Abstract
Connexins are members of a family of integral membrane proteins that provide a pathway for both electrical and metabolic coupling between cells. Astroglia express connexin 30 (Cx30)-GJB6 and Cx43-GJA1, while oligodendroglia express Cx29/Cx31.3-GJC3, Cx32-GJB1, and Cx47-GJC2. Connexins organize into hexameric hemichannels (homomeric if all subunits are identical or heteromeric if one or more differs). Hemichannels from one cell then form cell-cell channels with a hemichannel from an apposed cell. (These are termed homotypic if the hemichannels are identical and heterotypic if the hemichannels differ). Oligodendrocytes couple to each other through Cx32/Cx32 or Cx47/Cx47 homotypic channels and they couple to astrocytes via Cx32/Cx30 or Cx47/Cx43 heterotypic channels. Astrocytes couple via Cx30/Cx30 and Cx43/Cx43 homotypic channels. Though Cx32 and Cx47 may be expressed in the same cells, all available data suggest that Cx32 and Cx47 cannot interact heteromerically. Animal models wherein one or in some cases two different CNS glial connexins have been deleted have helped to clarify the role of these molecules in CNS function. Mutations in a number of different CNS glial connexin genes cause human disease. Mutations in GJC2 lead to three distinct phenotypes, Pelizaeus Merzbacher like disease, hereditary spastic paraparesis (SPG44) and subclinical leukodystrophy.
Keywords:
GJC2; Connexin 47; leukodystrophy; Pelizaeus Merzbacher like disease; PMLD; PMLD1; gap junction; SPG44; HLD2 1. Introduction
Connexins are members of a family of integral membrane proteins, almost all of which oligomerize to form gap junctions (GJs). Gap junction channels provide a pathway for both electrical and metabolic coupling between cells [1]. GJ coupling of glial cells creates a glial syncytium [2], allowing propagation of signals such as Ca2+ waves (see Scemes and Giaume [3] for more detail), or potentially underlying handling potassium generated by neural activity [4,5,6,7]. Connexin hexamers can also form functional membrane hemichannels, also called connexons, in the plasma membrane; these mediate cellular processes, including metabolite influx and efflux [8,9], ATP release and signaling [10,11,12], glutamate release [8,12], glutathione release, [13] and responses to spinal cord injury [14]. Homomeric hemichannels contain six identical connexin subunits and in heteromeric hemichannels at least one connexin differs from the others. Two opposed apposed hexameric hemichannels form a dodecameric cell-cell or gap junction channel. In homotypic cell-cell channels the two hemichannels are identical, while in heterotypic channels the two hemichannels differ.
There are at least 21 members of the human connexin family and at least 20 in mice [15]. A number of these, including connexin 26 (Cx26)/GJB2, Cx29/Cx31.3/GJC3, Cx30/GJB6, Cx32/GJB1, Cx36/GJD2, Cx43/GJA1, Cx45/GJC1 and Cx47/GJC2 are expressed in the central and/or peripheral nervous system and mutations in a number of these cause central and/or peripheral nervous system disease. For example, mutations in Cx43 are associated with oculodentodigital dysplasia [16,17], a pleiotropic disorder that includes variable neurological manifestations such as seizures, cognitive deficits, and white matter abnormalities on imaging, [18,19,20,21], and mutations in Cx32 cause the most common form of X-linked Charcot Marie Tooth disease (CMTX1). In this review we will focus on the role of mutations in Cx47/GJC2 in several different phenotypes including a severe early onset dysmyelinating disorder, Pelizaeus-Merzbacher-like disease (PMLD1 or HLD2) [22], a milder, later onset disorder, hereditary spastic paraplegia (SPG44) [23], and a recently described subclinical leukodystrophy [24]. Recent general reviews of ODDD are available [25,26,27]; readers interested in CNS manifestations of Cx43 mutations may also find two older reviews [28,29] of use. Cx32 associated neurologic disease have also been recently reviewed [30,31]. All in all, at least 11 different connexins are associated with a large number of human disease phenotypes [32,33,34,35].
2. Glial Connexins
Astrocytes (As) express Cx30 and Cx43, and we and others have demonstrated that these connexins form homotypic (Cx43/Cx43 and Cx30/Cx30) but not heterotypic (Cx43/Cx30) GJs in transfected cells [36,37]. (The nomenclature connexin A/connexin B refers to a cell pairing configuration where cell 1, expressing connexin A is paired with cell 2, expressing connexin B to produce a gap junction channel. Where junctional voltage is applied, the voltage stated is applied to cell 2 with respect to cell 1.) In mouse hippocampal slices, the deletion of Gja1 (encoding Cx43) alone results in about a 50% reduction in astrocytic coupling [38,39], while knockout of both Cx43 and Gjb6 (encoding Cx30) results in the complete loss of A/A coupling [39]. Histological approaches also provide evidence that A/A GJs are comprised of homotypic Cx30/Cx30 and Cx43/Cx43 channels [40,41,42,43]. Astrocytic Cx43-positive GJ plaques are found throughout the brain while in gray matter Cx30-positive GJ plaques are more prominent [41,43,44]. Some astrocytes may also express Cx26, but some contradictory data have been presented [45]. The contribution of Cx26 to A/A coupling is unclear given the complete loss of A/A coupling seen in the Cx43/Cx30 double knockout [39]. Mutations in GJB2 (encoding Cx26) and GJB6 (encoding Cx30) have been noted in patients with hearing loss [46] and skin diseases [47] but are not associated with CNS abnormalities in humans or mice.
Oligodendrocytes (Os) express Cx29/Cx31.3, Cx32, and Cx47 [4,43,48,49,50]. Both Cx32 and Cx47 form functional homotypic gap junctions [36,37,51,52]. However, coupling between Cx32 and Cx47 homomeric hemichannels has not been demonstrated [36,37]. In mice, Cx29 does not form gap junctions and is localized to the adaxonal membrane of CNS myelin sheaths, where it forms hexameric rosettes [50,53,54,55]. In transfected cells, Cx29 [56] and Cx31.3 [57] do not form functional cell-cell channels, but the human orthologue, Cx31.3, appears to form hemichannels [57]. In most cells the expression of connexins leads to formation of gap junctions between different cells. However, in both oligodendrocytes in the CNS [4] and Schwann cells in the PNS [58], Cx32 is localized to the noncompact myelin where it is presumed to form reflexive gap junctions, connecting adjacent loops of myelin and shortening the pathway for diffusion between the ab- and adaxonal compartments. Experiments in rodents have also shown that Cx47 and Cx32 are found in GJ plaques that are localized on the cell bodies of oligodendrocytes [50]. Based on both conventional electron microscopy (EM) [59,60] and freeze replica immune labeling (FRIL) [5,42], it was concluded that O/O coupling did not occur to any significant degree. However, more recently two groups have reported functional O/O coupling in the mouse corpus callosum [61,62]. This O/O coupling is lost in mice that lack both Cx32 and Cx47. In addition, EM of the corpus callosum demonstrated morphologic gap junctions between oligodendrocytes [61].
We have recently examined whether Cx32 and Cx47, which are both expressed in oligodendrocytes and show substantial overlap in expression pattern in the CNS [43,49,50], can form functional heteromeric channels. Here we present some of our recently published data [63], underlying our conclusion that this is not the case. We first paired cells expressing Cx47 and Cx32 (Cx32+Cx47) or Cx32 or Cx47 alone with cells expressing either Cx30, Cx32, Cx43 or Cx47. We found that the strength of coupling for the Cx32+Cx47/Cx32 pairing was similar to that of the Cx32/Cx32 pairings and the strength of the Cx32+Cx47/Cx47 pairing was similar to the coupling produced by the Cx47/Cx47 homotypic pairing. A hallmark of homotypic gap junctions is that they produce a symmetric normalized steady state conductance voltage (Gj-Vj) relationship when examined by dual whole cell patch clamp recording. As shown in Figure 1a,b the Gj-Vj plots for the Cx32+Cx47/Cx32 and Cx32+Cx47/Cx47 are symmetric and overlap almost perfectly with the Cx32/Cx32 and Cx47/Cx47 Gj-Vj plots. This is the expected result if Cx32+Cx47 heteromers are not contributing to the cell-cell channels. Similarly, in Figure 1c,d, the Cx32+Cx47/Cx43 and Cx32+Cx47/Cx30 Gj-Vj plots are asymmetric since they represent heterotypic channels. These Gj-Vj plots are superimposable on those produced by Cx32/Cx47 and Cx32/Cx30 cell-cell channels, suggesting that there is no contribution from Cx32+Cx47 heteromers. Additional physiologic data arguing against heteromer formation are available [63]. Since an overlapping expression pattern is a prerequisite for heteromer formation, we also examined whether localizations of Cx32 and Cx47 were correlated when exogenously expressed in the same cells. When we performed this experiment using Cx32 and Cx30, two closely related connexins predicted to show heteromeric interactions (Koval et al., 2014), a relatively high degree of overlap in the pattern of staining was seen, as evidenced by a Pearson correlation coefficient of almost 0.5 (Figure 2). On the other hand, when Cx32WT (wild-type) was co-expressed with Cx47WT, little overlap is seen and the average correlation coefficient was very close to 0.0 (Figure 2). Figure 3 shows examples of confocal planar images used to do the analysis shown in Figure 2. (See Abrams et al. 2021 [63] for further details). In summary, the bulk of the evidence suggests that wild-type Cx47 and Cx32 do not form functional heteromers.
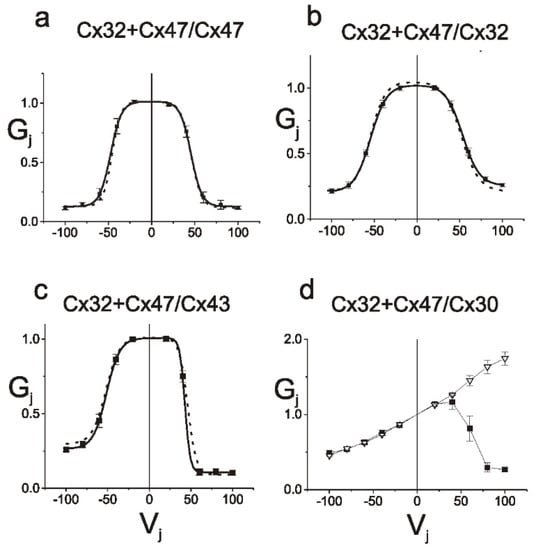
Figure 1.
