Brain Monoamine Dysfunction in Response to Predator Scent Stress Accompanies Stress-Susceptibility in Female Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
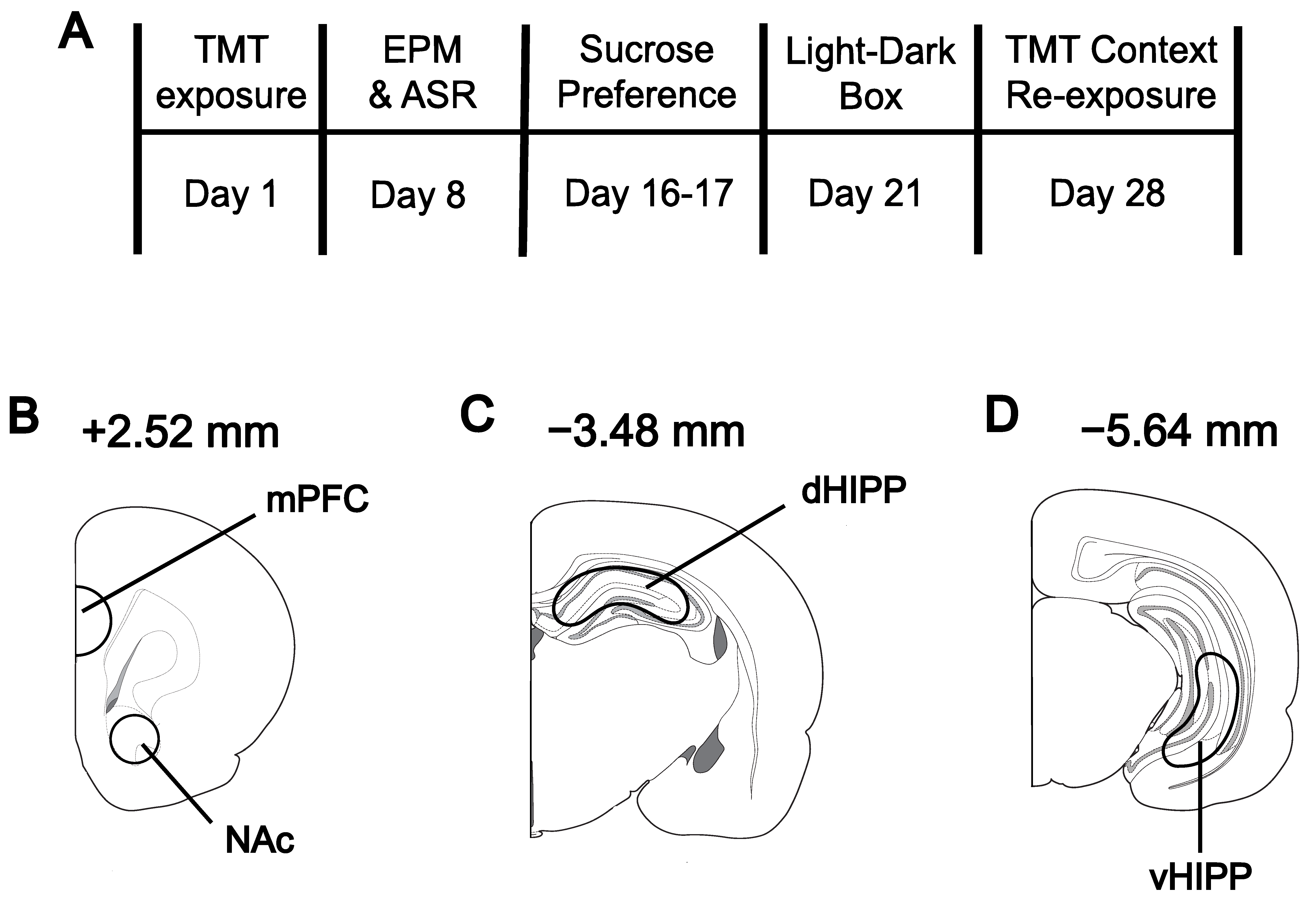
2.2. Stress Exposure
2.3. Elevated Plus Maze and Acoustic Startle Response
2.4. Sucrose Preference Test
2.5. Light–Dark Box
2.6. Stress Context Re-Exposure
2.7. Tissue Collection and High-Performance Liquid Chromatography
2.8. Statistical Analysis
3. Results
3.1. Effect of PSS on Behavior
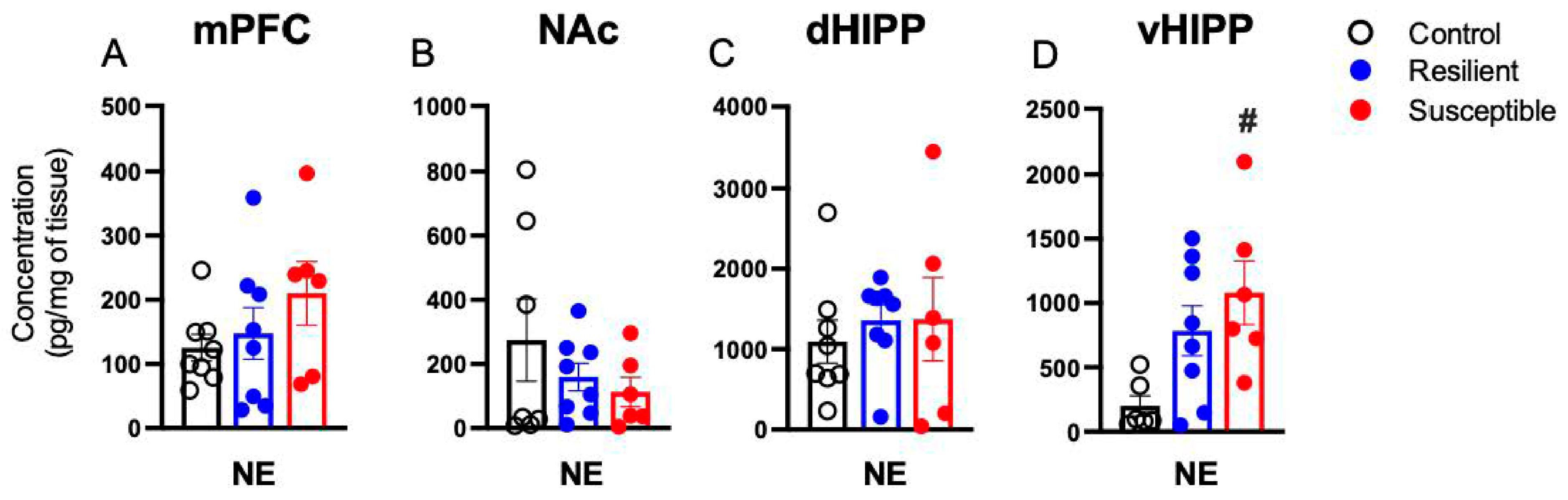
3.2. Effect of PSS on Brain Norepinephrine
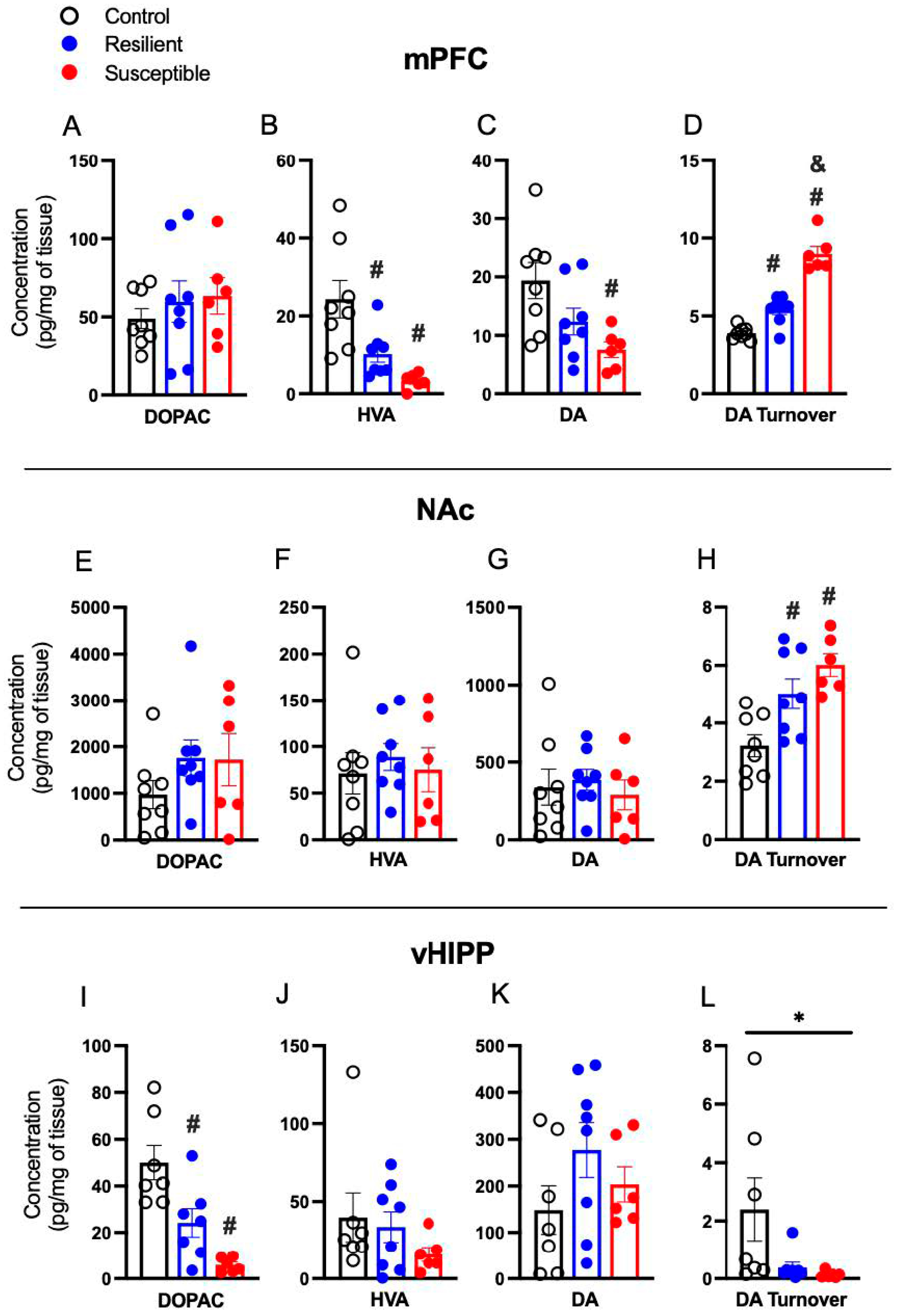
3.3. Effect of PSS on Brain Dopamine, Metabolites, and Dopamine Turnover
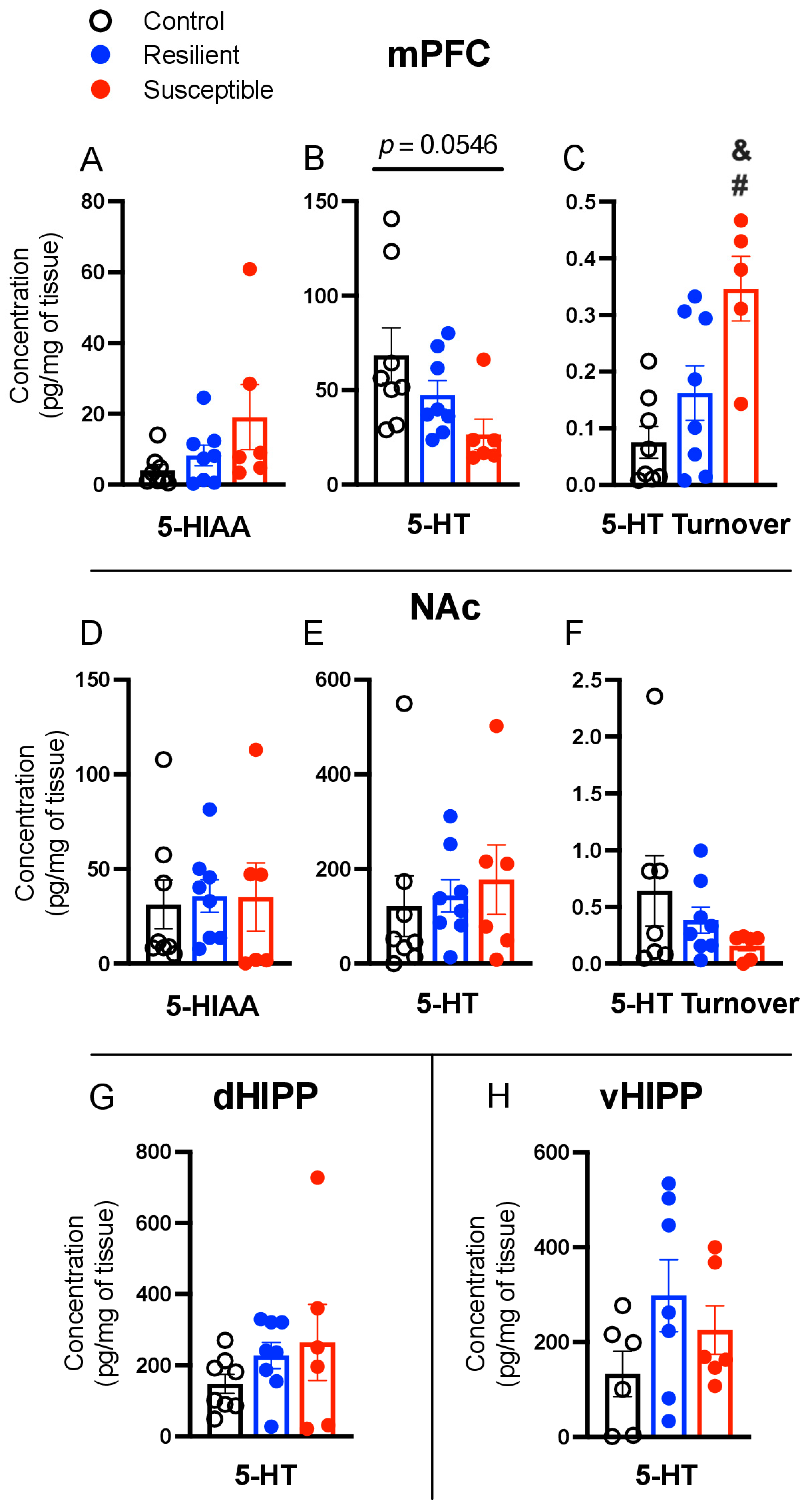
3.4. Effect of PSS on Brain Serotonin, 5-Hydroxyindoleacetic Acid, and Serotonin Turnover
4. Discussion
4.1. Norepinephrine
4.2. Dopamine
4.3. Serotonin
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Merikangas, K.R.; Walters, E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Koenen, K.C.; Ratanatharathorn, A.; Ng, L.; McLaughlin, K.A.; Bromet, E.J.; Stein, D.J.; Karam, E.G.; Meron Ruscio, A.; Benjet, C.; Scott, K.; et al. Posttraumatic stress disorder in the World Mental Health Surveys. Psychol. Med. 2017, 47, 2260–2274. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Sonnega, A.; Bromet, E.; Hughes, M.; Nelson, C.B. Posttraumatic stress disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry 1995, 52, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, D.G.; Resnick, H.S.; Milanak, M.E.; Miller, M.W.; Keyes, K.M.; Friedman, M.J. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J. Trauma Stress 2013, 26, 537–547. [Google Scholar] [CrossRef]
- Olff, M.; Langeland, W.; Draijer, N.; Gersons, B.P.R. Gender differences in posttraumatic stress disorder. Psychol. Bull. 2007, 133, 183–204. [Google Scholar] [CrossRef]
- Morina, N.; Wicherts, J.M.; Lobbrecht, J.; Priebe, S. Remission from post-traumatic stress disorder in adults: A systematic review and meta-analysis of long term outcome studies. Clin. Psychol. Rev. 2014, 34, 249–255. [Google Scholar] [CrossRef]
- Katzman, M.A.; Struzik, L.; Vivian, L.L.; Vermani, M.; McBride, J.C. Pharmacotherapy of post-traumatic stress disorder: A family practitioners guide to management of the disease. Expert Rev. Neurother. 2005, 5, 129–139. [Google Scholar] [CrossRef]
- Arora, R.C.; Fichtner, C.G.; O’Connor, F.; Crayton, J. Paroxetine binding in the blood platelets of post-traumatic stress disorder patients. Life Sci. 1993, 53, 919–928. [Google Scholar] [CrossRef]
- Kitaichi, Y.; Inoue, T.; Nakagawa, S.; Boku, S.; Kakuta, A.; Izumi, T.; Koyama, T. Sertraline increases extracellular levels not only of serotonin, but also of dopamine in the nucleus accumbens and striatum of rats. Eur. J. Pharmacol. 2010, 647, 90–96. [Google Scholar] [CrossRef]
