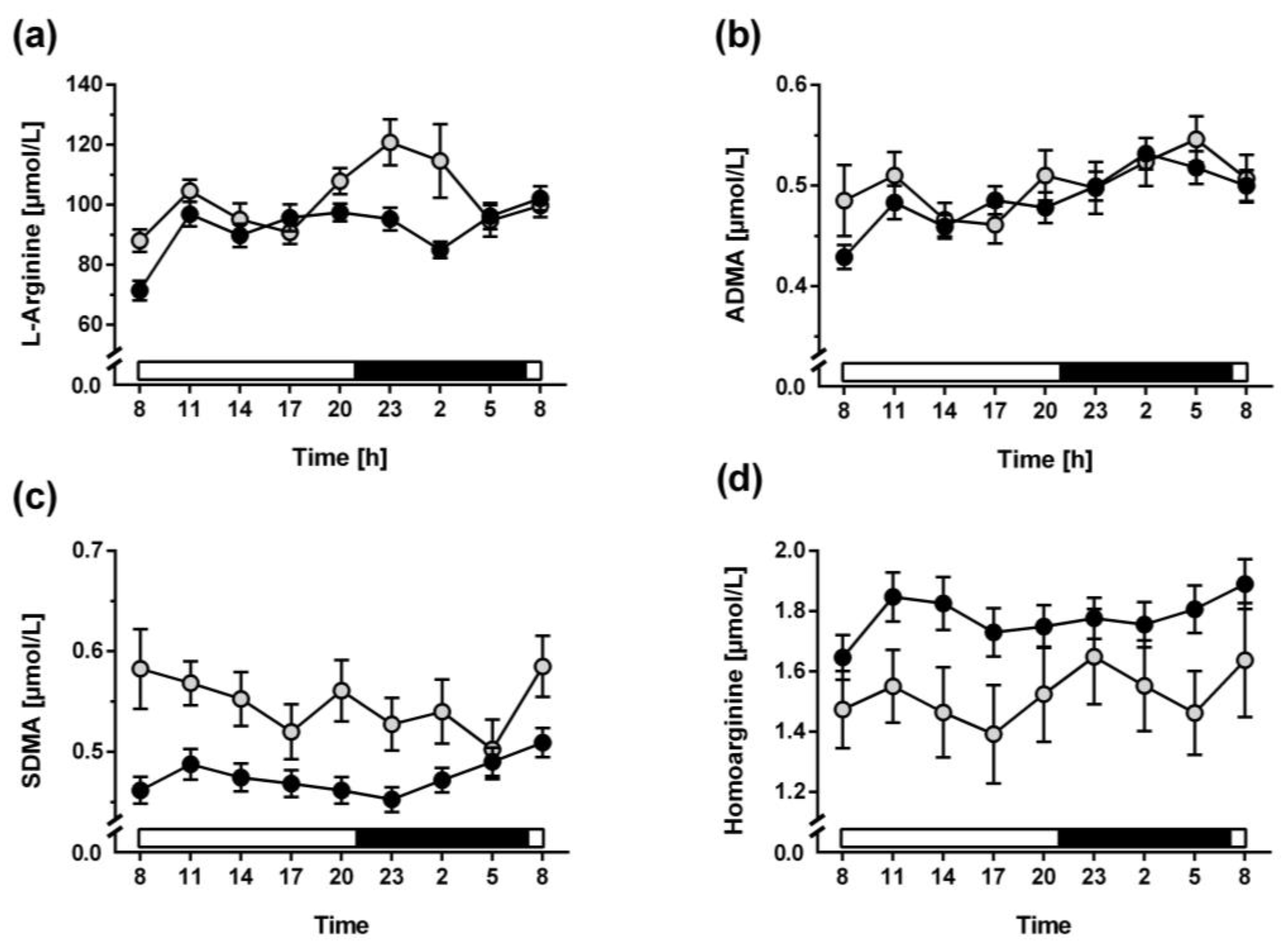
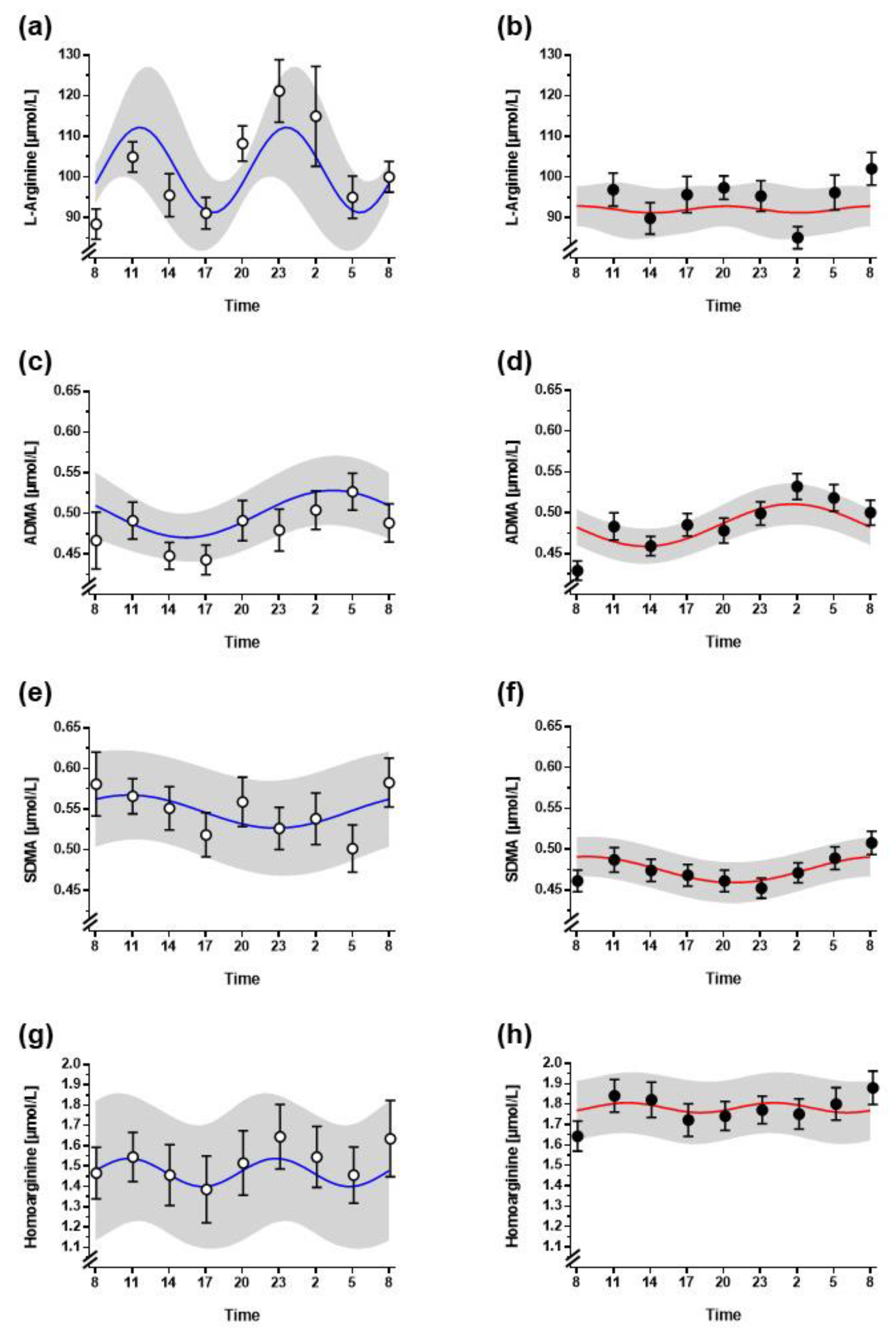
3.2. Diurnal Variation of L-Arginine and Its Metabolites
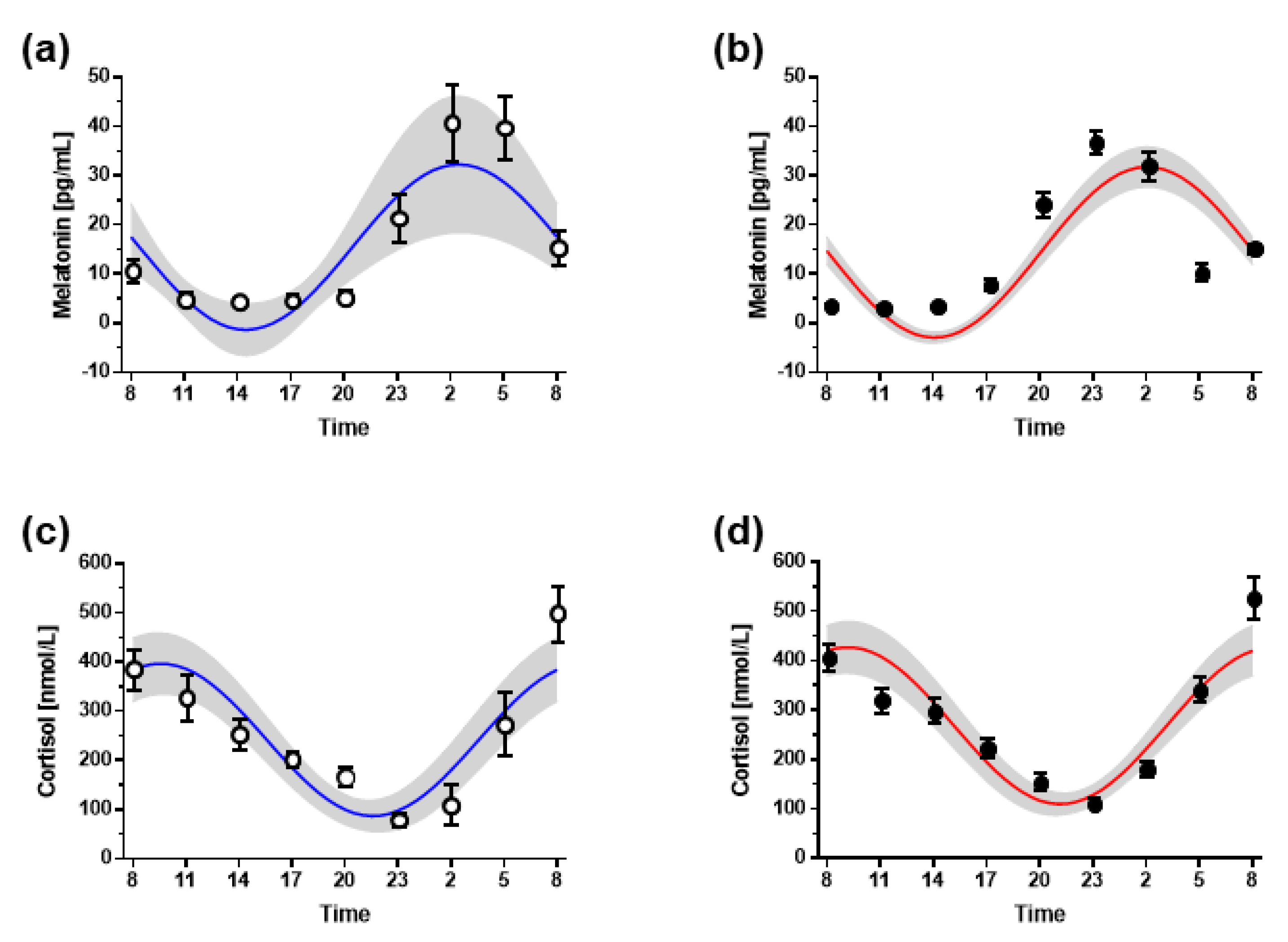
3.3. Hormone Markers of Circadian Timing
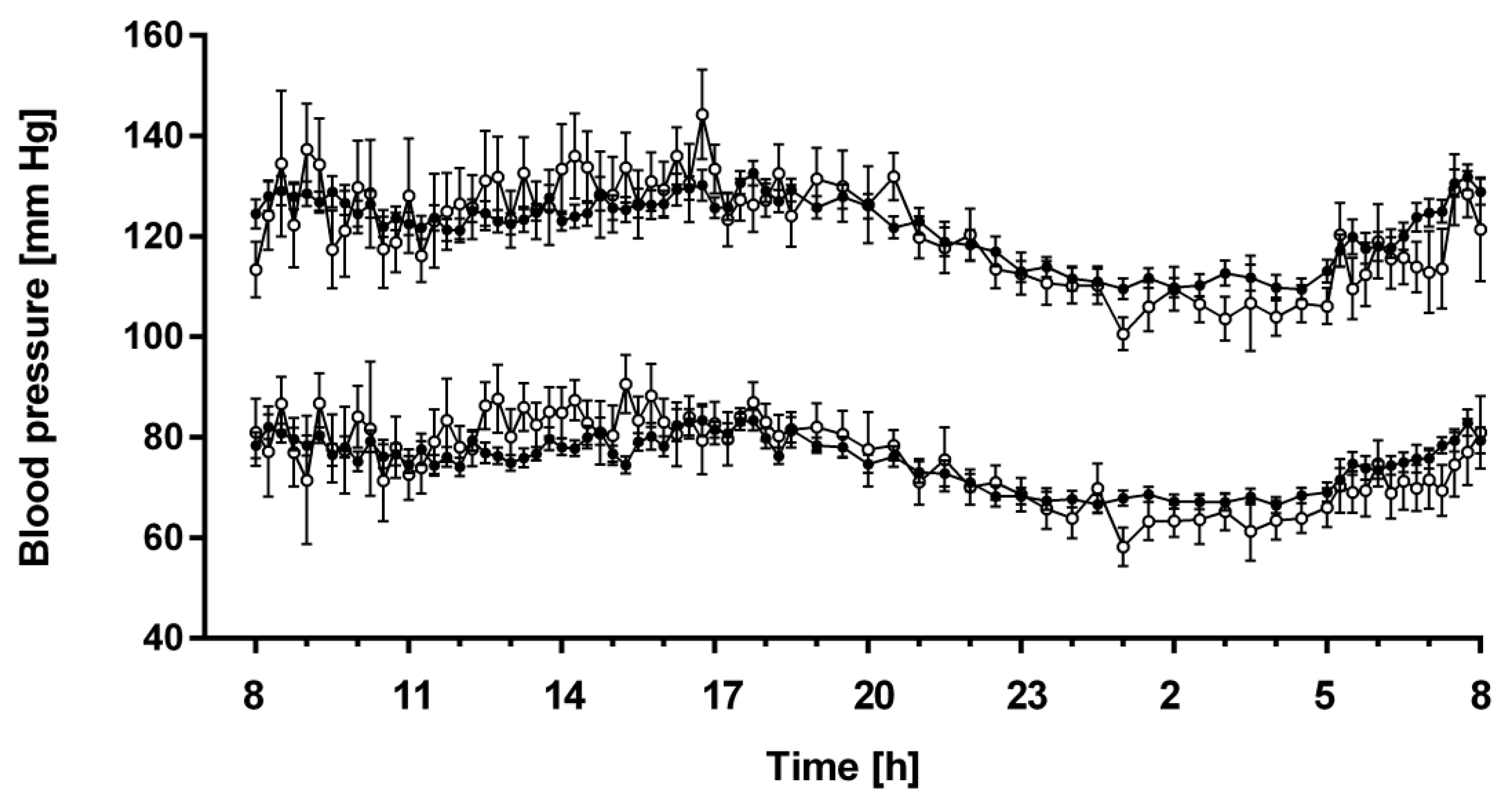
3.4. 24-h Profiles of Blood Pressure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aschoff, J. Circadian Rhythms in Man. Science 1965, 148, 1427–1432. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.Y.; Lenn, N.J. A Retinohypothalamic Projection in the Rat. J. Comp. Neurol. 1972, 146, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lewy, A.J.; Wehr, T.A.; Goodwin, F.K.; Newsome, D.A.; Markey, S.P. Light Suppresses Melatonin Secretion in Humans. Science 1980, 210, 1267–1269. [Google Scholar] [CrossRef] [PubMed]
- Saba, G.C.; Materazzi, F.; Hoet, J.J. Diurnal Rhythm of the Adrenal Cortial Activity and of the Cortisol Metabolism in Normal Subjects. Folia Endocrinol. 1963, 16, 611–616. [Google Scholar]
- Millar-Craig, M.W.; Bishop, C.N.; Raftery, E.B. Circadian Variation of Blood-Pressure. Lancet 1978, 1, 795–797. [Google Scholar] [CrossRef]
- Kollias, E.G.; Stamatelopoulos, K.S.; Papaioannou, T.G.; A Zakopoulos, N.; Alevizaki, M.; Alexopoulos, G.P.; Kontoyannis, A.D.; Karga, H.; Koroboki, E.; Lekakis, J.P.; et al. Diurnal variation of endothelial function and arterial stiffness in hypertension. J. Hum. Hypertens. 2009, 23, 597–604. [Google Scholar] [CrossRef]
- Bastianini, S.; Silvani, A.; Berteotti, C.; Martire, V.L.; Zoccoli, G. Mice Show Circadian Rhythms of Blood Pressure During Each Wake-Sleep State. Chrono. Int. 2012, 29, 82–86. [Google Scholar] [CrossRef]
- Hermida, R.C.; Ayala, D.E.; Mojón, A.; Fernández, J.R. Blunted Sleep-Time Relative Blood Pressure Decline Increases Cardiovascular Risk Independent of Blood Pressure Level—The “Normotensive Non-Dipper” Paradox. Chrono. Int. 2013, 30, 87–98. [Google Scholar] [CrossRef]
- Salles, G.F.; Reboldi, G.; Fagard, R.H.; Cardoso, C.R.; Pierdomenico, S.D.; Verdecchia, P.; Eguchi, K.; Kario, K.; Hoshide, S.; Polonia, J.; et al. Prognostic Effect of the Nocturnal Blood Pressure Fall in Hypertensive Patients: The Ambulatory Blood Pressure Collaboration in Patients with Hypertension (Abc-H) Meta-Analysis. Hypertension 2016, 67, 693–700. [Google Scholar] [CrossRef]