Properties of junctions between cells expressing Cx32 and Cx47 paired with cells expressing (a) Cx47, (b) Cx32, (c) Cx43 or (d) Cx30 alone. Average normalized steady state Gj-Vj relations for Cx32+Cx47/Cx47, Cx32+Cx47/Cx32, Cx32+Cx47/Cx43 and Cx32+Cx47/Cx30 pairings are shown. The average normalized steady-state (filled squares) junctional conductance (Gj) at each Vj was calculated as described [63]. The solid lines are the best fit of the data to a double Boltzmann distribution as described in the Methods, and the dotted lines are the best fit of data for Cx47/Cx47, Cx32/Cx32, or Cx47/43 heterotypic junctions. Gj-Vj relations for Cx32+Cx47/Cx47, Cx32+Cx47/Cx32, Cx32+Cx47/Cx43, and Cx32+Cx47/Cx30 pairings are indistinguishable from those for Cx32/Cx32, Cx47/Cx47, Cx47/Cx43 and Cx32/Cx30 junctions respectively. The error bars represent SEM. Figure and legend modified from Abrams et al., 2021 [63]. Used with permission of Wiley Periodicals LLC Copyright © 2023.
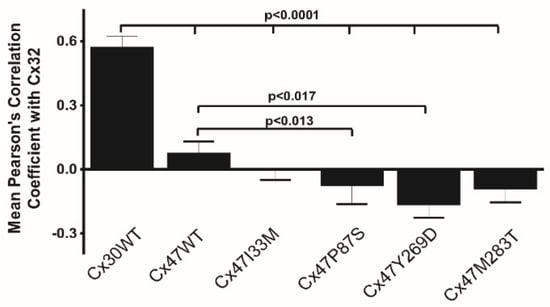
Figure 2.
Graphic representation of results of experiments to determine colocalization of Cx32WT with Cx47WT and mutants. As shown, cells expressing Cx30WT and Cx32WT show significantly more correlation in the overlap of staining than do any of the other pairs of co-expressed connexins. Cells expressing Cx32WT and Cx47WT showed essentially no correlation of staining for the two connexins and cells expressing Cx32WT with either Cx47I33M, Cx47P87S, or Cx47M283T showed only a slight and non-significant negative correlation of staining. Cells expressing Cx47Y269D and Cx32WT did show a statistically significant negative correlation of staining. Figure and legend modified from Abrams et al. 2021 [63]. Used with permission of Wiley Periodicals LLC Copyright © 2023.
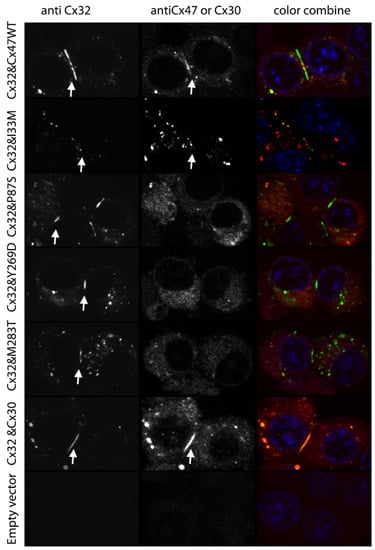
Figure 3.
Examples of images used for determination of colocalization as summarized in Figure 2. Arrows show selected plaques for Cx30WT, Cx32WT, Cx47WT and Cx47I33M. A few non-junctional areas in Cx32+Cx47WT and Cx32+Cx47I33M expressing cells show overlap between staining for Cx32 and Cx47. Non interacting connexins can participate in the same junction; however, they are regionally distinct within that junction [64]. The areas of overlap may represent internalized (annular) gap junctions [65] which often tend to be engulfed en masse, or connexin containing organelles that are part of the degradation pathway [66]. Scale bar 20 microns. Figure and legend modified from Abrams et al. 2021 [63]. Used with permission of Wiley Periodicals LLC Copyright © 2023.
Connexins also underlie oligodendrocyte/astrocyte coupling. Our work in transfected cell lines shows that Cx43/Cx47 and Cx30/Cx32 (but not Cx43/Cx32 or Cx30/Cx47) form morphologic and functional GJs that have distinct electrophysiological properties, [36] but using a different approach, another group [37] showed that Cx30 and Cx47 can form functional channels. However, data showing that loss of Cx30 has no effect on localization of Cx47 in mouse brain suggest that Cx47 and Cx30 do not interact in vivo [67]. It is worth pointing out that data from Abrams et al. 2021, including that shown in Figure 1 also support our conclusion that Cx30 and Cx47 do not form functional heterotypic channels. Our finding that the Cx32+Cx47/Cx30 and Cx32/Cx30 pairs are indistinguishable, argues against both a heterotypic interaction between Cx47 and Cx30 and a heteromeric interaction between Cx32 and Cx47. Anatomical studies [4,39,41,43] make a strong case that A/O coupling consist predominantly of Cx43/Cx47 and Cx30/Cx32 heterotypic channels. (There is no evidence for a role for Cx26 in A/O coupling). A/O coupling is weak [37] or absent [61] in the corpus callosum. In the cerebral cortex, A/O coupling is dependent on Cx47, but not on Cx32, indicating that Cx43/Cx47 channels (and not Cx30/Cx32 channels) are required for A/O coupling in mouse cortex [61].
The GJC2 gene contains two ATG codons at the upstream end of the coding region, separated by two intervening codons. Arguments have been made for assigning either the upstream [68] or the downstream [69] ATG as the start codon, but whether one of the two or both are used in vivo remains unresolved. In a recent study comparing the function of a form of Cx47 containing the longer (M1 and M4) and the shorter (M4) N-terminal domain, Fasciani and colleagues [70] show that the Cx47(M1 and M4) shows permeability differences between a positive and negatively charged dye, while the Cx47(M4) is equally permeable to both. Cx32/Cx30 and Cx43/Cx47 channels show permeability and rectification properties that may have an impact on their function in O/A coupling. For example, we have shown that Cx32/Cx30 channels are highly permeable to the monovalent anionic dye Alexafluor 350 but permeability to Lucifer Yellow (a divalent anionic dye) was not demonstrable. On the other hand, Cx47(M4)/Cx43 channels were equally permeable to both [36]. These differences in permeability may affect which signaling molecules or metabolites are able to traverse O/O and O/A junctions. Furthermore, O/A channels (Cx32/30 and Cx47(M1 and M4)/Cx43) show rectification with instantaneous (open channel) current increasing when the cell expressing Cx32 or Cx47(M1 and M4) is more positive. Fasciani et al. [70] suggest that this rectification may act to reduce potassium movement from oligodendrocyte to astrocyte during periods of neural activity where the same voltage gradient (positive in oligodendrocyte relative to astrocyte) that causes potassium to flow from O to A also leads to a reduction in open channel current and potassium flux for both Cx32/Cx30 and Cx47(M1 and M4)/Cx43 channels. They suggest that this in turn could result in increased local redistribution among coupled oligodendrocytes. It is worth noting that these considerations would not apply to Cx47(M4)/Cx43 channels where little or no rectification is seen [69,70].
3. Mouse Models for the Study of Glial Connexins
Genetically modified mice with targeted deletion or mutation of oligodendrocyte connexins have contributed to our understanding of the roles of these connexins in the CNS. Cx32 (Gjb1) null (KO) mice develop a demyelinating peripheral neuropathy after 3 months of age [71,72], with axonal changes evident by two months, [73] but only subtle changes in CNS myelin thickness are seen [74,75]. However, alterations in the expression of Cx32 in the CNS may cause a predisposition to increased intensity of response to inflammatory stimuli. For example, the Cx32KO mouse is somewhat more susceptible to EAE than the WT [76,77], and a mouse in which the Cx32 p.T55I transgene is expressed on a Cx32KO mouse background show greater susceptibility to LPS than does the WT or the Cx32KO [78]. However, to date, no baseline CNS behavioral abnormalities have been described in the Cx32KO mouse. Similarly, Cx47KO mice show no overt behavioral abnormalities. While one model shows no baseline pathological changes, [49] a different mouse model does show minimal, regionally limited myelin abnormalities [48]. Of note, loss of Cx47 has been shown to increase susceptibility to EAE [77,79] and loss of Cx32 increases susceptibility to both EAE and LPS mediated neuroinflammation [76,77,78]. The basis for the susceptibilities of both the Cx32KO and Cx47KO mice has not been fully clarified, but may be related to increased blood brain barrier permeability in these mice. Both Cx43 and Cx47 have C-terminal PDZ-binding domains and bind ZO-1, one of the defining members of PDZ family [80,81,82,83,84,85]. In Cx47KO mice, ZO-1, ZO-1–associated nucleic acid binding proteins (ZONAB), and multi-PDZ domain protein 1 (MUPP1) [86] lose their Cx47 dependent junctional localization. The resulting nuclear translocation of ZONAB, a Y-box transcription factor, may repress ErbB2 [87], a tyrosine kinase receptor implicated in oligodendrocyte development, differentiation, and survival [88,89,90]. ZONAB also regulates a number of cell cycle regulatory genes [91,92]. Thus, appropriate localization of Cx47 in the oligodendrocyte may be important for stabilization/organization of the oligodendrocyte membrane junctional complex, regulation of gene expression and control of the cell cycle.
Mice lacking both Cx32 and Cx47 show a floridly abnormal phenotype that includes abnormal movements, seizures, and death by around six weeks of age. CNS pathology includes demyelinated and remyelinated axons, enlarged extracellular spaces separating the axon from its myelin sheath, and significant oligodendrocyte apoptosis. These findings, as well as those described below, have led to the speculation that this vacuolization might be due to increased periaxonal accumulation of potassium due to failure of connexin dependent spatial buffering in the double KO [49]. Other data supporting the potassium buffering hypothesis include: (1) targeted deletion of. Kir4.1 [93], an inwardly rectifying K+ channel [94,95], leads to vacuolization of white matter similar to that seen in the Cx32-Cx47 dKO [93]; (2) the null allele of Kir4.1 genetically interacts with the null alleles of Cx32 and Cx47,suggesting that these three molecules are active in a common pathway [7]; and (3) reduced retinal ganglion cell activity reduces optic nerve vacuoles in the Cx32-Cx47 double knockout mice while increased activity causes increased vacuolization [7].
Cx29 (the mouse ortholog of human Cx31.3) is widely expressed in the CNS during development [96], and is found small myelinated fibers of mature brain and spinal cord [50,53,55,83]. No CNS phenotype, including normal brainstem auditory evoked potentials, was noted in mice with targeted ablation of Cx29 [97], though another group found that peripheral auditory pathways are affected [98]. A number of variants in GJC3 have been identified in patients with deafness [99,100,101]. A few of these variants have been evaluated in exogenous expression systems and show alterations in trafficking (p.W77S) [102], channel function (p.R15G and p.L23H) [103,104], or channel structure by cryo-EM (p.R15G) [103]. However, to date no human variant of GJC3 has been listed in ClinVar (version 20230306) [105] as pathogenic or likely pathogenic. Thus, whether GJC3 mutations are a cause of nonsyndromic deafness requires further clarification [106].
