Eosinophils, Basophils, and Neutrophils in Bullous Pemphigoid
Abstract
1. Introduction
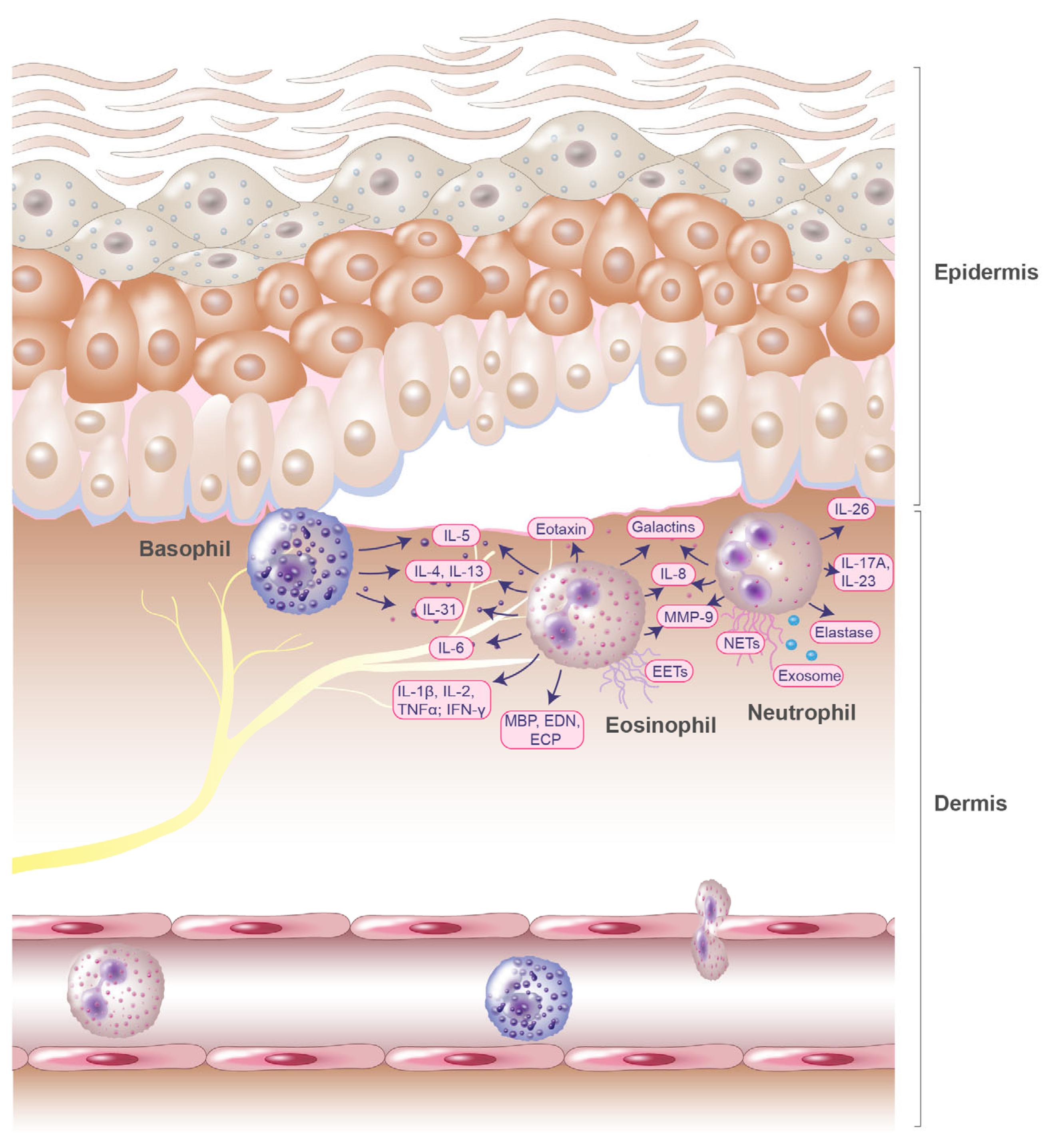
2. Role of Eosinophils in the Pathogenesis of Bullous Pemphigoid
3. Role of Basophils in the Pathogenesis of Bullous Pemphigoid
4. Role of Neutrophils in the Pathogenesis of Bullous Pemphigoid
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raap, U.; Werfel, T. Images in Clinical Medicine. Bullous Pemphigoid. N. Engl. J. Med. 2015, 373, 1659. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Limberg, M.M.; Gehring, M.; Kotnik, N.; Kapp, A.; Gibbs, B.F.; Raap, U. Neurological disorders are associated with bullous pemphigoid. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 925–929. [Google Scholar] [CrossRef]
- Holtsche, M.M.; Boch, K.; Schmidt, E. Autoimmune bullous dermatoses. J. Dtsch. Dermatol. Ges. 2023, 21, 405–412. [Google Scholar] [CrossRef]
- Stanley, J.R.; Hawley-Nelson, P.; Yuspa, S.H.; Shevach, E.M.; Katz, S.I. Characterization of bullous pemphigoid antigen: A unique basement membrane protein of stratified squamous epithelia. Cell 1981, 24, 897–903. [Google Scholar] [CrossRef]
- Diaz, L.A.; Ratrie HI, I.I.; Saunders, W.S.; Futamura, S.; Squiquera, H.L.; Anhalt, G.J.; Giudice, G. Isolation of a human epidermal cDNA corresponding to the 180-kD autoantigen recognized by bullous pemphigoid and herpes gestationis sera. Immunolocalization of this protein to the hemidesmosome. J. Clin. Investig. 1990, 86, 1088–1094. [Google Scholar] [CrossRef]
- Stanley, J.R. Cell adhesion molecules as targets of autoantibodies in pemphigus and pemphigoid, bullous diseases due to defective epidermal cell adhesion. Adv. Immunol. 1993, 53, 291–325. [Google Scholar]
- Ehrlich, P. Beiträge zur Kenntniss der Anilinfärbungen und ihrer Verwendung in der mikroskopischen Technik. Arch. Für Mikrosk. Anat. 1877, 13, 263–277. [Google Scholar] [CrossRef]
- Osgood, E.E.; Brownlee, I.E.; Osgood, M.W.; Ellis, D.M.; Cohen, W. Total differential and absolute leukocyte counts and sedimentation rates: Determined for healthy persons nineteen years of age and over. Arch. Intern. Med. 1939, 64, 105–120. [Google Scholar] [CrossRef]
- Yousefi, S.; Gold, J.A.; Andina, N.; Lee, J.J.; Kelly, A.M.; Kozlowski, E.; Schmid, I.; Straumann, A.; Reichenbach, J.; Gleich, G.J.; et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 2008, 14, 949–953. [Google Scholar] [CrossRef]
- Morshed, M.; Yousefi, S.; Stöckle, C.; Simon, H.U.; Simon, D. Thymic stromal lymphopoietin stimulates the formation of eosinophil extracellular traps. Allergy 2012, 67, 1127–1137. [Google Scholar] [CrossRef]
- Engineer, L.; Bhol, K.; Kumari, S.; Razzaque Ahmed, A. Bullous pemphigoid: Interaction of interleukin 5, anti-basement membrane zone antibodies and eosinophils. A preliminary observation. Cytokine 2001, 13, 32–38. [Google Scholar] [CrossRef]
- Ernst, N.; Friedrich, M.; Bieber, K.; Kasperkiewicz, M.; Gross, N.; Sadik, C.D.; Zillikens, D.; Schmidt, E.; Ludwig, R.J.; Hartmann, K. Expression of PD-1 and Tim-3 is increased in skin of patients with bullous pemphigoid and pemphigus vulgaris. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 486–492. [Google Scholar] [CrossRef]
