Abstract
Gap junctions (GJs) are not static bridges; instead, GJs as well as the molecular building block connexin (Cx) proteins undergo major expression changes in the degenerating retinal tissue. Various progressive diseases, including retinitis pigmentosa, glaucoma, age-related retinal degeneration, etc., affect neurons of the retina and thus their neuronal connections endure irreversible changes as well. Although Cx expression changes might be the hallmarks of tissue deterioration, GJs are not static bridges and as such they undergo adaptive changes even in healthy tissue to respond to the ever-changing environment. It is, therefore, imperative to determine these latter adaptive changes in GJ functionality as well as in their morphology and Cx makeup to identify and distinguish them from alterations following tissue deterioration. In this review, we summarize GJ alterations that take place in healthy retinal tissue and occur on three different time scales: throughout the entire lifespan, during daily changes and as a result of quick changes of light adaptation.
1. Introduction
The mammalian retina is a sheet of nervous tissue that covers the posterior aspect of the eye. Its neurons are organized into three cellular layers incorporating the light-sensitive photoreceptors (PRs), three interneuron populations including bipolar cells (BCs) horizontal cells (HCs) and amacrine cells (ACs), as well as the retinal ganglion cells (RGCs) that provide the sole output of the retina towards the brain. Neurons in the retina like in other brain areas communicate via chemical and/or electrical synapses. Following the initial descriptions [1,2,3,4], electrical synapses (also called gap junctions—GJ) were considered scarce and were thought to play insignificant roles, but their essential interplay in neuronal signaling became evident just recently. Experimental work over the past 3 decades also showed that GJs are just as important in the retina as in any other brain area [5,6]. Although the retinal distribution pattern of the retinal connexins (Cx; molecular building blocks of GJs) gained firm support, it is well documented that their expression levels are subject to changes in the external and internal milieu. The sections of this review will summarize some of these changes.
1.1. GJs and Connexin Building Blocks
GJs serve as conduits allowing for the free transcellular diffusion of ions and small molecules up to 1 kD [1,2], which paves the way for a molecular exchange between cells. In addition to small molecules, charged ions pass through GJs as well, thereby altering the membrane potential of both cells, which is a process that is utilized by neurons to perform fast and two-directional signaling. Due to this latter phenomenon, GJs are also called electrical synapses. At GJ sites, the membranes of neighboring cells form close appositions leaving only a very thin (1–2 nm) synaptic space in between. These intimate physical contacts harbor a piece of molecular machinery whose integral parts are the so-called connexons/hemichannels on each side. A functional channel is formed by two opposing hemichannels that are located in plasma membranes of neighboring cells. Each connexon is formed by a hexameric conglomerate of six membrane-spanning connexin (Cx) protein subunits. Cx-s are formed by four transmembrane domains: two extracellular and one intracellular loops and cytoplasmic C- and N-terminal endings. The amino acid sequence and the Cx makeup determine the molecular permeability and unitary conductance of each pore. The functional properties of the pore can also be modified by the intracellular molecular milieu, and thus the on- and offset of various signal-transduction pathways [7]. In the human and mouse genomes that have been studied most extensively, over 20 Cx genes were identified and sequenced [8]. Cx proteins are named simply after their molecular weights expressed in kilodaltons (kDa; ranging between 21–70 kDa, including Cx43, Cx40, Cx26, etc.). The Cx phylogenetic tree has three main branches comprising α-connexins (e.g., Cx38 and Cx40, Cx43, Cx45, Cx46, Cx50) with long intracellular C- and N-terminal chains, small β-connexins (e.g., Cx26, Cx30.2, Cx31 and Cx32) with short intracellular N-terminal [7] and medium-sized γ-connexins (e.g., mammalian Cx36, skate, perch and zebrafish Cx35, perch Cx34.7). In contrast to α- and/or β-Cx-s, pores formed by γ-Cx-s have unique features as they have low (or no) voltage- and pH-sensitivity and are also unable to form heterologous channels with α- and β-Cx subunits. It has now been firmly established that Cx-s are important in development, differentiation and growth control in all organs, and every vertebrate class uses a unique set of Cx-s to build GJs [9].
1.2. GJs in the Retina
GJs are abundant in all brain areas including the mammalian retina (Figure 1), where all major neuron classes have been shown to form GJs to couple neighbor cells [6,10,11,12,13,14]. In addition to the great variety of connecting cell types, the diverse Cx makeup also suggests that retinal GJs play a number of roles in signal processing. The best-studied GJ sites in the vertebrate retina are those highly conductive GJs that couple HCs into extensive laterally oriented syncytia. GJs of this circuit are used to average signals of the ambient background light across the coupled HC array, the signal of which can be then utilized by BCs to detect contrasts prevailing against this uniform background. Homologous GJ synapses (connecting neurons of the same subtype) are commonly used to average noise, thereby increasing the signal-to-noise ratio. Such noise-reducing GJs exist in both plexiform layers, including the cone–cone GJs in the outer retina as well as those that couple AII ACs in extended homologous arrays in the inner retina [15]. On the other hand, heterologous GJs connecting rods to cone PRs provide an alternative route for rod-mediated signals [6,16,17,18,19,20,21]. Finally, a cohort of studies showed that GJs formed between RGC neighbors or connecting RGCs to nearby ACs partake in the correlation of the RGC spike output sent towards the brain [22,23,24,25,26]. Overall, these data indicate that electrical synaptic circuitry in the retina is as complex and dynamic as the well-described circuits formed by chemical neurotransmitters.
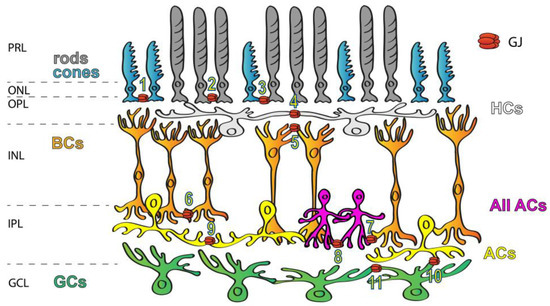
Figure 1.
Expression of GJs in the mammalian retina. GJs connecting retinal neurons are ubiquitous and they form a variety of connections, including rod/rod (1), cone/cone (2), rod/cone (3), HC/HC (4), dendritic BC/BC (5), axonal BC/BC (6), AII AC/ON BC (7), AII AC/AII AC (8), AC/AC (9), AC/RGC (10) and RGC/RGC (11) GJs. Labels to the left mark the layers of the retinal tissue: PRL—photoreceptors layer, ONL—outer nuclear layer, OPL—outer plexiform layer, INL—inner nuclear layer, IPL—inner plexiform layer and GCL—ganglion cells layer.
1.3. Connexin Composition of Retinal GJs
Cx36 has been found in both plexiform layers [27] of the retina of various mammalian species, whereas other subunits are restricted to either plexiform layer (Figure 2). Cx45 for example can be found mostly in the inner retina [28], whereas both Cx50 and Cx57 are expressed by the outer retinal HCs and thus their distribution is confined to the OPL [29,30]. Cx36 GJs have been reported in multiple sites of the rod pathways, including AII AC junctions and those formed between rod and cone PRs [27,31,32,33,34,35,36]. In the proximal retina, Cx45 has been localized to some of the axon terminals of ON cone BCs at sites where they form GJs with the Cx36-expressing AII ACs. While these latter connections are clearly heterotypic, the rest of the AII AC/ON cone BC connections are homotypic and show the presence of Cx36 in both neuron populations [37,38]. Therefore, both Cx36 and Cx45 are essential subunits for transmitting rod-mediated signals for night vision pathways. Apart from mediating signals of the night vision pathways, both Cx36 and Cx45 participate in RGC circuits to serve visual feature encoding. It has been shown for instance that Cx36 is expressed by α-GCs and that Cx45 comprises GJs of ON–OFF direction-selective RGCs, serving spike synchronization and encoding of the direction of movement, respectively [39,40,41,42]. In addition to Cx36 and Cx45, only one other subunit, Cx30.2, has been shown to be expressed by A1 type of RGCs [43]. Besides the retinal distribution pattern of the Cx protein subunits, the expression levels of corresponding Cx36 mRNA transcripts have been studied as well, and their distribution patterns correlated with those of the subunit proteins [44,45,46]. However, GJs are not static bridges but, similar to chemical synapses, their prevalence and gating properties are highly regulated by changes throughout the postnatal development by external factors like adaptation to various light conditions and/or by the circadian master clock [38,47]. Many of the rapid changes in GJ coupling appear to be due to changes in post-translational modification (i.e., phosphorylation) rather than changes in protein expression, but longer lasting intrinsic and/or environmental alterations also induce expression changes as well. The following sections will point out the milestones in the corresponding field of research.
