Single Mutations in Cytochrome P450 Oxidoreductase Can Alter the Specificity of Human Cytochrome P450 1A2-Mediated Caffeine Metabolism
Abstract
1. Introduction
2. Materials and Methods
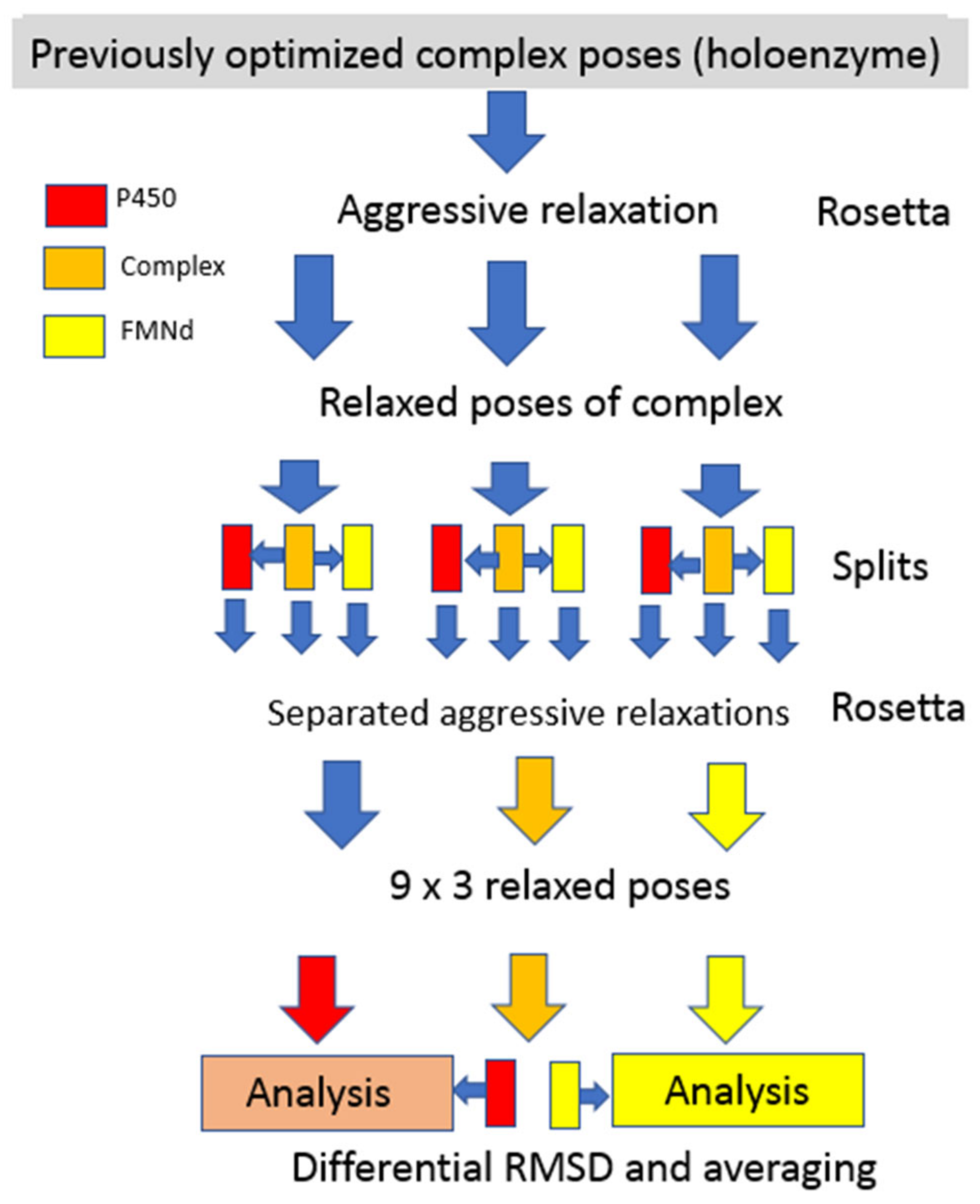
Computational Simulations
3. Results
3.1. Isolation and Characterization of the Membrane Fractions
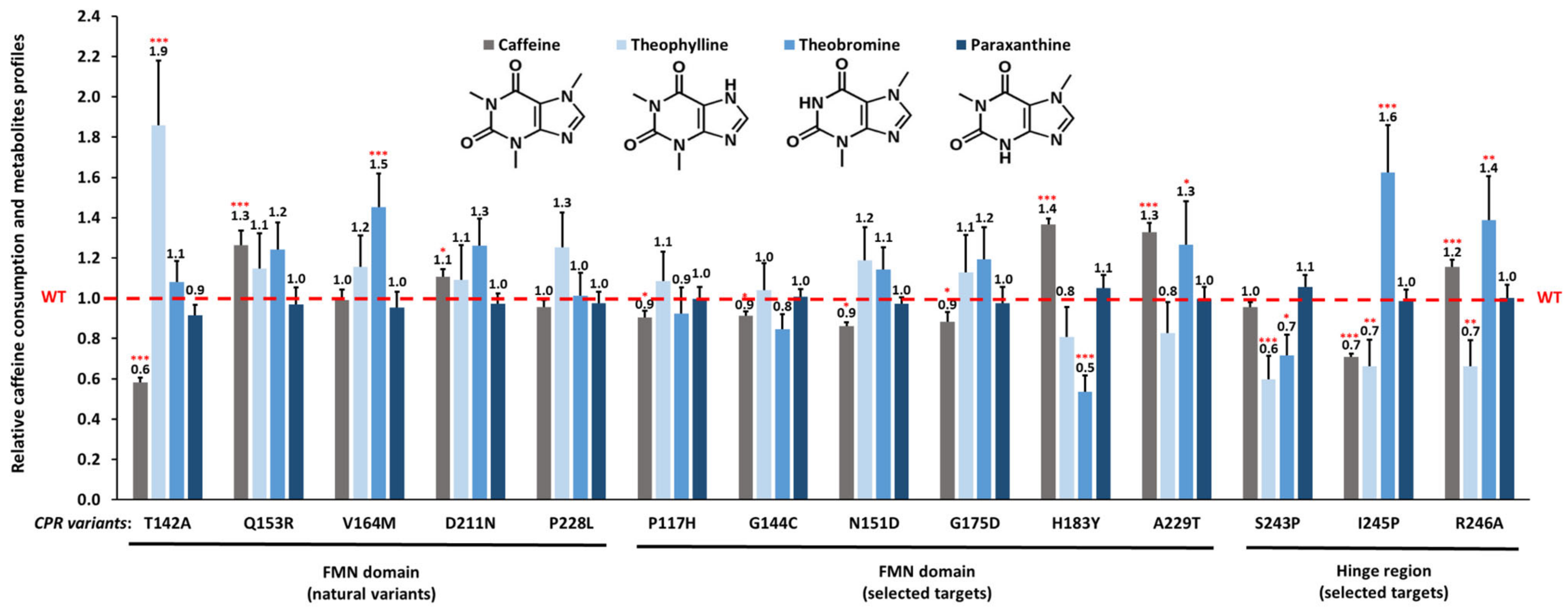
3.2. Caffeine Consumption
3.3. Effect of CPR Variants on CYP1A2-Caffeine Metabolite Pattern
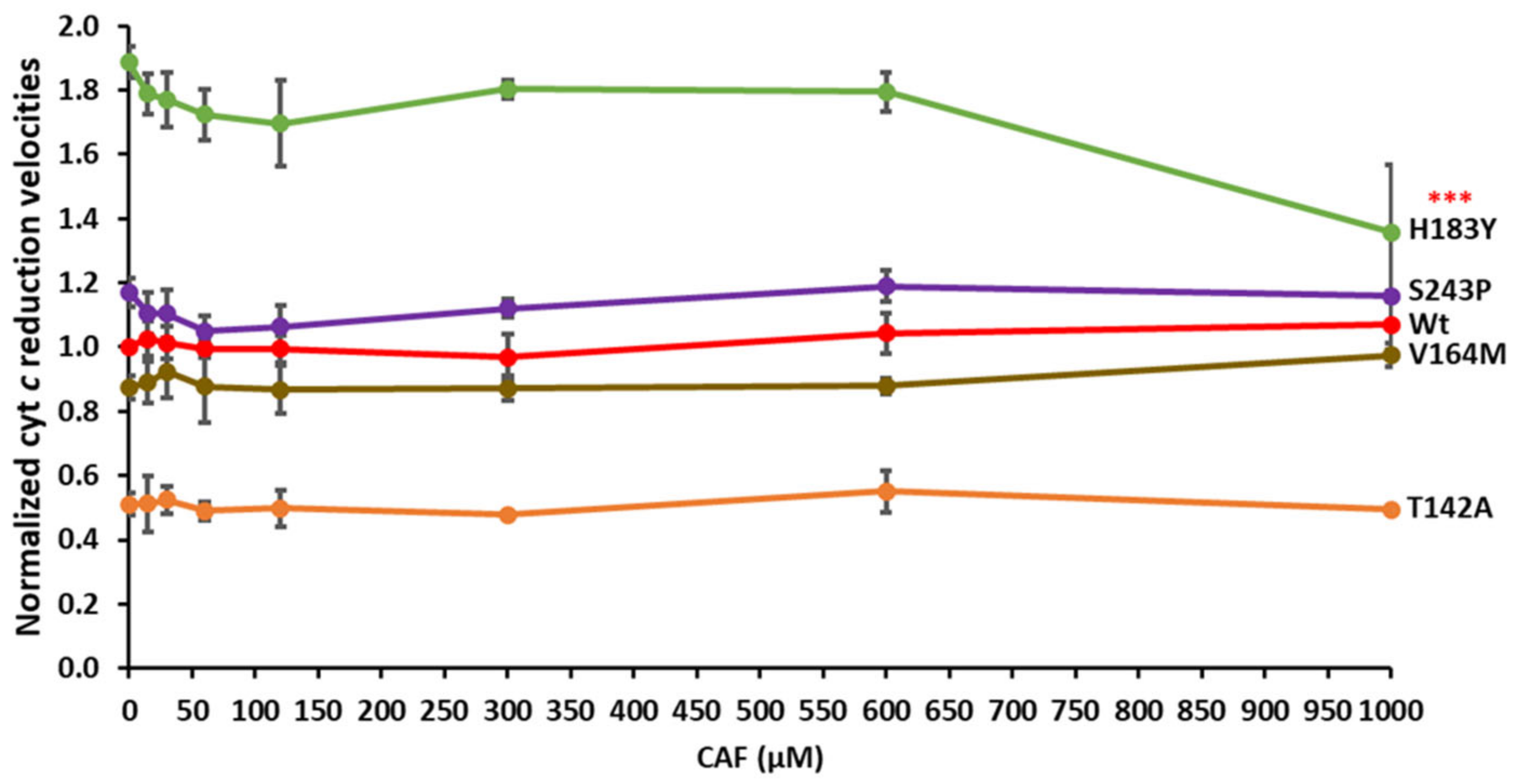
3.4. Effect of CPR Variants on ROS Production in CYP1A2-Mediated Caffeine Metabolism
3.5. Cytochrome c Competition in the CPRvar/CYP1A2–Caffeine Reaction
3.6. Interaction of CYP1A2 with CPR
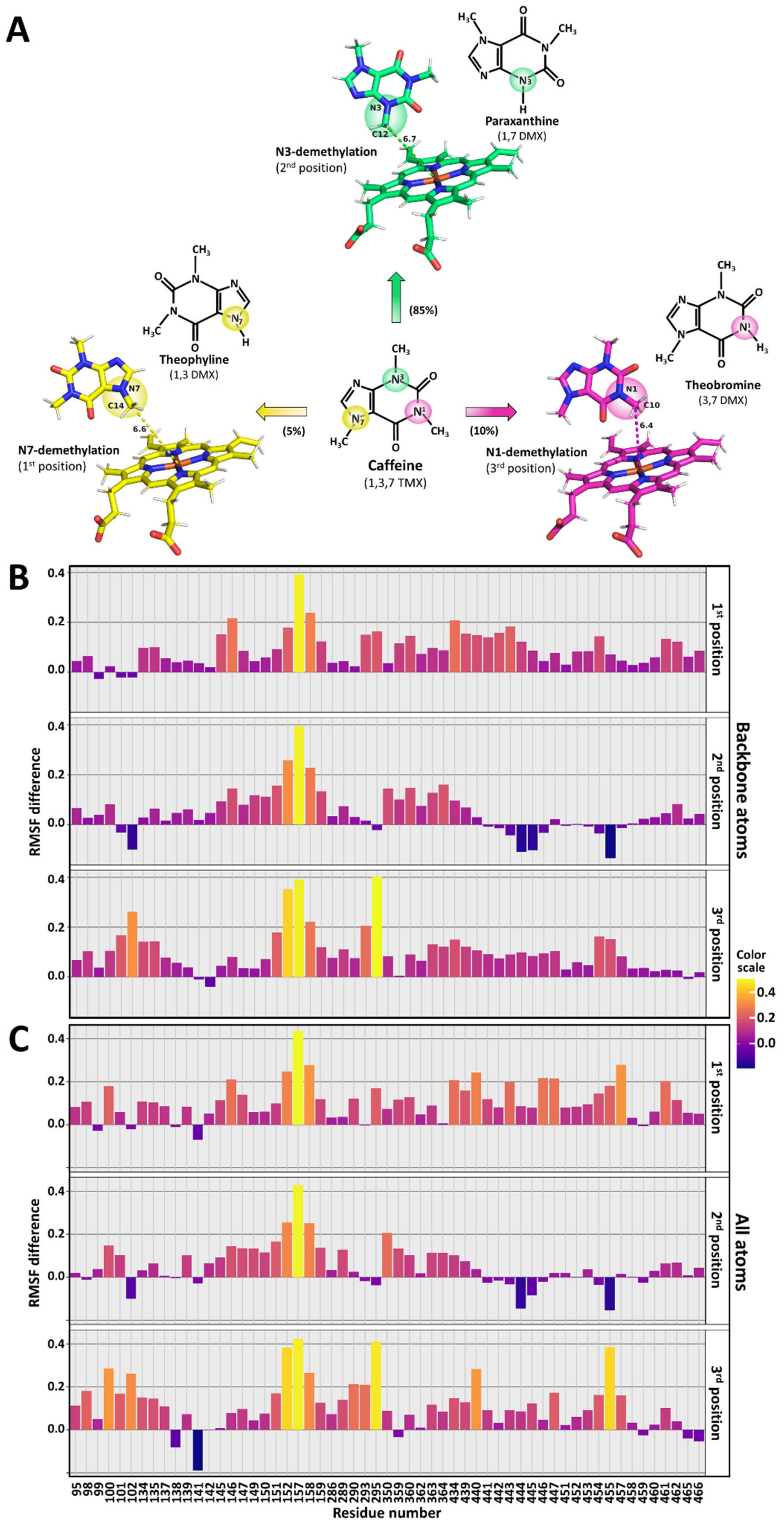
3.7. Caffeine Docking to CYP1A2
3.8. Molecular Dynamics of Caffeine-Free or Caffeine-Bound CYP1A2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guengerich, F. Human Cytochrome P450 Enzymes. In Cytochrome P450: Structure, Mechanism, and Biochemistry; Ortiz de Montellano, P.R., Ed.; Springer International Publishing: Basel, Switzerland, 2015. [Google Scholar]
- Waskell, L.; Kim, J.J. Electron Transfer Partners of Cytochrome P450. In Cytochrome P450: Structure, Mechanism, and Biochemistry; Ortiz de Montellano, P.R., Ed.; Springer International Publishing: Basel, Switzerland, 2015; pp. 33–68. [Google Scholar]
- Esteves, F.; Rueff, J.; Kranendonk, M. The Central Role of Cytochrome P450 in Xenobiotic Metabolism—A Brief Review on a Fascinating Enzyme Family. J. Xenobiotics 2021, 11, 94–114. [Google Scholar] [CrossRef] [PubMed]
- Schacter, B.A.; Nelson, E.B.; Marver, H.S.; Masters, B.S.S. Immunochemical Evidence for an Association of Heme Oxygenase with the Microsomal Electron Transport System. J. Biol. Chem. 1972, 247, 3601–3607. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Nakazono, K.; Kosaka, H. Purification and partial characterization of squalene epoxidase from rat liver microsomes. Biochim. Biophys. Acta—Protein Struct. Mol. Enzym. 1982, 709, 84–90. [Google Scholar] [CrossRef]
- Pandey, A.V.; Flück, C.E. NADPH P450 Oxidoreductase: Structure, Function, and Pathology of Diseases. Pharmacol. Ther. 2013, 138, 229–254. [Google Scholar] [CrossRef]
- Xia, C.; Hamdane, D.; Shen, A.L.; Choi, V.; Kasper, C.B.; Pearl, N.M.; Zhang, H.; Im, S.-C.; Waskell, L.; Kim, J.-J.P. Conformational Changes of NADPH-Cytochrome P450 Oxidoreductase Are Essential for Catalysis and Cofactor Binding. J. Biol. Chem. 2011, 286, 16246–16260. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Roberts, D.L.; Paschke, R.; Shea, T.M.; Masters, B.S.; Kim, J.J. Three-Dimensional Structure of NADPH-Cytochrome P450 Reductase: Prototype for FMN- and FAD-Containing Enzymes. Proc. Natl. Acad. Sci. USA 1997, 94, 8411–8416. [Google Scholar] [CrossRef]