- Kimura, R.; Sugita, K.; Horie, T.; Yamamoto, O. Dual role of basophils in the pathogenesis of bullous pemphigoid elucidated by pathological and ultrastructural studies. Eur. J. Dermatol. 2022, 32, 322–333. [Google Scholar] [CrossRef]
- Simon, D.; Borradori, L.; Simon, H.U. Eosinophils as putative therapeutic targets in bullous pemphigoid. Exp. Dermatol. 2017, 26, 1187–1192. [Google Scholar] [CrossRef]
- Kasperkiewicz, M.; Zillikens, D. The pathophysiology of bullous pemphigoid. Clin. Rev. Allergy Immunol. 2007, 33, 67–77. [Google Scholar] [CrossRef]
- Amber, K.T.; Valdebran, M.; Kridin, K.; Grando, S.A. The Role of Eosinophils in Bullous Pemphigoid: A Developing Model of Eosinophil Pathogenicity in Mucocutaneous Disease. Front. Med. 2018, 5, 201. [Google Scholar] [CrossRef]
- Engmann, J.; Rüdrich, U.; Behrens, G.; Papakonstantinou, E.; Gehring, M.; Kapp, A.; Raap, U. Increased Activity and Apoptosis of Eosinophils in Blister Fluids, Skin and Peripheral Blood of Patients with Bullous Pemphigoid. Acta Derm. Venereol. 2017, 97, 464–471. [Google Scholar] [CrossRef]
- Inaoki, M.; Takehara, K. Increased serum levels of interleukin (IL)-5, IL-6 and IL-8 in bullous pemphigoid. J. Dermatol. Sci. 1998, 16, 152–157. [Google Scholar] [CrossRef]
- Ameglio, F.; D’Auria, L.; Bonifati, C.; Ferraro, C.; Mastroianni, A.; Giacalone, B. Cytokine pattern in blister fluid and serum of patients with bullous pemphigoid: Relationships with disease intensity. Br. J. Dermatol. 1998, 138, 611–614. [Google Scholar] [CrossRef]
- Rico, M.J.; Benning, C.; Weingart, E.S.; Streilein, R.D.; Hall, R.P., III. Characterization of skin cytokines in bullous pemphigoid and pemphigus vulgaris. Br. J. Dermatol. 1999, 140, 1079–1086. [Google Scholar] [CrossRef]
- Wakugawa, M.; Nakamura, K.; Hino, H.; Toyama, K.; Hattori, N.; Okochi, H.; Yamada, H.; Hirai, K.; Tamaki, K.; Furue, M. Elevated levels of eotaxin and interleukin-5 in blister fluid of bullous pemphigoid: Correlation with tissue eosinophilia. Br. J. Dermatol. 2000, 143, 112–116. [Google Scholar] [CrossRef]
- Salz, M.; Haeberle, S.; Hoffmann, J.; Enk, A.H.; Hadaschik, E.N. Elevated IL-31 serum levels in bullous pemphigoid patients correlate with eosinophil numbers and are associated with BP180-IgE. J. Dermatol. Sci. 2017, 87, 309–311. [Google Scholar] [CrossRef]
- Rüdrich, U.; Gehring, M.; Papakonstantinou, E.; Illerhaus, A.; Engmann, J.; Kapp, A.; Hartmann, K.; Meyer, N.H.; Gibbs, B.F.; Raap, U. Eosinophils are a Major Source of Interleukin-31 in Bullous Pemphigoid. Acta Derm. Venereol. 2018, 98, 766–771. [Google Scholar] [CrossRef]
- Czech, W.; Schaller, J.; Schöpf, E.; Kapp, A. Granulocyte activation in bullous diseases: Release of granular proteins in bullous pemphigoid and pemphigus vulgaris. J. Am. Acad. Dermatol. 1993, 29, 210–215. [Google Scholar] [CrossRef]
- Caproni, M.; Palleschi, G.M.; Falcos, D.; D’Agata, A.; Cappelli, G.; Fabbri, P. Serum eosinophil cationic protein (ECP) in bullous pemphigoid. Int. J. Dermatol. 1995, 34, 177–180. [Google Scholar] [CrossRef]