Mice with global knockout of Cx43 are not viable due to cardiac defects [107]. Astrocyte specific knockout of Cx43 (Cx43Astro) does not cause overt behavioral changes but does lead to poorer performance on behavioral testing (e.g., water maze, plus maze, open field activity) [108], 50% reduction in A/A coupling [39], and increased velocity of spreading depression [109]. Mice lacking Cx30 [110] also show subtle behavioral abnormalities on detailed testing (e.g., spatial memory test and open field test). Mice with global loss of Cx30 and targeted deletion of astrocyte Cx43 showed no A/A coupling and reduced hippocampal K+ buffering [39], as well as motor abnormalities including increased falls and crossing time on balance beam and reduced latency to fall on rotorod testing [111]; life expectancy was not notably reduced, [39,111] indicating that loss of A/O and O/O coupling has greater impact on CNS function than does loss of A/A and A/O coupling. In contrast to the modest effects on behavior and normal life expectancy, Lutz et al. [111] found prominent white matter abnormalities in Cx43Astro-Cx30 dKO mice that are similar to those noted in Cx32-Cx47 dKO and Kir4.1 KO mice. In addition, work by Rouach et al. utilizing the Cx30-Cx43Astro dKO mouse demonstrates that A/A channels play a role in activity dependent intercellular trafficking of potassium [112].
Several other CNS connexin double knockout mice have been examined. Magnotti and colleagues [113] investigated the Cx32-Cx43Astro dKO mouse. These mice show white matter vacuolation in the corpus callosum, anterior commissure, and cerebellum as well as progressive loss of cerebellar astrocytes without loss of oligodendrocytes or microglia. They also exhibit increased foot slippage on balance beam by 6 weeks, seizures between 8 and 11 weeks and early mortality with ~85% dying before 20 weeks of age. Using a different promotor (mouse GFAP) than that used for the Magnotti studies (hGFAP) [113] to activate the Cre-recombinase to achieve astrocyte specific knockout, May et al. [114] found activated microglia and reactive gliosis in the corpus callosum and cingulum but not cerebellum, and reduced numbers of Olig2+ cells in the cingulum of Cx32-Cx43Astro mice. They also found that loss of astrocytic Cx43 led to reductions in Cx47 protein levels without changes in Cx47 mRNA suggesting that Cx43 had posttranslational effects on Cx47 localization and stability.
The findings in the Cx47-Cx30 dKO mice are particularly notable [115]. There is a complete loss of O/A coupling but maintenance of O/O coupling. About 40% develop severe motor impairments and die between 42 and 90 days, but the balance live relatively normal spans and have milder motor impairment and action tremors. After p45, all mice showed vacuolation and reduced numbers of myelinated fibers in both the cortex and cerebellum. In addition, the more severely affected mice showed focal areas of tissue damage, gliosis and increased microglial staining. This variability remains unexplained but may reflect variability in the genetic background since the mice used were not fully backcrossed (~85% C57Bl/6) or be due to uncontrolled environmental factors. In contrast, Cx47-Cx43Astro double knockouts show no pathology and have a normal life span [113].
Thus, the order of phenotypic severity of double connexin knockout mice seems to be Cx47/Cx32 > Cx47/30 ≈ Cx32/Cx43 > Cx43/Cx30 ≫ Cx47/Cx43 with no data in the literature about Cx32/Cx30 double knockout. This suggests that loss of all O/O and O/A coupling may have severe developmental effects not seen when some element of O/O coupling is preserved. In addition, complete loss of A/A coupling in itself is apparently less severe than when accompanied by loss of one of the connexins mediating O/A and/or O/O coupling, and partial preservation of O/O, O/A and A/A coupling seems sufficient to prevent the development of an overt phenotype.
4. Manifestations of GJC2 Mutations
Homozygous or compound heterozygous mutations in GJC2 lead to a number of different neurological phenotypes [31]. These include: (1) a severe early onset central nervous system dysmyelinating disorder, Pelizaeus-Merzbacher-like disease (PMLD1 or HLD2) [22]; (2) a milder, later onset disorder, Hereditary Spastic Paraplegia (SPG44) [23]; and (3) a recently described subclinical leukodystrophy [24]. PMLD is named for its similarities to X-linked Pelizaeus-Merzbacher disease (PMD), caused by mutations in PLP1, encoding proteolipoprotein (PLP), the major protein of CNS myelin [116]. Dominant mutations in GJC2 also lead to hereditary (primary) lymphedema [117,118] and some variants in GJC2 are a susceptibility factor for secondary lymphedema after breast cancer surgery [119]. The dominant inheritance pattern of GJC2 associated lymphedema makes it likely that this disorder is due to a gain of function. There is no known overlap between mutations causing CNS disease and those causing lymphedema. Readers interested in further information on the role of GJC2 and other connexins in lymphatic function and lymphedema are referred to a recent review on this topic [120].
5. PMLD (HLD2)
Pelizaeus-Merzbacher-like disease was originally conceptualized as a group of diseases causing a PMD phenotype but lacking PLP1 mutations. However, as discussed below it is now often used to refer exclusively to severe hypomyelinating disease resulting from mutations in GJC2. In 2004 Uhlenberg et al. [22] identified GJC2 mutations in 3 of 6 PMLD families examined. A few years later Henneke and colleagues [121] showed that among patients with a PMD phenotype but lacking mutations in the PLP1 gene, about 8% were found to have biallelic mutations in GJC2. However, patients classified as not GJC2 related may actually carry mutations in portions of the GJC2 gene not sequenced in this study, such as the promotor region [122]. In addition, while significant clinical information about the patients with GJC2 mutations is provided, there is less information about the phenotypes of the GJC2 mutation negative patients. Some PLP1 mutation negative patients might have had additional phenotypic features that, with our expanding understanding of the spectrum of hereditary hypomyelinating disorders, would now result in their being distinguished from those with a PMD phenotype. Thus, it appears that the term PMLD was originally used fairly broadly as a synonym for non-PLP1 associated hereditary leukodystrophy (HLD), but this seems to have fallen out of favor. For example, the original description of patients with HLD3 due to AIMP1/p43 mutations classified these as PMLD. [123] However the use of the term PMLD has been questioned in this context [124,125]. A search for PMLD in OMIM only reveals GJC2 and a single report of SNAP29 mutations [126] (the cause of cerebral dysgenesis, neuropathy, ichthyosis, and keratoderma syndrome (CEDNIK)) as causative of PMLD. The NORD Rare disease database https://rarediseases.org/rare-diseases/pelizaeus-merzbacher-disease/ (accessed on 11 March 2023) separates PMLD (caused by GJC2 mutations) from their list of other diseases that need to be distinguished from PMD. Nonetheless, the term PMLD has also been used recently to describe both an unusual case associated with mutations in NPC1, the gene associated with Niemann Pick disease types C1 and D [127] and a RARS mutation associated hypomyelinating phenotype [128].
Cardinal manifestations of PMLD include nystagmus within the first 6 months of life, cerebellar ataxia by 4 years, and spasticity by 6 years of age [22,129,130,131]. All patients with PMLD have extensive signal changes on MRI consistent with abnormal myelination; relative sparing of the corticospinal tracts and involvement of the brainstem white matter has been reported [22,129,130,131,132,133,134,135]. Axonal damage appears to be less prominent since MRI spectroscopy studies in two patients showed normal or near normal choline, N-acetyl aspartate and creatine levels [131]. (Abnormal N-acetyl aspartate levels indicate axonal damage.) While imaging studies are informative, our understanding of this disorder has been limited by the lack of published autopsy studies of the brains of patients with GJC2 associated diseases.
To date, at least 45 different variants in the coding region of GJC2 have been associated with recessively inherited CNS disease [22,24,121,129,130,131,132,133,136,137,138,139,140,141,142,143,144]. These include missense, nonsense, and frameshift mutations. Two promoter mutations c.-167A>G [122,145,146] and c. -170A>G [147] and one mutation in the 5’ noncoding region at a predicted highly conserved splice site [148] have also been identified.
The advent of the genome aggregation database (gnomAD, https://gnomad.broadinstitute.org/) (accessed on 11 March 2023) and other databases of population genetic variability have reinforced the idea that not all detected variants are likely to be pathogenic. This idea arises because some variants found in these population databases are simply too common to contribute to the disease caused by mutations in those same genes. However, to make such assertions one needs to have a reasonable estimate of the population frequency of the relevant disorder. The prevalence of PMD is thought to range from 1:200,000–1:500,000 in the US [149], with incidence possibly as high as between one [150] and 1.9 per 100,000 male live birth [151]. A recent study from China [137] suggests that PMD is roughly 10 times more common than GJC2 associated disorders. Thus, the incidence of GJC2 associated leukodystrophy is likely no more than 1 in 500,000 births. Thus, for a recessive disorder such as PMLD, any variant appearing with a frequency greater than about 1/7000 alleles would be highly unlikely to be pathogenic, and alleles appearing with a frequency of 1/1000 or more would have to be responsible for at least half of all PMLD. Thus, variants such as p.G146S [138] (in this review, the residue numbering scheme for GJC2 follows that used in Orthmann-Murphy et al. 2007 [69]) and p.T395I [121] are likely not pathogenic.
6. Hereditary Spastic Paraparesis (SPG44)
Orthmann-Murphy et al. [23] described a large family with three affected members carrying a homozygous I33M mutation of GJC2 [23]. Three individuals (two brothers and a cousin) had a phenotype similar to that of other patients with hereditary spastic paraparesis (HSP); GJC2 related HSP is designated as SPG44. All three patients were ambulatory until at least the age of 30 and all three showed spasticity, hyperreflexia, and intention tremor on exam; none had nystagmus. All heterozygous individuals in the family were normal. More recently, two siblings from a second family were described with late onset ataxia, tremor, and pyramidal signs. The symptomatic family members were both found to be homozygous for the p.V271L mutation [152]. In summary, Cx47 associated HSP is a much milder than is PMLD. Consistent with this, white matter changes seen on cerebral MRI are much milder than those seen in PMLD [23].
7. Subclinical Leukodystrophy
At least one patient with a subclinical GJC2 related leukodystrophy has been reported [24]. The patient, who carried a previously unreported homozygous mutation in Cx47, p.R98L, had mild diffuse white matter abnormalities on MRI imaging, but lacked clinical neurological findings.
8. Mechanisms of GJC2 Associated Diseases
All CNS diseases associated with GJC2 are inherited in an autosomal recessive pattern, suggesting that this disorder is characterized by the loss of function of GJC2. However, this alone would not explain the differences in severity seen in different forms of GJC2 related disease and would be discordant with the lack of overt phenotype seen in the Cx47 knockout mouse [48,49]. (We acknowledge that a mouse model is not always able to fully recapitulate all aspects of human disease.) There are several possible reasons why some mutations in GJC2 cause a severe, PMLD phenotype while others do not.