- Vincent, B.; Morellet, N.; Fatemi, F.; Aigrain, L.; Truan, G.; Guittet, E.; Lescop, E. The Closed and Compact Domain Organization of the 70-KDa Human Cytochrome P450 Reductase in Its Oxidized State as Revealed by NMR. J. Mol. Biol. 2012, 420, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Aigrain, L.; Pompon, D.; Truan, G.; Moréra, S. Cloning, purification, crystallization and preliminary X-ray analysis of a chimeric NADPH-cytochrome P450 reductase. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2009, 65, 210–212. [Google Scholar] [CrossRef]
- Ellis, J.; Gutierrez, A.; Barsukov, I.L.; Huang, W.-C.; Grossmann, J.G.; Roberts, G.C.K. Domain Motion in Cytochrome P450 Reductase: Conformational Equilibria Revealed by NMR and Small-Angle x-Ray Scattering. J. Biol. Chem. 2009, 284, 36628–36637. [Google Scholar] [CrossRef]
- Hamdane, D.; Xia, C.; Im, S.-C.; Zhang, H.; Kim, J.-J.P.; Waskell, L. Structure and Function of an NADPH-Cytochrome P450 Oxidoreductase in an Open Conformation Capable of Reducing Cytochrome P450. J. Biol. Chem. 2009, 284, 11374–11384. [Google Scholar] [CrossRef]
- Sündermann, A.; Oostenbrink, C. Molecular Dynamics Simulations Give Insight into the Conformational Change, Complex Formation, and Electron Transfer Pathway for Cytochrome P450 Reductase. Protein Sci. 2013, 22, 1183–1195. [Google Scholar] [CrossRef]
- Frances, O.; Fatemi, F.; Pompon, D.; Guittet, E.; Sizun, C.; Pérez, J.; Lescop, E.; Truan, G. A Well-Balanced Preexisting Equilibrium Governs Electron Flux Efficiency of a Multidomain Diflavin Reductase. Biophys. J. 2015, 108, 1527–1536. [Google Scholar] [CrossRef]
- Quast, R.B.; Fatemi, F.; Kranendonk, M.; Margeat, E.; Truan, G. Accurate Determination of Human CPR Conformational Equilibrium by SmFRET Using Dual Orthogonal Noncanonical Amino Acid Labeling. Chembiochem 2019, 20, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Ellis, J.; Moody, P.C.E.; Raven, E.L.; Roberts, G.C.K. Redox-Linked Domain Movements in the Catalytic Cycle of Cytochrome P450 Reductase. Structure 2013, 21, 1581–1589. [Google Scholar] [CrossRef]
- Voznesensky, A.I.; Schenkman, J.B. The Cytochrome P450 2B4-NADPH Cytochrome P450 Reductase Electron Transfer Complex Is Not Formed by Charge-Pairing. J. Biol. Chem. 1992, 267, 14669–14676. [Google Scholar] [CrossRef] [PubMed]
- Campelo, D.; Lautier, T.; Urban, P.; Esteves, F.; Bozonnet, S.; Truan, G.; Kranendonk, M. The Hinge Segment of Human NADPH-Cytochrome P450 Reductase in Conformational Switching: The Critical Role of Ionic Strength. Front. Pharmacol. 2017, 8, 755. [Google Scholar] [CrossRef] [PubMed]
