Metal-Based Nanoparticles in Food Packaging and Coating Technologies: A Review
Abstract
1. Introduction
Types of Nanomaterial for Food Packaging
2. General Synthetic Approaches for the Preparation of Nanomaterials
3. Metal-Based Nanoparticles in Food Packaging Technology
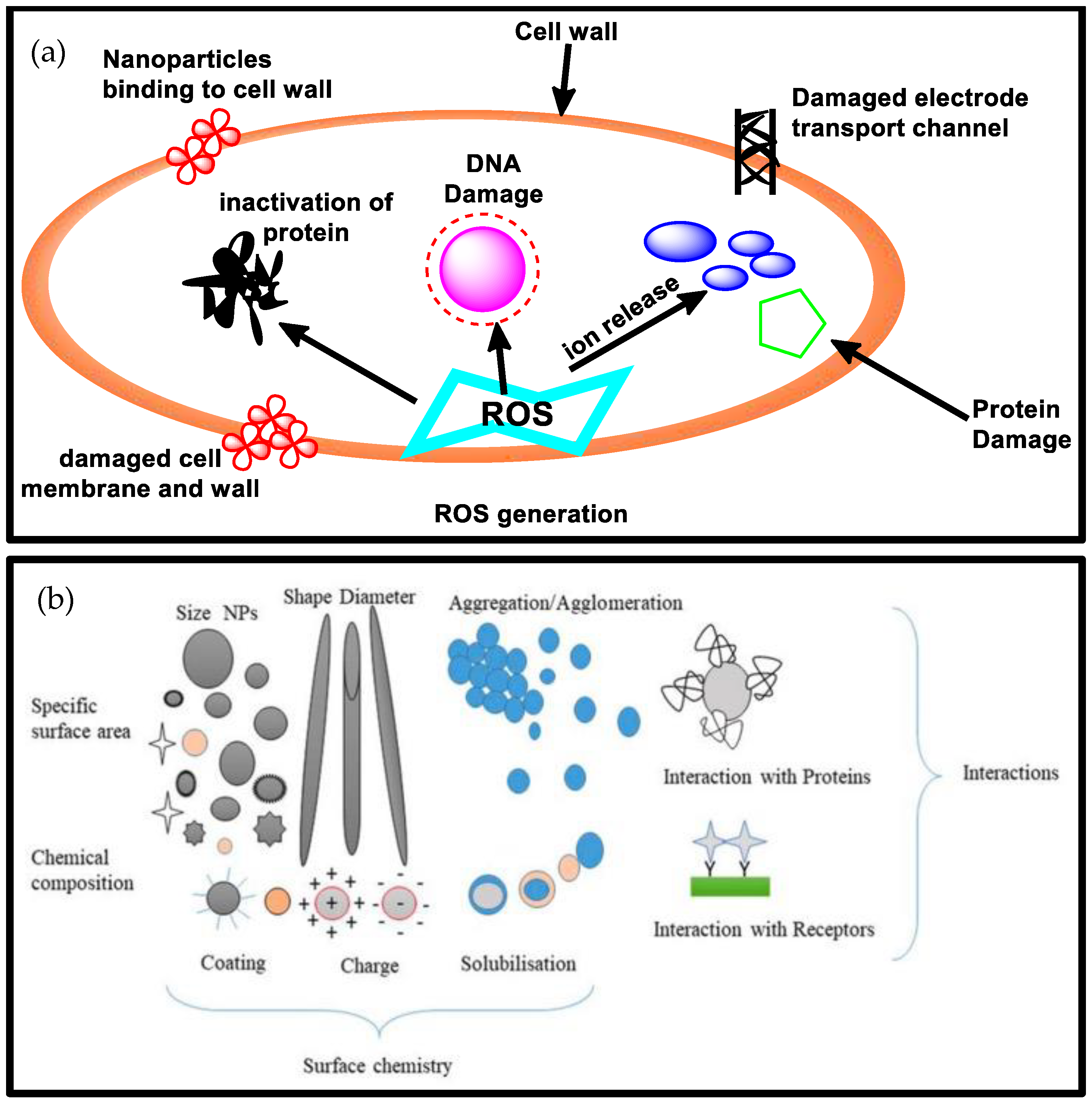
3.1. The Physicochemical Properties, Roles, and Biological Properties of Metal-Based Nanoparticles and Their Relevance in Food Packaging
3.2. Prominent Examples of Metal-Based Nanoparticle and Their Food Packaging Applications
3.3. Practical Application of Metal-Based Nanoparticles Composites to Food Materials
4. Limitation of Nanotechnology in Food Packaging
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNCTD. The Role of Science, Technology and Innovation in Ensuring Food Security by 2030; United Nations: Geneva, Switzerland, 2017. [Google Scholar]
- Mukhopadhyay, S.S. Nanotechnology in Agriculture: Prospects and Constraints. Nanotechnol. Sci. Appl. 2014, 7, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention Estimates of Foodborne Illness in the United States. Available online: http://www.cdc.gov/Features/dsFoodborneEstimates/ (accessed on 15 January 2023).
- Tripathi, P.; Dubey, N.K. Exploitation of Natural Products as an Alternative Strategy to Control Postharvest Fungal Rotting of Fruit and Vegetables. Postharvest Biol. Technol. 2004, 32, 235–245. [Google Scholar] [CrossRef]
- Sukainah, H.; Masod, M.Y. The Role of Food Packaging Grade Materials. In Global Challenges and Innovation in Science and Management; KAAV Publications: Delhi, India, 2009; p. 2009. ISBN 9789386789853. [Google Scholar]
- Marsh, K.; Bugusu, B. Food Packaging? Roles, Materials, and Environmental Issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef] [PubMed]
- Espitia, P.J.P.; de Fátima Ferreira Soares, N.; dos Reis Coimbra, J.S.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioproc. Tech. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Restuccia, D.; Spizzirri, U.G.; Parisi, O.I.; Cirillo, G.; Curcio, M.; Iemma, F.; Puoci, F.; Vinci, G.; Picci, N. New EU Regulation Aspects and Global Market of Active and Intelligent Packaging for Food Industry Applications. Food Control 2010, 21, 1425–1435. [Google Scholar] [CrossRef]
- Soares, N.; de Sá Silva, C.; Santiago-Silva, P.; Espitia, P.; Gonçalves, M.; Lopez, M.; Miltz, J.; Cerqueira, M.; Vicente, A.; Teixeira, J.; et al. Active and Intelligent Packaging for Milk and Milk Products. In Engineering Aspects of Milk and Dairy Products; CRC Press: Boca Raton, FL, USA, 2009; pp. 175–199. ISBN 9781420090390. [Google Scholar]
- Schirmer, B.C.; Heiberg, R.; Eie, T.; Møretrø, T.; Maugesten, T.; Carlehøg, M.; Langsrud, S. A Novel Packaging Method with a Dissolving CO2 Headspace Combined with Organic Acids Prolongs the Shelf Life of Fresh Salmon. Int. J. Food Microbiol. 2009, 133, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Appendini, P.; Hotchkiss, J.H. Immobilization of Lysozyme on Food Contact Polymers as Potential Antimicrobial Films. Packag. Technol. Sci. 1997, 10, 271–279. [Google Scholar] [CrossRef]
- Gálvez, A.; Abriouel, H.; López, R.L.; Omar, N. Ben Bacteriocin-Based Strategies for Food Biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef]
- Sahoo, B.; Rath, S.K.; Mahanta, S.K.; Arakha, M. Nanotechnology Mediated Detection and Control of Phytopathogens. In Bio-Nano Interface; Springer: Singapore, 2022; pp. 109–125. ISBN 9789811625169. [Google Scholar]
- Neme, K.; Nafady, A.; Uddin, S.; Tola, Y.B. Application of Nanotechnology in Agriculture, Postharvest Loss Reduction and Food Processing: Food Security Implication and Challenges. Heliyon 2021, 7, e08539. [Google Scholar] [CrossRef]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in Sustainable Agriculture: An Emerging Opportunity. J. Control. Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological Synthesis of Metallic Nanoparticles. Nanomedicine 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver Nanoparticles as a New Generation of Antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Sawai, J. Quantitative Evaluation of Antibacterial Activities of Metallic Oxide Powders (ZnO, MgO and CaO) by Conductimetric Assay. J. Microbiol. Methods 2003, 54, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, A.; Ijaz, I.; Gilani, E.; Nazir, A.; Zain, H.; Saeed, R.; Alarfaji, S.S.; Hussain, S.; Aftab, R.; Naseer, Y. Green Synthesis of Metal and Metal Oxide Nanoparticles Using Different Plants’ Parts for Antimicrobial Activity and Anticancer Activity: A Review Article. Coatings 2021, 11, 1374. [Google Scholar] [CrossRef]
- Bradley, E.L.; Castle, L.; Chaudhry, Q. Applications of Nanomaterials in Food Packaging with a Consideration of Opportunities for Developing Countries. Trends Food Sci. Technol. 2011, 22, 604–610. [Google Scholar] [CrossRef]
- Chaudhry, Q.; Scotter, M.; Blackburn, J.; Ross, B.; Boxall, A.; Castle, L.; Aitken, R.; Watkins, R. Applications and Implications of Nanotechnologies for the Food Sector. Food Addit. Contam. Part A 2008, 25, 241–258. [Google Scholar] [CrossRef]
- Cioffi, N.; Torsi, L.; Ditaranto, N.; Tantillo, G.; Ghibelli, L.; Sabbatini, L.; Bleve-Zacheo, T.; D’Alessio, M.; Zambonin, P.G.; Traversa, E. Copper Nanoparticle/Polymer Composites with Antifungal and Bacteriostatic Properties. Chem. Mater. 2005, 17, 5255–5262. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Wang, C.; Mathiyalagan, R.; Yang, D.C. The Development of a Green Approach for the Biosynthesis of Silver and Gold Nanoparticles by Using Panax Ginseng Root Extract, and Their Biological Applications. Artif. Cells Nanomed. Biotechnol. 2015, 44, 1150–1157. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Wang, C.; Mathiyalagan, R.; El-Agamy Farh, M.; Yang, D.C. Biogenic Silver and Gold Nanoparticles Synthesized Using Red Ginseng Root Extract, and Their Applications. Artif. Cells Nanomed. Biotechnol. 2015, 44, 811–816. [Google Scholar] [CrossRef]
- Meena Kumari, M.; Jacob, J.; Philip, D. Green Synthesis and Applications of Au–Ag Bimetallic Nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 137, 185–192. [Google Scholar] [CrossRef]
- Gopinath, K.; Kumaraguru, S.; Bhakyaraj, K.; Mohan, S.; Venkatesh, K.S.; Esakkirajan, M.; Kaleeswarran, P.; Alharbi, N.S.; Kadaikunnan, S.; Govindarajan, M.; et al. Green Synthesis of Silver, Gold and Silver/Gold Bimetallic Nanoparticles Using the Gloriosa Superba Leaf Extract and Their Antibacterial and Antibiofilm Activities. Microb. Pathog. 2016, 101, 1–11. [Google Scholar] [CrossRef]