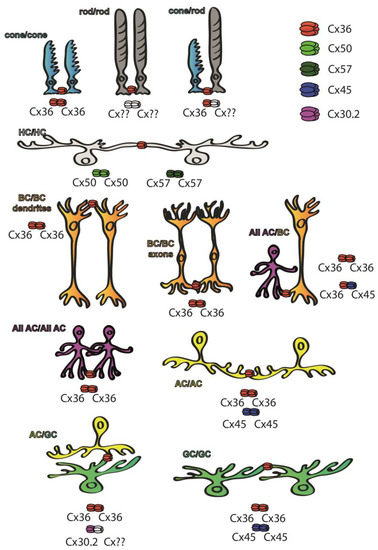
Figure 2.
Cx subunit composition of GJs connecting neurons of the mouse retina. While Cx36 subunits are ubiquitous throughout the retina [27], Cx50 and Cx57 subunits are only found in outer retinal HC GJ connections [29,30], whereas Cx45 is majorly expressed in the inner retina [28]. Some of the retinal GJs are homotypic thus connecting neurons that express the same Cx subunits in their hemichannels, including cone/cone GJs [27,31,32,33,34,35,36], AII GJs [31,32,33] that comprise Cx36 or ON–OFF DS RGCs that express Cx45 [41]. Other connections are clearly heterotypic, thus connecting neurons expressing at least two different Cx subunits. The best known such connection is formed between BC axon terminals and AII ACs, in which AII cells express Cx36 while BCs express either Cx36 or Cx45 depending on their cellular subtype [37,38]. A third cohort of connections comprise a connexon whose identity has been confirmed, while the connecting hemichannel has not been determined yet (marked by ??). These latter connections include rod hemichannels [27,31,32,33,34,35,36], GJs that connect α-GCs into an array [39,40,42] or the ACs that form GJs with Cx30.2-expressing A1 type RGCs [43].
2. Postnatal Changes in Connexin Expression
Over 50 years ago, Potter and his colleagues [48] provided electrophysiological evidence that the squid embryo maintained low-resistance conduits between certain cells during development that are subsequently lost in the adult animal. Soon after this first demonstration, it became clear that this phenomenon is not unique to the squid embryo, but it is rather ubiquitous in the animal kingdom. GJs have been found in both invertebrates including the sea urchin, starfish, ascidians (note that invertebrates utilize innexin proteins to build GJs) and various species from all vertebrate classes [49]. Since those early days, it has repeatedly been shown that the expression of GJ-forming Cx transcripts display a temporal pattern during organogenesis both outside the brain [50,51,52] and in many brain areas [53,54,55,56,57]. Cx36 expression in the murine retina for example displayed an increased expression from P1 to adult retinal stages in the two plexiform layers [58]. Quantitative polymerase chain reaction (qPCR) and western blot (WB) measurements in the rat retina also showed a gradual increase in Cx36 expression in both the mRNA transcript and protein levels [46], suggesting that the described developmental expression changes are ubiquitous among mammalian species. It appears that Cx36 immunoreactive puncta appear first in a few cells in the presumptive ganglion cell layer at P1 [45] and become abundant in the two plexiform layers at P10 [46]. This latter developmental stage also coincides with a peculiar surge in Cx36 protein expression in WB measurements and just precedes the maximum of Cx36 mRNA expression at P15 [45,46]. The Cx36 expression peak also overlaps with the observable colocalizations between Cx36 puncta and parvalbumin (PV)-expressing rat AII amacrine cell dendrites in strata 3 and 4, whereas those located on AII amacrine cell dendritic branches in stratum 5 appear later at P15 and become pronounced only by P20 [46]. This thus insinuates that contrary to the presence of Cx36 in many retinal microcircuits, its expression maximum is closely linked to the formation of retinal night vision pathways. Although most studies followed the Cx36 expression changes during the early postnatal (P1–P20) and adult (P30–P60) life, little is known about the aging (>P300) retina (note that all the postnatal timepoints here refer to the murine retina as the most popular experimental models of retinal research). Based on our own unpublished observations, the aging mouse retina seems to lose much of the Cx36 immunoreactivity in terms of the number of antiserum-stained plaques in the IPL. However, it is unclear if this is related to the loss of Cx36 GJs and the corresponding function, or if it is due to other issues including: (i) Cx36 being replaced by other Cx subtypes in the aging retina; (ii) the a-Cx36 antiserum not recognizing the protein that might change the active conformation with time; (iii) the Cx36 GJs reducing in size over time and most of them reducing beyond the detection level or (iv) the aging tissue being more sensitive to the experimental manipulations and samples start disintegrating when the same experimental protocol is utilized. Thus, future work should clarify this question. In contrast to the monotonous increase in Cx36 throughout postnatal development, the expression profile of Cx45, the other major subunit utilized by retinal neurons, displays a continuous decrement from P1 to adulthood [58]. This correlates with the increasing downregulation of the Cx45 mRNA level during development in the rat retina [45,59] (our own unpublished observations, Figure 3a,b). This differential expression pattern of these two Cx subunits (Cx36 and Cx45) could be explained by the replacement or substitution of some of the Cx45 GJs by Cx36 connections during the early postnatal life.
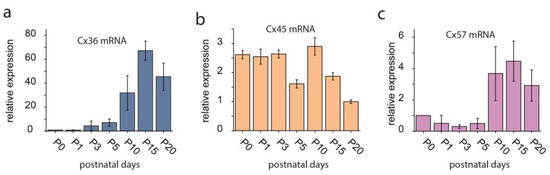
Figure 3.
Expression of the Cx36 (a), Cx45 (b) and Cx57 (c) mRNA transcript in the rat retina throughout the early postnatal life (P0–P20). Adopted and modified from Kovács-Öller et al., 2014 and Kovács-Öller PhD thesis [46,60]. Error bars represent SD for data from n = 3 independent samples.
Besides the above two prevalent Cx subunits, Cx30.2 is also expressed in some RGC connections [43], whereas Cx50 and Cx57 subunits form horizontal cell GJs [29,61,62]. The developmental changes of these latter Cx subunits have not been established yet; although, our unpublished observations indicate that the Cx57 mRNA transcript level is monotonously increasing during postnatal development P0-P15 and displays a plateau in the adult tissue (unpublished observations, Figure 3c). Since this latter Cx is specifically expressed by retinal HCs, its expression level likely follows the maturation of the outer retinal wiring especially those formed by horizontal cells. One other well-studied subunit is Cx43 whose expression, similar to those of Cx36, displayed an increased expression from early postnatal to adult retinal stages [58]. Cx43 is almost absent in retinal progenitor cells at P1, but it becomes prominent at P10. Much of this label at this stage seems to coincide with vimentin-positive Muller cell processes. In addition to protein expression, quantitative PCR showed that Cx43 and vimentin transcripts also shared very similar temporal expression patterns indicating the strong correlation between Muller cell maturation and Cx43 functioning. Besides Muller cells, Cx43 expression was also prevalent in the nerve ganglion cell layers later at P5–P15 [58]. At least some of this latter staining can be accounted for by the Cx43 expression of astrocytes, based on the colocalization of Cx43 with GFAP from P15 [45]. The importance of Cx43 is also highlighted by the expression profile alterations in different diseases like in ischemia [63] and in diabetic retinopathy in the retinal vasculature [64,65] In addition to their roles in the adult retina, some GJs have been suggested to play a role in neuronal development as well. It has been shown, using neuronal precursor cells, that Cx43 expression levels increased with postnatal age in the subventricular zone. This suggested that Cx43 might be a negative regulator of cell proliferation, even though it promotes proliferation during embryonic development [66]. In addition, Cx45 has been hypothesized to enhance synaptogenesis based on its frequent colocalization with Synaptophysin and the elevated number of heterotypic Cx45/Cx36 gap junctions prior to eye opening [67].