- Thomas, D.N.; Nutt, D.J.; Holman, R.B. Sertraline, a selective serotonin reuptake inhibitor modulates extracellular noradrenaline in the rat frontal cortex. J. Psychopharmacol. 1998, 12, 366–370. [Google Scholar] [CrossRef]
- De Berardis, D.; Marini, S.; Serroni, N.; Iasevoli, F.; Tomasetti, C.; de Bartolomeis, A.; Mazza, M.; Tempesta, D.; Valchera, A.; Fornaro, M.; et al. Targeting the Noradrenergic System in Posttraumatic Stress Disorder: A Systematic Review and Meta-Analysis of Prazosin Trials. Curr. Drug Targets 2015, 16, 1094–1106. [Google Scholar] [CrossRef]
- Daly, C.M.; Doyle, M.E.; Radkind, M.; Raskind, E.; Daniels, C. Clinical case series: The use of Prazosin for combat-related recurrent nightmares among Operation Iraqi Freedom combat veterans. Mil. Med. 2005, 170, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Vaiva, G.; Ducrocq, F.; Jezequel, K.; Averland, B.; Lestavel, P.; Brunet, A.; Marmar, C.R. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol. Psychiatry 2003, 54, 947–949. [Google Scholar] [CrossRef] [PubMed]
- Geracioti, T.D.; Baker, D.G.; Ekhator, N.N.; West, S.A.; Hill, K.K.; Bruce, A.B.; Schmidt, D.; Rounds-Kugler, B.; Yehuda, R.; Keck, P.E.; et al. CSF norepinephrine concentrations in posttraumatic stress disorder. Am. J. Psychiatry 2001, 158, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Southwick, S.M.; Giller, E.L.M.; Mason, J.W.M. Urinary catecholamine excretion and severity of PTSD symptoms in Vietnam combat veterans. J. Nerv. Ment. Dis. 1992, 180, 321–325. [Google Scholar] [CrossRef]
- Glover, D.A.; Powers, M.B.; Bergman, L.; Smits, J.A.; Telch, M.J.; Stuber, M. Urinary dopamine and turn bias in traumatized women with and without PTSD symptoms. Behav. Brain Res. 2003, 144, 137–141. [Google Scholar] [CrossRef]
- Cohen, H.; Zohar, J.; Matar, M.A.; Zeev, K.; Loewenthal, U.; Richter-Levin, G. Setting apart the affected: The use of behavioral criteria in animal models of post traumatic stress disorder. Neuropsychopharmacology 2004, 29, 1962–1970. [Google Scholar] [CrossRef]
- Schwendt, M.; Shallcross, J.; Hadad, N.A.; Namba, M.D.; Hiller, H.; Wu, L.; Krause, E.G.; Knackstedt, L.A. A novel rat model of comorbid PTSD and addiction reveals intersections between stress susceptibility and enhanced cocaine seeking with a role for mGlu5 receptors. Transl. Psychiatry 2018, 8, 209. [Google Scholar] [CrossRef]
- Shallcross, J.; Hámor, P.; Bechard, A.R.; Romano, M.; Knackstedt, L.; Schwendt, M. The divergent effects of CDPPB and cannabidiol on fear extinction and anxiety in a predator scent stress model of PTSD in rats. Front. Behav. Neurosci. 2019, 13, 91. [Google Scholar] [CrossRef]
- Shallcross, J.; Wu, L.; Wilkinson, C.S.; Knackstedt, L.A.; Schwendt, M. Increased mGlu5 mRNA expression in BLA glutamate neurons facilitates resilience to the long-term effects of a single predator scent stress exposure. Brain Struct. Funct. 2021, 226, 2279–2293. [Google Scholar] [CrossRef]