- Dobrucka, R.; Długaszewska, J. Biosynthesis and Antibacterial Activity of ZnO Nanoparticles Using Trifolium Pratense Flower Extract. Saudi J. Biol. Sci. 2016, 23, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Gunalan, S.; Sivaraj, R.; Rajendran, V. Green Synthesized ZnO Nanoparticles against Bacterial and Fungal Pathogens. Prog. Nat. Sci. Mater. Int. 2012, 22, 693–700. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Sreekanth, T.V.M.; Tettey, C.O.; Jun, Y.I.; Mook, S.H. Characterization, Antibacterial, Antioxidant, and Cytotoxic Activities of ZnO Nanoparticles Using Coptidis Rhizoma. Bioorg. Med. Chem. Lett. 2014, 24, 4298–4303. [Google Scholar] [CrossRef] [PubMed]
- Pulit-Prociak, J.; Chwastowski, J.; Kucharski, A.; Banach, M. Functionalization of Textiles with Silver and Zinc Oxide Nanoparticles. Appl. Surf. Sci. 2016, 385, 543–553. [Google Scholar] [CrossRef]
- Agarwal, H.; Venkat Kumar, S.; Rajeshkumar, S. A Review on Green Synthesis of Zinc Oxide Nanoparticles—An Eco-Friendly Approach. Resour.-Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Sharma, C.; Dhiman, R.; Rokana, N.; Panwar, H. Nanotechnology: An Untapped Resource for Food Packaging. Front. Microbiol. 2017, 8, 1735. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Fatima, F.; Kumar, A. Relevance of Nanomaterials in Food Packaging and Its Advanced Future Prospects. J. Inorg. Organomet. Polym. Mater. 2020, 30, 5180–5192. [Google Scholar] [CrossRef]
- Suh, S.; Meng, X.; Ko, S. Proof of Concept Study for Different-Sized Chitosan Nanoparticles as Carbon Dioxide (CO2) Indicators in Food Quality Monitoring. Talanta 2016, 161, 265–270. [Google Scholar] [CrossRef]
- Wang, Y.C.; Lu, L.; Gunasekaran, S. Biopolymer/Gold Nanoparticles Composite Plasmonic Thermal History Indicator to Monitor Quality and Safety of Perishable Bioproducts. Biosens. Bioelectron. 2017, 92, 109–116. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites Materials for Food Packaging Applications: Concepts and Future Outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef]
- Dash, K.K.; Deka, P.; Bangar, S.P.; Chaudhary, V.; Trif, M.; Rusu, A. Applications of Inorganic Nanoparticles in Food Packaging: A Comprehensive Review. Polymers 2022, 14, 521. [Google Scholar] [CrossRef]
- Gregor-Svetec, D. Intelligent Packaging. In Nanomaterials for Food Packaging: Materials, Processing Technologies, and Safety Issues; Elsevier: Amsterdam, The Netherlands, 2018; pp. 203–247. ISBN 9780323512718. [Google Scholar]
- Vanderroost, M.; Ragaert, P.; Devlieghere, F.; De Meulenaer, B. Intelligent Food Packaging: The next Generation. Trends Food Sci. Technol. 2014, 39, 47–62. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Fagioli, L.; Campana, R.; Lam, J.K.W.; Baffone, W.; Palmieri, G.F.; Casettari, L.; Bonacucina, G. Chitosan-Based Nanosystems and Their Exploited Antimicrobial Activity. Eur. J. Pharm. Sci. 2018, 117, 8–20. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Wilkanowicz, S.; Melendez-Rodriguez, B.; Lagaron, J.M. Nanoencapsulation of Aloe Vera in Synthetic and Naturally Occurring Polymers by Electrohydrodynamic Processing of Interest in Food Technology and Bioactive Packaging. J. Agric. Food Chem. 2017, 65, 4439–4448. [Google Scholar] [CrossRef]
- Hoseinnejad, M.; Jafari, S.M.; Katouzian, I. Inorganic and Metal Nanoparticles and Their Antimicrobial Activity in Food Packaging Applications. Crit. Rev. Microbiol. 2018, 44, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Almasi, H.; Jafarzadeh, P.; Mehryar, L. Fabrication of Novel Nanohybrids by Impregnation of CuO Nanoparticles into Bacterial Cellulose and Chitosan Nanofibers: Characterization, Antimicrobial and Release Properties. Carbohydr. Polym. 2018, 186, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, A.; Khursheed, N.; Fatima, S.; Anjum, Z.; Younis, K. Application of Nanotechnology in Food Packaging: Pros and Cons. J. Agric. Food Res. 2022, 7, 100270. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of Metallic Nanoparticles Using Plant Extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. “Green” Synthesis of Metals and Their Oxide Nanoparticles: Applications for Environmental Remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Das, R.K.; Pachapur, V.L.; Lonappan, L.; Naghdi, M.; Pulicharla, R.; Maiti, S.; Cledon, M.; Dalila, L.M.A.; Sarma, S.J.; Brar, S.K. Biological Synthesis of Metallic Nanoparticles: Plants, Animals and Microbial Aspects. Nanotechnol. Environ. Eng. 2017, 2, 18. [Google Scholar] [CrossRef]
- Meyers, M.A.; Mishra, A.; Benson, D.J. Mechanical Properties of Nanocrystalline Materials. Prog. Mater. Sci. 2006, 51, 427–556. [Google Scholar] [CrossRef]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.V.; Alam, M.; Kumar, R.; et al. Fungus-Mediated Synthesis of Silver Nanoparticles and Their Immobilization in the Mycelial Matrix: A Novel Biological Approach to Nanoparticle Synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Devatha, C.P.; Thalla, A.K. Green Synthesis of Nanomaterials; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 9780081019757. [Google Scholar]
- Watt, J.; Cheong, S.; Tilley, R.D. How to Control the Shape of Metal Nanostructures in Organic Solution Phase Synthesis for Plasmonics and Catalysis. Nano Today 2013, 8, 198–215. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Oriola, A.O.; Onwudiwe, D.C.; Oyedeji, A.O. Plant Extracts Mediated Metal-Based Nanoparticles: Synthesis and Biological Applications. Biomolecules 2022, 12, 627. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Huang, Y.; Parsons, J.G.; Zhao, L.; Lopez-Moreno, L.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L. Plant-Based Green Synthesis of Metallic Nanoparticles: Scientific Curiosity or a Realistic Alternative to Chemical Synthesis? Nanotechnol. Environ. Eng. 2016, 1, 4. [Google Scholar] [CrossRef]
- Shukla, P.; Chaurasia, P.; Younis, K.; Qadri, O.S.; Faridi, S.A.; Srivastava, G. Nanotechnology in Sustainable Agriculture: Studies from Seed Priming to Post-Harvest Management. Nanotechnol. Environ. Eng. 2019, 4, 11. [Google Scholar] [CrossRef]
- Primožič, M.; Knez, Ž.; Leitgeb, M. (Bio)Nanotechnology in Food Science—Food Packaging. Nanomaterials 2021, 11, 292. [Google Scholar] [CrossRef]
- Eric Kunz Global Smart Packaging Market Size to Reach over USD 33.00 Billion by 2028. Available online: https://www.globenewswire.com/news-release/2022/02/15/2384878/0/en/Global-Smart-Packaging-Market-Size-to-Reach-over-USD-33-00-Billion-by-2028-Vantage-Market-Research.html (accessed on 15 January 2023).
