Vitamin D and Autoimmune Rheumatic Diseases
Abstract
1. Introduction
2. Vitamin D and Immunity
2.1. Vitamin D and Innate Immunity
2.2. Vitamin D and Dendritic Cells
2.3. Vitamin D and Adaptive Immune System
3. Vitamin D and Autoimmunity
4. Rheumatoid Arthritis and Vitamin D
5. Systemic Lupus Erythematosus and Vitamin D
6. Ankylosing Spondylitis and Vitamin D
7. Psoriatic Arthritis and Vitamin D
8. Sjogren’s Syndrome and Vitamin D
9. Systemic Sclerosis and Vitamin D
10. Inflammatory Myopathies and Vitamin D
11. Vitamin D Supplementation
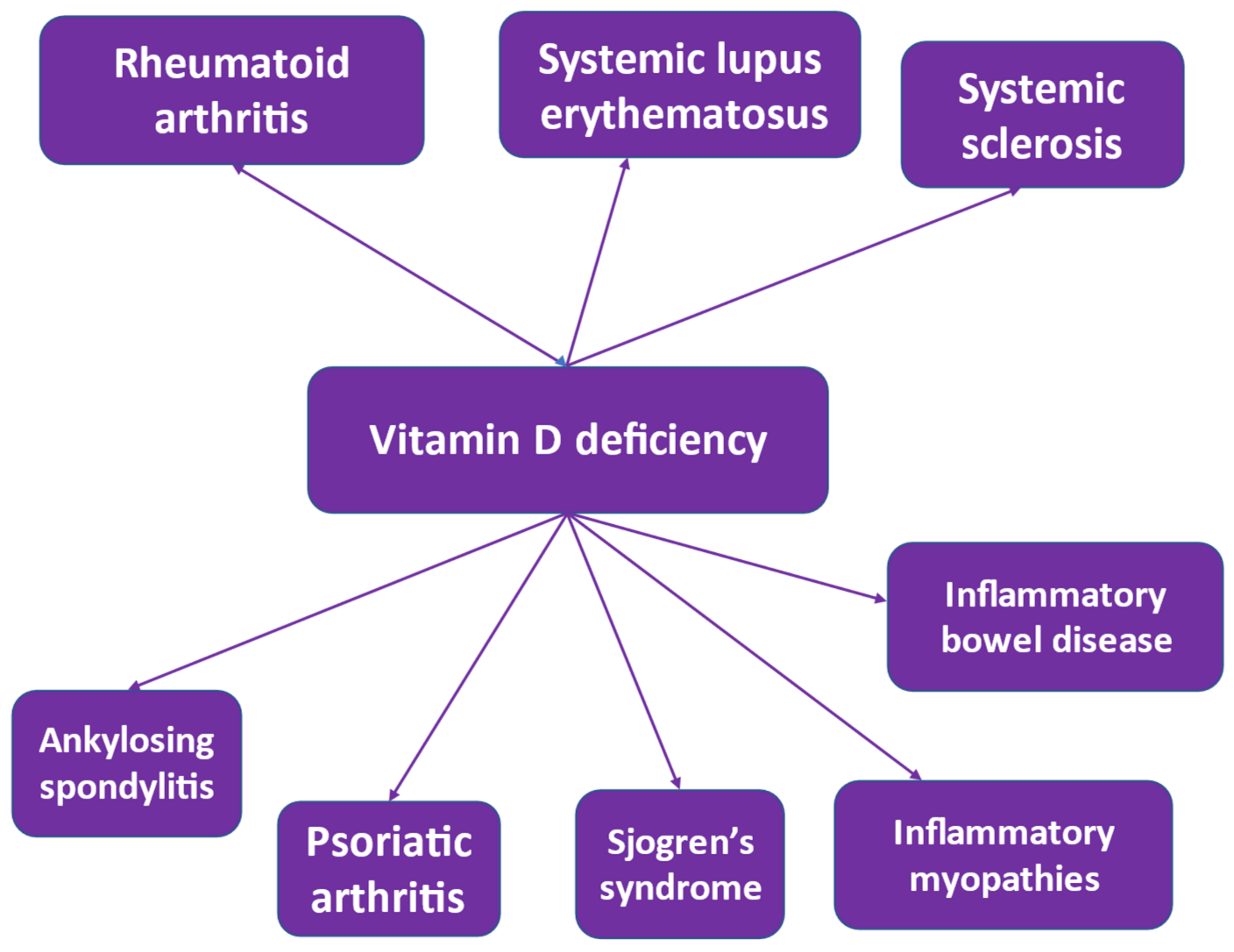
12. Vitamin D Deficiency in Autoimmune Rheumatic Diseases
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Li, S.; De La Cruz, J.; Bikle, D.D. New developments in our understanding of vitamin metabolism, action and treatment. Metabolism 2019, 98, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Williams, C. On the use and administration of cod-liver oil in pulmonary consumption. Lond. J. Med. 1849, 1, 50. [Google Scholar] [CrossRef]
- Finsen, N. Nobel prize presentation speech by professor the count KAH Morner. Rector R. Caroline Inst. Dec. 1903, 10, 1903. [Google Scholar]
- Moller, K.I.; Kongshoj, B.; Philipsen, P.A.; Thomsen, V.O.; Wulf, H.C. How Finsen’s light cured lupus vulgaris. Photodermatol. Photoimmunol. Photomed. 2005, 21, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Shapira, Y.; Agmon-Levin, N.; Shoenfeld, Y. Mycobacterium tuberculosis, autoimmunity, and vitamin D. Clin. Rev. Allergy Immunol. 2010, 38, 169–177. [Google Scholar] [CrossRef]
- Rook, G.A.; Steele, J.; Fraher, L.; Barker, S.; Karmali, R.; O’Riordan, J.; Stanford, J. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology 1986, 57, 159–163. [Google Scholar]
- Soeharto, D.A.; Rifai, D.A.; Marsudidjadja, S.; Roekman, A.E.; Assegaf, C.K.; Louisa, M. Vitamin D as an Adjunctive Treatment to Standard Drugs in Pulmonary Tuberculosis Patients: An Evidence-Based Case Report. Adv. Prev. Med. 2019, 2019, 5181847. [Google Scholar] [CrossRef]
- Oliveira, A.L.G.; Chaves, A.T.; Menezes, C.A.S.; Guimaraes, N.S.; Bueno, L.L.; Fujiwara, R.T.; Rocha, M. Vitamin D receptor expression and hepcidin levels in the protection or severity of leprosy: A systematic review. Microbes Infect. 2017, 19, 311–322. [Google Scholar] [CrossRef]
- Singh, I.; Lavania, M.; Pathak, V.K.; Ahuja, M.; Turankar, R.P.; Singh, V.; Sengupta, U. VDR polymorphism, gene expression and vitamin D levels in leprosy patients from North Indian population. PLoS Negl. Trop. Dis. 2018, 12, e0006823. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, T.; Wang, C.; Ji, Y. The association between vitamin D and COPD risk, severity, and exacerbation: An updated systematic review and meta-analysis. Int. J. Chron. Obs. Pulmon. Dis. 2016, 11, 2597–2607. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, V.; Kamolvit, W.; Herthelius, M.; Luthje, P.; Brauner, A.; Chromek, M. Association between vitamin D, antimicrobial peptides and urinary tract infection in infants and young children. Acta Paediatr. 