- Tedeschi, A.; Marzano, A.V.; Lorini, M.; Balice, Y.; Cugno, M. Eosinophil cationic protein levels parallel coagulation activation in the blister fluid of patients with bullous pemphigoid. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 813–817. [Google Scholar] [CrossRef]
- Simon, D.; Hoesli, S.; Roth, N.; Staedler, S.; Yousefi, S.; Simon, H.U. Eosinophil extracellular DNA traps in skin diseases. J. Allergy Clin. Immunol. 2011, 127, 194–199. [Google Scholar] [CrossRef]
- Solimani, F.; Didona, D.; Li, J.; Bao, L.; Patel, P.M.; Gasparini, G.; Kridin, K.; Cozzani, E.; Hertl, M.; Amber, K.T. Characterizing the proteome of bullous pemphigoid blister fluid utilizing tandem mass tag labeling coupled with LC-MS/MS. Arch. Dermatol. Res. 2022, 314, 921–928. [Google Scholar] [CrossRef]
- Pruessmann, J.; Pruessmann, W.; Holtsche, M.M.; Linnemann, B.; Hammers, C.M.; van Beek, N.; Zillikens, D.; Schmidt, E.; Sadik, C.D. Immunomodulator Galectin-9 is Increased in Blood and Skin of Patients with Bullous Pemphigoid. Acta Derm. Venereol. 2021, 101, adv00419. [Google Scholar] [CrossRef]
- Ståhle-Bäckdahl, M.; Inoue, M.; Guidice, G.J.; Parks, W.C. 92-kD gelatinase is produced by eosinophils at the site of blister formation in bullous pemphigoid and cleaves the extracellular domain of recombinant 180-kD bullous pemphigoid autoantigen. J. Clin. Investig. 1994, 93, 2022–2030. [Google Scholar] [CrossRef]
- Raap, U.; Gehring, M.; Kleiner, S.; Rüdrich, U.; Eiz-Vesper, B.; Haas, H.; Kapp, A.; Gibbs, B.F. Human basophils are a source of-and are differentially activated by-IL-31. Clin. Exp. Allergy 2017, 47, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Giusti, D.; Bini, E.; Terryn, C.; Didier, K.; Le Jan, S.; Gatouillat, G.; Durlach, A.; Nesmond, S.; Muller, C.; Bernard, P.; et al. NET Formation in Bullous Pemphigoid Patients With Relapse Is Modulated by IL-17 and IL-23 Interplay. Front. Immunol. 2019, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Shibata, S.; Ito, Y.; Taira, H.; Sugimoto, E.; Awaji, K.; Sato, S. Interleukin-26-DNA complexes promote inflammation and dermal-epidermal separation in a modified human cryosection model of bullous pemphigoid. Front. Immunol. 2022, 13, 1013382. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Shao, S.; Jiang, M.; Dang, E.; Shen, S.; Zhang, J.; Qiao, P.; Li, C.; Wang, G. Proinflammatory role of blister fluid-derived exosomes in bullous pemphigoid. J. Pathol. 2018, 245, 114–125. [Google Scholar] [CrossRef]
- Riani, M.; Le Jan, S.; Plée, J.; Durlach, A.; Le Naour, R.; Haegeman, G.; Bernard, P.; Antonicelli, F. Bullous pemphigoid outcome is associated with CXCL10-induced matrix metalloproteinase 9 secretion from monocytes and neutrophils but not lymphocytes. J. Allergy Clin. Immunol. 2017, 139, 863–872.e863. [Google Scholar] [CrossRef]
- Fang, H.; Shao, S.; Xue, K.; Yuan, X.; Qiao, P.; Zhang, J.; Cao, T.; Luo, Y.; Bai, X.; Li, W.; et al. Neutrophil extracellular traps contribute to immune dysregulation in bullous pemphigoid via inducing B-cell differentiation and antibody production. FASEB J. 2021, 35, e21746. [Google Scholar] [CrossRef]