8.1. Mislocalization
Work from our lab in both cell lines and primary oligodendrocyte cultures suggests that both the HSP and PMLD mutants are unable to form normally functioning gap junctions when assessed by dual whole cell recording [23,69,153]. In addition, we found that mutations associated with PMLD lead to loss of normal subcellular localization to gap junctions while a mutation associated with the milder HSP phenotype shows no such mis-localization [23]. Studies from our lab in HeLa and Neuro2a cell lines showed that expression of WT Cx47 or the mild HSP related mutant resulted in intercellular puncta consistent with the formation of gap junction plaques, while Cx47 mutations associated with PMLD resulted in intracellular staining colocalizing with Grp94, an ER marker [69] and few (p.M283T) or no (p.P87S and p.Y269D) intercellular plaques. This suggests the possibility that localization of Cx47 to the gap junction is important independent of the effects on channel function, and that milder mutations represent a partial loss of function (no gap junctional coupling but preserved localization to plaques) while more severe mutations affect both. This hypothesis is also consistent with findings in our studies of the p.R98L variant associated with an extremely mild subclinical leukodystrophy [24]. In that case, the protein localized to intercellular plaques and showed a reduced level of homotypic coupling, while heterotypic coupling with Cx43 was not significantly reduced. The notion that preservation of normal subcellular localization leads to reduced levels of pathogenicity independent of the ability to support gap junction communication is concordant with the observations noted above regarding the importance of Cx47 in proper localization of ZO-1, ZO-1-associated nucleic acid binding proteins (ZONAB), and multi-PDZ domain protein 1 (MUPP1) [86]. However, a study by Kim et al. [68] raises the possibility that differential localization to intercellular plaques is not a feature distinguishing mutations causing more or less severe phenotypes. They examined four variants associated with PMLD and found that these did form plaques but failed to induce intercellular coupling; however, these were GFP tagged, which may have normalized the trafficking. (Unpublished work from my laboratory suggests that the addition of GFP to the C-terminus of mutant forms of Cx32 showing abnormal trafficking rescues their ability to form plaques). Two additional GFP tagged variants, p.G149S and p.T398I showed plaque formation and significant intercellular coupling; this is consistent with the possibility, discussed above, that these are not pathogenic variants.
8.2. Activation of the Unfolded Protein Response (UPR)
Several GJC2 variants associated with PMLD show retention of mutant protein in the ER; this would be expected to cause ER stress and activation of the UPR pathway, but was not demonstrable in our original studies expressing PMLD mutants in HeLa cell lines [69]. We [153] then expressed CX47WT and mutants in primary oligodendrocytes lacking endogenous Cx47; in this system, the three PMLD associated mutants (p.P87S, p.Y269D and p.M283T) show ER retention (see Figure 4) and evidence of activation of the UPR and apoptotic pathways. On the other hand, the milder SPG44 associated mutation (p.I33M) shows a wild-type-like subcellular distribution and no activation of the UPR or apoptotic pathways. Using exogenous expression in MO3.13 oligodendrocyte cell lines, Chen and colleagues have demonstrated that two additional PMLD associated mutations, p.P70S and p.I43M, also triggered ER stress [154].
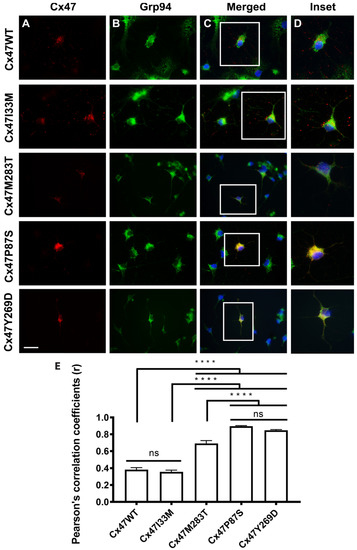
Figure 4.
Expression of Cx47WT and mutants in primary OLs. (A) Cx47 staining (red) for Cx47WT and p.I33M show a punctate staining pattern while PMLD associated mutations show diffuse cytoplasmic staining (p.P87S, p.Y269D) or both punctate and diffuse staining (p.M283T). Empty vector control showed no Cx47 staining. (B) Staining for GRP94 (green), a marker of the ER compartment. (C) Staining for Cx47 (red), GRP94 (green) and DAPI (blue) shows colocalization of p.P87S and p.Y269D and slightly reduced colocalization of GRP94 with p.M283T. Scale bar = 25 μm. (D) Insets of indicated areas in C. (E) Quantitation of colocalization with Pearson’s correlation coefficient. ANOVA p < 0.05, Tukey post-hoc test, **** p < 0.0001, ns = not statistically significant. Figure and legend from Flores-Obando 2022 [153] used with permission of Elsevier. Copyright © 2023.
8.3. Differential Interactions with Other Connexin Isoforms
Another possibility is that in GJC2 related disorders, interactions between mutant forms of Cx47 and wild-type Cx32 may contribute to the disease phenotypes. For example, the greater severity of mutations causing the more severe PMLD phenotype might be due to abnormal interactions with Cx32, not seen with Cx47WT or the mutation causing the milder HSP phenotype. Such interactions might produce a situation where both Cx32 and Cx47 function was reduced creating a severe phenotype such as that seen in the Cx32-Cx47 dKO mouse described above [48,49]. To examine the possibility that differential heteromeric interactions between Cx32 and the p.I33M, p.P87S, p.Y269D, and p.M283T mutants of Cx47 might explain the different severities of disease caused by these mutations, we examined pairs of cells wherein one cell was expressing both Cx32 and one of these Cx47 mutants, and the other cell expressed Cx30 or Cx32 alone [63]. We saw no evidence of a quantitative or qualitative effect of the expression of the mutant on the magnitudes of coupling or the normalized conductance voltage relations produced by these channels. Colocalization of Cx32 and Cx47 mutants would be a prerequisite for the formation of heteromeric channels. Therefore, we examined the distribution of Cx32 and a mutant form of Cx47 when co-expressed in cell pairs. As shown in Figure 2, and noted above, when we expressed Cx32 and Cx30 in the same cell, these two closely related connexins showed significant overlap in staining. (Pearson correlation coefficient was almost 0.5). This finding is consistent with the known heteromeric interactions between Cx30 and Cx32 [155]. When Cx32WT was co-expressed with Cx47WT the correlation coefficient was close to 0, while three of the mutants showed a slight, non-significant negative correlation with Cx32; however, the p.Y269D mutant showed a significant negative correlation with Cx32, suggesting that the overlap of this connexin with Cx32 is less than that predicted by chance. Examples of planar confocal images representative of those used for these studies are shown in Figure 3.
9. Conclusions
This review highlights the fact that we still have much to learn about the roles of connexins in the central nervous system in normal and pathological states. In particular, I have focused on the role of mutations in GJC2, the gene encoding Cx47, in hypomyelinating diseases of the CNS. There is still much to learn about these disorders, and progress will likely require the development of more robust cellular or animal model systems for the study of these diseases. As noted above, the Cx47 KO mouse has a very mild phenotype, and the phenotype of the only current PMLD “knockin” mouse is also not very robust. It is likely that further progress in our understanding of these disorders will require the use of other model systems such as differentiated human stem cells expressing the relevant mutant proteins. The establishment of such a system should also allow for better evaluation of treatments for these disorders.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Underlying data from the author’s lab is available upon reasonable request.
Conflicts of Interest
The author declares no conflict of interest.
References
- Kumar, N.M.; Gilula, N.B. The gap junction communication channel. Cell 1996, 84, 381–388. [Google Scholar] [CrossRef]
- Mugnaini, E. Cell junctions of astrocytes, ependyma, and related cells in the mammalian central nervous system, with emphasis on the hypothesis of a generalized functional syncytium of supporting cells. In Astrocytes, Vol I; Fedoroff, S., Vernadakis, A., Eds.; Academic Press: New York, NY, USA, 1986; pp. 329–371. [Google Scholar]
- Scemes, E.; Giaume, C. Astrocyte calcium waves: What they are and what they do. Glia 2006, 54, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Kamasawa, N.; Sik, A.; Morita, M.; Yasumura, T.; Davidson, K.G.; Nagy, J.I.; Rash, J.E. Connexin-47 and connexin-32 in gap junctions of oligodendrocyte somata, myelin sheaths, paranodal loops and Schmidt-Lanterman incisures: Implications for ionic homeostasis and potassium siphoning. Neuroscience 2005, 136, 65–86. [Google Scholar] [CrossRef] [PubMed]
- Rash, J.E. Molecular disruptions of the panglial syncytium block potassium siphoning and axonal saltatory conduction: Pertinence to neuromyelitis optica and other demyelinating diseases of the central nervous system. Neuroscience 2010, 168, 982–1008. [Google Scholar] [CrossRef] [PubMed]