- Hannemann, J.; Laing, A.; Middleton, B.; Cridland, J.; Staels, B.; Marx, N.; Grant, P.J.; Federici, M.; Stenberg, T.; Skene, D.J.; et al. Light Therapy Improves Diurnal Blood Pressure Control in Night Shift Workers Via Reduction of Catecholamines: The EuRhythDia Study. J. Hypertens. 2021, 39, 1678–1688. [Google Scholar] [CrossRef]
- Hayter, E.A.; Wehrens, S.M.T.; Van Dongen, H.P.A.; Stangherlin, A.; Gaddameedhi, S.; Crooks, E.; Barron, N.J.; Venetucci, L.A.; O’Neill, J.S.; Brown, T.M.; et al. Distinct Circadian Mechanisms Govern Cardiac Rhythms and Susceptibility to Arrhythmia. Nat. Commun. 2021, 12, 2472. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.J.; London, B.; Block, G.D.; Menaker, M. Cardiovascular Tissues Contain Independent Circadian Clocks. Clin. Exp. Hypertens. 2005, 27, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Viswambharan, H.; Carvas, J.M.; Antic, V.; Marecic, A.; Jud, C.; Zaugg, C.E.; Ming, X.-F.; Montani, J.-P.; Albrecht, U.; Yang, Z.; et al. Mutation of the Circadian Clock Gene Per2 Alters Vascular Endothelial Function. Circulation 2007, 115, 2188–2195. [Google Scholar] [CrossRef]
- Muller, J.E.; Stone, P.H.; Turi, Z.G.; Rutherford, J.D.; Czeisler, C.A.; Parker, C.; Poole, W.K.; Passamani, E.; Roberts, R.; Robertson, T.; et al. Circadian Variation in the Frequency of Onset of Acute Myocardial Infarction. N. Engl. J. Med. 1985, 313, 1315–1322. [Google Scholar] [CrossRef]
- Willich, S.N.; Goldberg, R.J.; Maclure, M.; Perriello, L.; Muller, J.E. Increased Onset of Sudden Cardiac Death in the First Three Hours after Awakening. Am. J. Cardiol. 1992, 70, 65–68. [Google Scholar] [CrossRef]
- Böger, R.H.; Maas, R.; Schulze, F.; Schwedhelm, E. Asymmetric Dimethylarginine (ADMA) as a Prospective Marker of Cardiovascular Disease and Mortality—An Update on Patient Populations with a Wide Range of Cardiovascular Risk. Pharmacol. Res. 2009, 60, 481–487. [Google Scholar] [CrossRef]
- Boger, R.H.; Sullivan, L.M.; Schwedhelm, E.; Wang, T.J.; Maas, R.; Benjamin, E.J.; Schulze, F.; Xanthakis, V.; Benndorf, R.A.; Vasan, R.S. Plasma Asymmetric Dimethylarginine and Incidence of Cardiovascular Disease and Death in the Community. Circulation 2009, 119, 1592–1600. [Google Scholar] [CrossRef]