- Campelo, D.; Esteves, F.; Brito Palma, B.; Costa Gomes, B.; Rueff, J.; Lautier, T.; Urban, P.; Truan, G.; Kranendonk, M. Probing the Role of the Hinge Segment of Cytochrome P450 Oxidoreductase in the Interaction with Cytochrome P450. Int. J. Mol. Sci. 2018, 19, 3914. [Google Scholar] [CrossRef]
- Pikuleva, I.A.; Cao, C.; Waterman, M.R. An Additional Electrostatic Interaction between Adrenodoxin and P450c27 (CYP27A1) Results in Tighter Binding than between Adrenodoxin and P450scc (CYP11A1). J. Biol. Chem. 1999, 274, 2045–2052. [Google Scholar] [CrossRef]
- Esteves, F.; Campelo, D.; Gomes, B.C.; Urban, P.; Bozonnet, S.; Lautier, T.; Rueff, J.; Truan, G.; Kranendonk, M. The Role of the FMN-Domain of Human Cytochrome P450 Oxidoreductase in Its Promiscuous Interactions with Structurally Diverse Redox Partners. Front. Pharmacol. 2020, 11, 299. [Google Scholar] [CrossRef] [PubMed]
- Hlavica, P. Mechanistic Basis of Electron Transfer to Cytochromes P450 by Natural Redox Partners and Artificial Donor Constructs. Adv. Exp. Med. Biol. 2015, 851, 247–297. [Google Scholar] [CrossRef] [PubMed]
- Esteves, F.; Urban, P.; Rueff, J.; Truan, G.; Kranendonk, M. Interaction Modes of Microsomal Cytochrome P450s with Its Reductase and the Role of Substrate Binding. Int. J. Mol. Sci. 2020, 21, 6669. [Google Scholar] [CrossRef]
- Shaik, S.; Dubey, K.D. The Catalytic Cycle of Cytochrome P450: A Fascinating Choreography. Trends Chem. 2021, 3, 1027–1044. [Google Scholar] [CrossRef]
- Kandel, S.E.; Lampe, J.N. Role of Protein-Protein Interactions in Cytochrome P450-Mediated Drug Metabolism and Toxicity. Chem. Res. Toxicol. 2014, 27, 1474–1486. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.F.; Stout, C.D. Structural Diversity of Eukaryotic Membrane Cytochrome P450s. J. Biol. Chem. 2013, 288, 17082–17090. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.F.; Connick, J.P.; Reed, J.R.; Backes, W.L.; Desai, M.C.; Xu, L.; Estrada, D.F.; Laurence, J.S.; Scott, E.E. Correlating Structure and Function of Drug-Metabolizing Enzymes: Progress and Ongoing Challenges. Drug Metab. Dispos. 2014, 42, 9–22. [Google Scholar] [CrossRef]
- Grzegorzewski, J.; Bartsch, F.; Köller, A.; König, M. Pharmacokinetics of Caffeine: A Systematic Analysis of Reported Data for Application in Metabolic Phenotyping and Liver Function Testing. Front. Pharmacol. 2021, 12, 752826. [Google Scholar] [CrossRef]
- Nehlig, A. Interindividual Differences in Caffeine Metabolism and Factors Driving Caffeine Consumption. Pharmacol. Rev. 2018, 70, 384–411. [Google Scholar] [CrossRef]