- Rash, J.E.; Vanderpool, K.G.; Yasumura, T.; Hickman, J.; Beatty, J.T.; Nagy, J.I. KV1 channels identified in rodent myelinated axons, linked to Cx29 in innermost myelin: Support for electrically active myelin in mammalian saltatory conduction. J. Neurophysiol. 2016, 115, 1836–1859. [Google Scholar] [CrossRef] [PubMed]
- Menichella, D.M.; Majdan, M.; Awatramani, R.; Goodenough, D.A.; Sirkowski, E.; Scherer, S.S.; Paul, D.L. Genetic and physiological evidence that oligodendrocyte gap junctions contribute to spatial buffering of potassium released during neuronal activity. J. Neurosci. 2006, 26, 10984–10991. [Google Scholar] [CrossRef]
- Ye, Z.C.; Wyeth, M.S.; Baltan-Tekkok, S.; Ransom, B.R. Functional hemichannels in astrocytes: A novel mechanism of glutamate release. J. Neurosci. 2003, 23, 3588–3596. [Google Scholar] [CrossRef]
- Quan, Y.; Du, Y.; Wu, C.; Gu, S.; Jiang, J.X. Connexin hemichannels regulate redox potential via metabolite exchange and protect lens against cellular oxidative damage. Redox Biol. 2021, 46, 102102. [Google Scholar] [CrossRef]
- Dale, N. Dynamic ATP signalling and neural development. J. Physiol. 2008, 586, 2429–2436. [Google Scholar] [CrossRef]
- Stout, C.E.; Costantin, J.L.; Naus, C.C.; Charles, A.C. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J. Biol. Chem. 2002, 277, 10482–10488. [Google Scholar] [CrossRef]
- Orellana, J.A.; Froger, N.; Ezan, P.; Jiang, J.X.; Bennett, M.V.; Naus, C.C.; Giaume, C.; Saez, J.C. ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J. Neurochem. 2011, 118, 826–840. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Dringen, R. Gap junction hemichannel-mediated release of glutathione from cultured rat astrocytes. Neurosci. Lett. 2007, 415, 45–48. [Google Scholar] [CrossRef] [PubMed]
- O’Carroll, S.J.; Alkadhi, M.; Nicholson, L.F.; Green, C.R. Connexin 43 mimetic peptides reduce swelling, astrogliosis, and neuronal cell death after spinal cord injury. Cell Commun. Adhes 2008, 15, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Sohl, G.; Willecke, K. Gap junctions and the connexin protein family. Cardiovasc. Res. 2004, 62, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Paznekas, W.A.; Boyadjiev, S.A.; Shapiro, R.E.; Daniels, O.; Wollnik, B.; Keegan, C.E.; Innis, J.W.; Dinulos, M.B.; Christian, C.; Hannibal, M.C.; et al. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Amer. J. Hum. Genet. 2003, 72, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Gorlin, R.J.; Meskin, L.H.; St. Geme, J.W. Oculodentodigital dysplasia. J. Ped. 1963, 63, 69–75. [Google Scholar] [CrossRef]
- Loddenkemper, T.; Grote, K.; Evers, S.; Oelerich, M.; Stogbauer, F. Neurological manifestations of the oculodentodigital dysplasia syndrome. J. Neurol. 2002, 249, 584–595. [Google Scholar] [CrossRef]
- Stanislaw, C.L.; Narvaez, C.; Roger, R.G.; Woodard, C.S. Oculodentodigital dysplasia with cerebral white matter abnormalies: An additional case. Proc. Greenwood Genet. Center 1998, 17, 20–24. [Google Scholar]
- Schrander-Stumpel, C.T.; Franke, C.L. Central nervous system abnormalities in oculodentodigital dysplasia. Genet. Couns. 1996, 7, 233–235. [Google Scholar]
- Cox, D.R.; DiSalvo, M.; Hall, B.D. Neurologic abnormalities in oculodentodigital dysplasia: A new finding. Clin. Res. 1978, 26, 193A. [Google Scholar]
- Uhlenberg, B.; Schuelke, M.; Ruschendorf, F.; Ruf, N.; Kaindl, A.M.; Henneke, M.; Thiele, H.; Stoltenburg-Didinger, G.; Aksu, F.; Topaloglu, H.; et al. Mutations in the gene encoding gap junction protein alpha 12 (connexin 46.6) cause Pelizaeus-Merzbacher-like disease. Am. J. Hum. Genet. 2004, 75, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Orthmann-Murphy, J.L.; Salsano, E.; Abrams, C.K.; Bizzi, A.; Uziel, G.; Freidin, M.M.; Lamantea, E.; Zeviani, M.; Scherer, S.S.; Pareyson, D. Hereditary spastic paraplegia is a novel phenotype for GJA12/GJC2 mutations. Brain 2009, 132, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Abrams, C.K.; Scherer, S.S.; Flores-Obando, R.; Freidin, M.M.; Wong, S.; Lamantea, E.; Farina, L.; Scaioli, V.; Pareyson, D.; Salsano, E. A new mutation in GJC2 associated with subclinical leukodystrophy. J. Neurol. 2014, 261, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Taşdelen, E.; Durmaz, C.D.; Karabulut, H.G. Autosomal Recessive Oculodentodigital Dysplasia: A Case Report and Review of the Literature. Cytogenet. Genome Res. 2018, 154, 181–186. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, L.; Wang, P.; Chen, C.; Zhang, A.; Wang, W.; Ding, X. Novel ocular findings in oculodentodigital dysplasia (ODDD): A case report and literature review. Ophthalmic Genet. 2019, 40, 54–59. [Google Scholar] [CrossRef]
- Choi, J.; Yang, A.; Song, A.; Lim, M.; Kim, J.; Jang, J.H.; Park, K.T.; Cho, S.; Jin, D.K. Oculodentodigital Dysplasia with a Novel Mutation in GJA1 Diagnosed by Targeted Gene Panel Sequencing: A Case Report and Literature Review. Ann. Clin. Lab. Sci. 2018, 48, 776–781. [Google Scholar]
- De Bock, M.; Kerrebrouck, M.; Wang, N.; Leybaert, L. Neurological manifestations of oculodentodigital dysplasia: A Cx43 channelopathy of the central nervous system? Front. Pharmacol. 2013, 4, 120. [Google Scholar] [CrossRef]
- Abrams, C.K.; Scherer, S.S. Gap junctions in inherited human disorders of the central nervous system. Biochim. Biophys. Acta. 2012, 1818, 2030–2047. [Google Scholar] [CrossRef]
- Abrams, C.K. GJB1 Disorders: Charcot-Marie-Tooth Neuropathy (CMT1X) and Central Nervous System Phenotypes. In GeneReviews(®); Adam, M.P., Everman, D.B., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Abrams, C.K. Diseases of connexins expressed in myelinating glia. Neurosci. Lett. 2019, 695, 91–99. [Google Scholar] [CrossRef]
- Srinivas, M.; Verselis, V.K.; White, T.W. Human diseases associated with connexin mutations. Biochim. Biophys. Acta Biomembr. 2018, 1860, 192–201. [Google Scholar] [CrossRef]
- Delmar, M.; Laird, D.W.; Naus, C.C.; Nielsen, M.S.; Verselis, V.K.; White, T.W. Connexins and Disease. Cold Spring Harb. Perspect. Biol. 2018, 10, a029348. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.W.; Lampe, P.D. Cellular mechanisms of connexin-based inherited diseases. Trends Cell Biol. 2022, 32, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zheng, J.; Chen, S.; Sun, Y. Connexin Mutations and Hereditary Diseases. Int. J. Mol. Sci. 2022, 23, 4255. [Google Scholar] [CrossRef] [PubMed]
- Orthmann-Murphy, J.L.; Freidin, M.; Fischer, E.; Scherer, S.S.; Abrams, C.K. Two distinct heterotypic channels mediate gap junction coupling between astrocyte and oligodendrocyte connexins. J. Neurosci. 2007, 27, 13949–13957. [Google Scholar] [CrossRef] [PubMed]
- Magnotti, L.M.; Goodenough, D.A.; Paul, D.L. Functional heterotypic interactions between astrocyte and oligodendrocyte connexins. Glia 2011, 59, 26–34. [Google Scholar] [CrossRef]
- Clasadonte, J.; Scemes, E.; Wang, Z.; Boison, D.; Haydon, P.G. Connexin 43-Mediated Astroglial Metabolic Networks Contribute to the Regulation of the Sleep-Wake Cycle. Neuron 2017, 95, 1365–1380. [Google Scholar] [CrossRef]
- Wallraff, A.; Kohling, R.; Heinemann, U.; Theis, M.; Willecke, K.; Steinhauser, C. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J. Neurosci. 2006, 26, 5438–5447. [Google Scholar] [CrossRef]
- Nagy, J.I.; Patel, D.; Ochalski, P.A.; Stelmack, G.L. Connexin30 in rodent, cat and human brain: Selective expression in gray matter astrocytes, co-localization with connexin43 at gap junctions and late developmental appearance. Neuroscience 1999, 88, 447–468. [Google Scholar] [CrossRef]
- Nagy, J.I.; Ionescu, A.V.; Lynn, B.D.; Rash, J.E. Coupling of astrocyte connexins Cx26, Cx30, Cx43 to oligodendrocyte Cx29, Cx32, Cx47: Implications from normal and connexin32 knockout mice. Glia 2003, 44, 205–218. [Google Scholar] [CrossRef]
- Rash, J.E.; Yasumura, T.; Dudek, F.E.; Nagy, J.I. Cell-specific expression of connexins and evidence of restricted gap junctional coupling between glial cells and between neurons. J. Neurosci. 2001, 21, 1983–2000. [Google Scholar] [CrossRef]
- Altevogt, B.M.; Paul, D.L. Four classes of intercellular channels between glial cells in the CNS. J. Neurosci. 2004, 24, 4313–4323. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.I.; Li, X.; Rempel, J.; Stelmack, G.; Patel, D.; Staines, W.A.; Yasumura, T.; Rash, J.E. Connexin26 in adult rodent central nervous system: Demonstration at astrocytic gap junctions and colocalization with connexin30 and connexin43. J. Comp. Neurol. 2001, 441, 302–323. [Google Scholar] [CrossRef] [PubMed]
- Filippov, M.A.; Hormuzdi, S.G.; Fuchs, E.C.; Monyer, H. A reporter allele for investigating connexin 26 gene expression in the mouse brain. Eur. J. Neurosci. 2003, 18, 3183–3192. [Google Scholar] [CrossRef]
- Wingard, J.C.; Zhao, H.B. Cellular and Deafness Mechanisms Underlying Connexin Mutation-Induced Hearing Loss - A Common Hereditary Deafness. Front. Cell. Neurosci. 2015, 9, 202. [Google Scholar] [CrossRef]