- Böger, R.H.; Bode-Böger, S.M.; Szuba, A.; Tsao, P.S.; Chan, J.R.; Tangphao, O.; Blaschke, T.F.; Cooke, J.P. Asymmetric Dimethylarginine (ADMA): A Novel Risk Factor for Endothelial Dysfunction: Its Role in Hypercholesterolemia. Circulation 1998, 98, 1842–1847. [Google Scholar] [CrossRef]
- Banjarnahor, S.; Rodionov, R.N.; König, J.; Maas, R. Transport of L-Arginine Related Cardiovascular Risk Markers. J. Clin. Med. 2020, 9, 3975. [Google Scholar] [CrossRef]
- Lüneburg, N.; von Holten, R.-A.; Töpper, R.F.; Schwedhelm, E.; Maas, R.; Böger, R.H. Symmetric Dimethylarginine Is a Marker of Detrimental Outcome in the Acute Phase after Ischaemic Stroke: Role of Renal Function. Clin. Sci. 2012, 122, 105–111. [Google Scholar] [CrossRef]
- Schulze, F.; Carter, A.M.; Schwedhelm, E.; Ajjan, R.; Maas, R.; von Holten, R.-A.; Atzler, D.; Grant, P.J.; Böger, R.H. Symmetric Dimethylarginine Predicts All-Cause Mortality Following Ischemic Stroke. Atherosclerosis 2010, 208, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Bahls, M.; Atzler, D.; Markus, M.R.; Friedrich, N.; Böger, R.H.; Völzke, H.; Felix, S.B.; Schwedhelm, E.; Dörr, M. Low-Circulating Homoarginine Is Associated with Dilatation and Decreased Function of the Left Ventricle in the General Population. Biomolecules 2018, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Choe, C.U.; Atzler, D.; Wild, P.S.; Carter, A.M.; Böger, R.H.; Ojeda, F.; Simova, O.; Stockebrand, M.; Lackner, K.; Nabuurs, C.; et al. Homoarginine Levels Are Regulated by L-Arginine:Glycine Amidinotransferase and Affect Stroke Outcome: Results from Human and Murine Studies. Circulation 2013, 128, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Schwedhelm, E.; Maas, R.; Tan-Andresen, J.; Schulze, F.; Riederer, U.; Böger, R.H. High-Throughput Liquid Chromatographic-tandem Mass Spectrometric Determination of Arginine and Dimethylated Arginine Derivatives in Human and Mouse Plasma. J. Chromatogr. B 2007, 851, 211–219. [Google Scholar] [CrossRef]
- Gunn, P.J.; Middleton, B.; Davies, S.K.; Revell, V.L.; Skene, D.J. Sex Differences in the Circadian Profiles of Melatonin and Cortisol in Plasma and Urine Matrices under Constant Routine Conditions. Chrono. Int. 2016, 33, 39–50. [Google Scholar] [CrossRef]