3. Light Adaptation-Induced Changes in Connexin Expression Levels
During the daily darkness/brightness cycle, the retina operates over a ~50-billion-fold change in illumination and maintains a broad dynamic range. This involves a tremendous capacity of light adaptation including several different mechanisms. One of these involves a shift of signaling between the rod and cone photoreceptor-initiated pathways. This rerouting of visual signals within the retina happens slowly during dusk and dawn but also occurs more quickly when one enters a dark room during daylight (or exits from a dark room to a bright exterior). For the smooth transition between signaling systems, the retina utilizes an intermediary route, called the secondary rod pathway, which relies on Cx36 GJs between rods and cones. The Cx protein subunit turnover takes only a few hours, leaving an effective time window for a potential light adaptation-induced Cx transcript and protein expression change [68]. However, the sudden light condition changes require considerably faster mechanisms than the expression of new channel-forming Cx subunits to increase GJ conductance. Therefore, rather than offsetting the Cx biosynthesis/degradation balance, the opening probability of GJs increases (GJs open) in darkness and dim light and decreases (GJs close) in bright light. When open, rod/cone GJs transmit rod signals through cones to cone bipolar cells that eventually target RGCs. The key step in this process is the alteration of the GJ opening probability, which is performed by the gradual phosphorylation/dephosphorylation of the Cx36 protein subunits. When Cx36 subunits are phosphorylated, the Cx36 GJ conductance between cones and rods increases [69]. In daylight, activation of dopamine D2/D4 receptors in cone photoreceptors inhibits adenylate cyclase activity and prevents the cAMP-dependent protein kinase A (PKA)-mediated Cx36 phosphorylation. Therefore, Cx36 channels are kept dephosphorylated and closed during bright light conditions. During darkness, on the other hand, the inhibition of adenylate cyclase is relieved and phosphorylation of Cx36 results in an increase in GJ conductance. In addition to dopamine, whose release is induced by light, intracellular adenosine levels also play a role in regulating photoreceptor GJ coupling. When adenosine levels are high at night, adenosine opposes the dopamine effect and enhances coupling, whereas it reinforces the dopamine effect during the day when both compounds suppress coupling [70]. Adaptation to scotopic (rod-mediated) vision, besides changes in the photoreceptor GJ gating, occurs in the rest of the retina as well. The electrical circuitry in the retina for example is dynamically regulated by light, suggesting that it plays a major role in adaptation [11,71,72,73,74,75,76,77,78]. For example, adaptational changes have been shown to modulate the extent of both tracer and electrical coupling of HCs that are maximized under dim ambient light conditions and diminish as the retina is dark- or light-adapted from this level [77,79,80,81,82,83,84]. Light adaptation increases expression of Cx57 [85] allowing for the slow adaptation of circuits to changing light conditions. A faster mechanism through mediators like dopamine or NO, both of which are released in the retina on a light-dependent matter, also affects signaling across the extensively coupled HC array. Dopamine, for example, decreases the HC GJ conductance acting through the intracellular messenger cAMP, thereby producing a concomitant reduction in receptive field size [86,87,88,88]. Through a separate intracellular cascade, NO also alters intracellular levels of cGMP and increases HC electrical coupling [89,90]. Similarly, under dark-adapted conditions, AII ACs in the proximal retina are coupled in relatively small groups, but the size of coupled AII cell groups dramatically increases following exposure to dim background light. This then reverts and AII cells uncouple secondarily when the retina is further adapted to even more intense background light [72]. AII cells form homologous GJs with nearby AIIs using Cx36 subunits [31,32], whereas their heterologous GJ contacts with ON cone bipolar cells comprises a mixture of Cx36 and Cx45 [31,32,34,91,92]. These differences in the subunit composition of the two sets of AII GJ populations can explain their different conductance and pharmacology. While homologous AII GJs are modulated by levels of the intracellular cAMP, those connecting AIIs to ON cone bipolar cells are modulated by cGMP [11]. Although, many other retinal interneurons (BCs and ACs) display GJ connections with their neighbors [15,93], the light-dependent changes in their conductance and/or Cx expression levels have not been studied yet. Besides GJs in PRs and the retinal interneurons, the light-modulated GJ coupling of the retinal output neuron RGCs has been studied for some of the subtypes. RGCs were found to be extensively coupled in the inner retina and light adaptation induces a monotonous expansion of coupled RGC arrays [43,94,95]. It has been proven for example that among ON–OFF direction-selective ganglion cells (DSGCs), superior preferring cells exhibited a broadening in their direction tuning at low light levels; however, the responsible intracellular mechanisms are not known [96]. On the other hand, it is well documented that light adaptation activated intracellular cascades that eventually lead to PKA-induced phosphorylation of Cx36 in OFF α-GCs arrays, whereas light adaptation initiated another intracellular cascade in the G1 RGC coupled network where a PKC-mediated phosphorylation of Cx30.2 occurred [43,97,98,99]. Less is known about the light adaptation-induced changes in the levels of Cx mRNA transcripts and corresponding proteins. It has been shown that Cx57, Cx45, Cx36 and Cx43 mRNA transcript levels were repressed by prolonged darkness (occurring between 3 h and 7 days). These changes were accompanied by a similar dark-induced downregulation of Cx36 and Cx43 protein subunits [45,58]. These results, therefore, indicate that the time window provided by the timescale of illumination changes during the night/day cycle could be effectively utilized to modulate the Cx transcript and protein expression changes [68] and corresponding GJ functioning in the retina.
4. Circadian Rhythmicity and GJ Function
Previous sections described how retinal GJs and their Cx building blocks endure expression changes throughout the lifespan of an organism and summarized how quick environmental changes affect the intracellular milieu and corresponding GJ gating in various retinal cells. The responses of the retinal tissue to changes on these two timescales overlap considerably. For example, adaptation to altered light conditions initially involves only the on- or offset of intracellular signaling mechanisms and the corresponding change in GJ gating. However, as pointed out in the previous section, longer lasting light adaptation eventually exerts Cx expression changes as well [45,58]. Thus, the daily cyclic alterations of the environment occur on overlapping timescales that evoke both quick molecular responses and longer-lasting mRNA and protein expression changes in neurons of the retina. That said, the daily light–dark cycle regulates neuronal activity in the retina, and a corresponding switch in signaling through rod- and cone-specific retinal circuitry occurs (see above). In addition to the rerouting of retinal signals, the expression levels of melatonin and dopamine [100] are also in the counter phase [101], serving as reciprocally inhibitory messengers for darkness and light, respectively [95,102]. These initial light/dark-induced changes in melatonin and dopamine levels mediate the expression of a cohort of other molecules, including the upregulation of protein kinase C in BCs in daylight conditions and the upregulation of parvalbumin in AII ACs in dark conditions [103,104]. Many of these changes can be explained by the diurnal rhythm and adaptation to ambient light levels (see above). However, maintained rhythmicity of melatonin synthesis has been observed in retinal cultures even in constant darkness [102,105,106]. Clearly, mechanisms under the control of the circadian clock also act on this overlapping time scale and thus contribute to some of the previously presented quick molecular changes and induce alterations in mRNA and protein expression with a slower onset. In this context, “diurnal rhythm” refers to responses to environmental cues (e.g., changes in the levels of background light), whereas a circadian rhythm is instead controlled by an endogenous oscillator. Besides melatonin, expression of certain retinal proteins, including iodopsin in cones [107], parvalbumin in AII ACs [104] and tryptophan hydroxylase in ACs [108], have been reported to follow a rhythm independent of the light-induced diurnal cycle. Moreover, many genes in the retina follow circadian