- Duarte, M.P.; Palma, B.B.; Gilep, A.A.; Laires, A.; Oliveira, J.S.; Usanov, S.A.; Rueff, J.; Kranendonk, M. The stimulatory role of human cytochrome b5 in the bioactivation activities of human CYP1A2, 2A6 and 2E1: A new cell expression system to study cytochrome P450-mediated biotransformation (a corrigendum report on Duarte et al. (2005) Mutagenesis 20, 93–100). Mutagenesis 2006, 22, 75–81. [Google Scholar] [CrossRef]
- Kranendonk, M.; Marohnic, C.C.; Panda, S.P.; Duarte, M.P.; Oliveira, J.S.; Masters, B.S.S.; Rueff, J. Impairment of Human CYP1A2-Mediated Xenobiotic Metabolism by Antley-Bixler Syndrome Variants of Cytochrome P450 Oxidoreductase. Arch. Biochem. Biophys. 2008, 475, 93–99. [Google Scholar] [CrossRef]
- Moutinho, D.; Marohnic, C.C.; Panda, S.P.; Rueff, J.; Masters, B.S.; Kranendonk, M. Altered Human CYP3A4 Activity Caused by Antley-Bixler Syndrome-Related Variants of NADPH-Cytochrome P450 Oxidoreductase Measured in a Robust in Vitro System. Drug Metab. Dispos. 2012, 40, 754–760. [Google Scholar] [CrossRef]
- Palma, B.B.; Silva, E.; Sousa, M.; Urban, P.; Rueff, J.; Kranendonk, M. Functional Characterization of Eight Human CYP1A2 Variants: The Role of Cytochrome B5. Pharmacogenet. Genom. 2013, 23, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Esteves, F.; Campelo, D.; Urban, P.; Bozonnet, S.; Lautier, T.; Rueff, J.; Truan, G.; Kranendonk, M. Human Cytochrome P450 Expression in Bacteria: Whole-Cell High-Throughput Activity Assay for CYP1A2, 2A6 and 3A4. Biochem. Pharmacol. 2018, 158, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Tassaneeyakul, W.; Birkett, D.J.; McManus, M.E.; Tassaneeyakul, W.; Veronese, M.E.; Andersson, T.; Tukey, R.H.; Miners, J.O. Caffeine Metabolism by Human Hepatic Cytochromes P450: Contributions of 1A2, 2E1 and 3A Isoforms. Biochem. Pharmacol. 1994, 47, 1767–1776. [Google Scholar] [CrossRef]
- Almeida, C.M.M. Overview of Sample Preparation and Chromatographic Methods to Analysis Pharmaceutical Active Compounds in Waters Matrices. Separations 2021, 8, 16. [Google Scholar] [CrossRef]
- Silva, S.; Cardoso, V.V.; Duarte, L.; Carneiro, R.N.; Almeida, C.M.M. Characterization of Five Portuguese Wastewater Treatment Plants: Removal Efficiency of Pharmaceutical Active Compounds through Conventional Treatment Processes and Environmental Risk. Appl. Sci. 2021, 11, 7388. [Google Scholar] [CrossRef]