- Sletten, T.L.; Revell, V.L.; Middleton, B.; Lederle, K.A.; Skene, D.J. Age-Related Changes in Acute and Phase-Advancing Responses to Monochromatic Light. J. Biol. Rhythm. 2009, 24, 73–84. [Google Scholar] [CrossRef]
- Molcan, L. Time Distributed Data Analysis by Cosinor.Online Application. bioRxiv 2023. [Google Scholar] [CrossRef]
- Cornelissen, G. Cosinor-based rhythmometry. Theor. Biol. Med. Model. 2014, 11, 11–16. [Google Scholar] [CrossRef]
- Visek, W.J. Arginine Needs, Physiological State and Usual Diets. A Reevaluation. J. Nutr. 1986, 116, 36–46. [Google Scholar] [CrossRef]
- Walser, M. Urea Cycle Disorders and Other Hereditary Hyperammonemic Syndromes. In The Metabolic Basis of Inherited Disease; Stanbury, J.B., Wyngaarden, J.B., Fredrickson, D.S., Goldstein, J.L., Brown, M.S., Eds.; McGraw-Hill: New York, NY, USA, 1983; pp. 402–438. [Google Scholar]
- Möller, P.; Bergström, J.; Eriksson, S.; Fürst, P.; Hellström, K. Effect of Aging on Free Amino Acids and Electrolytes in Leg Skeletal Muscle. Clin. Sci. 1979, 56, 427–432. [Google Scholar] [CrossRef]
- Hov, G.G.; Sagen, E.; Bigonah, A.; Asberg, A. Health-Associated Reference Values for Arginine, Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA) Measured with High-Performance Liquid Chromatography. Scand. J. Clin. Lab. Investig. 2007, 67, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Lüneburg, N.; Xanthakis, V.; Schwedhelm, E.; Sullivan, L.M.; Maas, R.; Anderssohn, M.; Riederer, U.; Glazer, N.L.; Vasan, R.S.; Böger, R.H. Reference Intervals for Plasma L-Arginine and the L-Arginine:Asymmetric Dimethylarginine Ratio in the Framingham Offspring Cohort. J. Nutr. 2011, 141, 2186–2190. [Google Scholar] [CrossRef] [PubMed]
- Balieiro, L.C.T.; Rossato, L.T.; Waterhouse, J.; Paim, S.L.; Mota, M.C.; Crispim, C.A. Nutritional Status and Eating Habits of Bus Drivers During the Day and Night. Chrono. Int. 2014, 31, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, A.; Lowson, E.; Arber, S.; Griffin, B.A.; Skene, D.J. Dietary Patterns of Nurses on Rotational Shifts Are Marked by Redistribution of Energy into the Nightshift. Nutrients 2020, 12, 1053. [Google Scholar] [CrossRef]