rhythmicity, including core circadian clock genes (Per1, Per2, Cry1, Cry2 and Bmal1; [109], transcriptional activators Rora, Rorb and Rorc; 51; [110], as well as miRNAs miR-103, miR-106b, miR-124a, miR-182, miR-422a and miR-422b51), which modulate the translation of mRNA transcripts into functional proteins. This converging evidence indicates that the retina has a biological circadian clock and that the switch from rod to cone vision involves the diurnal and also the circadian rhythm. The outer nuclear, inner nuclear and ganglion cell layers of the retina have been found to each contain an independent layer-specific circadian oscillator (93). It has been demonstrated for example that the relatively weak rod–cone electrical coupling in the retina is under circadian regulation [111,112]. It is known that during the day, the decreased release of melatonin results in increased dopamine release from dopaminergic ACs [111,112]. Dopamine then activates D2-like receptors on rods and cones, which results in decreased intracellular cAMP levels, decreased PKA activation and decreased Cx36 phosphorylation in the PRs [113,114,115,116,117,118,119,120,121]. Decreased Cx36 phosphorylation is correlated with decreased rod–cone GJ conductance [122]. In contrast, during the night, the retinal clock increases melatonin synthesis, which reduces extracellular dopamine levels and D2-like receptor activation, resulting in increased Cx36 phosphorylation [112,117]. Increased Cx36 phosphorylation is correlated with increased rod–cone GJ coupling [122,123], allowing the convergence of rod signals into cone pathways [69]. Whether the circadian rhythm regulates Cx36 mRNA transcript and protein levels in addition to Cx36 phosphorylation seems to be a controversial question. One study reported higher levels of Cx36 mRNA transcript and protein during the subjective night than during the day [124], but other studies have not found such circadian regulation [99,123]. Interestingly, in addition to the increased rod–cone GJ coupling during the night, it has been found that rod–rod GJ coupling is also under circadian regulation in the mouse retina, with more coupling during the night than during the day [125,126]. This trend is observed in the melatonin-proficient CBA/Ca mouse strain but not in the melatonin-deficient C57BL/6 mouse, indicating that the circadian regulation of rod–rod GJ coupling is also dependent on the melatonin/dopamine neuromodulator interaction [126]. In contrast to PR coupling, gating of other retinal GJs may not be under the control of the circadian clock. It appears for example that activation of D1 receptors, which causes GJ uncoupling between HCs, is not under circadian regulation as the extent of GJ coupling between horizontal cells has been found to be equal during night and during the day in the fish retina [127]. Independent of the melatonin/dopamine interaction, extracellular levels of adenosine are also under circadian regulation, such that extracellular adenosine levels are high during the night and lower during the day [112,128]. It has been demonstrated in the goldfish retina that the activation of adenosine A2A receptors on PRs during the night also contributes to increased rod–cone GJ coupling, thereby further enhancing the difference in rod–cone GJ coupling between night and day [128]. Thus, adenosine and dopamine both play a role in the circadian variation of rod–cone GJ coupling, but they do so with opposite effects on the PKA pathway and on Cx36 phosphorylation [122,128]. In addition to the D2-like and A2A receptors, the cannabinoid CB1 receptors on PRs have also been found to play a role in enhancing the difference in rod–cone GJ coupling between night and day. During the day, when the D2-like receptors are activated, endogenous activation of CB1 receptors decreases rod–cone GJ coupling. During the night, when the D2-like receptors are not activated, CB1 receptor activation increases rod–cone GJ coupling. Thus, it appears that the effect of CB1 activation on the extent of rod–cone GJ coupling depends on the activation state of the D2-like receptors [129]. The above cases exemplify that the GJ function is strongly modulated by not only the level of background light but also by the status of the circadian biological clock. Besides a few well-studied GJ connections (those connecting PRs or those between AII ACs), however, there is little or no information on similar daily changes in the rest of the electrical synapses in the retina. It is also unknown if the above-indicated molecular changes mediated by both diurnal and circadian regulators are accompanied by Cx transcriptional modifications. According to the scarce literature [122,123,124] and our own unpublished observations, such Cx expression responses do exist [46,60]. In the rat retina, we observed daily fluctuations of the Cx36 mRNA levels that reached the maximum both in the middle of the day and the middle of the night, while it was downregulated during dusk and dawn (Figure 4).
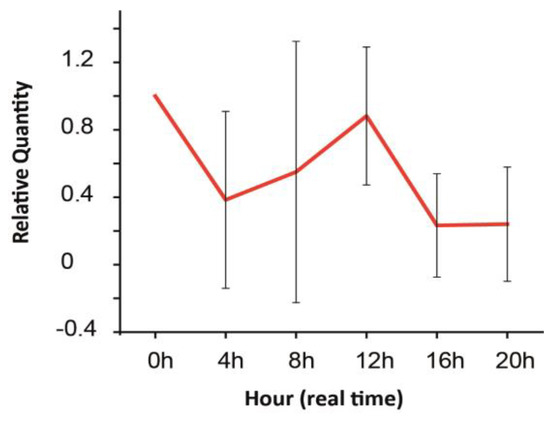
Figure 4.
Expression of the Cx36 mRNA transcript in the rat retina throughout an entire 24 h day period. Adopted and modified from Kovács-Öller et al., 2014 and Kovács-Öller PhD thesis [46,60]. Error bars represent SD for data from n = 3 independent samples.
Although the observed Cx36 mRNA level changes were not robust (and were not significant), they may correspond to the increasing GJ gating and Cx36 protein need in the coming dusk and dawn periods (note that mRNA expressions should precede protein level elevations thus mRNA transcript levels peak hours prior to when protein expression needs a boost). It is unknown, however, how and to what extent external diurnal and internal circadian factors contribute to these changes. Future work will enlighten these details along with those describing how other retinal Cx transcript and protein levels are controlled by the daily rhythm and if circadian mechanisms partake in that process. It has also been demonstrated that when the retina is treated with a GJ blocker, the circadian period of each layer-specific oscillator increases significantly compared to the normal circadian period of the whole retina (93). This thus reflects that GJs and Cx building blocks are not just under the control of daily cyclic changes but also partake in the functioning of the circadian clock. This indicates that an important mutual interaction exists between GJ coupling and the retinal circadian rhythm.
5. Conclusions
In this present review, we summarized latest advances in the field of GJ signaling in the mammalian retina with a focus on the expression alterations of GJ-forming retinal Cx subunits as a response to intrinsic and environmental changes. Such changes include the cyclic modulation of the intracellular molecular milieu driven by the circadian clock as well as cyclic diurnal and monotonous alterations throughout the postnatal life of an organism. While the Cx mRNA and protein expression alterations appear more prominent, changes occur on a longer timescale (throughout postnatal life or extended light/dark adaptation) and they also seem to follow quick changes of the internal and external environment, including circadian rhythmicity and diurnal light level alterations, respectively. By demonstrating these changes in GJ signaling as well as expression levels of the Cx building blocks, this work exemplifies how signaling through a sensory organ adapts to the altered environmental conditions. In addition, this converging evidence also pinpoints the importance of the standardization of experimental conditions, such as the age of the animal models, the timing of experiments and also the light conditions.
Author Contributions
Conceptualization, B.V. and T.K.-Ö.; validation, T.K.-Ö. and B.V.; formal analysis, T.K.-Ö.; investigation, G.H., G.V., L.P., G.S. and L.R.; resources, B.V.; data curation, T.K.-Ö.; writing—original draft preparation, G.H., G.V., L.P., G.S. and L.R.; writing—review and editing, L.R., G.S. and B.V.; visualization, T.K.-Ö. and B.V.; supervision, B.V.; project administration, B.V., G.H. and G.S.; funding acquisition, B.V. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the NKFIH and the European Union under the action of the ERA-NET COFUND (2019-2.1.7-ERANET-2021–00018; NEURON (NEURON-066 Rethealthsi) to B.V. The study was also financially supported by the NKFI (OTKA NN128293) (B.V.) from the European Union and the State of Hungary, co-financed by the European Social Fund in the framework of TKP2020 IKA-07 National Excellence Program (BV, T.K.-Ö.). In addition, this research was also financed by the Thematic Excellence Program 2021 Health Sub-programme of the Ministry for Innovation and Technology in Hungary, within the framework of the EGA-16 project of the University of Pécs. Finally, this work was supported by the ÚNKP-22-3-II-PTE-1414 (G.S.) New National Excellence Program of the Ministry of Human Capacities.