- Dierksmeier, U.; Frosch, P.J.; Czarnetzki, B.M. Eosinophil chemotactic factor (ECF) in blister fluid of dermatological diseases. Br. J. Dermatol. 1980, 102, 43–48. [Google Scholar] [CrossRef]
- Valdebran, M.; Kowalski, E.H.; Kneiber, D.; Li, J.; Kim, J.; Doan, L.; De Feraudy, S.; Amber, K.T. Epidermal expression of eotaxins and thymic stromal lymphopoietin in eosinophil rich dermatoses. Arch. Dermatol. Res. 2019, 311, 705–710. [Google Scholar] [CrossRef]
- Amber, K.T.; Chernyavsky, A.; Agnoletti, A.F.; Cozzani, E.; Grando, S.A. Mechanisms of pathogenic effects of eosinophil cationic protein and eosinophil-derived neurotoxin on human keratinocytes. Exp. Dermatol. 2018, 27, 1322–1327. [Google Scholar] [CrossRef]
- Pope, S.M.; Brandt, E.B.; Mishra, A.; Hogan, S.P.; Zimmermann, N.; Matthaei, K.I.; Foster, P.S.; Rothenberg, M.E. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. J. Allergy Clin. Immunol. 2001, 108, 594–601. [Google Scholar] [CrossRef]
- Alonso-Llamazares, J.; Rogers, R.S., 3rd; Oursler, J.R.; Calobrisi, S.D. Bullous pemphigoid presenting as generalized pruritus: Observations in six patients. Int. J. Dermatol. 1998, 37, 508–514. [Google Scholar] [PubMed]
- Bingham, E.A.; Burrows, D.; Sandford, J.C. Prolonged pruritus and bullous pemphigoid. Clin. Exp. Dermatol. 1984, 9, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, S.; Miyasato, M.; Iryo, K.; Nakama, T.; Kato, K.; Sasai, Y. Eosinophil phenotypes in bullous pemphigoid. J. Dermatol. 1992, 19, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Straub, C.; Pazdrak, K.; Young, T.W.; Stafford, S.J.; Wu, Z.; Wiktorowicz, J.E.; Haag, A.M.; English, R.D.; Soman, K.V.; Kurosky, A. Toward the Proteome of the Human Peripheral Blood Eosinophil. Proteom. Clin. Appl. 2009, 3, 1151–1173. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, S.J.; Weil, G.J.; Gleich, G.J. Formation of Charcot-Leyden crystals by human basophils. J. Exp. Med. 1982, 155, 1597–1609. [Google Scholar] [CrossRef]
- Niimi, Y.; Pawankar, R.; Kawana, S. Increased expression of matrix metalloproteinase-2, matrix metalloproteinase-9 and matrix metalloproteinase-13 in lesional skin of bullous pemphigoid. Int. Arch. Allergy Immunol. 2006, 139, 104–113. [Google Scholar] [CrossRef]
- Schwingshackl, A.; Duszyk, M.; Brown, N.; Moqbel, R. Human eosinophils release matrix metalloproteinase-9 on stimulation with TNF-alpha. J. Allergy Clin. Immunol. 1999, 104, 983–989. [Google Scholar] [CrossRef]
- Oikarinen, A.I.; Zone, J.J.; Ahmed, A.R.; Kiistala, U.; Uitto, J. Demonstration of collagenase and elastase activities in the blister fluids from bullous skin diseases. Comparison between dermatitis herpetiformis and bullous pemphigoid. J. Investig. Dermatol. 1983, 81, 261–266. [Google Scholar] [CrossRef]
- Okada, S.; Kita, H.; George, T.J.; Gleich, G.J.; Leiferman, K.M. Migration of eosinophils through basement membrane components in vitro: Role of matrix metalloproteinase-9. Am. J. Respir. Cell Mol. Biol. 1997, 17, 519–528. [Google Scholar] [CrossRef]
- de Graauw, E.; Sitaru, C.; Horn, M.; Borradori, L.; Yousefi, S.; Simon, H.U.; Simon, D. Evidence for a role of eosinophils in blister formation in bullous pemphigoid. Allergy 2017, 72, 1105–1113. [Google Scholar] [CrossRef]