- Li, X.; Ji, N.; Qiu, C.; Xia, M.; Xiong, L.; Sun, Q. The Effect of Peanut Protein Nanoparticles on Characteristics of Protein- and Starch-Based Nanocomposite Films: A Comparative Study. Ind. Crop. Prod. 2015, 77, 565–574. [Google Scholar] [CrossRef]
- Tirado-Kulieva, V.A.; Sánchez-Chero, M.; Jimenez, D.P.P.; Sánchez-Chero, J.; Cruz, A.G.Y.S.; Velayarce, H.H.M.; Suclupe, L.A.P.; Garcia, L.O.C. A Critical Review on The Integration of Metal Nanoparticles in Biopolymers: An Alternative for Active and Sustainable Food Packaging. Curr. Res. Nutr. Food Sci. 2022, 10, 1–18. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of Silver Nanoparticles with Different Shapes. Arab. J. Chem. 2019, 12, 1823–1838. [Google Scholar] [CrossRef]
- Rahme, K.; Holmes, J.D. Gold Nanoparticles: Synthesis, Characterization, and Bioconjugation. In Dekker Encyclopedia of Nanoscience and Nanotechnology, Third Edition; Taylor & Francis: New York, NY, USA, 2015; pp. 1–11. [Google Scholar]
- Adeyemi, J.O.; Elemike, E.E.; Onwudiwe, D.C.; Singh, M. Bio-Inspired Synthesis and Cytotoxic Evaluation of Silver-Gold Bimetallic Nanoparticles Using Kei-Apple (Dovyalis Caffra) Fruits. Inorg. Chem. Commun. 2019, 109, 107569. [Google Scholar] [CrossRef]
- Azhdari, S.; Sarabi, R.E.; Rezaeizade, N.; Mosazade, F.; Heidari, M.; Borhani, F.; Abdollahpour-Alitappeh, M.; Khatami, M. Metallic SPIONP/AgNP Synthesis Using a Novel Natural Source and Their Antifungal Activities. RSC Adv. 2020, 10, 29737–29744. [Google Scholar] [CrossRef]
- Dehghani, S.; Hadi Peighambardoust, S.; Peighambardoust, S.J.; Fasihnia, S.H.; Khosrowshahi, N.K.; Gullón, B.; Lorenzo, J.M. Optimization of the Amount of ZnO, CuO, and Ag Nanoparticles on Antibacterial Properties of Low-Density Polyethylene (LDPE) Films Using the Response Surface Method. Food Anal. Methods 2021, 14, 98–107. [Google Scholar] [CrossRef]
- Yu, J.; Yang, J.; Liu, B.; Ma, X. Preparation and Characterization of Glycerol Plasticized-Pea Starch/ZnO-Carboxymethylcellulose Sodium Nanocomposites. Bioresour. Technol. 2009, 100, 2832–2841. [Google Scholar] [CrossRef]
- Ebrahimi, Y.; Peighambardoust, S.J.; Peighambardoust, S.H.; Karkaj, S.Z. Development of Antibacterial Carboxymethyl Cellulose-Based Nanobiocomposite Films Containing Various Metallic Nanoparticles for Food Packaging Applications. J. Food Sci. 2019, 84, 2537–2548. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Peighambardoust, S.H.; Pournasir, N.; Mohammadzadeh Pakdel, P. Properties of Active Starch-Based Films Incorporating a Combination of Ag, ZnO and CuO Nanoparticles for Potential Use in Food Packaging Applications. Food Packag. Shelf Life 2019, 22, 100420. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ahmed, J.; Hiremath, N.; Auras, R.; Joseph, A. Thermo-Mechanical, Rheological, Structural and Antimicrobial Properties of Bionanocomposite Films Based on Fish Skin Gelatin and Silver-Copper Nanoparticles. Food Hydrocoll. 2017, 62, 191–202. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Sarbon, N.M. A Comparative Study: Physical, Mechanical and Antibacterial Properties of Bio-Composite Gelatin Films as Influenced by Chitosan and Zinc Oxide Nanoparticles Incorporation. Food Biosci. 2021, 43, 101250. [Google Scholar] [CrossRef]
- Wang, H.; Gong, X.; Miao, Y.; Guo, X.; Liu, C.; Fan, Y.Y.; Zhang, J.; Niu, B.; Li, W. Preparation and Characterization of Multilayer Films Composed of Chitosan, Sodium Alginate and Carboxymethyl Chitosan-ZnO Nanoparticles. Food Chem. 2019, 283, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Azizi-Lalabadi, M.; Alizadeh-Sani, M.; Divband, B.; Ehsani, A.; McClements, D.J. Nanocomposite Films Consisting of Functional Nanoparticles (TiO2 and ZnO) Embedded in 4A-Zeolite and Mixed Polymer Matrices (Gelatin and Polyvinyl Alcohol). Food Res. Int. 2020, 137, 109716. [Google Scholar] [CrossRef] [PubMed]
- Noshirvani, N.; Ghanbarzadeh, B.; Mokarram, R.R.; Hashemi, M.; Coma, V. Preparation and Characterization of Active Emulsified Films Based on Chitosan-Carboxymethyl Cellulose Containing Zinc Oxide Nano Particles. Int. J. Biol. Macromol. 2017, 99, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, T.; Tao, Y.; Ling, Z.; Huang, C.; Lai, C.; Yong, Q. Fabrication of Anti-Bacterial, Hydrophobic and UV Resistant Galactomannan-Zinc Oxide Nanocomposite Films. Polymer 2021, 215, 123412. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W.; Won, K. Preparation of Poly(Lactide)/Lignin/Silver Nanoparticles Composite Films with UV Light Barrier and Antibacterial Properties. Int. J. Biol. Macromol. 2018, 107, 1724–1731. [Google Scholar] [CrossRef]
- Figueroa-López, K.J.; Torres-Giner, S.; Enescu, D.; Cabedo, L.; Cerqueira, M.A.; Pastrana, L.M.; Lagaron, J.M. Electrospun Active Biopapers of Food Waste Derived Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with Short-Term and Long-Term Antimicrobial Performance. Nanomaterials 2020, 10, 506. [Google Scholar] [CrossRef]
- Huang, Y.; Mei, L.; Chen, X.; Wang, Q. Recent Developments in Food Packaging Based on Nanomaterials. Nanomaterials 2018, 8, 830. [Google Scholar] [CrossRef]
- Nowack, B.; Krug, H.F.; Height, M. 120 Years of Nanosilver History: Implications for Policy Makers. Environ. Sci. Technol. 2011, 45, 1177–1183. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver Nanoparticles as Antimicrobial Agent: A Case Study on E. Coli as a Model for Gram-Negative Bacteria. J. Colloid. Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A Review of the Antibacterial Effects of Silver Nanomaterials and Potential Implications for Human Health and the Environment. J. Nanoparticle Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- López-Rubio, A.; Fabra, M.J.; Martínez-Sanz, M. Food Packaging Based on Nanomaterials. Nanomaterials 2019, 9, 1224. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Satapathy, M.; Mukhopahayay, A.; Das, P. Leaf Extract Mediated Green Synthesis of Silver Nanoparticles from Widely Available Indian Plants: Synthesis, Characterization, Antimicrobial Property and Toxicity Analysis. Bioresour. Bioprocess. 2014, 1, 3. [Google Scholar] [CrossRef]
- Masum, M.I.; Siddiqa, M.M.; Ali, K.A.; Zhang, Y.; Abdallah, Y.; Ibrahim, E.; Qiu, W.; Yan, C.; Li, B. Biogenic Synthesis of Silver Nanoparticles Using Phyllanthus Emblicafruit Extract and Its Inhibitory Action against the Pathogen Acidovorax Oryzaestrain RS-2 of Rice Bacterial Brown Stripe. Front. Microbiol. 2019, 10, 820. [Google Scholar] [CrossRef]
- Krithiga, N.; Rajalakshmi, A.; Jayachitra, A. Green Synthesis of Silver Nanoparticles Using Leaf Extracts of Clitoria Ternatea and Solanum Nigrum and Study of Its Antibacterial Effect against Common Nosocomial Pathogens. J. Nanosci. 2015, 2015, 928204. [Google Scholar] [CrossRef]
- Kumar, R.; Münstedt, H. Silver Ion Release from Antimicrobial Polyamide/Silver Composites. Biomaterials 2005, 26, 2081–2088. [Google Scholar] [CrossRef]
- Duncan, T.V. Applications of Nanotechnology in Food Packaging and Food Safety: Barrier Materials, Antimicrobials and Sensors. J. Colloid. Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef]
- Cavaliere, E.; De Cesari, S.; Landini, G.; Riccobono, E.; Pallecchi, L.; Rossolini, G.M.; Gavioli, L. Highly Bactericidal Ag Nanoparticle Films Obtained by Cluster Beam Deposition. Nanomedicine 2015, 11, 1417–1423. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Evaluation of Nanocomposite Packaging Containing Ag and ZnO on Shelf Life of Fresh Orange Juice. Innov. Food Sci. Emerg. Technol. 2010, 11, 742–748. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Mohamad Ibrahim, M.N. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef]
- Misra, S.K.; Dybowska, A.; Berhanu, D.; Luoma, S.N.; Valsami-Jones, E. The Complexity of Nanoparticle Dissolution and Its Importance in Nanotoxicological Studies. Sci. Total Environ. 2012, 438, 225–232. [Google Scholar] [CrossRef]
- Correa, M.G.; Martínez, F.B.; Vidal, C.P.; Streitt, C.; Escrig, J.; de Dicastillo, C.L. Antimicrobial Metal-Based Nanoparticles: A Review on Their Synthesis, Types and Antimicrobial Action. Beilstein J. Nanotechnol. 2020, 11, 1450–1469. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Urnukhsaikhan, E.; Bold, B.E.; Gunbileg, A.; Sukhbaatar, N.; Mishig-Ochir, T. Antibacterial Activity and Characteristics of Silver Nanoparticles Biosynthesized from Carduus Crispus. Sci. Rep. 2021, 11, 21047. [Google Scholar] [CrossRef]