- Lilly, E.; Sellitto, C.; Milstone, L.M.; White, T.W. Connexin channels in congenital skin disorders. Semin. Cell Dev. Biol. 2016, 50, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Odermatt, B.; Wellershaus, K.; Wallraff, A.; Seifert, G.; Degen, J.; Euwens, C.; Fuss, B.; Bussow, H.; Schilling, K.; Steinhauser, C.; et al. Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. J. Neurosci. 2003, 23, 4549–4559. [Google Scholar] [CrossRef] [PubMed]
- Menichella, D.M.; Goodenough, D.A.; Sirkowski, E.; Scherer, S.S.; Paul, D.L. Connexins are critical for normal myelination in the CNS. J. Neurosci. 2003, 23, 5963–5973. [Google Scholar] [CrossRef]
- Kleopa, K.A.; Orthmann, J.L.; Enriquez, A.; Paul, D.L.; Scherer, S.S. Unique distributions of the gap junction proteins connexin29, connexin32, and connexin47 in oligodendrocytes. Glia 2004, 47, 346–357. [Google Scholar] [CrossRef]
- Barrio, L.C.; Suchyna, T.; Bargiello, T.; Xu, L.X.; Roginski, R.S.; Bennett, M.V.; Nicholson, B.J. Gap junctions formed by connexins 26 and 32 alone and in combination are differently affected by applied voltage. Proc. Natl. Acad. Sci. USA 1991, 88, 8410–8414, erratum in Proc. Natl. Acad. Sci. USA 1992 89, 4220. [Google Scholar] [CrossRef]
- Teubner, B.; Odermatt, B.; Guldenagel, M.; Sohl, G.; Degen, J.; Bukauskas, F.; Kronengold, J.; Verselis, V.K.; Jung, Y.T.; Kozak, C.A.; et al. Functional expression of the new gap junction gene connexin47 transcribed in mouse brain and spinal cord neurons. J. Neurosci. 2001, 21, 1117–1126. [Google Scholar] [CrossRef]
- Altevogt, B.M.; Kleopa, K.A.; Postma, F.R.; Scherer, S.S.; Paul, D.L. Connexin29 is uniquely distributed within myelinating glial cells of the central and peripheral nervous systems. J. Neurosci. 2002, 22, 6458–6470. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.B.; Ichikawa, H.; Bechberger, J.F.; Valiunas, V.; Ohki, M.; Naus, C.C.; Kunimoto, T.; Tsuda, H.; Miller, W.T.; Goldberg, G.S. Normal cells control the growth of neighboring transformed cells independent of gap junctional communication and SRC activity. Cancer Res. 2004, 64, 1347–1358. [Google Scholar] [CrossRef]
- Nagy, J.I.; Ionescu, A.V.; Lynn, B.D.; Rash, J.E. Connexin29 and connexin32 at oligodendrocyte and astrocyte gap junctions and in myelin of the mouse central nervous system. J. Comp. Neurol. 2003, 464, 356–370. [Google Scholar] [CrossRef]
- Ahn, M.; Lee, J.; Gustafsson, A.; Enriquez, A.; Lancaster, E.; Sul, J.Y.; Haydon, P.G.; Paul, D.L.; Huang, Y.; Abrams, C.K.; et al. Cx29 and Cx32, two connexins expressed by myelinating glia, do not interact and are functionally distinct. J. Neurosci. Res. 2008, 86, 992–1006. [Google Scholar] [CrossRef]
- Sargiannidou, I.; Ahn, M.; Enriquez, A.D.; Peinado, A.; Reynolds, R.; Abrams, C.; Scherer, S.S.; Kleopa, K.A. Human oligodendrocytes express Cx31.3: Function and interactions with Cx32 mutants. Neurobiol. Dis. 2008, 30, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Scherer, S.S.; Deschenes, S.M.; Xu, Y.T.; Grinspan, J.B.; Fischbeck, K.H.; Paul, D.L. Connexin32 is a myelin-related protein in the PNS and CNS. J. Neurosci. 1995, 15, 8281–8294. [Google Scholar] [CrossRef] [PubMed]
- Massa, P.T.; Mugnaini, E. Cell junctions and intramembrane particles of astrocytes and oligodendrocytes: A freeze-fracture study. Neuroscience 1982, 7, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Massa, P.T.; Mugnaini, E. Cell-cell junctional interactions and characteristic plasma membrane features of cultured rat glial cells. Neuroscience 1985, 14, 695–709. [Google Scholar] [CrossRef]
- Wasseff, S.K.; Scherer, S.S. Cx32 and Cx47 mediate oligodendrocyte: Astrocyte and oligodendrocyte:oligodendrocyte gap junction coupling. Neurobiol. dis. 2011, 42, 506–513. [Google Scholar] [CrossRef]
- Maglione, M.; Tress, O.; Haas, B.; Karram, K.; Trotter, J.; Willecke, K.; Kettenmann, H. Oligodendrocytes in mouse corpus callosum are coupled via gap junction channels formed by connexin47 and connexin32. Glia 2010, 58, 1104–1117. [Google Scholar] [CrossRef]
- Abrams, C.K.; Flores-Obando, R.E.; Dungan, G.D.; Cherepanova, E.; Freidin, M.M. Investigating oligodendrocyte connexins: Heteromeric interactions between Cx32 and mutant or wild-type forms of Cx47 do not contribute to or modulate gap junction function. Glia 2021, 69, 1882–1896. [Google Scholar] [CrossRef] [PubMed]
- Falk, M.M. Connexin-specific distribution within gap junctions revealed in living cells. J. Cell. Sci. 2000, 113 Pt 22, 4109–4120. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.; Chodock, R.; Hand, A.R.; Laird, D.W. The origin of annular junctions: A mechanism of gap junction internalization. J. Cell Sci. 2001, 114, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Carette, D.; Gilleron, J.; Denizot, J.-P.; Grant, K.; Pointis, G.; Segretain, D. New cellular mechanisms of gap junction degradation and recycling. Biol. Cell 2015, 107, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Lynn, B.D.; Tress, O.; May, D.; Willecke, K.; Nagy, J.I. Ablation of connexin30 in transgenic mice alters expression patterns of connexin26 and connexin32 in glial cells and leptomeninges. Eur. J. Neurosci. 2011, 34, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Gloor, G.B.; Bai, D. The distribution and functional properties of Pelizaeus-Merzbacher-like disease-linked Cx47 mutations on Cx47/Cx47 homotypic and Cx47/Cx43 heterotypic gap junctions. Biochem. J. 2013, 452, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Orthmann-Murphy, J.L.; Enriquez, A.D.; Abrams, C.K.; Scherer, S.S. Loss-of-function GJA12/Connexin47 mutations cause Pelizaeus-Merzbacher-like disease. Mol. Cell Neurosci. 2007, 34, 629–641. [Google Scholar] [CrossRef]
- Fasciani, I.; Pluta, P.; González-Nieto, D.; Martínez-Montero, P.; Molano, J.; Paíno, C.L.; Millet, O.; Barrio, L.C. Directional coupling of oligodendrocyte connexin-47 and astrocyte connexin-43 gap junctions. Glia 2018, 66, 2340–2352. [Google Scholar] [CrossRef]
- Anzini, P.; Neuberg, D.H.; Schachner, M.; Nelles, E.; Willecke, K.; Zielasek, J.; Toyka, K.V.; Suter, U.; Martini, R. Structural abnormalities and deficient maintenance of peripheral nerve myelin in mice lacking the gap junction protein connexin 32. J. Neurosci. 1997, 17, 4545–4551. [Google Scholar] [CrossRef]
- Scherer, S.S.; Xu, Y.T.; Nelles, E.; Fischbeck, K.; Willecke, K.; Bone, L.J. Connexin32-null mice develop demyelinating peripheral neuropathy. Glia 1998, 24, 8–20. [Google Scholar] [CrossRef]
- Vavlitou, N.; Sargiannidou, I.; Markoullis, K.; Kyriacou, K.; Scherer, S.S.; Kleopa, K.A. Axonal pathology precedes demyelination in a mouse model of X-linked demyelinating/type I Charcot-Marie Tooth neuropathy. J. Neuropathol. Exp. Neurol. 2010, 69, 945–958. [Google Scholar] [CrossRef]
- Sutor, B.; Schmolke, C.; Teubner, B.; Schirmer, C.; Willecke, K. Myelination defects and neuronal hyperexcitability in the neocortex of connexin 32-deficient mice. Cereb. Cortex. 2000, 10, 684–697. [Google Scholar] [CrossRef]
- Sargiannidou, I.; Vavlitou, N.; Aristodemou, S.; Hadjisavvas, A.; Kyriacou, K.; Scherer, S.S.; Kleopa, K.A. Connexin32 mutations cause loss of function in Schwann cells and oligodendrocytes leading to PNS and CNS myelination defects. J. Neurosci. 2009, 29, 4736–4749. [Google Scholar] [CrossRef]
- Markoullis, K.; Sargiannidou, I.; Gardner, C.; Hadjisavvas, A.; Reynolds, R.; Kleopa, K.A. Disruption of oligodendrocyte gap junctions in experimental autoimmune encephalomyelitis. Glia 2012, 60, 1053–1066. [Google Scholar] [CrossRef]
- Papaneophytou, C.P.; Georgiou, E.; Karaiskos, C.; Sargiannidou, I.; Markoullis, K.; Freidin, M.M.; Abrams, C.K.; Kleopa, K.A. Regulatory role of oligodendrocyte gap junctions in inflammatory demyelination. Glia 2018, 66, 2589–2603. [Google Scholar] [CrossRef]
- Olympiou, M.; Sargiannidou, I.; Markoullis, K.; Karaiskos, C.; Kagiava, A.; Kyriakoudi, S.; Abrams, C.K.; Kleopa, K.A. Systemic inflammation disrupts oligodendrocyte gap junctions and induces ER stress in a model of CNS manifestations of X-linked Charcot-Marie-Tooth disease. Acta. Neuropathol. Commun. 2016, 4, 95. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulos, F.; Georgiou, E.; Sargiannidou, I.; Kleopa, K.A. Dysregulation of Blood-Brain Barrier and Exacerbated Inflammatory Response in Cx47-Deficient Mice after Induction of EAE. Pharmaceuticals 2021, 14, 621. [Google Scholar] [CrossRef] [PubMed]
- Giepmans, B.N.; Moolenaar, W.H. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr. Biol. 1998, 8, 931–934. [Google Scholar] [CrossRef]
- Kausalya, P.J.; Reichert, M.; Hunziker, W. Connexin45 directly binds to ZO-1 and localizes to the tight junction region in epithelial MDCK cells. FEBS Lett. 2001, 505, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.A.; Baruch, A.; Shestopalov, V.I.; Giepmans, B.N.; Dunia, I.; Benedetti, E.L.; Kumar, N.M. Lens connexins alpha3Cx46 and alpha8Cx50 interact with zonula occludens protein-1 (ZO-1). Mol. Biol. Cell 2003, 14, 2470–2481. [Google Scholar] [CrossRef]
- Li, X.; Ionescu, A.V.; Lynn, B.D.; Lu, S.; Kamasawa, N.; Morita, M.; Davidson, K.G.; Yasumura, T.; Rash, J.E.; Nagy, J.I. Connexin47, connexin29 and connexin32 co-expression in oligodendrocytes and Cx47 association with zonula occludens-1 (ZO-1) in mouse brain. Neuroscience 2004, 126, 611–630. [Google Scholar] [CrossRef] [PubMed]