- Ständer, S.; Hammers, C.M.; Vorobyev, A.; Schmidt, E.; Zillikens, D.; Ghorbanalipoor, S.; Bieber, K.; Ludwig, R.J.; Kridin, K. The impact of lesional inflammatory cellular infiltrate on the phenotype of bullous pemphigoid. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1702–1711. [Google Scholar] [CrossRef]
- Lin, L.; Hwang, B.J.; Culton, D.A.; Li, N.; Burette, S.; Koller, B.H.; Messingham, K.A.; Fairley, J.A.; Lee, J.J.; Hall, R.P.; et al. Eosinophils Mediate Tissue Injury in the Autoimmune Skin Disease Bullous Pemphigoid. J. Investig. Dermatol. 2018, 138, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, A.; Kotnik, N.; Diercks, G.F.H.; Meijer, J.M.; Di Zenzo, G.; Pas, H.H.; Jonkman, M.F.; Gibbs, B.F.; Raap, U.; Horváth, B. IgE autoantibodies in serum and skin of non-bullous and bullous pemphigoid patients. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 973–980. [Google Scholar] [CrossRef]
- Garrido, P.M.; Aguado-Lobo, M.; Espinosa-Lara, P.; Soares-Almeida, L.; Filipe, P. Association of Peripheral Blood and Cutaneous Eosinophils With Bullous Pemphigoid Disease Severity and Treatment Outcomes. Actas Dermo-Sifiliogr. 2022, 113, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Baum, S.; Engler Markowitz, M.; Lyakhovitsky, A.; Gershon, R.; Aviv, H.; Segal, Z.; Barzilai, A. Skin Eosinophil Counts in Bullous Pemphigoid as a Prognostic Factor for Disease Severity and Treatment Response. Acta Derm. Venereol. 2022, 103, adv00850. [Google Scholar] [CrossRef]
- Ehrlich, P. Beiträge zur kenntniss der granulirten bindegewebszellen und der eosinophilen leukocythen. Arch. Anat. Physiol. 1879, 3, 166–169. [Google Scholar]
- Ishizaka, K.; Tomioka, H.; Ishizaka, T. Mechanisms of passive sensitization. I. Presence of IgE and IgG molecules on human leukocytes. J. Immunol. 1970, 105, 1459–1467. [Google Scholar] [CrossRef]
- Ishizaka, T.; De Bernardo, R.; Tomioka, H.; Lichtenstein, L.M.; Ishizaka, K. Identification of basophil granulocytes as a site of allergic histamine release. J. Immunol. 1972, 108, 1000–1008. [Google Scholar] [CrossRef]
- Brunner, T.; Heusser, C.H.; Dahinden, C.A. Human peripheral blood basophils primed by interleukin 3 (IL-3) produce IL-4 in response to immunoglobulin E receptor stimulation. J. Exp. Med. 1993, 177, 605–611. [Google Scholar] [CrossRef]
- Piccinni, M.P.; Macchia, D.; Parronchi, P.; Giudizi, M.G.; Bani, D.; Alterini, R.; Grossi, A.; Ricci, M.; Maggi, E.; Romagnani, S. Human bone marrow non-B, non-T cells produce interleukin 4 in response to cross-linkage of Fc epsilon and Fc gamma receptors. Proc. Natl. Acad. Sci. USA 1991, 88, 8656–8660. [Google Scholar] [CrossRef]
- Li, H.; Sim, T.C.; Alam, R. IL-13 released by and localized in human basophils. J. Immunol. 1996, 156, 4833–4838. [Google Scholar] [CrossRef]