- Varier, K.M.; Gudeppu, M.; Chinnasamy, A.; Thangarajan, S.; Balasubramanian, J.; Li, Y.; Gajendran, B. Nanoparticles: Antimicrobial Applications and Its Prospects; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Jiang, Y.; Zheng, W.; Tran, K.; Kamilar, E.; Bariwal, J.; Ma, H.; Liang, H. Hydrophilic Nanoparticles That Kill Bacteria While Sparing Mammalian Cells Reveal the Antibiotic Role of Nanostructures. Nat. Commun. 2022, 13, 197. [Google Scholar] [CrossRef]
- Khezerlou, A.; Alizadeh-Sani, M.; Azizi-Lalabadi, M.; Ehsani, A. Nanoparticles and Their Antimicrobial Properties against Pathogens Including Bacteria, Fungi, Parasites and Viruses. Microb. Pathog. 2018, 123, 505–526. [Google Scholar] [CrossRef]
- Vieira, A.C.F.; de Matos Fonseca, J.; Menezes, N.M.C.; Monteiro, A.R.; Valencia, G.A. Active Coatings Based on Hydroxypropyl Methylcellulose and Silver Nanoparticles to Extend the Papaya (Carica papaya L.) Shelf Life. Int. J. Biol. Macromol. 2020, 164, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Sahareen, T. Investigation of Cellulosic Packets Impregnated with Silver Nanoparticles for Enhancing Shelf-Life of Vegetables. LWT 2017, 86, 116–122. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Hu, X.; Chelliah, R.; Oh, D.H.; Kathiresan, K.; Wang, M.H. Biogenic Silver Nanoparticles-Polyvinylpyrrolidone Based Glycerosomes Coating to Expand the Shelf Life of Fresh-Cut Bell Pepper (Capsicum annuum L. var. grossum (L.) Sendt). Postharvest Biol. Technol. 2020, 160, 111039. [Google Scholar] [CrossRef]
- Sharifan, H.; Noori, A.; Bagheri, M.; Moore, J.M. Postharvest Spraying of Zinc Oxide Nanoparticles Enhances Shelf Life Qualities and Zinc Concentration of Tomato Fruits. Crop Pasture Sci. 2021, 73, 22–31. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, W.; Pang, C.; Deng, W.; Xu, C.; Wang, X. Multifunctional Chitosan-Based Coating with Liposomes Containing Laurel Essential Oils and Nanosilver for Pork Preservation. Food Chem. 2019, 295, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, W.; Zhu, B.; Chen, H.; Chi, H.; Li, L.; Qin, Y.; Xue, J. The Quality Evaluation of Postharvest Strawberries Stored in Nano-Ag Packages at Refrigeration Temperature. Polymers 2018, 10, 894. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Snigdha, S.; Mathew, J.; Radhakrishnan, E.K. Biodegradable and Active Nanocomposite Pouches Reinforced with Silver Nanoparticles for Improved Packaging of Chicken Sausages. Food Packag. Shelf Life 2019, 19, 155–166. [Google Scholar] [CrossRef]
- Li, L.; Zhao, C.; Zhang, Y.; Yao, J.; Yang, W.; Hu, Q.; Wang, C.; Cao, C. Effect of Stable Antimicrobial Nano-Silver Packaging on Inhibiting Mildew and in Storage of Rice. Food Chem. 2017, 215, 477–482. [Google Scholar] [CrossRef]
- Dairi, N.; Ferfera-Harrar, H.; Ramos, M.; Garrigós, M.C. Cellulose Acetate/AgNPs-Organoclay and/or Thymol Nano-Biocomposite Films with Combined Antimicrobial/Antioxidant Properties for Active Food Packaging Use. Int. J. Biol. Macromol. 2019, 121, 508–523. [Google Scholar] [CrossRef]
- Zare, M.; Namratha, K.; Ilyas, S.; Sultana, A.; Hezam, A.; Sunil, L.; Surmeneva, M.A.; Surmenev, R.A.; Nayan, M.B.; Ramakrishna, S.; et al. Emerging Trends for ZnO Nanoparticles and Their Applications in Food Packaging. ACS Food Sci. Technol. 2022, 2, 763–781. [Google Scholar] [CrossRef]
- Rossi, L.; Fedenia, L.N.; Sharifan, H.; Ma, X.; Lombardini, L. Effects of Foliar Application of Zinc Sulfate and Zinc Nanoparticles in Coffee (Coffea arabica L.) Plants. Plant Physiol. Biochem. 2019, 135, 160–166. [Google Scholar] [CrossRef]
- Jalal, R.; Goharshadi, E.K.; Abareshi, M.; Moosavi, M.; Yousefi, A.; Nancarrow, P. ZnO Nanofluids: Green Synthesis, Characterization, and Antibacterial Activity. Mater. Chem. Phys. 2010, 121, 198–201. [Google Scholar] [CrossRef]
- Salehi, H.; De Diego, N.; Chehregani Rad, A.; Benjamin, J.J.; Trevisan, M.; Lucini, L. Exogenous Application of ZnO Nanoparticles and ZnSO4 Distinctly Influence the Metabolic Response in Phaseolus vulgaris L. Sci. Total Environ. 2021, 778, 146331. [Google Scholar] [CrossRef]
- Hsan, N.; Dutta, P.K.; Kumar, S.; Bera, R.; Das, N. Chitosan Grafted Graphene Oxide Aerogel: Synthesis, Characterization and Carbon Dioxide Capture Study. Int. J. Biol. Macromol. 2019, 125, 300–306. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan Based ZnO Nanoparticles Loaded Gallic-Acid Films for Active Food Packaging. Food Chem. 2021, 334. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Mehrotra, G.K.; Bhartiya, P.; Singh, A.; Dutta, P.K. Preparation, Physicochemical and Biological Evaluation of Quercetin Based Chitosan-Gelatin Film for Food Packaging. Carbohydr. Polym. 2020, 227, 115348. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M. Chitosan for Biomaterials III; Advances in Polymer Science; Jayakumar, R., Prabaharan, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; Volume 287, ISBN 978-3-030-83806-5. [Google Scholar]
- Youssef, A.M.; El-Nahrawy, A.M.; Abou Hammad, A.B. Sol-Gel Synthesis and Characterizations of Hybrid Chitosan-PEG/Calcium Silicate Nanocomposite Modified with ZnO-NPs and (E102) for Optical and Antibacterial Applications. Int. J. Biol. Macromol. 2017, 97, 561–567. [Google Scholar] [CrossRef]
- Pirsa, S.; Shamusi, T.; Kia, E.M. Smart Films Based on Bacterial Cellulose Nanofibers Modified by Conductive Polypyrrole and Zinc Oxide Nanoparticles. J. Appl. Polym. Sci. 2018, 135, 46617. [Google Scholar] [CrossRef]
- Roy, S.; Kim, H.C.; Panicker, P.S.; Rhim, J.W.; Kim, J. Cellulose Nanofiber-Based Nanocomposite Films Reinforced with Zinc Oxide Nanorods and Grapefruit Seed Extract. Nanomaterials 2021, 11, 877. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.W. Carboxymethyl Cellulose-Based Antioxidant and Antimicrobial Active Packaging Film Incorporated with Curcumin and Zinc Oxide. Int. J. Biol. Macromol. 2020, 148, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, A.; Isopencu, G.; Busuioc, C.; Popa, O.M.; Dietrich, P.; Socaciu-Siebert, L. Bacterial Cellulose Films with ZnO Nanoparticles and Propolis Extracts: Synergistic Antimicrobial Effect. Sci. Rep. 2019, 9, 17687. [Google Scholar] [CrossRef]
- Jayakumar, A.; Heera, K.V.; Sumi, T.S.; Joseph, M.; Mathew, S.; Praveen, G.; Nair, I.C.; Radhakrishnan, E.K. Starch-PVA Composite Films with Zinc-Oxide Nanoparticles and Phytochemicals as Intelligent PH Sensing Wraps for Food Packaging Application. Int. J. Biol. Macromol. 2019, 136, 395–403. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kim, S.M.; Rhim, J.W. Carboxymethyl Cellulose-Based Multifunctional Film Combined with Zinc Oxide Nanoparticles and Grape Seed Extract for the Preservation of High-Fat Meat Products. Sustain. Mater. Technol. 2021, 29, e00325. [Google Scholar] [CrossRef]
- Hari, K.D.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Improvement of the UV Barrier and Antibacterial Properties of Crosslinked Pectin/Zinc Oxide Bionanocomposite Films. Polymers 2021, 13, 2403. [Google Scholar] [CrossRef]
- Zhang, W.; Rhim, J.-W. Titanium Dioxide (TiO2) for the Manufacture of Multifunctional Active Food Packaging Films. Food Packag. Shelf Life 2022, 31, 100806. [Google Scholar] [CrossRef]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current Trends and Challenges in Cancer Management and Therapy Using Designer Nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Sani, M.; Khezerlou, A.; Ehsani, A. Fabrication and Characterization of the Bionanocomposite Film Based on Whey Protein Biopolymer Loaded with TiO2 Nanoparticles, Cellulose Nanofibers and Rosemary Essential Oil. Ind. Crop. Prod. 2018, 124, 300–315. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Alizadeh-Sani, M.; Khezerlou, A.; Mirzanajafi-Zanjani, M.; Zolfaghari, H.; Bagheri, V.; Divband, B.; Ehsani, A. Nanoparticles and Zeolites: Antibacterial Effects and Their Mechanism against Pathogens. Curr. Pharm. Biotechnol. 2019, 20, 1074–1086. [Google Scholar] [CrossRef]
- Alizadeh Sani, M.; Maleki, M.; Eghbaljoo-Gharehgheshlaghi, H.; Khezerlou, A.; Mohammadian, E.; Liu, Q.; Jafari, S.M. Titanium Dioxide Nanoparticles as Multifunctional Surface-Active Materials for Smart/Active Nanocomposite Packaging Films. Adv. Colloid. Interface Sci. 2022, 300, 102593. [Google Scholar] [CrossRef]