- Peplonska, B.; Kaluzny, P.; Trafalska, E. Rotating Night Shift Work and Nutrition of Nurses and Midwives. Chrono. Int. 2019, 36, 945–954. [Google Scholar] [CrossRef]
- Silva, C.M.; Teixeira, B.S.; Wright, K.P.; Maia, Y.C.P., Jr.; Crispim, C.A. Time-Related Eating Patterns Are Associated with the Total Daily Intake of Calories and Macronutrients in Day and Night Shift Workers. Nutrients 2022, 14, 2202. [Google Scholar] [CrossRef]
- Elherik, K.; Khan, F.; McLaren, M.; Kennedy, G.; Belch, J.J.F. Circadian Variation in Vascular Tone and Endothelial Cell Function in Normal Males. Clin. Sci. 2002, 102, 547–552. [Google Scholar] [CrossRef]
- Bergheanu, S.C.; van der Laarse, A.; van der Bom, J.G.; van der Hoeven, B.L.; le Cessie, S.; de Jong, M.G.; Liem, S.S.; Schalij, M.J.; Jukema, J.W. Asymmetric Dimethylarginine (ADMA) Levels Display a Morning Peak in Patients with Acute Myocardial Infarction. Dis. Markers 2011, 30, 245–552. [Google Scholar] [CrossRef]
- Tofler, G.H.; Brezinski, D.; Schafer, A.I.; Czeisler, C.A.; Rutherford, J.D.; Willich, S.N.; Gleason, R.E.; Williams, G.H.; Muller, J.E. Concurrent Morning Increase in Platelet Aggregability and the Risk of Myocardial Infarction and Sudden Cardiac Death. N. Engl. J. Med. 1987, 316, 1514–1518. [Google Scholar] [CrossRef]
- Bode-Böger, S.M.; Böger, R.H.; Kielstein, J.T.; Löffler, M.; Schäffer, J.; Frölich, J.C. Role of Endogenous Nitric Oxide in Circadian Blood Pressure Regulation in Healthy Humans and in Patients with Hypertension or Atherosclerosis. J. Investig. Med. 2000, 48, 125–132. [Google Scholar]
- Zhdanova, I.V.; Simmons, M.; Marcus, J.N.; Busza, A.C.; Leclair, O.U.; Taylor, J.A. Nocturnal Increase in Plasma cGMP Levels in Humans. J. Biol. Rhythm. 1999, 14, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Hannemann, J.; Böger, R. Regulation|Transcriptional and Post-Translational Regulation of the Dimethylarginines ADMA and SDMA and Their Impact on the L-Arginine—Nitric Oxide Pathway. In Encyclopedia of Biological Chemistry; Elsevier: Oxford, UK, 2021; pp. 674–687. [Google Scholar]