- Brömme, H.-J.; Zühlke, L.; Silber, R.-E.; Simm, A. DCFH2 Interactions with Hydroxyl Radicals and Other Oxidants—Influence of Organic Solvents. Exp. Gerontol. 2008, 43, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Reiniers, M.J.; van Golen, R.F.; Bonnet, S.; Broekgaarden, M.; van Gulik, T.M.; Egmond, M.R.; Heger, M. Preparation and Practical Applications of 2’,7’-Dichlorodihydrofluorescein in Redox Assays. Anal. Chem. 2017, 89, 3853–3857. [Google Scholar] [CrossRef]
- Krieger, E.; Vriend, G. New Ways to Boost Molecular Dynamics Simulations. J. Comput. Chem. 2015, 36, 996–1007. [Google Scholar] [CrossRef]
- Krieger, E.; Joo, K.; Lee, J.; Lee, J.; Raman, S.; Thompson, J.; Tyka, M.; Baker, D.; Karplus, K. Improving Physical Realism, Stereochemistry, and Side-Chain Accuracy in Homology Modeling: Four Approaches That Performed Well in CASP8. Proteins 2009, 77 (Suppl. S9), 114–122. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. Ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from Ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Paine, M.F.; Khalighi, M.; Fisher, J.M.; Shen, D.D.; Kunze, K.L.; Marsh, C.L.; Perkins, J.D.; Thummel, K.E. Characterization of Interintestinal and Intraintestinal Variations in Human CYP3A-Dependent Metabolism. J. Pharmacol. Exp. Ther. 1997, 283, 1552–1562. [Google Scholar] [PubMed]
- Venkatakrishnan, K.; von Moltke, L.L.; Court, M.H.; Harmatz, J.S.; Crespi, C.L.; Greenblatt, D.J. Comparison between Cytochrome P450 (CYP) Content and Relative Activity Approaches to Scaling from CDNA-Expressed CYPs to Human Liver Microsomes: Ratios of Accessory Proteins as Sources of Discrepancies between the Approaches. Drug Metab. Dispos. 2000, 28, 1493–1504. [Google Scholar]
- McCammon, K.M.; Panda, S.P.; Xia, C.; Kim, J.-J.P.; Moutinho, D.; Kranendonk, M.; Auchus, R.J.; Lafer, E.M.; Ghosh, D.; Martasek, P.; et al. Instability of the Human Cytochrome P450 Reductase A287P Variant Is the Major Contributor to Its Antley-Bixler Syndrome-like Phenotype. J. Biol. Chem. 2016, 291, 20487–20502. [Google Scholar] [CrossRef] [PubMed]
- Udhane, S.S.; Parween, S.; Kagawa, N.; Pandey, A.V. Altered CYP19A1 and CYP3A4 Activities Due to Mutations A115V, T142A, Q153R and P284L in the Human P450 Oxidoreductase. Front. Pharmacol. 2017, 8, 580. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Gonzalez, F.J.; Kalow, W.; Tang, B.K. Biotransformation of Caffeine, Paraxanthine, Theobromine and Theophylline by CDNA-Expressed Human CYP1A2 and CYP2E1. Pharmacogenetics 1992, 2, 73–77. [Google Scholar] [CrossRef]
- Shen, A.L.; Kasper, C.B. Role of Acidic Residues in the Interaction of NADPH-Cytochrome P450 Oxidoreductase with Cytochrome P450 and Cytochrome c. J. Biol. Chem. 1995, 270, 27475–27480. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, M.; Rwere, F.; Waskell, L.; Ramamoorthy, A. Kinetic and Structural Characterization of the Interaction between the FMN Binding Domain of Cytochrome P450 Reductase and Cytochrome c. J. Biol. Chem. 2015, 290, 4843–4855. [Google Scholar] [CrossRef]