- Chen, Z.; Han, S.; Zhou, S.; Feng, H.; Liu, Y.; Jia, G. Review of Health Safety Aspects of Titanium Dioxide Nanoparticles in Food Application. NanoImpact 2020, 18, 100224. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Mohammadian, E.; McClements, D.J. Eco-Friendly Active Packaging Consisting of Nanostructured Biopolymer Matrix Reinforced with TiO2 and Essential Oil: Application for Preservation of Refrigerated Meat. Food Chem. 2020, 322, 126782. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; De la Mora, Z.V.; Rodríguez-Barajas, N.; Sandoval-Contreras, T.; Nuño, K.; López-De la Mora, D.A.; Pérez-Larios, A.; Montalvo-González, E. Protein–Tio2: A Functional Hybrid Composite with Diversified Applications. Coatings 2020, 10, 1194. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Rhim, J.-W.; Azizi-Lalabadi, M.; Hemmati-Dinarvand, M.; Ehsani, A. Preparation and Characterization of Functional Sodium Caseinate/Guar Gum/TiO2/Cumin Essential Oil Composite Film. Int. J. Biol. Macromol. 2020, 145, 835–844. [Google Scholar] [CrossRef]
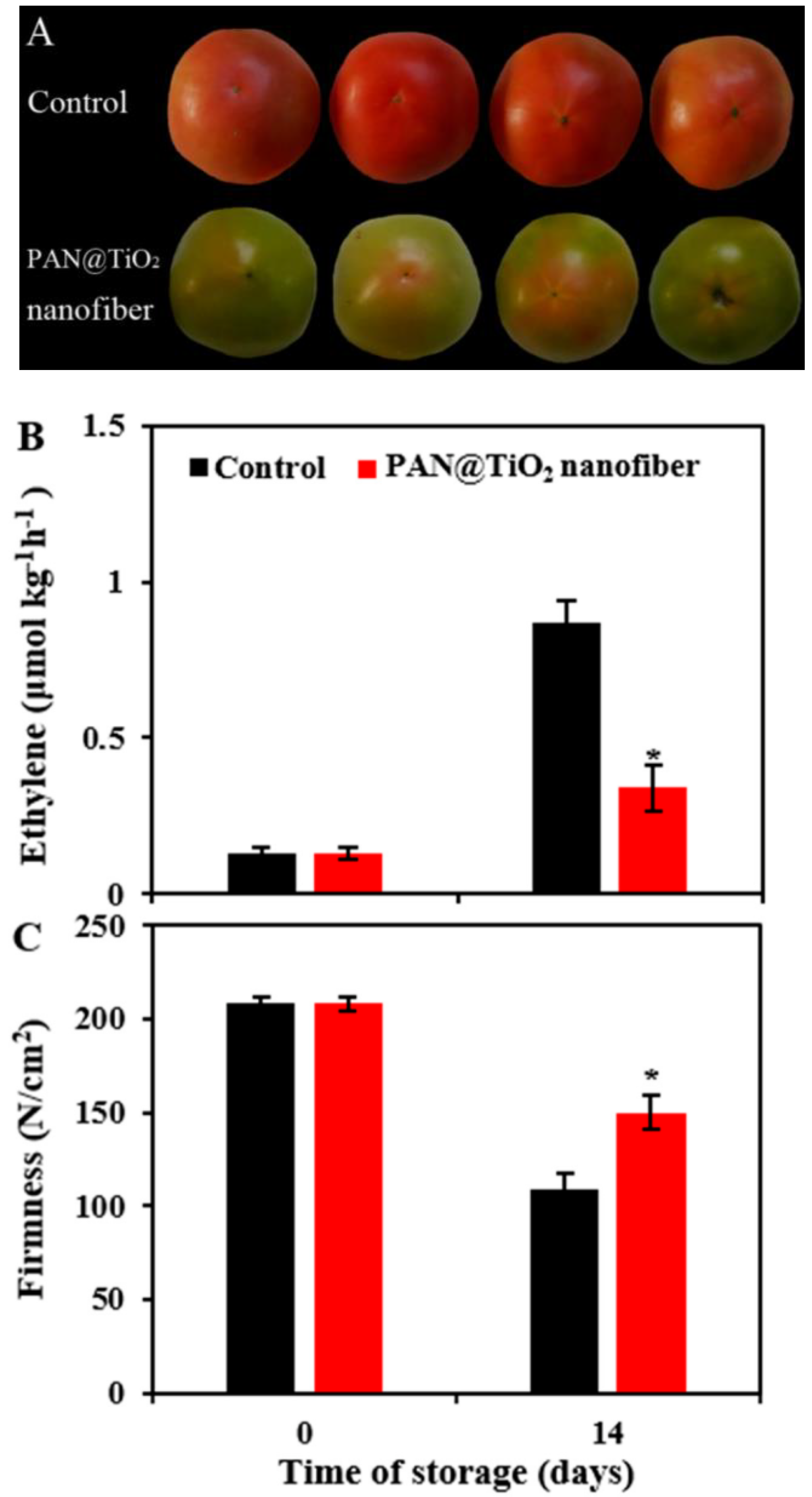
- Böhmer-Maas, B.W.; Fonseca, L.M.; Otero, D.M.; da Rosa Zavareze, E.; Zambiazi, R.C. Photocatalytic Zein-TiO2 Nanofibers as Ethylene Absorbers for Storage of Cherry Tomatoes. Food Packag. Shelf Life 2020, 24, 100508. [Google Scholar] [CrossRef]
- De Matos Fonseca, J.; Pabón, N.Y.L.; Valencia, G.A.; Nandi, L.G.; Dotto, M.E.R.; de Fátima Peralta Muniz Moreira, R.; Monteiro, A.R. Ethylene Scavenging Properties from Hydroxypropyl Methylcellulose-TiO2 and Gelatin-TiO2 Nanocomposites on Polyethylene Supports for Fruit Application. Int. J. Biol. Macromol. 2021, 178, 154–169. [Google Scholar] [CrossRef]
- De Chiara, M.L.V.; Pal, S.; Licciulli, A.; Amodio, M.L.; Colelli, G. Photocatalytic Degradation of Ethylene on Mesoporous TiO2/SiO2 Nanocomposites: Effects on the Ripening of Mature Green Tomatoes. Biosyst. Eng. 2015, 132, 61–70. [Google Scholar] [CrossRef]
- Alizadeh Sani, M.; Tavassoli, M.; Salim, S.A.; Azizi-lalabadi, M.; McClements, D.J. Development of Green Halochromic Smart and Active Packaging Materials: TiO2 Nanoparticle- and Anthocyanin-Loaded Gelatin/κ-Carrageenan Films. Food Hydrocoll. 2022, 124, 107324. [Google Scholar] [CrossRef]
- Goudarzi, V.; Shahabi-Ghahfarrokhi, I.; Babaei-Ghazvini, A. Preparation of Ecofriendly UV-Protective Food Packaging Material by Starch/TiO2 Bio-Nanocomposite: Characterization. Int. J. Biol. Macromol. 2017, 95, 306–313. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Guo, X.; Li, W.; Chen, J.; Liu, Q.; Xu, Q.; Wang, Q.; Yang, H.; Shui, Y.; et al. Effects of Different Tio2 Nanoparticles Concentrations on the Physical and Antibacterial Activities of Chitosan-Based Coating Film. Nanomaterials 2020, 10, 1365. [Google Scholar] [CrossRef] [PubMed]
- Vejdan, A.; Ojagh, S.M.; Adeli, A.; Abdollahi, M. Effect of TiO2 Nanoparticles on the Physico-Mechanical and Ultraviolet Light Barrier Properties of Fish Gelatin/Agar Bilayer Film. LWT 2016, 71, 88–95. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Kim, S.S.; Lee, J.; Lee, J. Effect of TiO2 on Highly Elastic, Stretchable UV Protective Nanocomposite Films Formed by Using a Combination of k-Carrageenan, Xanthan Gum and Gellan Gum. Int. J. Biol. Macromol. 2019, 123, 1020–1027. [Google Scholar] [CrossRef]
- Zolfi, M.; Khodaiyan, F.; Mousavi, M.; Hashemi, M. Development and Characterization of the Kefiran-Whey Protein Isolate-TiO2 Nanocomposite Films. Int. J. Biol. Macromol. 2014, 65, 340–345. [Google Scholar] [CrossRef]
- Salama, H.E.; Abdel Aziz, M.S. Optimized Alginate and Aloe Vera Gel Edible Coating Reinforced with NTiO2 for the Shelf-Life Extension of Tomatoes. Int. J. Biol. Macromol. 2020, 165, 2693–2701. [Google Scholar] [CrossRef]
- Kochkina, N.E.; Butikova, O.A. Effect of Fibrous TiO2 Filler on the Structural, Mechanical, Barrier and Optical Characteristics of Biodegradable Maize Starch/PVA Composite Films. Int. J. Biol. Macromol. 2019, 139, 431–439. [Google Scholar] [CrossRef] [PubMed]
- De Matos Fonseca, J.; Valencia, G.A.; Soares, L.S.; Dotto, M.E.R.; Campos, C.E.M.; de Fátima Peralta Muniz Moreira, R.; Fritz, A.R.M. Hydroxypropyl Methylcellulose-TiO2 and Gelatin-TiO2 Nanocomposite Films: Physicochemical and Structural Properties. Int. J. Biol. Macromol. 2020, 151, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Kaewklin, P.; Siripatrawan, U.; Suwanagul, A.; Lee, Y.S. Active Packaging from Chitosan-Titanium Dioxide Nanocomposite Film for Prolonging Storage Life of Tomato Fruit. Int. J. Biol. Macromol. 2018, 112, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Riahi, Z.; Priyadarshi, R.; Rhim, J.W.; Bagheri, R. Gelatin-Based Functional Films Integrated with Grapefruit Seed Extract and TiO2 for Active Food Packaging Applications. Food Hydrocoll. 2021, 112, 106314. [Google Scholar] [CrossRef]
- Arezoo, E.; Mohammadreza, E.; Maryam, M.; Abdorreza, M.N. The Synergistic Effects of Cinnamon Essential Oil and Nano TiO2 on Antimicrobial and Functional Properties of Sago Starch Films. Int. J. Biol. Macromol. 2020, 157, 743–751. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Y.; Liu, F.; Ren, F.; Zhao, G.; Leng, X. Fabrication and Characterization of TiO2/Whey Protein Isolate Nanocomposite Film. Food Hydrocoll. 2011, 25, 1098–1104. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Y.; Cai, X.; Wang, S. Fabrication of Gelatin-TiO2 Nanocomposite Film and Its Structural, Antibacterial and Physical Properties. Int. J. Biol. Macromol. 2016, 84, 153–160. [Google Scholar] [CrossRef]
- Dash, K.K.; Ali, N.A.; Das, D.; Mohanta, D. Thorough Evaluation of Sweet Potato Starch and Lemon-Waste Pectin Based-Edible Films with Nano-Titania Inclusions for Food Packaging Applications. Int. J. Biol. Macromol. 2019, 139, 449–458. [Google Scholar] [CrossRef]
- Razali, M.H.; Ismail, N.A.; Mat Amin, K.A. Titanium Dioxide Nanotubes Incorporated Gellan Gum Bio-Nanocomposite Film for Wound Healing: Effect of TiO2 Nanotubes Concentration. Int. J. Biol. Macromol. 2020, 153, 1117–1135. [Google Scholar] [CrossRef]
- Shao, X.; Sun, H.; Zhou, R.; Zhao, B.; Shi, J.; Jiang, R.; Dong, Y. Effect of Bovine Bone Collagen and Nano-TiO2 on the Properties of Hydroxypropyl Methylcellulose Films. Int. J. Biol. Macromol. 2020, 158, 937–944. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Kaewklin, P. Fabrication and Characterization of Chitosan-Titanium Dioxide Nanocomposite Film as Ethylene Scavenging and Antimicrobial Active Food Packaging. Food Hydrocoll. 2018, 84, 125–134. [Google Scholar] [CrossRef]
- Salama, H.E.; Abdel Aziz, M.S. Optimized Carboxymethyl Cellulose and Guanidinylated Chitosan Enriched with Titanium Oxide Nanoparticles of Improved UV-Barrier Properties for the Active Packaging of Green Bell Pepper. Int. J. Biol. Macromol. 2020, 165, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.R.; Savadkoohi, B.; Zahedi, Y.; Hatami, M.; Ako, K. Fabrication and Characterization of Hybrid Sodium Montmorillonite/TiO2 Reinforced Cross-Linked Wheat Starch-Based Nanocomposites. Int. J. Biol. Macromol. 2019, 131, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Mesgari, M.; Aalami, A.H.; Sathyapalan, T.; Sahebkar, A. A Comprehensive Review of the Development of Carbohydrate Macromolecules and Copper Oxide Nanocomposite Films in Food Nanopackaging. Bioinorg. Chem. Appl. 2022, 2022, 7557825. [Google Scholar] [CrossRef] [PubMed]
- Mahato, N.; Sharma, K.; Sinha, M.; Cho, M.H. Citrus Waste Derived Nutra-/Pharmaceuticals for Health Benefits: Current Trends and Future Perspectives. J. Funct. Foods 2018, 40, 307–316. [Google Scholar] [CrossRef]
- Errokh, A.; Ferraria, A.M.; Conceição, D.S.; Vieira Ferreira, L.F.; Botelho Do Rego, A.M.; Rei Vilar, M.; Boufi, S. Controlled Growth of Cu2O Nanoparticles Bound to Cotton Fibres. Carbohydr. Polym. 2016, 141, 229–237. [Google Scholar] [CrossRef]
- Garcia, C.V.; Shin, G.H.; Kim, J.T. Metal Oxide-Based Nanocomposites in Food Packaging: Applications, Migration, and Regulations. Trends Food Sci. Technol. 2018, 82, 21–31. [Google Scholar] [CrossRef]
- Dobrucka, R. Antioxidant and Catalytic Activity of Biosynthesized CuO Nanoparticles Using Extract of Galeopsidis Herba. J. Inorg. Organomet. Polym. Mater. 2018, 28, 812–819. [Google Scholar] [CrossRef]
- Gvozdenko, A.A.; Siddiqui, S.A.; Blinov, A.V.; Golik, A.B.; Nagdalian, A.A.; Maglakelidze, D.G.; Statsenko, E.N.; Pirogov, M.A.; Blinova, A.A.; Sizonenko, M.N.; et al. Synthesis of CuO Nanoparticles Stabilized with Gelatin for Potential Use in Food Packaging Applications. Sci. Rep. 2022, 12, 12843. [Google Scholar] [CrossRef]
- Jana, R.; Dey, A.; Das, M.; Datta, J.; Das, P.; Ray, P.P. Improving Performance of Device Made up of CuO Nanoparticles Synthesized by Hydrothermal over the Reflux Method. Appl. Surf. Sci. 2018, 452, 155–164. [Google Scholar] [CrossRef]
- Singh, P.K.; Das, A.K.; Hatui, G.; Nayak, G.C. Shape Controlled Green Synthesis of CuO Nanoparticles through Ultrasonic Assisted Electrochemical Discharge Process and Its Application for Supercapacitor. Mater. Chem. Phys. 2017, 198, 16–34. [Google Scholar] [CrossRef]