- Danan, D.; Matar, M.A.; Kaplan, Z.; Zohar, J.; Cohen, H. Blunted basal corticosterone pulsatility predicts post-exposure susceptibility to PTSD phenotype in rats. Psychoneuroendocrinology 2018, 87, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Kozlovsky, N.; Matar, M.A.; Kaplan, Z.; Kotler, M.; Zohar, J.; Cohen, H. The immediate early gene Arc is associated with behavioral resilience to stress exposure in an animal model of posttraumatic stress disorder. Eur. Neuropsychopharmacol. 2008, 18, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Modrak, C.G.; Wilkinson, C.S.; Blount, H.L.; Schwendt, M.; Knackstedt, L.A. The role of mGlu receptors in susceptibility to stress-induced anhedonia, fear, and anxiety-like behavior. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Morrow, B.A.; Redmond, A.J.; Roth, R.H.; Elsworth, J.D. The predator odor, TMT, displays a unique, stress-like pattern of dopaminergic and endocrinological activation in the rat. Brain Res. 2000, 864, 146–151. [Google Scholar] [CrossRef]
- De La Garza, R.; Mahoney, J.J. A distinct neurochemical profile in WKY rats at baseline and in response to acute stress: Implications for animal models of anxiety and depression. Brain Res. 2004, 1021, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Hayley, S.; Borowski, T.; Merali, Z.; Anisman, H. Central monoamine activity in genetically distinct strains of mice following a psychogenic stressor: Effects of predator exposure. Brain Res. 2001, 892, 293–300. [Google Scholar] [CrossRef]
- Han, X.; Wang, W.; Shao, F.; Li, N. Isolation rearing alters social behaviors and monoamine neurotransmission in the medial prefrontal cortex and nucleus accumbens of adult rats. Brain Res. 2011, 1385, 175–181. [Google Scholar] [CrossRef]
- Wilson, C.B.; Ebenezer, P.J.; McLaughlin, L.D.; Francis, J. Predator exposure/psychosocial stress animal model of post-traumatic stress disorder modulates neurotransmitters in the rat hippocampus and prefrontal cortex. PLoS ONE 2014, 9, e89104. [Google Scholar] [CrossRef]
- Tseilikman, V.; Komelkova, M.; Lapshin, M.; Alliluev, A.; Tseilikman, O.; Karpenko, M.; Pestereva, N.; Manukhina, E.; Downey, H.F.; Kondashevskaya, M.; et al. High and low anxiety phenotypes in a rat model of complex post-traumatic stress disorder are associated with different alterations in regional brain monoamine neurotransmission. Psychoneuroendocrinology 2020, 117, 104691. [Google Scholar] [CrossRef]
- Isingrini, E.; Perret, L.; Rainer, Q.; Amilhon, B.; Guma, E.; Tanti, A.; Martin, G.; Robinson, J.; Moquin, L.; Marti, F.; et al. Resilience to chronic stress is mediated by noradrenergic regulation of dopamine neurons. Nat. Neurosci. 2016, 19, 560–563. [Google Scholar] [CrossRef]
- Muneoka, K.; Oda, Y.; Iwata, M.; Iyo, M.; Hashimoto, K.; Shirayama, Y. Monoaminergic balances predict non-depression-like phenotype in Learned Helplessness Paradigm. Neuroscience 2020, 440, 290–298. [Google Scholar] [CrossRef]