Institutional Review Board Statement
Animal handling, housing and experimental procedures were reviewed and approved by the ethics committee of the University of Pécs (BA/35/51-42/2016 and BA02/2000-69/2017). All animals were treated in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. All efforts were made to minimize pain and discomfort during the experiments, and all procedures were carried out by obeying the 3R law.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Farquhar, M.G.; Palade, G.E. Cell junctions in amphibian skin. J. Cell Biol. 1965, 26, 263–291. [Google Scholar] [CrossRef]
- Furshpan, E.J.; Potter, D.D. Mechanism of Nerve-Impulse Transmission at a Crayfish Synapse. Nature 1957, 180, 342–343. [Google Scholar] [CrossRef] [PubMed]
- Rosenbluth, J. Smooth Muscle: An Ultrastructural Basis for the Dynamics of Its Contraction. Science 1965, 148, 1337–1339. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A. The interaction of electrical activity among neurons of lobster cardiac ganglion. Jpn. J. Physiol. 1958, 8, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Brightman, M.W.; Reese, T.S. Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell Biol. 1969, 40, 648–677. [Google Scholar] [CrossRef]
- Raviola, E.; Gilula, N.B. Gap Junctions between Photoreceptor Cells in the Vertebrate Retina. Proc. Natl. Acad. Sci. USA 1973, 70, 1677–1681. [Google Scholar] [CrossRef]
- Cruciani, V.; Mikalsen, S.-O. The Vertebrate Connexin Family. Cell. Mol. Life Sci. 2006, 63, 1125–1140. [Google Scholar] [CrossRef] [PubMed]
- Söhl, G.; Willecke, K. An Update on Connexin Genes and Their Nomenclature in Mouse and Man. Cell Commun. Adhes. 2003, 10, 173–180. [Google Scholar] [CrossRef]
- De Boer, T.P.; Van Der Heyden, M.A.G. Xenopus Connexins: How Frogs Bridge the Gap. Differentiation 2005, 73, 330–340. [Google Scholar] [CrossRef]
- Marc, R.E.; Liu, W.L.; Muller, J.F. Gap Junctions in the Inner Plexiform Layer of the Goldfish Retina. Vis. Res. 1988, 28, 9–24. [Google Scholar] [CrossRef]
- Mills, S.L.; Massey, S.C. Differential Properties of Two Gap Junctional Pathways Made by AII Amacrine Cells. Nature 1995, 377, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Raviola, E.; Gilula, N.B. Intramembrane Organization of Specialized Contacts in the Outer Plexiform Layer of the Retina. A Freeze-Fracture Study in Monkeys and Rabbits. J. Cell Biol. 1975, 65, 192–222. [Google Scholar] [CrossRef] [PubMed]
- Vaney, D.I. Many Diverse Types of Retinal Neurons Show Tracer Coupling When Injected with Biocytin or Neurobiotin. Neurosci. Lett. 1991, 125, 187–190. [Google Scholar] [CrossRef]
- Xin, D.; Bloomfield, S.A. Tracer Coupling Pattern of Amacrine and Ganglion Cells in the Rabbit Retina. J. Comp. Neurol. 1997, 383, 512–528. [Google Scholar] [CrossRef]
- Bloomfield, S.A.; Völgyi, B. The Diverse Functional Roles and Regulation of Neuronal Gap Junctions in the Retina. Nat. Rev. Neurosci. 2009, 10, 495–506. [Google Scholar] [CrossRef]
- Bloomfield, S.A.; Völgyi, B. Function and Plasticity of Homologous Coupling between AII Amacrine Cells. Vis. Res. 2004, 44, 3297–3306. [Google Scholar] [CrossRef] [PubMed]
- Dacheux, R.; Raviola, E. Horizontal Cells in the Retina of the Rabbit. J. Neurosci. 1982, 2, 1486–1493. [Google Scholar] [CrossRef]
- Nelson, R. Cat Cones Have Rod Input: A Comparison of the Response Properties of Cones and Horizontal Cell Bodies in the Retina of the Cat. J. Comp. Neurol. 1977, 172, 109–135. [Google Scholar] [CrossRef]
- Schneeweis, D.; Schnapf, J. Photovoltage of Rods and Cones in the Macaque Retina. Science 1995, 268, 1053–1056. [Google Scholar] [CrossRef]
- Smith, R.G.; Vardi, N. Simulation of the Aii Amacrine Cell of Mammalian Retina: Functional Consequences of Electrical Coupling and Regenerative Membrane Properties. Vis. Neurosci. 1995, 12, 851–860. [Google Scholar] [CrossRef]
- Völgyi, B.; Deans, M.R.; Paul, D.L.; Bloomfield, S.A. Convergence and Segregation of the Multiple Rod Pathways in Mammalian Retina. J. Neurosci. 2004, 24, 11182–11192. [Google Scholar] [CrossRef]
- Brivanlou, I.H.; Warland, D.K.; Meister, M. Mechanisms of Concerted Firing among Retinal Ganglion Cells. Neuron 1998, 20, 527–539. [Google Scholar] [CrossRef]
- DeVries, S.H. Correlated Firing in Rabbit Retinal Ganglion Cells. J. Neurophysiol. 1999, 81, 908–920. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.H.; Bloomfield, S.A. Gap Junctional Coupling Underlies the Short-Latency Spike Synchrony of Retinal α Ganglion Cells. J. Neurosci. 2003, 23, 6768–6777. [Google Scholar] [CrossRef] [PubMed]
- Mastronarde, D.N. Correlated Firing of Cat Retinal Ganglion Cells. I. Spontaneously Active Inputs to X- and Y-Cells. J. Neurophysiol. 1983, 49, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Völgyi, B.; Pan, F.; Paul, D.L.; Wang, J.T.; Huberman, A.D.; Bloomfield, S.A. Gap Junctions Are Essential for Generating the Correlated Spike Activity of Neighboring Retinal Ganglion Cells. PLoS ONE 2013, 8, e69426. [Google Scholar] [CrossRef]
- Güldenagel, M.; Söhl, G.; Plum, A.; Traub, O.; Teubner, B.; Weiler, R.; Willecke, K. Expression Patterns of Connexin Genes in Mouse Retina. J. Comp. Neurol. 2000, 425, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Petrasch-Parwez, E.; Habbes, H.-W.; Weickert, S.; Löbbecke-Schumacher, M.; Striedinger, K.; Wieczorek, S.; Dermietzel, R.; Epplen, J.T. Fine-Structural Analysis and Connexin Expression in the Retina of a Transgenic Model of Huntington’s Disease: Retinal Degeneration in R6/2 Transgenic Mice. J. Comp. Neurol. 2004, 479, 181–197. [Google Scholar] [CrossRef]
- Hombach, S.; Janssen-Bienhold, U.; Sohl, G.; Schubert, T.; Bussow, H.; Ott, T.; Weiler, R.; Willecke, K. Functional Expression of Connexin57 in Horizontal Cells of the Mouse Retina. Eur. J. Neurosci. 2004, 19, 2633–2640. [Google Scholar] [CrossRef]
- Massey, S.C.; O’Brien, J.J.; Trexler, E.B.; Li, W.; Keung, J.W.; Mills, S.L.; O’Brien, J. Multiple Neuronal Connexins in the Mammalian Retina. Cell Commun. Adhes. 2003, 10, 425–430. [Google Scholar] [CrossRef]
- Deans, M.R.; Volgyi, B.; Goodenough, D.A.; Bloomfield, S.A.; Paul, D.L. Connexin36 Is Essential for Transmission of Rod-Mediated Visual Signals in the Mammalian Retina. Neuron 2002, 36, 703–712. [Google Scholar] [CrossRef]
- Feigenspan, A.; Teubner, B.; Willecke, K.; Weiler, R. Expression of Neuronal Connexin36 in AII Amacrine Cells of the Mammalian Retina. J. Neurosci. 2001, 21, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Feigenspan, A.; Janssen-Bienhold, U.; Hormuzdi, S.; Monyer, H.; Degen, J.; Söhl, G.; Willecke, K.; Ammermüller, J.; Weiler, R. Expression of Connexin36 in Cone Pedicles and OFF-Cone Bipolar Cells of the Mouse Retina. J. Neurosci. 2004, 24, 3325–3334. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Massey, S.C. Electrical Synapses in Retinal ON Cone Bipolar Cells: Subtype-Specific Expression of Connexins. Proc. Natl. Acad. Sci. USA 2005, 102, 13313–13318. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-J.; Han, J.-W.; Kim, H.-J.; Kim, I.-B.; Lee, M.-Y.; Oh, S.-J.; Chung, J.-W.; Chun, M.-H. The Immunocytochemical Localization of Connexin 36 at Rod and Cone Gap Junctions in the Guinea Pig Retina. Eur. J. Neurosci. 2003, 18, 2925–2934. [Google Scholar] [CrossRef]