- Mukherjee, G.; Nandekar, P.P.; Wade, R.C. An Electron Transfer Competent Structural Ensemble of Membrane-Bound Cytochrome P450 1A1 and Cytochrome P450 Oxidoreductase. Commun. Biol. 2021, 4, 55. [Google Scholar] [CrossRef]
- Shimada, T.; Mernaugh, R.L.; Guengerich, F.P. Interactions of Mammalian Cytochrome P450, NADPH-Cytochrome P450 Reductase, and Cytochrome b(5) Enzymes. Arch. Biochem. Biophys. 2005, 435, 207–216. [Google Scholar] [CrossRef]
- Sansen, S.; Yano, J.K.; Reynald, R.L.; Schoch, G.A.; Griffin, K.J.; Stout, C.D.; Johnson, E.F. Adaptations for the Oxidation of Polycyclic Aromatic Hydrocarbons Exhibited by the Structure of Human P450 1A2. J. Biol. Chem. 2007, 282, 14348–14355. [Google Scholar] [CrossRef] [PubMed]
- Jandova, Z.; Gill, S.C.; Lim, N.M.; Mobley, D.L.; Oostenbrink, C. Binding Modes and Metabolism of Caffeine. Chem. Res. Toxicol. 2019, 32, 1374–1383. [Google Scholar] [CrossRef]
- Regal, K.A.; Nelson, S.D. Orientation of Caffeine within the Active Site of Human Cytochrome P450 1A2 Based on NMR Longitudinal (T1) Relaxation Measurements. Arch. Biochem. Biophys. 2000, 384, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Kot, M.; Daniel, W.A. The Relative Contribution of Human Cytochrome P450 Isoforms to the Four Caffeine Oxidation Pathways: An in Vitro Comparative Study with CDNA-Expressed P450s Including CYP2C Isoforms. Biochem. Pharmacol. 2008, 76, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Gentry, K.A.; Anantharamaiah, G.M.; Ramamoorthy, A. Probing Protein-Protein and Protein-Substrate Interactions in the Dynamic Membrane-Associated Ternary Complex of Cytochromes P450, B5, and Reductase. Chem. Commun. 2019, 55, 13422–13425. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Pandey, A.V.; Agrawal, V.; Reardon, W.; Lapunzina, P.D.; Mowat, D.; Jabs, E.W.; Van Vliet, G.; Sack, J.; Flück, C.E.; et al. Diversity and Function of Mutations in P450 Oxidoreductase in Patients with Antley-Bixler Syndrome and Disordered Steroidogenesis. Am. J. Hum. Genet. 2005, 76, 729–749. [Google Scholar] [CrossRef]
- Tomková, M.; Marohnic, C.C.; Gurwitz, D.; Seda, O.; Masters, B.S.S.; Martásek, P. Identification of Six Novel P450 Oxidoreductase Missense Variants in Ashkenazi and Moroccan Jewish Populations. Pharmacogenomics 2012, 13, 543–554. [Google Scholar] [CrossRef]