- Blount, H.L.; Dee, J.; Wu, L.; Schwendt, M.; Knackstedt, L.A. Stress resilience-associated behaviors following predator scent stress are accompanied by upregulated nucleus accumbens mGlu5 transcription in female Sprague Dawley rats. Behav. Brain Res. 2023, 436, 114090. [Google Scholar] [CrossRef] [PubMed]
- Brenes, J.C.; Fornaguera, J. The effect of chronic fluoxetine on social isolation-induced changes on sucrose consumption, immobility behavior, and on serotonin and dopamine function in hippocampus and ventral striatum. Behav. Brain Res. 2009, 198, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Brenes Sáenz, J.C.; Villagra, O.R.; Fornaguera Trías, J. Factor analysis of Forced Swimming test, Sucrose Preference test and Open Field test on enriched, social and isolated reared rats. Behav. Brain Res. 2006, 169, 57–65. [Google Scholar] [CrossRef]
- Sun, H.; Guan, L.; Zhu, Z.; Li, H. Reduced levels of NR1 and NR2A with depression-like behavior in different brain regions in prenatally stressed juvenile offspring. PLoS ONE 2013, 8, e81775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kindel, G.H.; Wülfert, E.; Hanin, I. Effects of immobilization stress on hippocampal monoamine release: Modification by mivazerol, a new α2-adrenoceptor agonist. Neuropharmacology 1995, 34, 1661–1672. [Google Scholar] [CrossRef]
- Imperato, A.; Puglisi-Allegra, S.; Zocchi, A.; Scrocco, M.G.; Casolini, P.; Angelucci, L. Stress activation of limbic and cortical dopamine release is prevented by ICS 205-930 but not by diazepam. Eur. J. Pharmacol. 1990, 175, 211–214. [Google Scholar] [CrossRef]
- Sorg, B.A.; Kalivas, P.W. Effects of cocaine and footshock stress on extracellular dopamine levels in the medial prefrontal cortex. Neuroscience 1993, 53, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Abercrombie, E.D.; Keefe, K.A.; DiFrischia, D.S.; Zigmond, M.J. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J. Neurochem. 1989, 52, 1655–1658. [Google Scholar] [CrossRef]
- Feenstra, M.G.; Botterblom, M.H.; van Uum, J.F. Novelty-induced increase in dopamine release in the rat prefrontal cortex in vivo: Inhibition by diazepam. Neurosci. Lett. 1995, 189, 81–84. [Google Scholar] [CrossRef]
- Martinez, R.C.R.; Oliveira, A.R.; Macedo, C.E.; Molina, V.A.; Brandão, M.L. Involvement of dopaminergic mechanisms in the nucleus accumbens core and shell subregions in the expression of fear conditioning. Neurosci. Lett. 2008, 446, 112–116. [Google Scholar] [CrossRef]
- Bland, S.T.; Hargrave, D.; Pepin, J.L.; Amat, J.; Watkins, L.R.; Maier, S.F. Stressor controllability modulates stress-induced dopamine and serotonin efflux and morphine-induced serotonin efflux in the medial prefrontal cortex. Neuropsychopharmacology 2003, 28, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Yadid, G.; Overstreet, D.H.; Zangen, A. Limbic dopaminergic adaptation to a stressful stimulus in a rat model of depression. Brain Res. 2001, 896, 43–47. [Google Scholar] [CrossRef]