- Mills, S.L.; O’Brien, J.J.; Li, W.; O’Brien, J.; Massey, S.C. Rod Pathways in the Mammalian Retina Use Connexin 36. J. Comp. Neurol. 2001, 436, 336–350. [Google Scholar] [CrossRef]
- Lin, B.; Jakobs, T.C.; Masland, R.H. Different Functional Types of Bipolar Cells Use Different Gap-Junctional Proteins. J. Neurosci. 2005, 25, 6696–6701. [Google Scholar] [CrossRef]
- Maxeiner, S.; Dedek, K.; Janssen-Bienhold, U.; Ammermüller, J.; Brune, H.; Kirsch, T.; Pieper, M.; Degen, J.; Krüger, O.; Willecke, K.; et al. Deletion of Connexin45 in Mouse Retinal Neurons Disrupts the Rod/Cone Signaling Pathway between AII Amacrine and ON Cone Bipolar Cells and Leads to Impaired Visual Transmission. J. Neurosci. 2005, 25, 566–576. [Google Scholar] [CrossRef]
- Hidaka, S.; Akahori, Y.; Kurosawa, Y. Dendrodendritic Electrical Synapses between Mammalian Retinal Ganglion Cells. J. Neurosci. 2004, 24, 10553–10567. [Google Scholar] [CrossRef]
- Schubert, T.; Degen, J.; Willecke, K.; Hormuzdi, S.G.; Monyer, H.; Weiler, R. Connexin36 Mediates Gap Junctional Coupling of Alpha-Ganglion Cells in Mouse Retina. J. Comp. Neurol. 2005, 485, 191–201. [Google Scholar] [CrossRef]
- Schubert, T.; Maxeiner, S.; Krüger, O.; Willecke, K.; Weiler, R. Connexin45 Mediates Gap Junctional Coupling of Bistratified Ganglion Cells in the Mouse Retina. J. Comp. Neurol. 2005, 490, 29–39. [Google Scholar] [CrossRef]
- Völgyi, B.; Abrams, J.; Paul, D.L.; Bloomfield, S.A. Morphology and Tracer Coupling Pattern of Alpha Ganglion Cells in the Mouse Retina. J. Comp. Neurol. 2005, 492, 66–77. [Google Scholar] [CrossRef]
- Pérez De Sevilla Müller, L.; Dedek, K.; Janssen-Bienhold, U.; Meyer, A.; Kreuzberg, M.M.; Lorenz, S.; Willecke, K.; Weiler, R. Expression and Modulation of Connexin30.2, a Novel Gap Junction Protein in the Mouse Retina. Vis. Neurosci. 2010, 27, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Kihara, A.H.; Santos, T.O.; Osuna-Melo, E.J.; Paschon, V.; Vidal, K.S.M.; Akamine, P.S.; Castro, L.M.; Resende, R.R.; Hamassaki, D.E.; Britto, L.R.G. Connexin-mediated Communication Controls Cell Proliferation and Is Essential in Retinal Histogenesis. Int. J. Dev. Neurosci. 2010, 28, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Kihara, A.H.; Mantovani De Castro, L.; Belmonte, M.A.; Yan, C.Y.I.; Moriscot, A.S.; Hamassaki, D.E. Expression of Connexins 36, 43, and 45 during Postnatal Development of the Mouse Retina. J. Neurobiol. 2006, 66, 1397–1410. [Google Scholar] [CrossRef]
- Kovács-Öller, T.; Raics, K.; Orbán, J.; Nyitrai, M.; Völgyi, B. Developmental Changes in the Expression Level of Connexin36 in the Rat Retina. Cell Tissue Res. 2014, 358, 289–302. [Google Scholar] [CrossRef]
- Güldenagel, M.; Ammermüller, J.; Feigenspan, A.; Teubner, B.; Degen, J.; Söhl, G.; Willecke, K.; Weiler, R. Visual Transmission Deficits in Mice with Targeted Disruption of the Gap Junction Gene Connexin36. J. Neurosci. 2001, 21, 6036–6044. [Google Scholar] [CrossRef]
- Potter, D.D.; Furshpan, E.J.; Lennox, E.S. Connections between Cells of the Developing Squid as Revealed by Electrophysiological Methods. Proc. Natl. Acad. Sci. USA 1966, 55, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Warner, A. Gap Junctions in Development—A Perspective. Semin. Cell Biol. 1992, 3, 81–91. [Google Scholar] [CrossRef]
- Gimlich, R.L.; Kumar, N.M.; Gilula, N.B. Sequence and Developmental Expression of MRNA Coding for a Gap Junction Protein in Xenopus. J. Cell Biol. 1988, 107, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Gimlich, R.L.; Kumar, N.M.; Gilula, N.B. Differential Regulation of the Levels of Three Gap Junction MRNAs in Xenopus Embryos. J. Cell Biol. 1990, 110, 597–605. [Google Scholar] [CrossRef]
- Nishi, M.; Kumar, N.M.; Gilula, N.B. Developmental Regulation of Gap Junction Gene Expression during Mouse Embryonic Development. Dev. Biol. 1991, 146, 117–130. [Google Scholar] [CrossRef]
- Belliveau, D.J.; Kidder, G.M.; Naus, C.C.G. Expression of Gap Junction Genes during Postnatal Neural Development. Dev. Genet. 1991, 12, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Arai, Y.; Urano, A.; Hyodo, S. Androgen Regulates Gap Junction MRNA Expression in Androgen-Sensitive Motoneurons in the Rat Spinal Cord. Neurosci. Lett. 1991, 131, 159–162. [Google Scholar] [CrossRef]
- Matsumoto, A.; Arai, Y.; Urano, A.; Hyodo, S. Cellular Localization of Gap Junction MRNA in the Neonatal Rat Brain. Neurosci. Lett. 1991, 124, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Micevych, P.E.; Abelson, L. Distribution of MRNAs Coding for Liver and Heart Gap Junction Proteins in the Rat Central Nervous System. J. Comp. Neurol. 1991, 305, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Shiosaka, S.; Whittaker, M.E.; Hertzberg, E.L.; Nagy, J.I. Gap Junction Protein in Rat Hippocampus: Light Microscope Immunohistochemical Localization. J. Comp. Neurol. 1989, 281, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Kihara, A.H.; Mantovani De Castro, L.; Moriscot, A.S.; Hamassaki, D.E. Prolonged Dark Adaptation Changes Connexin Expression in the Mouse Retina. J. Neurosci. Res. 2006, 83, 1331–1341. [Google Scholar] [CrossRef]
- Kihara, A.H.; Paschon, V.; Akamine, P.S.; Saito, K.C.; Leonelli, M.; Jiang, J.X.; Hamassaki, D.E.; Britto, L.R.G. Differential Expression of Connexins during Histogenesis of the Chick Retina. Dev. Neurobiol. 2008, 68, 1287–1302. [Google Scholar] [CrossRef]
- Kovács-Öller, T. Factors Influencing the Expression of Gap Junction Forming Connexin Proteins in the Retina of Vertebrate Animals. Ph.D. Thesis, University of Pécs, Pécs, Hungary, 2015. [Google Scholar]
- Pan, F.; Keung, J.; Kim, I.-B.; Snuggs, M.B.; Mills, S.L.; O’Brien, J.; Massey, S.C. Connexin 57 Is Expressed by the Axon Terminal Network of B-Type Horizontal Cells in the Rabbit Retina. J. Comp. Neurol. 2012, 520, 2256–2274. [Google Scholar] [CrossRef]
- Dorgau, B.; Herrling, R.; Schultz, K.; Greb, H.; Segelken, J.; Ströh, S.; Bolte, P.; Weiler, R.; Dedek, K.; Janssen-Bienhold, U. Connexin50 Couples Axon Terminals of Mouse Horizontal Cells by Homotypic Gap Junctions: Distribution of Cx50 in Mouse Horizontal Cell. J. Comp. Neurol. 2015, 523, 2062–2081. [Google Scholar] [CrossRef]
- Toychiev, A.H.; Ivanova, E.; Yee, C.W.; Sagdullaev, B.T. Block of Gap Junctions Eliminates Aberrant Activity and Restores Light Responses during Retinal Degeneration. J. Neurosci. 2013, 33, 13972–13977. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.; Kovacs-Oller, T.; Sagdullaev, B.T. Vascular Pericyte Impairment and Connexin43 Gap Junction Deficit Contribute to Vasomotor Decline in Diabetic Retinopathy. J. Neurosci. 2017, 37, 7580–7594. [Google Scholar] [CrossRef]