- Leiper, J.M.; Santa Maria, J.; Chubb, A.; MacAllister, R.J.; Charles, I.G.; Whitley, G.S.; Vallance, P. Identification of Two Human Dimethylarginine Dimethylaminohydrolases with Distinct Tissue Distributions and Homology with Microbial Arginine Deiminases. Biochem. J. 1999, 343 Pt 1, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gill, P.S.; Chabrashvili, T.; Onozato, M.L.; Raggio, J.; Mendonca, M.; Dennehy, K.; Li, M.; Modlinger, P.; Leiper, J.; et al. Isoform-Specific Regulation by N(G),N(G)-Dimethylarginine Dimethylaminohydrolase of Rat Serum Asymmetric Dimethylarginine and Vascular Endothelium-Derived Relaxing Factor/No. Circ. Res. 2007, 101, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Lüneburg, N.; Lieb, W.; Zeller, T.; Chen, M.-H.; Maas, R.; Carter, A.M.; Xanthakis, V.; Glazer, N.L.; Schwedhelm, E.; Seshadri, S.S.; et al. Genome-Wide Association Study of L-Arginine and Dimethylarginines Reveals Novel Metabolic Pathway for Symmetric Dimethylarginine. Circ. Cardiovasc. Genet. 2014, 7, 864–872. [Google Scholar] [CrossRef]
- Hannemann, J.; Cordts, K.; Seniuk, A.; Choe, C.U.; Schmidt-Hutten, L.; Duque Escobar, J.; Weinberger, F.; Böger, R.; Schwedhelm, E. Arginine:Glycine Amidinotransferase Is Essential for Creatine Supply in Mice During Chronic Hypoxia. Front. Physiol. 2021, 12, 703069. [Google Scholar] [CrossRef] [PubMed]
- Atzler, D.; Rosenberg, M.; Anderssohn, M.; Choe, C.-U.; Lutz, M.; Zugck, C.; Böger, R.H.; Frey, N.; Schwedhelm, E. Homoarginine—An Independent Marker of Mortality in Heart Failure. Int. J. Cardiol. 2013, 168, 4907–4909. [Google Scholar] [CrossRef]
- Schwedhelm, E.; Song, R.J.; Vasan, R.S.; Heuvel, E.R.v.D.; Hannemann, J.; Xanthakis, V.; Böger, R. Association of Lower Plasma Homoarginine Concentrations with Greater Risk of All-Cause Mortality in the Community: The Framingham Offspring Study. J. Clin. Med. 2020, 9, 2016. [Google Scholar] [CrossRef]
- Faller, E.K.M.; Atzler, D.; McAndrew, D.J.; Zervou, S.; Whittington, H.J.; Simon, J.N.; Aksentijevic, D.; Hove, M.T.; Choe, C.-U.; Isbrandt, D.; et al. Impaired Cardiac Contractile Function in Arginine:Glycine Amidinotransferase Knockout Mice Devoid of Creatine Is Rescued by Homoarginine but Not Creatine. Cardiovasc. Res. 2018, 114, 417–430. [Google Scholar] [CrossRef]
- Atzler, D.; Appelbaum, S.; Cordts, K.; Ojeda, F.M.; Wild, P.S.; Münzel, T.; Blankenberg, S.; Böger, R.H.; Blettner, M.; Beutel, M.E.; et al. Reference Intervals of Plasma Homoarginine from the German Gutenberg Health Study. Clin. Chem. Lab. Med. 2016, 54, 1231–1237. [Google Scholar] [CrossRef]