- Lillrank, S.M.; Lipska, B.K.; Kolachana, B.S.; Weinberger, D.R. Attenuated extracellular dopamine levels after stress and amphetamine in the nucleus accumbens of rats with neonatal ventral hippocampal damage. J. Neural Transm. 1999, 106, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition, 6th ed.; Academic Press: Cambridge, MA, USA, 2006; p. 456. [Google Scholar]
- Perrine, S.A.; Ghoddoussi, F.; Michaels, M.S.; Hyde, E.M.; Kuhn, D.M.; Galloway, M.P. MDMA administration decreases serotonin but not N-acetylaspartate in the rat brain. Neurotoxicology 2010, 31, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Slotkin, T.A.; Kreider, M.L.; Tate, C.A.; Seidler, F.J. Critical prenatal and postnatal periods for persistent effects of dexamethasone on serotonergic and dopaminergic systems. Neuropsychopharmacology 2006, 31, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Sziray, N.; Leveleki, C.; Levay, G.; Markó, B.; Harsing, L.G., Jr.; Mikics, E.; Barsy, B.; Haller, J. Mechanisms underlying the long-term behavioral effects of traumatic experience in rats: The role of serotonin/noradrenaline balance and NMDA receptors. Brain Res. Bull. 2007, 71, 376–385. [Google Scholar] [CrossRef]
- Sigurdsson, T.; Duvarci, S. Hippocampal-prefrontal interactions in cognition, behavior and psychiatric disease. Front. Syst. Neurosci. 2015, 9, 190. [Google Scholar] [CrossRef]
- Jurgens, C.W.D.; Rau, K.E.; Knudson, C.A.; King, J.D.; Carr, P.A.; Porter, J.E.; Doze, V.A.; Hennan, J.K.; Elokdah, H.; Leal, M.; et al. Beta1 adrenergic receptor-mediated enhancement of hippocampal CA3 network activity. J. Pharmacol. Exp. Ther. 2005, 314, 552–560. [Google Scholar] [CrossRef]
- O’Carroll, R.E.; Drysdale, E.; Cahill, L.; Shajahan, P.; Ebmeier, K.P. Stimulation of the noradrenergic system enhances and blockade reduces memory for emotional material in man. Psychol Med. 1999, 29, 1083–1088. [Google Scholar] [CrossRef]
- Arnsten, A.F.T.; Steere, J.C.; Jentsch, D.J.; Li, B.M. Noradrenergic Influences on Prefrontal Cortical Cognitive Function: Opposing Actions at Postjunctional α1 Versus α2-Adrenergic Receptors. In Advances in Pharmacology; Elsevier: Amsterdam, The Netherlands, 1997; Volume 42, pp. 764–767. [Google Scholar] [CrossRef]
- Cuadra, G.; Zurita, A.; Lacerra, C.; Molina, V. Chronic stress sensitizes frontal cortex dopamine release in response to a subsequent novel stressor: Reversal by naloxone. Brain Res. Bull. 1999, 48, 303–308. [Google Scholar] [CrossRef]