- Kovacs-Oller, T.; Ivanova, E.; Bianchimano, P.; Sagdullaev, B.T. The Pericyte Connectome: Spatial Precision of Neurovascular Coupling Is Driven by Selective Connectivity Maps of Pericytes and Endothelial Cells and Is Disrupted in Diabetes. Cell Discov. 2020, 6, 39. [Google Scholar] [CrossRef]
- Swayne, L.A.; Bennett, S.A.L. Connexins and Pannexins in Neuronal Development and Adult Neurogenesis. BMC Cell Biol. 2016, 17, S10. [Google Scholar] [CrossRef] [PubMed]
- Hilgen, G. Connexin45 Colocalization Patterns in the Plexiform Layers of the Developing Mouse Retina. J. Anat. 2022, 243, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.W. Life Cycle of Connexins in Health and Disease. Biochem. J. 2006, 394, 527–543. [Google Scholar] [CrossRef]
- Zhang, S.; Lyuboslavsky, P.; Dixon, J.A.; Chrenek, M.A.; Sellers, J.T.; Hamm, J.M.; Ribelayga, C.P.; Zhang, Z.; Le, Y.Z.; Iuvone, P.M. Effects of Cone Connexin-36 Disruption on Light Adaptation and Circadian Regulation of the Photopic ERG. Investig. Ophthalmol. Vis. Sci. 2020, 61, 24. [Google Scholar] [CrossRef]
- Li, H.; Chuang, A.Z.; O’Brien, J. Regulation of Photoreceptor Gap Junction Phosphorylation by Adenosine in Zebrafish Retina. Vis. Neurosci. 2014, 31, 237–243. [Google Scholar] [CrossRef]
- Baldridge, W.H.; Vaney, D.I.; Weiler, R. The Modulation of Intercellular Coupling in the Retina. Semin. Cell Dev. Biol. 1998, 9, 311–318. [Google Scholar] [CrossRef]
- Bloomfield, S.A.; Xin, D.; Osborne, T. Light-Induced Modulation of Coupling between AII Amacrine Cells in the Rabbit Retina. Vis. Neurosci. 1997, 14, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Hampson, E.C.; Weiler, R.; Vaney, D.I. PH-Gated Dopaminergic Modulation of Horizontal Cell Gap Junctions in Mammalian Retina. Proc. Biol. Sci. 1994, 255, 67–72. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Weiler, R.; Vaney, D.I. Endogenous Dopaminergic Regulation of Horizontal Cell Coupling in the Mammalian Retina. J. Comp. Neurol. 2000, 418, 33–40. [Google Scholar] [CrossRef]
- Weiler, R.; He, S.; Vaney, D.I. Retinoic Acid Modulates Gap Junctional Permeability Between Horizontal Cells of The Mammalian Retina: Retinoic Acid and Gap Junction Coupling. Eur. J. Neurosci. 1999, 11, 3346–3350. [Google Scholar] [CrossRef]
- Weiler, R.; Pottek, M.; He, S.; Vaney, D.I. Modulation of Coupling between Retinal Horizontal Cells by Retinoic Acid and Endogenous Dopamine. Brain Res. Rev. 2000, 32, 121–129. [Google Scholar] [CrossRef]
- Xin, D.; Bloomfield, S.A. Comparison of the Responses of AII Amacrine Cells in the Dark- and Light-Adapted Rabbit Retina. Vis. Neurosci. 1999, 16, 653–665. [Google Scholar] [CrossRef]
- Xin, D.; Bloomfield, S.A. Effects of Nitric Oxide on Horizontal Cells in the Rabbit Retina. Vis. Neurosci. 2000, 17, 799–811. [Google Scholar] [CrossRef]
- Baldridge, W.H.; Ball, A.K. Background Illumination Reduces Horizontal Cell Receptive-Field Size in Both Normal and 6-Hydroxydopamine-Lesioned Goldfish Retinas. Vis. Neurosci. 1991, 7, 441–450. [Google Scholar] [CrossRef]
- Dong, C.J.; McReynolds, J.S. The Relationship between Light, Dopamine Release and Horizontal Cell Coupling in the Mudpuppy Retina. J. Physiol. 1991, 440, 291–309. [Google Scholar] [CrossRef]
- Mangel, S.C.; Dowling, J.E. Responsiveness and Receptive Field Size of Carp Horizontal Cells Are Reduced by Prolonged Darkness and Dopamine. Science 1985, 229, 1107–1109. [Google Scholar] [CrossRef]
- Mangel, S.C.; Dowling, J.E. The Interplexiform-Horizontal Cell System of the Fish Retina: Effects of Dopamine, Light Stimulation and Time in the Dark. Proc. R. Soc. Lond. B Biol. Sci. 1987, 231, 91–121. [Google Scholar] [CrossRef]
- Umino, O.; Lee, Y.; Dowling, J.E. Effects of Light Stimuli on the Release of Dopamine from Interplexiform Cells in the White Perch Retina. Vis. Neurosci. 1991, 7, 451–458. [Google Scholar] [CrossRef]
- Witkovsky, P.; Shi, X.-P. Slow Light and Dark Adaptation of Horizontal Cells in the Xenopus Retina: A Role for Endogenous Dopamine. Vis. Neurosci. 1990, 5, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Janssen-Bienhold, U.; Trümpler, J.; Hilgen, G.; Schultz, K.; De Sevilla Müller, L.P.; Sonntag, S.; Dedek, K.; Dirks, P.; Willecke, K.; Weiler, R. Connexin57 Is Expressed in Dendro-Dendritic and Axo-Axonal Gap Junctions of Mouse Horizontal Cells and Its Distribution Is Modulated by Light. J. Comp. Neurol. 2009, 513, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Lasater, E.M.; Dowling, J.E. Dopamine Decreases Conductance of the Electrical Junctions between Cultured Retinal Horizontal Cells. Proc. Natl. Acad. Sci. USA 1985, 82, 3025–3029. [Google Scholar] [CrossRef] [PubMed]
- Piccolino, F.C.; Zingirian, M.; Mosci, C. Classification of Proliferative Diabetic Retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 1987, 225, 245–250. [Google Scholar] [CrossRef]
- McHahon, D.G.; Knapp, A.G.; Dowling, J.E. Horizontal Cell Gap Junctions: Single-Channel Conductance and Modulation by Dopamine. Proc. Natl. Acad. Sci. USA 1989, 86, 7639–7643. [Google Scholar] [CrossRef]
- DeVries, S.H.; Schwartz, E.A. Hemi-Gap-Junction Channels in Solitary Horizontal Cells of the Catfish Retina. J. Physiol. 1992, 445, 201–230. [Google Scholar] [CrossRef]
- Pottek, M.; Schultz, K.; Weiler, R. Effects of Nitric Oxide on the Horizontal Cell Network and Dopamine Release in the Carp Retina. Vis. Res. 1997, 37, 1091–1102. [Google Scholar] [CrossRef]
- Roska, B.; Werblin, F. Vertical Interactions across Ten Parallel, Stacked Representations in the Mammalian Retina. Nature 2001, 410, 583–587. [Google Scholar] [CrossRef]
- Dedek, K.; Schultz, K.; Pieper, M.; Dirks, P.; Maxeiner, S.; Willecke, K.; Weiler, R.; Janssen-Bienhold, U. Localization of Heterotypic Gap Junctions Composed of Connexin45 and Connexin36 in the Rod Pathway of the Mouse Retina. Eur. J. Neurosci. 2006, 24, 1675–1686. [Google Scholar] [CrossRef] [PubMed]
- Kántor, O.; Varga, A.; Nitschke, R.; Naumann, A.; Énzsöly, A.; Lukáts, Á.; Szabó, A.; Németh, J.; Völgyi, B. Bipolar Cell Gap Junctions Serve Major Signaling Pathways in the Human Retina. Brain Struct. Funct. 2017, 222, 2603–2624. [Google Scholar] [CrossRef]
- Hu, E.H.; Pan, F.; Völgyi, B.; Bloomfield, S.A. Light Increases the Gap Junctional Coupling of Retinal Ganglion Cells: Light Increases Coupling between Retinal Ganglion Cells. J. Physiol. 2010, 588, 4145–4163. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.L.; Xia, X.-B.; Hoshi, H.; Firth, S.I.; Rice, M.E.; Frishman, L.J.; Marshak, D.W. Dopaminergic Modulation of Tracer Coupling in a Ganglion-Amacrine Cell Network. Vis. Neurosci. 2007, 24, 593–608. [Google Scholar] [CrossRef]
- Yao, X.; Cafaro, J.; McLaughlin, A.J.; Postma, F.R.; Paul, D.L.; Awatramani, G.; Field, G.D. Gap Junctions Contribute to Differential Light Adaptation across Direction-Selective Retinal Ganglion Cells. Neuron 2018, 100, 216–228.e6. [Google Scholar] [CrossRef]