- Marohnic, C.C.; Panda, S.P.; McCammon, K.; Rueff, J.; Masters, B.S.S.; Kranendonk, M. Human Cytochrome P450 Oxidoreductase Deficiency Caused by the Y181D Mutation: Molecular Consequences and Rescue of Defect. Drug Metab. Dispos. 2010, 38, 332–340. [Google Scholar] [CrossRef]
- Li, S.; Du, L.; Bernhardt, R. Redox Partners: Function Modulators of Bacterial P450 Enzymes. Trends Microbiol. 2020, 28, 445–454. [Google Scholar] [CrossRef]
- Durairaj, P.; Li, S. Functional Expression and Regulation of Eukaryotic Cytochrome P450 Enzymes in Surrogate Microbial Cell Factories. Eng. Microbiol. 2022, 2, 100011. [Google Scholar] [CrossRef]
- Kaczmarczyk-Sedlak, I.; Folwarczna, J.; Sedlak, L.; Zych, M.; Wojnar, W.; Szumińska, I.; Wyględowska-Promieńska, D.; Mrukwa-Kominek, E. Effect of Caffeine on Biomarkers of Oxidative Stress in Lenses of Rats with Streptozotocin-Induced Diabetes. Arch. Med. Sci. 2019, 15, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Estrada, D.F.; Laurence, J.S.; Scott, E.E. Cytochrome P450 17A1 Interactions with the FMN Domain of Its Reductase as Characterized by NMR. J. Biol. Chem. 2016, 291, 3990–4003. [Google Scholar] [CrossRef]
- Kumar, A.; Estrada, D.F. Structural basis of bidirectional allostery across the heme in a cytochrome P450 enzyme. J. Biol. Chem. 2023, 104977. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.F.; Müller, D.J.; Altman, R.B.; Klein, T.E. PharmGKB Summary: Clozapine Pathway, Pharmacokinetics. Pharmacogenet. Genom. 2018, 28, 214–222. [Google Scholar] [CrossRef] [PubMed]

| Membrane Fractions | Protein Contents | Protein Stability | Caffeine | Metabolites | |||||
|---|---|---|---|---|---|---|---|---|---|
| CPR Region | CPR Form | CYP 1A2 | CPR | CPR/CYP | Relative Cyt c Reduction Rates | Consumption | Theophylline | Theobromine | Paraxanthine |
| (pmol/mg Protein) | Ratios | (6 h/0 h) | (µM) | ||||||
| FMN domain (natural variants) | T142A | 145 ± 2 | 38.5 ± 0.9 | 1:4 | 1.01 ± 0.13 | 5.82 ± 0.18 | 0.85 ± 0.10 | 0.39 ± 0.01 | 4.58 ± 0.15 |
| Q153R | 267 ± 3 | 26.0 ± 0.4 | 1:10 | 0.85 ± 0.12 | 12.96 ± 0.66 | 1.16 ± 0.08 | 1.00 ± 0.04 | 10.80 ± 0.66 | |
| V164M | 259 ± 2 | 35.2 ± 0.8 | 1:7 | 0.94 ± 0.16 | 10.15 ± 0.48 | 0.92 ± 0.02 | 0.91 ± 0.05 | 8.32 ± 0.48 | |
| D211N | 211 ± 4 | 24.9 ± 0.7 | 1:8 | 1.03 ± 0.16 | 11.34 ± 0.31 | 0.97 ± 0.09 | 0.89 ± 0.05 | 9.49 ± 0.30 | |
| P228L | 451 ± 4 | 41.9 ± 1.1 | 1:11 | 0.95 ± 0.14 | 9.57 ± 0.30 | 0.94 ± 0.05 | 0.60 ± 0.04 | 8.03 ± 0.28 | |
| FMN domain (selected targets) | P117H a | 148 ± 1 | 12.2 ± 0.5 | 1:12 | 0.93 ± 0.14 | 9.29 ± 0.30 | 0.79 ± 0.03 | 0.53 ± 0.06 | 7.97 ± 0.30 |
| G144C a | 124 ± 2 | 14.1 ± 0.2 | 1:12 | 0.93 ± 0.17 | 9.39 ± 0.11 | 0.76 ± 0.03 | 0.49 ± 0.01 | 8.14 ± 0.10 | |