- Gibbs, B.F.; Haas, H.; Falcone, F.H.; Albrecht, C.; Vollrath, I.B.; Noll, T.; Wolff, H.H.; Amon, U. Purified human peripheral blood basophils release interleukin-13 and preformed interleukin-4 following immunological activation. Eur. J. Immunol. 1996, 26, 2493–2498. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Satoh, T.; Takayama, K.; Miyagishi, C.; Walls, A.F.; Yokozeki, H. Basophil recruitment and activation in inflammatory skin diseases. Allergy 2011, 66, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Asbrink, E.; Hovmark, A. Serum IgE levels in patients with bullous pemphigoid and its correlation to the activity of the disease and anti-basement membrane zone antibodies. Acta Derm. Venereol. 1984, 64, 243–246. [Google Scholar] [PubMed]
- Dvorak, A.M.; Mihm, M.C., Jr.; Osage, J.E.; Kwan, T.H.; Austen, K.F.; Wintroub, B.U. Bullous pemphigoid, an ultrastructural study of the inflammatory response: Eosinophil, basophil and mast cell granule changes in multiple biopsies from one patient. J. Investig. Dermatol. 1982, 78, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Kursewicz, C.D.; Fayne, R.A.; Nanda, S.; Shah, S.M.; Nattkemper, L.; Yokozeki, H.; Yosipovitch, G. Pathophysiologic mechanisms of itch in bullous pemphigoid. J. Am. Acad. Dermatol. 2020, 83, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Schultze, M. Ein heizbarer Objecttisch und seine Verwendung bei Untersuchungen des Blutes. Arch. Für Mikrosk. Anat. 1865, 1, 1–42. [Google Scholar] [CrossRef]
- Liew, P.X.; Kubes, P. The Neutrophil’s Role During Health and Disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, S.H.; Kim, J.H.; Kim, S.C. Circulating Eosinophil and Neutrophil Counts Correlate with Disease Severity in Bullous Pemphigoid. Ann. Dermatol. 2018, 30, 544–549. [Google Scholar] [CrossRef]
- Liu, Z.; Giudice, G.J.; Zhou, X.; Swartz, S.J.; Troy, J.L.; Fairley, J.A.; Till, G.O.; Diaz, L.A. A major role for neutrophils in experimental bullous pemphigoid. J. Clin. Investig. 1997, 100, 1256–1263. [Google Scholar] [CrossRef]
- Verraes, S.; Hornebeck, W.; Polette, M.; Borradori, L.; Bernard, P. Respective contribution of neutrophil elastase and matrix metalloproteinase 9 in the degradation of BP180 (type XVII collagen) in human bullous pemphigoid. J. Investig. Dermatol. 2001, 117, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shapiro, S.D.; Zhou, X.; Twining, S.S.; Senior, R.M.; Giudice, G.J.; Fairley, J.A.; Diaz, L.A. A critical role for neutrophil elastase in experimental bullous pemphigoid. J. Clin. Investig. 2000, 105, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Schulze, F.S.; Beckmann, T.; Nimmerjahn, F.; Ishiko, A.; Collin, M.; Köhl, J.; Goletz, S.; Zillikens, D.; Ludwig, R.; Schmidt, E. Fcγ receptors III and IV mediate tissue destruction in a novel adult mouse model of bullous pemphigoid. Am. J. Pathol. 2014, 184, 2185–2196. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Barnado, A.; Crofford, L.J.; Oates, J.C. At the Bedside: Neutrophil extracellular traps (NETs) as targets for biomarkers and therapies in autoimmune diseases. J. Leukoc. Biol. 2016, 99, 265–278. [Google Scholar] [CrossRef]
- Chakievska, L.; Holtsche, M.M.; Künstner, A.; Goletz, S.; Petersen, B.S.; Thaci, D.; Ibrahim, S.M.; Ludwig, R.J.; Franke, A.; Sadik, C.D.; et al. IL-17A is functionally relevant and a potential therapeutic target in bullous pemphigoid. J. Autoimmun. 2019, 96, 104–112. [Google Scholar] [CrossRef]