- Kothmann, W.W.; Li, X.; Burr, G.S.; O’Brien, J. Connexin 35/36 Is Phosphorylated at Regulatory Sites in the Retina. Vis. Neurosci. 2007, 24, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Kothmann, W.W.; Massey, S.C.; O’Brien, J. Dopamine-Stimulated Dephosphorylation of Connexin 36 Mediates AII Amacrine Cell Uncoupling. J. Neurosci. 2009, 29, 14903–14911. [Google Scholar] [CrossRef]
- Urschel, S.; Höher, T.; Schubert, T.; Alev, C.; Söhl, G.; Wörsdörfer, P.; Asahara, T.; Dermietzel, R.; Weiler, R.; Willecke, K. Protein Kinase A-Mediated Phosphorylation of Connexin36 in Mouse Retina Results in Decreased Gap Junctional Communication between AII Amacrine Cells. J. Biol. Chem. 2006, 281, 33163–33171. [Google Scholar] [CrossRef]
- Cahill, G.M.; Besharse, J.C. Circadian Clock Functions Localized in Xenopus Retinal Photoreceptors. Neuron 1993, 10, 573–577. [Google Scholar] [CrossRef]
- Adachi, A.; Nogi, T.; Ebihara, S. Phase-Relationship and Mutual Effects between Circadian Rhythms of Ocular Melatonin and Dopamine in the Pigeon1Published on the World Wide Web on 30 March 1998.1. Brain Res. 1998, 792, 361–369. [Google Scholar] [CrossRef]
- Cahill, G.M.; Grace, M.S.; Besharse, J.C. Rhythmic Regulation of Retinal Melatonin: Metabolic Pathways, Neurochemical Mechanisms, and the Ocular Circadian Clock. Cell. Mol. Neurobiol. 1991, 11, 529–560. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, R.; Lesauter, J.; Silver, R.; Garcia-España, A.; Witkovsky, P. Diurnal and Circadian Variation of Protein Kinase C Immunoreactivity in the Rat Retina: Rhythm of PKC Immunoreactivity in Rat Retina. J. Comp. Neurol. 2001, 439, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, R.; Lesauter, J.; Banvolgyi, T.; Petrovics, G.; Silver, R.; Witkovsky, P. AII Amacrine Neurons of the Rat Retina Show Diurnal and Circadian Rhythms of Parvalbumin Immunoreactivity. Cell Tissue Res. 2004, 315, 181–186. [Google Scholar] [CrossRef]
- Anderson, F.E.; Green, C.B. Symphony of Rhythms in the Xenopus laevis Retina. Microsc. Res. Tech. 2000, 50, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Tosini, G.; Menaker, M. Circadian Rhythms in Cultured Mammalian Retina. Science 1996, 272, 419–421. [Google Scholar] [CrossRef]
- Pierce, M.E.; Sheshberadaran, H.; Zhang, Z.; Fox, L.E.; Applebury, M.L.; Takahashi, J.S. Circadian Regulation of Lodopsin Gene Expression in Embryonic Photoreceptors in Retinal Cell Culture. Neuron 1993, 10, 579–584. [Google Scholar] [CrossRef]
- Green, C.B.; Cahill, G.M.; Besharse, J.C. Tryptophan Hydroxylase Is Expressed by Photoreceptors in Xenopus laevis Retina. Vis. Neurosci. 1995, 12, 663–670. [Google Scholar] [CrossRef]
- Ruan, G.-X.; Zhang, D.-Q.; Zhou, T.; Yamazaki, S.; McMahon, D.G. Circadian Organization of the Mammalian Retina. Proc. Natl. Acad. Sci. USA 2006, 103, 9703–9708. [Google Scholar] [CrossRef]
- Kamphuis, W.; Cailotto, C.; Dijk, F.; Bergen, A.; Buijs, R.M. Circadian Expression of Clock Genes and Clock-Controlled Genes in the Rat Retina. Biochem. Biophys. Res. Commun. 2005, 330, 18–26. [Google Scholar] [CrossRef]
- Iuvone, P.M.; Tosini, G.; Pozdeyev, N.; Haque, R.; Klein, D.C.; Chaurasia, S.S. Circadian Clocks, Clock Networks, Arylalkylamine N-Acetyltransferase, and Melatonin in the Retina. Prog. Retin. Eye Res. 2005, 24, 433–456. [Google Scholar] [CrossRef]
- Ribelayga, C.; Wang, Y.; Mangel, S.C. A Circadian Clock in the Fish Retina Regulates Dopamine Release via Activation of Melatonin Receptors: Retinal Circadian Clock Regulates Dopamine Release. J. Physiol. 2004, 554, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Barlow, R. Chapter 35 Circadian and Efferent Modulation of Visual Sensitivity. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2001; Volume 131, pp. 487–503. ISBN 978-0-444-51484-4. [Google Scholar]
- Green, C.B.; Besharse, J.C. Retinal Circadian Clocks and Control of Retinal Physiology. J. Biol. Rhythms 2004, 19, 91–102. [Google Scholar] [CrossRef]
- Hornstein, E.P.; Verweij, J.; Li, P.H.; Schnapf, J.L. Gap-Junctional Coupling and Absolute Sensitivity of Photoreceptors in Macaque Retina. J. Neurosci. 2005, 25, 11201–11209. [Google Scholar] [CrossRef]
- Krizaj, D.; Gábriel, R.; Owen, W.G.; Witkovsky, P. Dopamine D2 Receptor-Mediated Modulation of Rod–cone Coupling in the Xenopus Retina. J. Comp. Neurol. 1998, 398, 529–538. [Google Scholar] [CrossRef]
- Ribelayga, C.; Wang, Y.; Mangel, S.C. Dopamine Mediates Circadian Clock Regulation of Rod and Cone Input to Fish Retinal Horizontal Cells. J. Physiol. 2002, 544, 801–816. [Google Scholar] [CrossRef]
- Ribelayga, C.; Cao, Y.; Mangel, S.C. The Circadian Clock in the Retina Controls Rod–cone Coupling. Neuron 2008, 59, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mangel, S.C. A Circadian Clock Regulates Rod and Cone Input to Fish Retinal Cone Horizontal Cells. Proc. Natl. Acad. Sci. USA 1996, 93, 4655–4660. [Google Scholar] [CrossRef]
- Witkovsky, M.T. Psychiatric Problems of Youth in Primary Care: A Review. WMJ 2004, 103, 14–19. [Google Scholar]
- Yang, X.-L.; Wu, S.M. Modulation of Rod–cone Coupling by Light. Science 1989, 244, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Z.; Blackburn, M.R.; Wang, S.W.; Ribelayga, C.P.; O’Brien, J. Adenosine and Dopamine Receptors Coregulate Photoreceptor Coupling via Gap Junction Phosphorylation in Mouse Retina. J. Neurosci. 2013, 33, 3135–3150. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H.; Liu, X.; O’Brien, J.; Ribelayga, C.P. Circadian Clock Control of Connexin36 Phosphorylation in Retinal Photoreceptors of the CBA/CaJ Mouse Strain. Vis. Neurosci. 2015, 32, E009. [Google Scholar] [CrossRef]
- Katti, C.; Butler, R.; Sekaran, S. Diurnal and Circadian Regulation of Connexin 36 Transcript and Protein in the Mammalian Retina. Investig. Ophthalmol. Vis. Sci. 2013, 54, 821. [Google Scholar] [CrossRef]
- Jin, N.G.; Chuang, A.Z.; Masson, P.J.; Ribelayga, C.P. Rod Electrical Coupling Is Controlled by a Circadian Clock and Dopamine in Mouse Retina: Circadian Clock Control of Rod Electrical Coupling. J. Physiol. 2015, 593, 1597–1631. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.G.; Ribelayga, C.P. Direct Evidence for Daily Plasticity of Electrical Coupling between Rod Photoreceptors in the Mammalian Retina. J. Neurosci. 2016, 36, 178–184. [Google Scholar] [CrossRef]
- Ribelayga, C.; Mangel, S.C. Absence of Circadian Clock Regulation of Horizontal Cell Gap Junctional Coupling Reveals Two Dopamine Systems in the Goldfish Retina. J. Comp. Neurol. 2003, 467, 243–253. [Google Scholar] [CrossRef]
- Cao, J.; Ribelayga, C.P.; Mangel, S.C. A Circadian Clock in the Retina Regulates Rod–cone Gap Junction Coupling and Neuronal Light Responses via Activation of Adenosine A2A Receptors. Front. Cell. Neurosci. 2021, 14, 605067. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Mangel, S.C. Interactions of Cone Cannabinoid CB1 and Dopamine D4 Receptors Increase Day/Night Difference in Rod-cone Gap Junction Coupling in Goldfish Retina. J. Physiol. 2021, 599, 4085–4100. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).