- Thierry, A.M.; Tassin, J.P.; Blanc, G.; Glowinski, J. Selective activation of mesocortical DA system by stress. Nature 1976, 263, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Meiser, J.; Weindl, D.; Hiller, K. Complexity of dopamine metabolism. Cell Commun. Signal 2013, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Weickert, C.; Akil, M.; Lipska, B.; Hyde, T.; Herman, M.; Kleinman, J.; Weinberger, D. Catechol O-methyltransferase mRNA expression in human and rat brain: Evidence for a role in cortical neuronal function. Neuroscience 2003, 116, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Kolassa, I.-T.; Kolassa, S.; Ertl, V.; Papassotiropoulos, A.; De Quervain, D.J.-F. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-o-methyltransferase Val(158)Met polymorphism. Biol. Psychiatry 2010, 67, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.; Johansson, M.; Agren, H.; Friberg, P. Reduced brain norepinephrine and dopamine release in treatment-refractory depressive illness: Evidence in support of the catecholamine hypothesis of mood disorders. Arch. Gen. Psychiatry 2000, 57, 787–793. [Google Scholar] [CrossRef]
- Nawijn, L.; van Zuiden, M.; Frijling, J.L.; Koch, S.B.J.; Veltman, D.J.; Olff, M. Reward functioning in PTSD: A systematic review exploring the mechanisms underlying anhedonia. Neurosci. Biobehav. Rev. 2015, 51, 189–204. [Google Scholar] [CrossRef]
- Mehta, N.D.; Stevens, J.S.; Li, Z.; Gillespie, C.; Fani, N.; Michopoulos, V.; Felger, J.C. Inflammation, reward circuitry and symptoms of anhedonia and PTSD in trauma-exposed women. Soc. Cogn. Affect Neurosci. 2020, 15, 1046–1055. [Google Scholar] [CrossRef]
- Yamada, M.; Kawahara, Y.; Kaneko, F.; Kishikawa, Y.; Sotogaku, N.; Poppinga, W.J.; Folgering, J.H.; Dremencov, E.; Kawahara, H.; Nishi, A. Upregulation of the dorsal raphe nucleus-prefrontal cortex serotonin system by chronic treatment with escitalopram in hyposerotonergic Wistar-Kyoto rats. Neuropharmacology 2013, 72, 169–178. [Google Scholar] [CrossRef]
- Barton, D.A.; Esler, M.D.; Dawood, T.; Lambert, E.A.; Haikerwal, D.; Brenchley, C.; Socratous, F.; Hastings, J.; Guo, L.; Wiesner, G.; et al. Elevated brain serotonin turnover in patients with depression: Effect of genotype and therapy. Arch. Gen. Psychiatry 2008, 65, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Duchesne, A.; Dufresne, M.M.; Sullivan, R.M. Sex differences in corticolimbic dopamine and serotonin systems in the rat and the effect of postnatal handling. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 251–261. [Google Scholar] [CrossRef]
- Long, T.; Yao, J.K.; Li, J.; Kirshner, Z.Z.; Nelson, D.; Dougherty, G.G.; Gibbs, R.B. Comparison of transitional vs surgical menopause on monoamine and amino acid levels in the rat brain. Mol. Cell. Endocrinol. 2018, 476, 139–147. [Google Scholar] [CrossRef] [PubMed]
| Control (n = 8) + | Resilient (n = 8) | Susceptible (n = 6) | |
|---|---|---|---|
| Exposure freezing (s) | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Re-exposure freezing (s) | 7.83 ± 14.06 | 18.50 ± 52.33 | 4 ± 7.27 |
| EPM: time in OA (s) | 104.7 ± 14.08 | 88.01 ± 2.59 * | 43.49 ± 8.15 * |
| EPM: time in CA (s) | 114.5 ± 6.81 | 138.9 ± 12.34 | 175.1 ± 16.63 |
| EPM: OA entries (#) | 20.88 ± 5.12 | 17.38 ± 2.81 | 11.50 ± 3.37 |
| EPM: CA entries (#) | 25.25 ± 3.08 | 34.63 ± 3.95 | 34.76 ± 7.90 |