- Pestovsky, Y.S.; Martínez-Antonio, A. The Use of Nanoparticles and Nanoformulations in Agriculture. J. Nanosci. Nanotechnol. 2017, 17, 8699–8730. [Google Scholar] [CrossRef]
- Gautam, S.; Misra, P.; Shukla, P.K.; Ramteke, P.W. Effect of Copper Oxide Nanoparticle on the Germination, Growth and Chlorophyll in Soybean (Glycine max (L.). Vegetos 2016, 29, 157–160. [Google Scholar] [CrossRef]
- Mousa, A.M.; Abdel Aziz, O.A.; Al-Hagar, O.E.A.; Gizawy, M.A.; Allan, K.F.; Attallah, M.F. Biosynthetic New Composite Material Containing CuO Nanoparticles Produced by Aspergillus Terreus for 47Sc Separation of Cancer Theranostics Application from Irradiated Ca Target. Appl. Radiat. Isot. 2020, 166, 109389. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.P. Enhanced Surface Morphology of Copper Oxide (CuO) Nanoparticles and Its Antibacterial Activities. Mater. Today Proc. 2020, 50, 2865–2868. [Google Scholar] [CrossRef]
- Abdullah, A.S.; Essa, F.A.; Bacha, H.B.; Omara, Z.M. Improving the Trays Solar Still Performance Using Reflectors and Phase Change Material with Nanoparticles. J. Energy Storage 2020, 31, 101744. [Google Scholar] [CrossRef]
- Amalraj, S.; Michael, P.A. Synthesis and Characterization of Al2O3 and CuO Nanoparticles into Nanofluids for Solar Panel Applications. Results Phys. 2019, 15, 102797. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-hindi, R. Antimicrobial Food Packaging Based on Sustainable Bio-Based Materials for Reducing Foodborne Pathogens: A Review. Food Chem. 2020, 310, 125915. [Google Scholar] [CrossRef]
- Kalia, A.; Kaur, M.; Shami, A.; Jawandha, S.K.; Alghuthaymi, M.A.; Thakur, A.; Abd-Elsalam, K.A. Nettle-Leaf Extract Derived ZnO/CuO Nanoparticle-Biopolymer-Based Antioxidant and Antimicrobial Nanocomposite Packaging Films and Their Impact on Extending the Post-Harvest Shelf Life of Guava Fruit. Biomolecules 2021, 11, 224. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sathiyaseelan, A.; Mariadoss, A.V.A.; Xiaowen, H.; Wang, M.H. Physical and Bioactivities of Biopolymeric Films Incorporated with Cellulose, Sodium Alginate and Copper Oxide Nanoparticles for Food Packaging Application. Int. J. Biol. Macromol. 2020, 153, 207–214. [Google Scholar] [CrossRef]
- Dehghani, S.; Peighambardoust, S.H.; Peighambardoust, S.J.; Hosseini, S.V.; Regenstein, J.M. Improved Mechanical and Antibacterial Properties of Active LDPE Films Prepared with Combination of Ag, ZnO and CuO Nanoparticles. Food Packag. Shelf Life 2019, 22, 100391. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Zhang, Y.; Shang, Y.; Zhang, X.; Wen, Y. Preparation of PAN@TiO2 Nanofibers for Fruit Packaging Materials with Efficient Photocatalytic Degradation of Ethylene. Materials 2019, 12, 896. [Google Scholar] [CrossRef]
- Maneerat, C.; Hayata, Y. Efficiency Of TiO2 Photocatalytic Reaction On Delay Of Fruit Ripening And Removal Of Off-Flavors From The Fruit Storage Atmosphere. Trans. ASABE 2006, 49, 833–837. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Jiang, Y.; Ding, Y.; Yun, J.; Tang, Y.; Zhang, P. Effect of Nano-ZnO-Coated Active Packaging on Quality of Fresh-Cut “Fuji” Apple. Int. J. Food Sci. Technol. 2011, 46, 1947–1955. [Google Scholar] [CrossRef]
- Simpson, R.; Corevic, E.; Rojas, S. Modelling a Modified Atmosphere Packaging System for Fresh Scallops (Argopecten purpuratus). Packag. Technol. Sci. 2007, 20, 87–97. [Google Scholar] [CrossRef]
- Anugrah, D.S.B.; Alexander, H.; Pramitasari, R.; Hudiyanti, D.; Sagita, C.P. A Review of Polysaccharide-Zinc Oxide Nanocomposites as Safe Coating for Fruits Preservation. Coatings 2020, 10, 988. [Google Scholar] [CrossRef]
- Arroyo, B.J.; Bezerra, A.C.; Oliveira, L.L.; Arroyo, S.J.; de Melo, E.A.; Santos, A.M.P. Antimicrobial Active Edible Coating of Alginate and Chitosan Add ZnO Nanoparticles Applied in Guavas (Psidium guajava L.). Food Chem. 2020, 309, 125566. [Google Scholar] [CrossRef] [PubMed]
- Lavinia, M.; Hibaturrahman, S.N.; Harinata, H.; Wardana, A.A. Antimicrobial Activity and Application of Nanocomposite Coating from Chitosan and ZnO Nanoparticle to Inhibit Microbial Growth on Fresh-Cut Papaya. Food Res. 2020, 4, 307–311. [Google Scholar] [CrossRef]
- Emamifar, A.; Bavaisi, S. Nanocomposite Coating Based on Sodium Alginate and Nano-ZnO for Extending the Storage Life of Fresh Strawberries (Fragaria × Ananassa Duch.). J. Food Meas. Charact. 2020, 14, 1012–1024. [Google Scholar] [CrossRef]
- Meindrawan, B.; Suyatma, N.E.; Wardana, A.A.; Pamela, V.Y. Nanocomposite Coating Based on Carrageenan and ZnO Nanoparticles to Maintain the Storage Quality of Mango. Food Packag. Shelf Life 2018, 18, 140–146. [Google Scholar] [CrossRef]
- Koushesh Saba, M.; Amini, R. Nano-ZnO/Carboxymethyl Cellulose-Based Active Coating Impact on Ready-to-Use Pomegranate during Cold Storage. Food Chem. 2017, 232, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Romadhan, M.F.; Pujilestari, S. Sintesis Nanopartikel ZnO Dan Aplikasinya Sebagai Edible Coating Berbasis Pektin Untuk Memperpanjang Umur Simpan Buah Belimbing. J. Agroindustri Halal 2019, 5, 30–38. [Google Scholar] [CrossRef]
- Martínez-Abad, A.; Lagaron, J.M.; Ocio, M.J. Development and Characterization of Silver-Based Antimicrobial Ethylene–Vinyl Alcohol Copolymer (EVOH) Films for Food-Packaging Applications. J. Agric. Food Chem. 2012, 60, 5350–5359. [Google Scholar] [CrossRef] [PubMed]
- Kowsalya, E.; MosaChristas, K.; Balashanmugam, P.; Rani, J.C. Biocompatible Silver Nanoparticles/Poly(Vinyl Alcohol) Electrospun Nanofibers for Potential Antimicrobial Food Packaging Applications. Food Packag. Shelf Life 2019, 21, 100379. [Google Scholar] [CrossRef]
- Kumar, S.; Shukla, A.; Baul, P.P.; Mitra, A.; Halder, D. Biodegradable Hybrid Nanocomposites of Chitosan/Gelatin and Silver Nanoparticles for Active Food Packaging Applications. Food Packag. Shelf Life 2018, 16, 178–184. [Google Scholar] [CrossRef]
- Zhang, W.; Roy, S.; Rhim, J. Copper-based Nanoparticles for Biopolymer-based Functional Films in Food Packaging Applications. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1933–1952. [Google Scholar] [CrossRef]
- Gautam, G.; Mishra, P. Development and Characterization of Copper Nanocomposite Containing Bilayer Film for Coconut Oil Packaging. J. Food Process Preserv. 2017, 41, e13243. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Vats, C.; Sangwan, P.; Kumar, V.; Abhineet; Chauhan, P.; Chauhan, R.S.; Chaudhary, K. Recent Insights into Metallic Nanoparticles in Shelf-Life Extension of Agrifoods: Properties, Green Synthesis, and Major Applications. Front. Sustain. Food Syst. 2022, 6, 1025342. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Kalita, S.; Singh, N.; Katiyar, V.; Mitra, A.; Halder, D. Nanomaterials in Food Packaging. In Biopolymer-Based Food Packaging; Wiley: Hoboken, NJ, USA, 2022; pp. 336–367. ISBN 9781119702313. [Google Scholar]
- Gupta, R.; Xie, H. Nanoparticles in Daily Life: Applications, Toxicity and Regulations. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 209–230. [Google Scholar] [CrossRef]
- Paidari, S.; Tahergorabi, R.; Anari, E.S.; Nafchi, A.M.; Zamindar, N.; Goli, M. Migration of Various Nanoparticles into Food Samples: A Review. Foods 2021, 10, 2114. [Google Scholar] [CrossRef] [PubMed]
- Pradesh, U. Nanotechnology in Packaging of Fresh Fruits and Vegetables. In Emerging Postharvest Treatment of Fruits and Vegetables; Apple Academic Press: Waretown, NJ, USA, 2018; pp. 147–166. [Google Scholar]
- Dimitrijevic, M.; Karabasil, N.; Boskovic, M.; Teodorovic, V.; Vasilev, D.; Djordjevic, V.; Kilibarda, N.; Cobanovic, N. Safety Aspects of Nanotechnology Applications in Food Packaging. Procedia Food Sci. 2015, 5, 57–60. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, S.; Bing, X.; Gao, C.; Wang, T.; Yuan, B. Nanosilver Migrated into Food-Simulating Solutions from Commercially Available Food Fresh Containers. Packag. Technol. Sci. 2011, 24, 291–297. [Google Scholar] [CrossRef]
- Huang, J.Y.; Li, X.; Zhou, W. Safety Assessment of Nanocomposite for Food Packaging Application. Trends Food Sci. Technol. 2015, 45, 187–199. [Google Scholar] [CrossRef]
- Song, H.; Li, B.; Lin, Q.-B.; Wu, H.-J.; Chen, Y. Migration of Silver from Nanosilver–Polyethylene Composite Packaging into Food Simulants. Food Addit. Contam. Part A 2011, 28, 1758–1762. [Google Scholar] [CrossRef] [PubMed]
- Al-Ali, R.M.; Al-Hilifi, S.A.; Rashed, M.M.A. Fabrication, Characterization, and Anti-free Radical Performance of Edible Packaging-chitosan Film Synthesized from Shrimp Shell Incorporated with Ginger Essential Oil. J. Food Meas. Charact. 2021, 15, 2951–2962. [Google Scholar] [CrossRef]