- Le Jan, S.; Plée, J.; Vallerand, D.; Dupont, A.; Delanez, E.; Durlach, A.; Jackson, P.L.; Edwin Blalock, J.; Bernard, P.; Antonicelli, F. Innate immune cell-produced IL-17 sustains inflammation in bullous pemphigoid. J. Investig. Dermatol. 2014, 134, 2908–2917. [Google Scholar] [CrossRef]
- Margaroli, C.; Bradley, B.; Thompson, C.; Brown, M.R.; Giacalone, V.D.; Bhatt, L.; Stoff, B.; Ahuja, S.; Springman, E.; Tirouvanziam, R.; et al. Distinct compartmentalization of immune cells and mediators characterizes bullous pemphigoid disease. Exp. Dermatol. 2020, 29, 1191–1198. [Google Scholar] [CrossRef]
- Rai, P. Role of neutrophil-to-lymphocyte, neutrophil-to-eosinophil and platelet-to-lymphocyte ratios in the diagnosis of bullous pemphigoid and Pemphigus disease. Indian J. Pathol. Microbiol. 2023, 66, 70–74. [Google Scholar] [CrossRef]
- Lu, M.; DiBernardo, E.; Parks, E.; Fox, H.; Zheng, S.Y.; Wayne, E. The Role of Extracellular Vesicles in the Pathogenesis and Treatment of Autoimmune Disorders. Front. Immunol. 2021, 12, 566299. [Google Scholar] [CrossRef]
- Gasparini, G.; Tasso, R.; Palamà, M.E.F.; Ciferri, M.C.; Gentili, C.; Di Zenzo, G.; Provini, A.; Salemme, A.; Quarto, R.; Parodi, A.; et al. Pilot study investigating BP-180 in extracellular vesicles derived from blister fluid of bullous pemphigoid patients. Arch. Dermatol. Res. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.; Reimer, S.; Kruse, N.; Bröcker, E.B.; Zillikens, D. The IL-8 release from cultured human keratinocytes, mediated by antibodies to bullous pemphigoid autoantigen 180, is inhibited by dapsone. Clin. Exp. Immunol. 2001, 124, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.; Ambach, A.; Bastian, B.; Bröcker, E.B.; Zillikens, D. Elevated levels of interleukin-8 in blister fluid of bullous pemphigoid compared with suction blisters of healthy control subjects. J. Am. Acad. Dermatol. 1996, 34, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.M.; Lewis, H.A.; Cornelius, L.A.; Chen, D.Y.; Rosman, I.S. Neutrophil-predominant bullous pemphigoid induced by checkpoint inhibitors: A case series. J. Cutan. Pathol. 2020, 47, 742–746. [Google Scholar] [CrossRef] [PubMed]
- de Graauw, E.; Sitaru, C.; Horn, M.P.; Borradori, L.; Yousefi, S.; Simon, D.; Simon, H.U. Monocytes enhance neutrophil-induced blister formation in an ex vivo model of bullous pemphigoid. Allergy 2018, 73, 1119–1130. [Google Scholar] [CrossRef]
- Hellberg, L.; Samavedam, U.; Holdorf, K.; Hänsel, M.; Recke, A.; Beckmann, T.; Steinhorst, K.; Boehncke, W.H.; Kirchner, T.; Möckel, N.; et al. Methylprednisolone blocks autoantibody-induced tissue damage in experimental models of bullous pemphigoid and epidermolysis bullosa acquisita through inhibition of neutrophil activation. J. Investig. Dermatol. 2013, 133, 2390–2399. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ning, G.; Zhao, M.L.; Fleming, M.G.; Diaz, L.A.; Werb, Z.; Liu, Z. Mast cells play a key role in neutrophil recruitment in experimental bullous pemphigoid. J. Clin. Investig. 2001, 108, 1151–1158. [Google Scholar] [CrossRef]
| Immune Cells | Mediators | Upregulation and Functions | Ref. |
|---|---|---|---|