| Sucrose consumed (mL) | 82.13 ± 3.46 | 75.00 ± 1.77 * | 53.33 ± 5.00 * |
| Sucrose preference (%) | 89.64 ± 2.43 | 87.86 ± 2.38 | 82.58 ± 4.49 |
| L-D: time in dark (s) | 216.3 ± 35.15 | 298.4 ± 13.30 | 315 ± 31.17 |
| L-D: time in light (s) | 383.8 ± 35.15 | 301.6 ± 31.17 | 285.0 ± 31.17 |
| L-D: latency to dark (s) | 6.286 ± 2.41 | 13.38 ± 3.85 | 16.00 ± 6.29 |
| L-D: latency to light (s) | 24.75 ± 7.48 | 23.00 ± 6.36 | 27.00 ± 8.05 |
| Reference | Stress Model | Strain | Sex | NE | DA | DA Turnover | 5-HT | 5-HT Turnover | |
|---|---|---|---|---|---|---|---|---|---|
| mPFC | Morrow et al., 2000 [24] | Footshock | Sprague-Dawley rats | Male | ↔ stressed v. CTRL | ↑ stressed v. CTRL | |||
| Muneoka et al., 2020 [31] | Footshock | Sprague-Dawley | Male | ↔ Susceptible v. CTRL & Resilient | ↔ Susceptible v. CTRL & Resilient | ↔ Susceptible v. CTRL & Resilient | ↔ Susceptible v. CTRL & Resilient | ↔ Susceptible v. CTRL & Resilient | |
| De La Garza & Mahoney, 2004 [25] | Forced swim | Wistar and WKY rats | Male | ↑ stressed v. CTRL ↔ Susceptible v. Resilient | ↓ Susceptible v. CTRL & Resilient | ↑ Susceptible v. CTRL & Resilient | ↓ Susceptible v. CTRL & Resilient | ↑ Susceptible v. CTRL & Resilient | |
| Isingrini et al., 2016 [30] | Social defeat | C57BL/6 mice | Male | ↔ Susceptible v. CTRL & Resilient | |||||
| Hayley et al., 2001 [26] | Predator scent | C57BL/6ByJ & BALB/cByJ mice | Male | ↑ stressed v. CTRL | ↔ stressed v. CTRL | ↔ stressed v. CTRL | |||
| Han et al., 2011 [27] | Post-weaning isolation | Sprague-Dawley rats | Male | ↑ stressed v. CTRL | ↔ stressed v. CTRL | ↑ stressed v. CTRL | ↔ stressed v. CTRL | ||
| Wilson et al., 2014 [28] | Predator scent + psychosocial stress | Sprague-Dawley rats | Male | ↑ stressed v. CTRL | ↑ stressed v. CTRL | ↓ stressed v. CTRL | |||
| Tseilikman et al., 2020 [29] | Repeated predator scent | Wistar rats | Male | ↔ Susceptible v. CTRL ↑ Resilient v. CTRL | ↔ Susceptible & Resilient v. CTRL | ↑ Susceptible v. CTRL ↔ Resilient v. Susceptible & CTRL | ↓ Susceptible v. CTRL ↔ Resilient v. Susceptible & CTRL | ↓ Susceptible v. CTRL ↔ Resilient v. Susceptible & CTRL | |
| Morrow et al., 2000 [24] | Acute and repeated predator scent | Sprague-Dawley rats | Male | ↔ stressed v. CTRL | ↑ stressed v. CTRL | ||||
| Present Results | Predator scent | Sprague-Dawley | Female | ↔ Susceptible v. CTRL & Resilient | ↓Susceptible v. CTRL | ↑ Susceptible v. CTRL & Resilient ↑ Resilient v. CTRL | ↔ Susceptible v. CTRL & Resilient | ↑ Susceptible v. CTRL & Resilient | |
| NAc | Morrow et al., 2000 [24] | Footshock | Sprague-Dawley rats | Male | ↔ stressed v. CTRL | ↑ stressed v. CTRL | |||
| Muneoka et al., 2020 [31] | Footshock | Sprague-Dawley | Male | ↔ Susceptible v. CTRL & Resilient | ↔ Susceptible v. CTRL & Resilient | ↔ Susceptible v. CTRL & Resilient | ↓ Susceptible v. Resilient | ↔ Susceptible v. CTRL & Resilient | |