- Onyeaka, H.; Passaretti, P.; Miri, T.; Al-Sharify, Z.T. The Safety of Nanomaterials in Food Production and Packaging. Curr. Res. Food Sci. 2022, 5, 763–774. [Google Scholar] [CrossRef]
- Balfourier, A.; Marty, A.P.; Gazeau, F. Importance of Metal Biotransformation in Cell Response to Metallic Nanoparticles: A Transcriptomic Meta-Analysis Study. ACS Nanosci. Au 2023, 3, 46–57. [Google Scholar] [CrossRef]
- Song, W.; Zhang, J.; Guo, J.; Zhang, J.; Ding, F.; Li, L.; Sun, Z. Role of the Dissolved Zinc Ion and Reactive Oxygen Species in Cytotoxicity of ZnO Nanoparticles. Toxicol. Lett. 2010, 199, 389–397. [Google Scholar] [CrossRef]
- Xiu, Z.M.; Zhang, Q.B.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef]
- Loza, K.; Diendorf, J.; Sengstock, C.; Ruiz-Gonzalez, L.; Gonzalez-Calbet, J.M.; Vallet-Regi, M.; Köller, M.; Epple, M. The Dissolution and Biological Effects of Silver Nanoparticles in Biological Media. J. Mater. Chem. B 2014, 2, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, L.; Wang, C.; Zhang, S.; Liu, H.; Li, S.; Wang, X. Toxicity and Mechanisms of Action of Titanium Dioxide Nanoparticles in Living Organisms. J. Environ. Sci. 2019, 75, 40–53. [Google Scholar] [CrossRef]
- Zhang, N.; Xiong, G.; Liu, Z. Toxicity of Metal-Based Nanoparticles: Challenges in the Nano Era. Front. Bioeng. Biotechnol. 2022, 10, 1001572. [Google Scholar] [CrossRef] [PubMed]
- Creutzenberg, O.; Bellmann, B.; Korolewitz, R.; Koch, W.; Mangelsdorf, I.; Tillmann, T.; Schaudien, D. Change in Agglomeration Status and Toxicokinetic Fate of Various Nanoparticles in Vivo Following Lung Exposure in Rats. Inhal. Toxicol. 2012, 24, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Bruinink, A.; Wang, J.; Wick, P. Effect of Particle Agglomeration in Nanotoxicology. Arch. Toxicol. 2015, 89, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Rabbani, G.; Baig, M.H.; Lim, J.H.; Khan, M.E.; Lee, E.J.; Ashraf, G.M.; Choi, I. Nanoparticle-Based Drugs: A Potential Armamentarium of Effective Anti-Cancer Therapies. Curr. Drug Metab. 2018, 19, 839–846. [Google Scholar] [CrossRef]
- Andersson, P.O.; Lejon, C.; Ekstrand-Hammarström, B.; Akfur, C.; Ahlinder, L.; Bucht, A.; Österlund, L. Polymorph- and Size-Dependent Uptake and Toxicity of TiO2 Nanoparticles in Living Lung Epithelial Cells. Small 2011, 7, 514–523. [Google Scholar] [CrossRef]
- Bae, E.; Park, H.-J.; Lee, J.; Kim, Y.; Yoon, J.; Park, K.; Choi, K.; Yi, J. Bacterial Cytotoxicity of the Silver Nanoparticle Related to Physicochemical Metrics and Agglomeration Properties. Environ. Toxicol. Chem. 2010, 29, 2154–2160. [Google Scholar] [CrossRef]
- Karakoti, A.S.; Das, S.; Thevuthasan, S.; Seal, S. PEGylated Inorganic Nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 1980–1994. [Google Scholar] [CrossRef]
- Dunphy Guzman, K.A.; Finnegan, M.P.; Banfield, J.F. Influence of Surface Potential on Aggregation and Transport of Titania Nanoparticles. Environ. Sci. Technol. 2006, 40, 7688–7693. [Google Scholar] [CrossRef]
- Murugadoss, S.; Brassinne, F.; Sebaihi, N.; Petry, J.; Cokic, S.M.; Van Landuyt, K.L.; Godderis, L.; Mast, J.; Lison, D.; Hoet, P.H.; et al. Agglomeration of Titanium Dioxide Nanoparticles Increases Toxicological Responses in Vitro and in Vivo. Part. Fibre Toxicol. 2020, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Divya, K.; Jisha, M.S. Chitosan Nanoparticles Preparation and Applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Yousuf, O.; Islam, R.U.; Younis, K. Silver Nanoparticles as an Active Packaging Ingredient and Its Toxicity. Packag. Technol. Sci. 2021, 34, 653–663. [Google Scholar] [CrossRef]
- Naseer, B.; Srivastava, G.; Qadri, O.S.; Faridi, S.A.; Islam, R.U.; Younis, K. Importance and Health Hazards of Nanoparticles Used in the Food Industry. Nanotechnol. Rev. 2018, 7, 623–641. [Google Scholar] [CrossRef]

| Material Type | Form | Application Product | Function | Brand and Company |
|---|---|---|---|---|
| Nylon 6-nanoclay composite | Barrier nylon resins | Beer and flavoured alcoholic beverage bottles, PET | Oxygen scavenging | Aegis HFX Resin and OXCE Resin. Honeywell International Inc., Phoenix, AZ, USA |
| Iron Oxidation | Sachets & Film | Fried snacks | Oxygen scavenger | OxyGuard®, Clariant Ltd., Mutten, Swaziland |
| Allyl isothiocyanate (AIT) or scavenging molecular O2 (Listeria populations) | Tray/Pads | Ham, ready-to-eat meat product | CO2 emitter and antimicrobial pad | UltraZap R Xtenda Pak pads. Paper Pak Industries, Winnipeg, MB, Canada |
| Titanium Dioxide (Nanoencapsulation) | Powder | Powdered milk-based products | Anticaking | Carnation Instant food. Carnation Breakfast Essential, Vevey, Switzerland |
| Nanosilver | Bag, Spray | Fruits & Vegetables | Antimicrobial actions | Biomaster. Addmaster Limited, Monrovia, CA, USA |
| Nanoclay Al2O3 • 2SiO2 • 2H2O | Film | Dried Fruits, cheeses, Coffee | Gas Barrier | N-coat. Multifilm Packing Corporation, Elgin, IL, USA |
| Changing colour based on aromatic compounds (sensor) | stickers | Fruits | Freshness Indicators | RipeSense™. Ripesense Limited, Tauranga, New Zealand. |
| Cerium oxide | Film | Retort Products and hot fill of meat and fish products | Oxygen scavenger | OMAC® Imperm®. Mitsubishi Gas Chemical Inc., Chiyoda-ku, Japan |
| sodium carbonate/sodium glycinate | Sachets/ Labels | Strawberries, eggplant | CO2 scavenger | Ageless®. Mitsubishi Gas Chemical Inc., Chiyoda-ku, Japan |
| TTI based on enzyme, Lipase, and pH indicating dye | Stickers | Seafood, Oysters | Freshness (Based on color) | TimeStrip®. TimeStrip UK Ltd., Cambridge, UK. |
| Metal-Based Nanoparticles Enmeshed in Some Natural Biopolymers | Observed Properties Due to the Metal-Based Nanoparticles |
|---|---|
| Carboxymethyl cellulose/ZnO, CuO, and Ag | Enhanced rate of UV absorption. Decreased water vapor permeability (WVP). Improvement of Young’s modulus (YM), tensile strength (TS), and elongation at break (EB). [66] |
| Starch/Ag-ZnO-CuO | Decreased water solubility (WS), water vapor permeability (WVP), and elongation at break (EB). Increase of TS and YM. Optimum UV and visible absorbance. [67] |
| Fish skin gelatin/Ag-Cu | Enhanced thickness, TS, a* (red/green) and b* (yellow/blue) in value, total colour difference (ΔE), transparency, and thermal degradation temperature (TDT). Decreased EB and lightness (L*) value. Darker colour. [68] |
| Gelatin-starch/ZnO | Enhanced thickness, TS, and melting temperature. Decreased EB, WVP, and WS. [69] |
| Soy protein/ZnO | Decreased L* value, whiteness index, and OTR (oxygen transmission rate). Enhanced a* and b* value, transparency, ΔE, EB, and TS. Showed good barrier properties against UV and visible light. WVP isolate. [70] |
| Gelatin-PVA/TiO2-ZnO | Decreased oxygen permeability (OP), transparency, WVP, and EB. Enhanced YM, TS, and thickness. [71] |
| CMC-chitosan/ZnO | Decreased a*, b*, and L* value, YM, and TS. Enhanced b* value, chroma value, ∆E, EB, and contact angle reduction. [72] |
| Galactomannan/ZnO | Enhanced contact angle, TS, TDT, YM, UVA, and UVB absorption. Decreased OP and WVP. [73] |
| Nanolignin-PLLA/Ag, Ag2O, TiO2, WO3, Fe2O3 and ZnFe2O | Enhanced a* and b* value, TS, thickness, ∆E, and YM. Decreased EB, WVP, and L* value. [74] |
| Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/ZnO | Decreased a*, b*, and L* value and EB. Enhanced TS, TDT, transparency, and toughness. [75] |
| Polymer Matrix | Quantity/Percentage (%) Weight of Ag | Notable Properties | References |
|---|---|---|---|
| liposomes were used to encapsulate Laurel essential oil (LEO) and AgNPs, mixed with chitosan to coat polyethylene (PE) films. (PC-Lip/LEO/AgNPs) | Not indicated | PC-Lip/LEO/AgNPs films showed good useful antioxidant and antimicrobial properties. Its evaluation on pork meat showed the extension of shelf life from 9 days, which is for pure PE, to 15 days without cytotoxicity. | [101] |
| polylactic acid (PLA)/AgNPs | 1–10% | Preserved ascorbic acid in strawberries. Decreased the reduction rate of polyphenols in the same fruit. PLA/Ag 5% film showed better preservative properties than the other counterparts. | [102] |
| Polyvinyl alcohol/clay/AgNPs nanocomposites film | Not indicated | Enhanced mechanical, light barrier and water-resistant properties were observed. Antimicrobial action against S. Typhimurium and S. aureus enabled it as active food packaging material. Fabricated Pouches of PVA/clay/Ag nanocomposite prevented microbial spoilage in chicken sausages. | [103] |