| Eosinophils | IL-15, eosinophil colony-stimulating factor | Elevated in blister fluids of patients with BP | [16] |
| IL-2, IL-4, IFN-γ | Increased serum levels in patients with BP | [19] | |
| IL-1β, IL-8, TNFα | Increased serum levels in patients with BP; significant correlations with disease intensity | [19] | |
| IL-6, IL-8 | Increased serum levels in patients with BP | [18,19] | |
| Eotaxin | Upregulated in blister fluids; significantly correlated with the number of dermal infiltrating eosinophils | [21] | |
| IL-5 | Elevated serum levels; significant correlations with disease intensity; increased amounts in blister fluids; induces dermal–epidermal separation upon stimulation of eosinophils | [11,16,18,19,20,21] | |
| IL-31 | Increased concentrations in BP blister fluid; secreted by human eosinophils | [22,23] | |
| MBP, EDN, ECP | Significantly elevated in patients with BP | [22,24,25,26] | |
| EETs | Released in BP lesions | [27] | |
| Galectin-10 | Found in proteome of BP blister fluids | [28] | |
| Eosinophil peroxidase | Found in proteome of BP blister fluids | [28] | |
| Galectin-9 | Elevated in serum and in lesional skin; chemoattractant for eosinophils | [29] | |
| MMP-9 | Abundant component blister fluid MMP-9 is produced by eosinophils; degrades BP180 autoantigen | [30] | |
| Basophils | IL-4, IL-5, IL-13 | Detected in biopsies of lesional skin from patients with BP | [20] |
| IL-31 | Increased concentrations in BP blister fluids; released by human basophils | [22,31] | |
| Neutrophils | IL-17A, IL-23 | Elevated in serum of BP patients; enhance the release of NETs; IL-17A induces expression of MMP-9 and neutrophil elastase | [32] |
| IL-26, extracellular DNA | IL26–DNA complexes mediate dermal–epidermal separation and increase secretion of inflammatory mediators from neutrophils | [33] | |
| IL-8 | Derived from blister fluid exosomes; induces neutrophil recruitment | [34] | |
| MMP-9 | Increased induction by chemokine CXCL10 in BP | [35] | |
| Neutrophil elastase | Cleaves BP180 antigen into tripeptides | [34] | |
| NETs | Higher amounts in skin lesions, serum, and blister fluids in BP | [36] | |
| Galectin-9 | Increased in neutrophils in BP skin lesions | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limberg, M.M.; Weihrauch, T.; Gray, N.; Ernst, N.; Hartmann, K.; Raap, U. Eosinophils, Basophils, and Neutrophils in Bullous Pemphigoid. Biomolecules 2023, 13, 1019. https://doi.org/10.3390/biom13071019
Limberg MM, Weihrauch T, Gray N, Ernst N, Hartmann K, Raap U. Eosinophils, Basophils, and Neutrophils in Bullous Pemphigoid. Biomolecules. 2023; 13(7):1019. https://doi.org/10.3390/biom13071019
Chicago/Turabian StyleLimberg, Maren M., Tobias Weihrauch, Natalie Gray, Nancy Ernst, Karin Hartmann, and Ulrike Raap. 2023. "Eosinophils, Basophils, and Neutrophils in Bullous Pemphigoid" Biomolecules 13, no. 7: 1019. https://doi.org/10.3390/biom13071019
APA StyleLimberg, M. M., Weihrauch, T., Gray, N., Ernst, N., Hartmann, K., & Raap, U. (2023). Eosinophils, Basophils, and Neutrophils in Bullous Pemphigoid. Biomolecules, 13(7), 1019. https://doi.org/10.3390/biom13071019