| N151D a | 446 ± 4 | 27.7 ± 0.5 | 1:12 | 1.02 ± 0.13 | 8.85 ± 0.07 | 0.82 ± 0.50 | 0.63 ± 0.02 | 7.40 ± 0.05 | |
| G175D a | 379 ± 2 | 26.5 ± 0.4 | 1:14 | 1.01 ± 0.05 | 9.09 ± 0.45 | 0.80 ± 0.08 | 0.67 ± 0.05 | 7.62 ± 0.45 | |
| H183Y | 179 ± 2 | 14,9 ± 0.3 | 1:12 | 0.89 ± 0.06 | 14.05 ± 0.08 | 0.89 ± 0.12 | 0.47 ± 0.06 | 12.70 ± 0.64 | |
| A229T a | 71 ± 4 | 17.3 ± 0.2 | 1:4 | 0.99 ± 0.11 | 13.30 ± 0.28 | 0.86 ± 0.12 | 1.04 ± 0.15 | 11.40 ± 0.52 | |
| Hinge region (selected targets) | S243P a | 73 ± 4 | 7.7 ± 0.2 | 1:9 | 0.60 ± 0.06 ** | 9.81 ± 0.06 | 0.46 ± 0.07 | 0.44 ± 0.05 | 8.92 ± 0.40 |
| I245P a | 102 ± 1 | 6.1 ± 0.5 | 1:17 | 0.98 ± 0.12 | 7.28 ± 0.09 | 0.38 ± 0.06 | 0.73 ± 0.08 | 6.17 ± 0.28 | |
| R246A a | 91 ± 2 | 5.4 ± 0.2 | 1:17 | 0.84 ± 0.06 | 11.89 ± 0.23 | 0.62 ± 0.09 | 1.02 ± 0.13 | 10.25 ± 0.54 | |
| - | Wt (A) | 56 ± 3 | 23.3 ± 1.6 | 1:2 | 0.93 ± 0.05 | 10.02 ± 0.28 | 0.77 ± 0.09 | 0.63 ± 0.05 | 8.61 ± 0.08 |
| - | Wt (B) | 116 ± 3 | 12.4 ± 0.8 | 1:9 | 0.88 ± 0.07 | 10.25 ± 0.23 | 0.80 ± 0.10 | 0.63 ± 0.05 | 8.81 ± 0.20 |
| - | Wt (C) a | 54 ± 1 | 4.1 ± 1.5 | 1:13 | 0.84 ± 0.11 | 10.28 ± 0.21 | 0.82 ± 0.10 | 0.63 ± 0.06 | 8.83 ± 0.20 |
| - | CPRnull a | 85 ± 3 | ND | - | ND | ND | ND | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteves, F.; Almeida, C.M.M.; Silva, S.; Saldanha, I.; Urban, P.; Rueff, J.; Pompon, D.; Truan, G.; Kranendonk, M. Single Mutations in Cytochrome P450 Oxidoreductase Can Alter the Specificity of Human Cytochrome P450 1A2-Mediated Caffeine Metabolism. Biomolecules 2023, 13, 1083. https://doi.org/10.3390/biom13071083
Esteves F, Almeida CMM, Silva S, Saldanha I, Urban P, Rueff J, Pompon D, Truan G, Kranendonk M. Single Mutations in Cytochrome P450 Oxidoreductase Can Alter the Specificity of Human Cytochrome P450 1A2-Mediated Caffeine Metabolism. Biomolecules. 2023; 13(7):1083. https://doi.org/10.3390/biom13071083
Chicago/Turabian StyleEsteves, Francisco, Cristina M. M. Almeida, Sofia Silva, Inês Saldanha, Philippe Urban, José Rueff, Denis Pompon, Gilles Truan, and Michel Kranendonk. 2023. "Single Mutations in Cytochrome P450 Oxidoreductase Can Alter the Specificity of Human Cytochrome P450 1A2-Mediated Caffeine Metabolism" Biomolecules 13, no. 7: 1083. https://doi.org/10.3390/biom13071083
APA StyleEsteves, F., Almeida, C. M. M., Silva, S., Saldanha, I., Urban, P., Rueff, J., Pompon, D., Truan, G., & Kranendonk, M. (2023). Single Mutations in Cytochrome P450 Oxidoreductase Can Alter the Specificity of Human Cytochrome P450 1A2-Mediated Caffeine Metabolism. Biomolecules, 13(7), 1083. https://doi.org/10.3390/biom13071083