2019, 108, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, I.; Pawliczak, R. The Active Metabolite of Vitamin D3 as a Potential Immunomodulator. Scand. J. Immunol. 2016, 83, 83–91. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Wei, R.; Christakos, S. Mechanisms Underlying the Regulation of Innate and Adaptive Immunity by Vitamin D. Nutrients 2015, 7, 8251–8260. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M. Vitamin D or hormone D deficiency in autoimmune rheumatic diseases, including undifferentiated connective tissue disease. Arthritis Res. Ther. 2008, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Plebani, M.; Shoenfeld, Y.; Adorini, L.; Tincani, A. Vitamin D endocrine system and the immune response in rheumatic diseases. Vitam. Horm. 2011, 86, 327–351. [Google Scholar] [CrossRef]
- Cutolo, M.; Otsa, K.; Paolino, S.; Yprus, M.; Veldi, T.; Seriolo, B. Vitamin D involvement in rheumatoid arthritis and systemic lupus erythaematosus. Ann. Rheum. Dis. 2009, 68, 446–447. [Google Scholar] [CrossRef]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 97. [Google Scholar] [CrossRef]
- Hahn, J.; Cook, N.R.; Alexander, E.K.; Friedman, S.; Walter, J.; Bubes, V.; Kotler, G.; Lee, I.M.; Manson, J.E.; Costenbader, K.H. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ 2022, 376, e066452. [Google Scholar] [CrossRef] [PubMed]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.R.; Li, D.; Jeffery, L.E.; Raza, K.; Hewison, M. Vitamin D, Autoimmune Disease and Rheumatoid Arthritis. Calcif. Tissue Int. 2020, 106, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Bae, S.C. Vitamin D level in rheumatoid arthritis and its correlation with the disease activity: A meta-analysis. Clin. Exp. Rheumatol. 2016, 34, 827–833. [Google Scholar]
- Lee, W.L.; Lee, F.K.; Wang, P.H. Vitamin D and systemic lupus erythematous. J. Chin. Med. Assoc. 2022, 85, 811–812. [Google Scholar] [CrossRef]
- Amital, H.; Szekanecz, Z.; Szücs, G.; Dankó, K.; Nagy, E.; Csépány, T.; Kiss, E.; Rovensky, J.; Tuchynova, A.; Kozakova, D.; et al. Serum concentrations of 25-OH vitamin D in patients with systemic lupus erythematosus (SLE) are inversely related to disease activity: Is it time to routinely supplement patients with SLE with vitamin D? Ann. Rheum. Dis. 2010, 69, 1155–1157. [Google Scholar] [CrossRef]
- Ruaro, B.; Casabella, A.; Paolino, S.; Alessandri, E.; Patané, M.; Gotelli, E.; Sulli, A.; Cutolo, M. Trabecular Bone Score and Bone Quality in Systemic Lupus Erythematosus Patients. Front. Med. 2020, 7, 574842. [Google Scholar] [CrossRef]
- Gubatan, J.; Moss, A.C. Vitamin D in inflammatory bowel disease: More than just a supplement. Curr. Opin. Gastroenterol. 2018, 34, 217–225. [Google Scholar] [CrossRef]
- Vernia, F.; Valvano, M.; Longo, S.; Cesaro, N.; Viscido, A.; Latella, G. Vitamin D in Inflammatory Bowel Diseases. Mechanisms of Action and Therapeutic Implications. Nutrients 2022, 14, 269. [Google Scholar] [CrossRef]
- Pierrot-Deseilligny, C.; Souberbielle, J.C. Vitamin D and multiple sclerosis: An update. Mult. Scler. Relat. Disord. 2017, 14, 35–45. [Google Scholar] [CrossRef]