- Skene, D.J.; Skornyakov, E.; Chowdhury, N.R.; Gajula, R.P.; Middleton, B.; Satterfield, B.C.; Porter, K.I.; Van Dongen, H.P.A.; Gaddameedhi, S. Separation of Circadian- and Behavior-Driven Metabolite Rhythms in Humans Provides a Window on Peripheral Oscillators and Metabolism. Proc. Natl. Acad. Sci. USA 2018, 115, 7825–7830. [Google Scholar] [CrossRef]
| Night Shift Workers | Non-Night Shift Workers | p | |
|---|---|---|---|
| Demographics | |||
| No. of individuals | 60 | 12 | n.a. |
| Age (years) | 35.8 ± 11.7 | 37.1 ± 11.6 | n.s. |
| Sex (f/m) | 35/25 | 6/6 | n.s. |
| Anthropometrics | |||
| Height (cm) | 173.7 ± 8.5 | 176.3 ± 9.0 | n.s. |
| Weight (kg) | 81.6 ± 19.5 | 74.7 ± 14.6 | n.s. |
| BMI (kg/m2) | 27.1 ± 6.0 | 23.8 ± 2.7 | 0.07 |
| Systolic blood pressure (mm Hg) | 122.1 ± 10.9 | 116.8 ± 13.9 | n.s. |
| Diastolic blood pressure (mm Hg) | 73.6 ± 8.7 | 74.4 ± 9.2 | n.s. |
| Heart Rate (1/min) | 64.8 ± 11.5 | 68.6 ± 12.5 | n.s. |
| Work History | |||
| Night shifts since (years) | 10.8 ± 9.4 | - | n.a. |
| No. of night shifts/month | 7.9 ± 4.4 | - | n.a. |
| Laboratory Parameters | |||
| Glucose (mg/dL) | 88.5 ± 10.5 | 86.6 ± 5.9 | n.s. |
| HbA1c (%) | 5.1 ± 0.4 | 5.0 ± 0.3 | n.s. |
| Total cholesterol (mg/dL) | 179.7 ± 36.3 | 166.9 ± 33.2 | n.s. |
| Triglycerides (mg/dL) | 105.2 ± 46.0 | 86.3 ± 34.3 | n.s. |
| LDL cholesterol (mg/dL) | 99.8 ± 33.0 | 84.4 ± 33.2 | n.s. |
| HDL cholesterol (mg/dL) | 57.2 ± 17.9 | 65.2 ± 19.1 | n.s. |
| Creatinine (mg/dL) | 0.83 ± 0.16 | 0.75 ± 0.16 | n.s. |
| L-Arginine | ADMA | SDMA | Homoarginine | |
|---|---|---|---|---|
| Non-night shift workers | ||||
| Period (h) | 12 h | 24 h | 24 h | 12 h |
| Mesor [µmol/L] | 101.7 ± 14.9 | 0.50 ± 0.05 | 0.55 ± 0.09 | 1.47 ± 0.47 |
| Acrophase * | n.d. | 04:05 ± 2:03 | n.d. | n.d. |
| Nadir * | n.d. | 15:42 ± 2:07 | n.d. | n.d. |
| Amplitude [µmol/L] | 14.2 ± 10.2 | 0.04 ± 0.02 | 0.03 ± 0.02 | 0.18 ± 0.12 |
| Zero-amplitude test (p value) | 0.157 | 0.038 | 0.366 | 0.077 |
| Night shift workers | ||||
| Period (h) | 12 h | 24 h | 24 h | 12 h |
| Mesor [µmol/L] | 92.0 ± 18.9 | 0.49 ± 0.08 | 0.47 ± 0.08 | 1.76 ± 0.59 |
| Acrophase * | n.d. | 03:10 ± 1:04 | n.d. | 00:06 ± 3:42 12:06 ± 3:42 |