| Polyethylene/Ag/TiO2 | Ag/TiO2 nanopowder (9 g) | Showed strong antibacterial activity because of the interaction between Ag and TiO2. This film retarded the changes in the pasting qualities and texture of rice. | [104] |
| (AgNPs) encapsulated in gelatin-montmorillonite(M), cellulose acetate (CA), and/or thymol. CA/Ag/M film | 3–5% | tensile properties, UV blocking, and oxygen barrier properties of the films were enhanced. Good antioxidant activity was recorded, including those having thymol. Synergistic effects of AgNPs and thymol on the films’ antimicrobial and antifungal activities. | [105] |
| Polymer Matrix | Concentration/Percentage (%) Weight of ZnO | Notable Properties | References |
|---|---|---|---|
| Carboxymethyl cellulose (CMC)/curcumin/ZnO functional film | 1.0% | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) value increased to 7.5% and 92.5% upon the introduction of 1% of ZnO NPs and curcumin to the film, respectively. Improved UV barrier and mechanical properties upon the addition of 0.5 wt% of curcumin and 1 wt% of ZnO. CMC film with of 1 wt% ZnO and curcumin (CMC/Cur1.0/ZnO1.0) showed optimal functional films with antibacterial and antioxidant properties. | [117] |
| Bacteria cellulose (BC) Gluconacetobacter xylinum/ZnO (mediated propolis extract) Functional films | Not indicated | Showed antimicrobial properties with a minimum inhibition concentration (MIC) recorded at 0.438 mg/mL on Escherichia coli, Bacillus subtilis, and Candida albicans upon the addition of ZnO on the bacteria cellulose matrix. | [118] |
| Gelatin/starch/ZnO composite films | 12.5% of ZnO was used in preparing the nanocomposite | Incorporating ZnO enhanced the tensile strength (0.20–0.22 MPa) and reduced the elongation at breaks and film solubility. The bio-nanocomposite films showed antibacterial properties against Staphylococcus aureus and Escherichia coli. | [69] |
| Starch/polyvinyl alcohol (PVA)/ZnO composite films | 3.12 μg/mL (Minimum inhibitory concentration value was used) | Enhanced water barrier, UV barrier, mechanical and antimicrobial properties. In the antimicrobial study, an inhibition zone of 28 mm was recorded against Salmonella typhimurium. | [119] |
| Grape seed extract (GSE, 5 wt% of CMC)/ZnO composite films | 3% | The inclusion of GSE enhanced antioxidant activity to the CMC-based films, exhibiting about 95% and 25% scavenging activity against ABTS and DPPH oxidative free radicals. The film exhibited 100% UV protection. Furthermore, upon the addition of ZnO NPs, the composite film showed enhanced mechanical and water vapor barrier properties. Also, the composite film displayed potent antibacterial properties against foodborne pathogens of E. coli and L. monocytogenes. | [120] |
| Pectin/ZnO composite films | 0.5–1.5% | The UV-light barrier property of the pectin/ZnO films was significantly enhanced as the concentration of ZnO increased. | [121] |
| Polymer Matrix | Percentage (%) Weight of TiO2 in the Total Mixture of the Composite | Effects/Functions of TiO2 in the Prepared Food Packaging Material | References |
|---|---|---|---|
| Sodium caseinate/guar gum | 1–2 | Enhanced tensile strength and antimicrobial properties; reduced the permeability of water vapor and solubility. | [140] |
| Alginate and Aloe vera gel | 1–5 | Enhanced tensile strength, opacity, elongation-at-break, and antibacterial properties; Reduced water vapor permeability. | [141] |
| Starch/poly(vinyl alcohol) | 0.01–1 | Enhanced tensile strength and opacity; Reduced water vapor permeability. | [142] |
| Gelatin | 0.5–2 | Enhanced opacity. Reduced the water vapor permeability and water content. | [143] |
| Chitosan | 1 | Enhanced tensile strength and shelf life; reduced the permeability of water vapor. | [144] |
| Gelatin | 0.5–5 | Enhanced tensile strength and antimicrobial properties; reduced the permeability of water vapor. | [145] |
| Sago starch | 1–5 | Enhanced tensile strength, opacity, and antibacterial properties. Reduced the water vapor permeability, water content, and water solubility. | [146] |
| Whey protein isolate | 0.1–2 | Enhanced tensile strength, opacity, elongation-at-break, Reduced water content, and water solubility. | [147] |
| Hydroxypropyl methylcellulose | 0.5–2 | Enhanced opacity and elongation-at-break (EB). | [143] |
| k-carrageenan/xanthan gum/ gellan gum | 1–7 | Enhanced tensile strength and antimicrobial properties; reduced water vapor permeability and water content. | [139] |
| Gelatin | 3–5 | Enhanced tensile strength, opacity, elongation-at-break, and antibacterial properties; Reduced water vapor permeability. | [148] |
| Sweet potato starch/lemon- waste pectin | 0.5–4 | Enhanced tensile strength. Reduced the water vapor permeability, water content, and water solubility. | [149] |
| Gellan gum | 1–20 | Enhanced thickness, tensile strength, opacity, and antimicrobial properties; reduced the permeability of water vapor. | [150] |
| Hydroxypropyl methylcellulose | 0.04 | Enhanced elongation-at-break. | [151] |
| Chitosan | 0.25–2 | Enhanced tensile strength and antimicrobial properties; reduced the permeability of water vapor. | [152] |
| Kefiran/whey protein isolate | 1–5 | Enhanced elongation-at-break. Reduced the water vapor permeability, water content, and water solubility. | [131] |
| CMC/guanidinylatedchitosan | 1–5 | Enhanced tensile strength, opacity, and antibacterial properties. Reduced the water vapor permeability, water content, and water solubility. | [153] |
| Wheat starch | 1–4 | Enhanced opacity. Reduced the water vapor permeability and water solubility. | [154] |
| Polysaccharide Materials and ZnO Quantity | Study Conditions | Effect of Coating | References |
|---|---|---|---|
| 5% w/v of Chitosan 1%. ZnO v/v gel. | Dipping Stored at 21 ± 1 °C for 20 days and 80% RH Effect Guava | Reduced colour change and weight loss and maintained firmness. Ripening ratio index (SS/TA) was reduced. No external injuries were observed until end of storage. | [178] |
| 5% w/v of Alginate. ZnO 1% w/v gel. | Dipping 20 days at 21 ± 1 °C and RH 80 Guava | None | [178] |
| 5% w/v of Alginate–chitosan (90–10%). ZnO 1% w/v gel. | Dipping 20 days at 21 ± 1 °C and RH 80 Guava | Maintained firmness better and prevented external injuries. | [178] |
| 3 g of Chitosan in 0.4 L coating solution. ZnO at varying percentages between 0.005–0.027% w/w coating solution (611.30 nm). | Dipping 12 days, 10 °C Fresh-cut papaya | Microbial growth/action was reduced. | [179] |
| 1.5% w/v of Alginate. ZnO at varying concentrations between 0.25 and 1.25 g/L (30–50 nm). | Dipping 20 days, 1 °C, RH 95% Strawberry | Reduced colour change, weight loss, and maintain firmness. Increases antimicrobial and antioxidant activity by maintaining ascorbic acid. Control fruit maturity, improve Titrable acidity (TA) and prevent increase of Total soluble solids (TSS). Reduces the decrease in anthocyanin, phenolic, and peroxidase activity and decreases superoxide dismutase activity. Extended the storage life of fresh fruits by up to 20 days | [180] |
| 0.8 g of Carrageenan in 0.1 L solution. 1% w/w of carrageenan + ZnO 0.5%. | Dipping 20 °C and RH 61% Mango | Reduced the production of CO2 and weight loss. Maintained total acidity, colour, and textural appearance better. | [181] |
| 0.5% w/v of CMC. ZnO 0.1% and 0.2% w/v (30–100 nm). | Dipping 12 days, 4 °C and RH 90% Pomegranate arils | Reduced weight loss and loss of vitamin C. Reduced the loss of anthocyanin and phenolic content. Showed higher antioxidant activities | [182] |
| 10 g of Pectin in 1 L solution. ZnO 0.1 g/L in solution | Dipping 8 days at 25 °C Star fruit | Reduced browning index, redness value, and weight loss. Reduced physical damage. | [183] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeyemi, J.O.; Fawole, O.A. Metal-Based Nanoparticles in Food Packaging and Coating Technologies: A Review. Biomolecules 2023, 13, 1092. https://doi.org/10.3390/biom13071092
Adeyemi JO, Fawole OA. Metal-Based Nanoparticles in Food Packaging and Coating Technologies: A Review. Biomolecules. 2023; 13(7):1092. https://doi.org/10.3390/biom13071092
Chicago/Turabian StyleAdeyemi, Jerry O., and Olaniyi A. Fawole. 2023. "Metal-Based Nanoparticles in Food Packaging and Coating Technologies: A Review" Biomolecules 13, no. 7: 1092. https://doi.org/10.3390/biom13071092
APA StyleAdeyemi, J. O., & Fawole, O. A. (2023). Metal-Based Nanoparticles in Food Packaging and Coating Technologies: A Review. Biomolecules, 13(7), 1092. https://doi.org/10.3390/biom13071092