| Isingrini et al., 2016 [30] | Social defeat | C57BL/6 mice | Male | ↔ Susceptible v. CTRL & Resilient | |||||
| Han et al., 2011 [27] | Post-weaning isolation | Sprague-Dawley rats | Male | ↑ stressed v. CTRL | ↔ stressed v. CTRL | ↑ stressed v. CTRL | ↑ stressed v. CTRL | ||
| Morrow et al., 2000 [24] | Predator scent | Sprague-Dawley rats | Male | ↔ stressed v. CTRL | ↔ stressed v. CTRL | ||||
| Present Results | Predator scent | Sprague-Dawley | Female | ↔ Susceptible v. CTRL & Resilient | ↔ Susceptible v. CTRL & Resilient | ↑ Susceptible v. CTRL ↑ Resilient v. CTRL | ↔ Susceptible v. CTRL & Resilient | ↔ Susceptible v. CTRL & Resilient | |
| HIPP | Sziray et al., 2007 [49] | Footshock | Sprague-Dawley | Male | ↓ stressed v. CTRL | ↓ stressed v. CTRL | |||
| Muneoka et al., 2020 [31] | Footshock | Sprague-Dawley | Male | ↔ Susceptible v. CTRL & Resilient | ↔ Susceptible v. CTRL & Resilient | ↑ Susceptible v. CTRL & Resilient | ↔ Susceptible v. CTRL & Resilient | ↔ Susceptible v. CTRL & Resilient | |
| Hayley et al., 2001 [26] | Predator scent | C57BL/6ByJ & BALB/cByJ mice | Male | ↔ stressed v. CTRL | ↔ stressed v. CTRL | ↔ stressed v. CTRL | |||
| Wilson et al., 2014 [28] | Repeated PSS + psychosocial stress | Sprague-Dawley | Male | ↑ stressed v. CTRL | ↔ stressed v. CTRL | ↓ stressed v. CTRL | |||
| Tseilikman et al., 2020 [29] | Repeated predator scent | Wistar | Male | ↔ Susceptible v. CTRL & Resilient | ↓ Susceptible v. CTRL & Resilient | ↑ Susceptible v. CTRL & Resilient | ↓ Susceptible v. CTRL & Resilient | ↑ Susceptible v. CTRL & Resilient | |
| Present Results (vHIPP) | Predator scent | Sprague-Dawley | Female | ↑ Susceptible v. CTRL ↔ Resilient v. CTRL & Susceptible | ↔ Susceptible v. CTRL & Resilient | ↔ Susceptible v. CTRL & Resilient | ↔ Susceptible v. CTRL & Resilient | ↔ Susceptible v. CTRL & Resilient |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilkinson, C.S.; Blount, H.L.; Schwendt, M.; Knackstedt, L.A. Brain Monoamine Dysfunction in Response to Predator Scent Stress Accompanies Stress-Susceptibility in Female Rats. Biomolecules 2023, 13, 1055. https://doi.org/10.3390/biom13071055
Wilkinson CS, Blount HL, Schwendt M, Knackstedt LA. Brain Monoamine Dysfunction in Response to Predator Scent Stress Accompanies Stress-Susceptibility in Female Rats. Biomolecules. 2023; 13(7):1055. https://doi.org/10.3390/biom13071055
Chicago/Turabian StyleWilkinson, Courtney S., Harrison L. Blount, Marek Schwendt, and Lori A. Knackstedt. 2023. "Brain Monoamine Dysfunction in Response to Predator Scent Stress Accompanies Stress-Susceptibility in Female Rats" Biomolecules 13, no. 7: 1055. https://doi.org/10.3390/biom13071055
APA StyleWilkinson, C. S., Blount, H. L., Schwendt, M., & Knackstedt, L. A. (2023). Brain Monoamine Dysfunction in Response to Predator Scent Stress Accompanies Stress-Susceptibility in Female Rats. Biomolecules, 13(7), 1055. https://doi.org/10.3390/biom13071055