| Nadir * | n.d. | 14:40 ± 1:06 | n.d. | 06:06 ± 3:42 18:06 ± 3:42 |
| Amplitude [µmol/L] | 11.9 ± 11.3 | 0.06 ± 0.04 | 0.04 ± 0.03 | 0.16 ± 0.12 |
| Zero-amplitude test (p value) | 0.986 | 0.009 | 0.150 | 0.026 |
| Melatonin | Cortisol | |
|---|---|---|
| Non-night shift workers | ||
| Period (h) | 24 h | 24 h |
| Mesor [µmol/L] | 15.47 ± 8.97 | 241.9 ± 54.8 |
| Acrophase * | 02:53 ± 1:43 | 09:41 ± 1:40 |
| Nadir * | 14:21 ± 2:55 | 21:41 ± 1.40 |
| Amplitude [µmol/L] | 18.14 ± 1.41 | 166.5 ± 56.9 |
| Zero-amplitude test (p value) | 0.008 | 0.007 |
| Night shift workers | ||
| Period (h) | 24 h | 24 h |
| Mesor [µmol/L] | 13.24 ± 7.34 | 265.0 ± 117.6 |
| Acrophase * | 02:27 ± 3:06 | 09:09 ± 2:20 |
| Nadir * | 13:35 ± 2:07 | 21:09 ± 3:08 |
| Amplitude [µmol/L] | 17.10 ± 10.85 | 183.0 ± 92.3 |
| Zero-amplitude test (p value) | 0.005 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hannemann, J.; Skene, D.J.; Middleton, B.; Schwedhelm, E.; Laing, A.; Böger, R. Diurnal Variation of L-Arginine and the Cardiovascular Risk Markers Asymmetric and Symmetric Dimethylarginine and Homoarginine in Rotating Night Shift Workers and Controls. Biomolecules 2023, 13, 1282. https://doi.org/10.3390/biom13091282
Hannemann J, Skene DJ, Middleton B, Schwedhelm E, Laing A, Böger R. Diurnal Variation of L-Arginine and the Cardiovascular Risk Markers Asymmetric and Symmetric Dimethylarginine and Homoarginine in Rotating Night Shift Workers and Controls. Biomolecules. 2023; 13(9):1282. https://doi.org/10.3390/biom13091282
Chicago/Turabian StyleHannemann, Juliane, Debra J. Skene, Benita Middleton, Edzard Schwedhelm, Anika Laing, and Rainer Böger. 2023. "Diurnal Variation of L-Arginine and the Cardiovascular Risk Markers Asymmetric and Symmetric Dimethylarginine and Homoarginine in Rotating Night Shift Workers and Controls" Biomolecules 13, no. 9: 1282. https://doi.org/10.3390/biom13091282
APA StyleHannemann, J., Skene, D. J., Middleton, B., Schwedhelm, E., Laing, A., & Böger, R. (2023). Diurnal Variation of L-Arginine and the Cardiovascular Risk Markers Asymmetric and Symmetric Dimethylarginine and Homoarginine in Rotating Night Shift Workers and Controls. Biomolecules, 13(9), 1282. https://doi.org/10.3390/biom13091282