- Penes, M.C.; Li, X.; Nagy, J.I. Expression of zonula occludens-1 (ZO-1) and the transcription factor ZO-1-associated nucleic acid-binding protein (ZONAB)-MsY3 in glial cells and colocalization at oligodendrocyte and astrocyte gap junctions in mouse brain. Eur. J. Neurosci. 2005, 22, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Stout, C.; Goodenough, D.A.; Paul, D.L. Connexins: Functions without junctions. Curr. Opin. Cell Biol. 2004, 16, 507–512. [Google Scholar] [CrossRef]
- Li, X.; Penes, M.; Odermatt, B.; Willecke, K.; Nagy, J.I. Ablation of Cx47 in transgenic mice leads to the loss of MUPP1, ZONAB and multiple connexins at oligodendrocyte-astrocyte gap junctions. Eur. J. Neurosci. 2008, 28, 1503–1517. [Google Scholar] [CrossRef] [PubMed]
- Balda, M.S.; Matter, K. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. Embo. J. 2000, 19, 2024–2033. [Google Scholar] [CrossRef]
- Flores, A.I.; Mallon, B.S.; Matsui, T.; Ogawa, W.; Rosenzweig, A.; Okamoto, T.; Macklin, W.B. Akt-mediated survival of oligodendrocytes induced by neuregulins. J. Neurosci. 2000, 20, 7622–7630. [Google Scholar] [CrossRef]
- Park, S.K.; Miller, R.; Krane, I.; Vartanian, T. The erbB2 gene is required for the development of terminally differentiated spinal cord oligodendrocytes. J. Cell Biol. 2001, 154, 1245–1258. [Google Scholar] [CrossRef]
- Kim, J.Y.; Sun, Q.; Oglesbee, M.; Yoon, S.O. The role of ErbB2 signaling in the onset of terminal differentiation of oligodendrocytes in vivo. J. Neurosci. 2003, 23, 5561–5571. [Google Scholar] [CrossRef]
- Balda, M.S.; Garrett, M.D.; Matter, K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J. Cell Biol. 2003, 160, 423–432. [Google Scholar] [CrossRef]
- Sourisseau, T.; Georgiadis, A.; Tsapara, A.; Ali, R.R.; Pestell, R.; Matter, K.; Balda, M.S. Regulation of PCNA and cyclin D1 expression and epithelial morphogenesis by the ZO-1-regulated transcription factor ZONAB/DbpA. Mol. Cell Biol. 2006, 26, 2387–2398. [Google Scholar] [CrossRef]
- Neusch, C.; Rozengurt, N.; Jacobs, R.E.; Lester, H.A.; Kofuji, P. Kir4.1 potassium channel subunit is crucial for oligodendrocyte development and in vivo myelination. J. Neurosci. 2001, 21, 5429–5438. [Google Scholar] [CrossRef]
- Higashi, K.; Fujita, A.; Inanobe, A.; Tanemoto, M.; Doi, K.; Kubo, T.; Kurachi, Y. An inwardly rectifying K(+) channel, Kir4.1, expressed in astrocytes surrounds synapses and blood vessels in brain. Am. J. Physiol. Cell Physiol. 2001, 281, C922–C931. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Head, V.; Timpe, L.C. Identification of an inward rectifier potassium channel gene expressed in mouse cortical astrocytes. Glia 2001, 33, 57–71. [Google Scholar] [CrossRef]
- Sohl, G.; Eiberger, J.; Jung, Y.T.; Kozak, C.A.; Willecke, K. The mouse gap junction gene connexin29 is highly expressed in sciatic nerve and regulated during brain development. Biol. Chem. 2001, 382, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Eiberger, J.; Kibschull, M.; Strenzke, N.; Schober, A.; Bussow, H.; Wessig, C.; Djahed, S.; Reucher, H.; Koch, D.A.; Lautermann, J.; et al. Expression pattern and functional characterization of connexin29 in transgenic mice. Glia 2006, 53, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhang, Y.; Chang, Q.; Ahmad, S.; Dahlke, I.; Yi, H.; Chen, P.; Paul, D.L.; Lin, X. Connexin29 is highly expressed in cochlear Schwann cells, and it is required for the normal development and function of the auditory nerve of mice. J. Neurosci. 2006, 26, 1991–1999. [Google Scholar] [CrossRef] [PubMed]
- Adadey, S.M.; Esoh, K.K.; Quaye, O.; Amedofu, G.K.; Awandare, G.A.; Wonkam, A. GJB4 and GJC3 variants in non-syndromic hearing impairment in Ghana. Exp. Biol. Med. (Maywood) 2020, 245, 1355–1367. [Google Scholar] [CrossRef]
- Wang, W.H.; Yang, J.J.; Lin, Y.C.; Yang, J.T.; Chan, C.H.; Li, S.Y. Identification of novel variants in the Cx29 gene of nonsyndromic hearing loss patients using buccal cells and restriction fragment length polymorphism method. Audiol. Neurootol. 2010, 15, 81–87. [Google Scholar] [CrossRef]
- Yang, J.J.; Huang, S.H.; Chou, K.H.; Liao, P.J.; Su, C.C.; Li, S.Y. Identification of mutations in members of the connexin gene family as a cause of nonsyndromic deafness in Taiwan. Audiol. Neurootol. 2007, 12, 198–208. [Google Scholar] [CrossRef]
- Wong, S.H.; Wang, W.H.; Chen, P.H.; Li, S.Y.; Yang, J.J. Functional analysis of a nonsyndromic hearing loss-associated mutation in the transmembrane II domain of the GJC3 gene. Int. J. Med. Sci. 2017, 14, 246–256. [Google Scholar] [CrossRef]
- Lee, H.J.; Jeong, H.; Hyun, J.; Ryu, B.; Park, K.; Lim, H.H.; Yoo, J.; Woo, J.S. Cryo-EM structure of human Cx31.3/GJC3 connexin hemichannel. Sci. Adv. 2020, 6, eaba4996. [Google Scholar] [CrossRef] [PubMed]
- Su, C.C.; Li, S.Y.; Yen, Y.C.; Nian, J.H.; Liang, W.G.; Yang, J.J. Mechanism of two novel human GJC3 missense mutations in causing non-syndromic hearing loss. Cell Biochem. Biophys. 2013, 66, 277–286. [Google Scholar] [CrossRef]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic. Acids. Res. 2018, 46, D1062–D1067. [Google Scholar] [CrossRef] [PubMed]
- Adadey, S.M.; Wonkam-Tingang, E.; Twumasi Aboagye, E.; Nayo-Gyan, D.W.; Boatemaa Ansong, M.; Quaye, O.; Awandare, G.A.; Wonkam, A. Connexin Genes Variants Associated with Non-Syndromic Hearing Impairment: A Systematic Review of the Global Burden. Life 2020, 10, 258. [Google Scholar] [CrossRef] [PubMed]
- Reaume, A.G.; De Sousa, P.A.; Kulkarni, S.; Langille, B.L.; Zhu, D.; Davies, T.C.; Juneja, S.C.; Kidder, G.M.; Rossant, J. Cardiac malformation in neonatal mice lacking connexin43. Science 1995, 267, 1831–1834. [Google Scholar] [CrossRef]
- Frisch, C.; Theis, M.; De Souza Silva, M.A.; Dere, E.; Sohl, G.; Teubner, B.; Namestkova, K.; Willecke, K.; Huston, J.P. Mice with astrocyte-directed inactivation of connexin43 exhibit increased exploratory behaviour, impaired motor capacities, and changes in brain acetylcholine levels. Eur. J. Neurosci. 2003, 18, 2313–2318. [Google Scholar] [CrossRef]
- Theis, M.; Jauch, R.; Zhuo, L.; Speidel, D.; Wallraff, A.; Doring, B.; Frisch, C.; Sohl, G.; Teubner, B.; Euwens, C.; et al. Accelerated hippocampal spreading depression and enhanced locomotory activity in mice with astrocyte-directed inactivation of connexin43. J. Neurosci. 2003, 23, 766–776. [Google Scholar] [CrossRef]
- Dere, E.; De Souza-Silva, M.A.; Frisch, C.; Teubner, B.; Sohl, G.; Willecke, K.; Huston, J.P. Connexin30-deficient mice show increased emotionality and decreased rearing activity in the open-field along with neurochemical changes. Eur. J. Neurosci. 2003, 18, 629–638. [Google Scholar] [CrossRef]
- Lutz, S.E.; Zhao, Y.; Gulinello, M.; Lee, S.C.; Raine, C.S.; Brosnan, C.F. Deletion of astrocyte connexins 43 and 30 leads to a dysmyelinating phenotype and hippocampal CA1 vacuolation. J. Neurosci. 2009, 29, 7743–7752. [Google Scholar] [CrossRef]
- Rouach, N.; Koulakoff, A.; Abudara, V.; Willecke, K.; Giaume, C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science 2008, 322, 1551–1555. [Google Scholar] [CrossRef]
- Magnotti, L.M.; Goodenough, D.A.; Paul, D.L. Deletion of oligodendrocyte Cx32 and astrocyte Cx43 causes white matter vacuolation, astrocyte loss and early mortality. Glia 2011, 59, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- May, D.; Tress, O.; Seifert, G.; Willecke, K. Connexin47 protein phosphorylation and stability in oligodendrocytes depend on expression of Connexin43 protein in astrocytes. J. Neurosci. 2013, 33, 7985–7996. [Google Scholar] [CrossRef] [PubMed]
- Tress, O.; Maglione, M.; May, D.; Pivneva, T.; Richter, N.; Seyfarth, J.; Binder, S.; Zlomuzica, A.; Seifert, G.; Theis, M.; et al. Panglial gap junctional communication is essential for maintenance of myelin in the CNS. J. Neurosci. 2012, 32, 7499–7518. [Google Scholar] [CrossRef] [PubMed]
- Garbern, J.Y. Pelizaeus-Merzbacher disease: Genetic and cellular pathogenesis. Cell Mol. Life Sci. 2007, 64, 50–65. [Google Scholar] [CrossRef]
- Ferrell, R.E.; Baty, C.J.; Kimak, M.A.; Karlsson, J.M.; Lawrence, E.C.; Franke-Snyder, M.; Meriney, S.D.; Feingold, E.; Finegold, D.N. GJC2 missense mutations cause human lymphedema. Am. J. Hum. Genet. 2010, 86, 943–948. [Google Scholar] [CrossRef]
- Michelini, S.; Vettori, A.; Maltese, P.E.; Cardone, M.; Bruson, A.; Fiorentino, A.; Cappellino, F.; Sainato, V.; Guerri, G.; Marceddu, G.; et al. Genetic Screening in a Large Cohort of Italian Patients Affected by Primary Lymphedema Using a Next Generation Sequencing (NGS) Approach. Lymphology 2016, 49, 57–72. [Google Scholar]
- Finegold, D.N.; Baty, C.J.; Knickelbein, K.Z.; Perschke, S.; Noon, S.E.; Campbell, D.; Karlsson, J.M.; Huang, D.; Kimak, M.A.; Lawrence, E.C.; et al. Connexin 47 mutations increase risk for secondary lymphedema following breast cancer treatment. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 2382–2390. [Google Scholar] [CrossRef]
- Ehrlich, A.; Molica, F.; Hautefort, A.; Kwak, B.R. Lymphatic Connexins and Pannexins in Health and Disease. Int. J. Mol. Sci. 2021, 22, 5734. [Google Scholar] [CrossRef]
- Henneke, M.; Combes, P.; Diekmann, S.; Bertini, E.; Brockmann, K.; Burlina, A.P.; Kaiser, J.; Ohlenbusch, A.; Plecko, B.; Rodriguez, D.; et al. GJA12 mutations are a rare cause of Pelizaeus-Merzbacher-like disease. Neurology 2008, 70, 748–754. [Google Scholar] [CrossRef]
- Meyer, E.; Kurian, M.A.; Morgan, N.V.; McNeill, A.; Pasha, S.; Tee, L.; Younis, R.; Norman, A.; van der Knaap, M.S.; Wassmer, E.; et al. Promoter mutation is a common variant in GJC2-associated Pelizaeus-Merzbacher-like disease. Mol. Genet. Metab. 2011, 104, 637–643. [Google Scholar] [CrossRef]
- Feinstein, M.; Markus, B.; Noyman, I.; Shalev, H.; Flusser, H.; Shelef, I.; Liani-Leibson, K.; Shorer, Z.; Cohen, I.; Khateeb, S.; et al. Pelizaeus-Merzbacher-like disease caused by AIMP1/p43 homozygous mutation. Am. J. Hum. Genet. 2010, 87, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Biancheri, R.; Rossi, A.; Zara, F.; Filocamo, M. AIMP1/p43 mutation and PMLD. Am. J. Hum. Genet. 2011, 88, 391. [Google Scholar] [CrossRef] [PubMed]
- Boespflug-Tanguy, O.; Aubourg, P.; Dorboz, I.; Bégou, M.; Giraud, G.; Sarret, C.; Vaurs-Barrière, C. Neurodegenerative disorder related to AIMP1/p43 mutation is not a PMLD. Am. J. Hum. Genet. 2011, 88, 392–393; author reply 393–395. [Google Scholar] [CrossRef] [PubMed]
- Llaci, L.; Ramsey, K.; Belnap, N.; Claasen, A.M.; Balak, C.D.; Szelinger, S.; Jepsen, W.M.; Siniard, A.L.; Richholt, R.; Izat, T.; et al. Compound heterozygous mutations in SNAP29 is associated with Pelizaeus-Merzbacher-like disorder (PMLD). Hum. Genet. 2019, 138, 1409–1417. [Google Scholar] [CrossRef]
- Fu, H.; Wang, Q.; Liu, H. Novel Mutations in NPC1 are Associated with Pelizaeus-Merzbacher-Like Disease: A Case Report. Int. J. Gen. Med. 2021, 14, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Nafisinia, M.; Sobreira, N.; Riley, L.; Gold, W.; Uhlenberg, B.; Weiß, C.; Boehm, C.; Prelog, K.; Ouvrier, R.; Christodoulou, J. Mutations in RARS cause a hypomyelination disorder akin to Pelizaeus-Merzbacher disease. Eur. J. Hum. Genet. 2017, 25, 1134–1141. [Google Scholar] [CrossRef]
- Salviati, L.; Trevisson, E.; Baldoin, M.C.; Toldo, I.; Sartori, S.; Calderone, M.; Tenconi, R.; Laverda, A. A novel deletion in the GJA12 gene causes Pelizaeus-Merzbacher-like disease. Neurogenetics 2007, 8, 57–60. [Google Scholar] [CrossRef]
- Wolf, N.I.; Cundall, M.; Rutland, P.; Rosser, E.; Surtees, R.; Benton, S.; Chong, W.K.; Malcolm, S.; Ebinger, F.; Bitner-Glindzicz, M.; et al. Frameshift mutation in GJA12 leading to nystagmus, spastic ataxia and CNS dys-/demyelination. Neurogenetics 2007, 8, 39–44. [Google Scholar] [CrossRef]
- Bugiani, M.; Al Shahwan, S.; Lamantea, E.; Bizzi, A.; Bakhsh, E.; Moroni, I.; Balestrini, M.R.; Uziel, G.; Zeviani, M. GJA12 mutations in children with recessive hypomyelinating leukoencephalopathy. Neurology 2006, 67, 273–279. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Wang, Y.; Chen, T.; Wu, X.; Jiang, Y. Two novel gap junction protein alpha 12 gene mutations in two Chinese patients with Pelizaeus-Merzbacher-like disease. Brain Dev. 2010, 32, 236–243. [Google Scholar] [CrossRef]
- Biancheri, R.; Rosano, C.; Denegri, L.; Lamantea, E.; Pinto, F.; Lanza, F.; Severino, M.; Filocamo, M. Expanded spectrum of Pelizaeus-Merzbacher-like disease: Literature revision and description of a novel GJC2 mutation in an unusually severe form. Eur. J. Hum. Genet. 2013, 21, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Shimojima, K.; Tanaka, R.; Shimada, S.; Sangu, N.; Nakayama, J.; Iwasaki, N.; Yamamoto, T. A novel homozygous mutation of GJC2 derived from maternal uniparental disomy in a female patient with Pelizaeus-Merzbacher-like disease. J. Neurol. Sci. 2013. [Google Scholar] [CrossRef] [PubMed]
- Kammoun Jellouli, N.; Salem, I.H.; Ellouz, E.; Louhichi, N.; Tlili, A.; Kammoun, F.; Triki, C.; Fakhfakh, F. Molecular confirmation of founder mutation c.-167A>G in Tunisian patients with PMLD disease. Gene 2013, 513, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Li, D.; Wu, Y.; Xiao, J.; Ji, H.; Wu, X.; Wang, J.; Jiang, Y. Identification of GJC2 gene mutations in chinese patients with Pelizaeus-Merzbacher-like disease. Minerva Pediatr. 2016. [Google Scholar] [CrossRef]
- Ji, H.; Li, D.; Wu, Y.; Zhang, Q.; Gu, Q.; Xie, H.; Ji, T.; Wang, H.; Zhao, L.; Zhao, H.; et al. Hypomyelinating disorders in China: The clinical and genetic heterogeneity in 119 patients. PLoS ONE 2018, 13, e0188869. [Google Scholar] [CrossRef]
- Henneke, M.; Gegner, S.; Hahn, A.; Plecko-Startinig, B.; Weschke, B.; Gartner, J.; Brockmann, K. Clinical neurophysiology in GJA12-related hypomyelination vs Pelizaeus-Merzbacher disease. Neurology 2010, 74, 1785–1789. [Google Scholar] [CrossRef]
- Bilir, B.; Yapici, Z.; Yalcinkaya, C.; Baris, I.; Carvalho, C.M.; Bartnik, M.; Ozes, B.; Eraksoy, M.; Lupski, J.R.; Battaloglu, E. High frequency of GJA12/GJC2 mutations in Turkish patients with Pelizaeus-Merzbacher disease. Clin. Genet. 2013, 83, 66–72. [Google Scholar] [CrossRef]
- Strauss, K.A.; Puffenberger, E.G. Genetics, medicine, and the Plain people. Annu. Rev. Genomics. Hum. Genet. 2009, 10, 513–536. [Google Scholar] [CrossRef]
- Owczarek-Lipska, M.; Mulahasanovic, L.; Obermaier, C.D.; Hortnagel, K.; Neubauer, B.A.; Korenke, G.C.; Biskup, S.; Neidhardt, J. Novel mutations in the GJC2 gene associated with Pelizaeus-Merzbacher-like disease. Mol. Biol. Rep. 2019, 46, 4507–4516. [Google Scholar] [CrossRef]
- Diekmann, S.; Henneke, M.; Burckhardt, B.C.; Gartner, J. Pelizaeus-Merzbacher-like disease is caused not only by a loss of connexin47 function but also by a hemichannel dysfunction. Eur. J. Hum. Genet. 2010, 18, 985–992. [Google Scholar] [CrossRef]
- Karimzadeh, P.; Ahmadabadi, F.; Aryani, O.; Houshmand, M.; Khatami, A. New mutation of pelizaeus–Merzbacher-like disease; A report from Iran. Iran. J. Radiol. Q. J. Publ. Iran. Radiol. Soc. 2014, 11, e6913. [Google Scholar] [CrossRef]
- Javadikooshesh, S.; Zaimkohan, H.; Pourghorban, P.; Bahramim, F.; Ebadi, N. Pelizaeus-Merzbacher-Like Disease 1 Caused by a Novel Mutation in GJC2 Gene: A Case Report. Iran. J. Med. Sci. 2021, 46, 493–497. [Google Scholar] [CrossRef]
- Osaka, H.; Hamanoue, H.; Yamamoto, R.; Nezu, A.; Sasaki, M.; Saitsu, H.; Kurosawa, K.; Shimbo, H.; Matsumoto, N.; Inoue, K. Disrupted SOX10 regulation of GJC2 transcription causes Pelizaeus-Merzbacher-like disease. Ann. Neurol. 2010, 68, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Combes, P.; Kammoun, N.; Monnier, A.; Gonthier-Guéret, C.; Giraud, G.; Bertini, E.; Chahnez, T.; Fakhfakh, F.; Boespflug-Tanguy, O.; Vaurs-Barrière, C. Relevance of GJC2 promoter mutation in Pelizaeus-Merzbacher-like disease. Ann. Neurol. 2012, 71, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, L.; Inoue, K.; Helman, G.; Mora, S.; Maski, K.; Soul, J.S.; Bloom, M.; Evans, S.H.; Goto, Y.; Caldovic, L.; et al. GJC2 promoter mutations causing Pelizaeus-Merzbacher-like disease. Mol. Genet. Metab. 2014, 111, 393–398. [Google Scholar] [CrossRef]
- Al-Yahyaee, S.A.; Al-Kindi, M.; Jonghe, P.D.; Al-Asmi, A.; Al-Futaisi, A.; Vriendt, E.D.; Deconinck, T.; Chand, P. Pelizaeus-Merzbacher-Like Disease in a Family With Variable Phenotype and a Novel Splicing GJC2 Mutation. J. Child. Neurol. 2013, 28, 1467–1473. [Google Scholar] [CrossRef]
- Osório, M.J.; Goldman, S.A. Neurogenetics of Pelizaeus-Merzbacher disease. Handb. Clin. Neurol. 2018, 148, 701–722. [Google Scholar] [CrossRef]
- Bonkowsky, J.L.; Nelson, C.; Kingston, J.L.; Filloux, F.M.; Mundorff, M.B.; Srivastava, R. The burden of inherited leukodystrophies in children. Neurology 2010, 75, 718–725. [Google Scholar] [CrossRef]
- Singh, R.; Debopam, S. Pelizaeus-Merzbacher Disease. In StatPearls [Internet]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560522/ (accessed on 4 July 2022).
- Kuipers, D.J.S.; Tufekcioglu, Z.; Bilgiç, B.; Olgiati, S.; Dremmen, M.H.G.; van IJcken, W.F.J.; Breedveld, G.J.; Mancini, G.M.S.; Hanagasi, H.A.; Emre, M.; et al. Late-onset phenotype associated with a homozygous GJC2 missense mutation in a Turkish family. Park. Relat. Disord. 2019, 66, 228–231. [Google Scholar] [CrossRef]
- Flores-Obando, R.E.; Freidin, M.M.; Hernández, A.I.; Abrams, C.K. Activation of the unfolded protein response by Connexin47 mutations associated with Pelizaeus-Merzbacher-like disease. Mol. Cell. Neurosci. 2022, 120, 103716. [Google Scholar] [CrossRef]
- Chen, N.; Wang, J.; Jiang, Y.; Wu, Y.; Hao, H.; Ji, T. Different Mutations of Gap Junction Connexin 47 Lead to Discrepant Activation of Unfolded Protein Response Pathway in Pelizaeus-Merzbacher-Like Disease. Neuropediatrics 2017, 48, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Koval, M.; Molina, S.A.; Burt, J.M. Mix and match: Investigating heteromeric and heterotypic gap junction channels in model systems and native tissues. FEBS. Lett. 2014, 588, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).