- Trombetta, A.C.; Smith, V.; Gotelli, E.; Ghio, M.; Paolino, S.; Pizzorni, C.; Vanhaecke, A.; Ruaro, B.; Sulli, A.; Cutolo, M. Vitamin D deficiency and clinical correlations in systemic sclerosis patients: A retrospective analysis for possible future developments. PLoS ONE 2017, 12, e0179062. [Google Scholar] [CrossRef] [PubMed]
- Manousaki, D.; Harroud, A.; Mitchell, R.E.; Ross, S.; Forgetta, V.; Timpson, N.J.; Smith, G.D.; Polychronakos, C.; Richards, J.B. Vitamin D levels and risk of type 1 diabetes: A Mendelian randomization study. PLoS Med. 2021, 18, e1003536. [Google Scholar] [CrossRef]
- Cvek, M.; Kaličanin, D.; Barić, A.; Vuletić, M.; Gunjača, I.; Torlak Lovrić, V.; Škrabić, V.; Punda, A.; Boraska Perica, V. Vitamin D and Hashimoto’s Thyroiditis: Observations from CROHT Biobank. Nutrients 2021, 13, 2793. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D and the skin: Physiology and pathophysiology. Rev. Endocr. Metab. Disord. 2012, 13, 3–19. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Snyder, L.; Arora, J. Vitamin A and vitamin D regulate the microbial complexity, barrier function, and the mucosal immune responses to ensure intestinal homeostasis. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and immune function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef] [PubMed]
- Ismailova, A.; White, J.H. Vitamin D, infections and immunity. Rev. Endocr. Metab. Disord. 2022, 23, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Zhang, Z.; Yang, L.; Li, R.; Ma, Y. Combined effect of vitamin C and vitamin D(3) on intestinal epithelial barrier by regulating Notch signaling pathway. Nutr. Metab. 2021, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Chan, H.; Liang, Y.; Liu, X.; Zhang, L.; Li, Q.; Zhang, Y.; Zeng, J.; Ugwu, F.N.; Ho, I.H.T.; et al. Cathelicidin preserves intestinal barrier function in polymicrobial sepsis. Crit. Care 2020, 24, 47. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, T.; Shi, Y.; Tian, F.; Hu, H.; Deb, D.K.; Chen, Y.; Bissonnette, M.; Li, Y.C. Gut Epithelial Vitamin D Receptor Regulates Microbiota-Dependent Mucosal Inflammation by Suppressing Intestinal Epithelial Cell Apoptosis. Endocrinology 2018, 159, 967–979. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D Regulation of Immune Function. Curr. Osteoporos. Rep. 2022, 20, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M.; Freeman, L.; Hughes, S.V.; Evans, K.N.; Bland, R.; Eliopoulos, A.G.; Kilby, M.D.; Moss, P.A.; Chakraverty, R. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J. Immunol. 2003, 170, 5382–5390. [Google Scholar] [CrossRef] [PubMed]
- Agerberth, B.; Charo, J.; Werr, J.; Olsson, B.; Idali, F.; Lindbom, L.; Kiessling, R.; Jornvall, H.; Wigzell, H.; Gudmundsson, G.H. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood 2000, 96, 3086–3093. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, B.; Davis, E.G.; Ross, C.R.; Blecha, F. Cathelicidins: Microbicidal activity, mechanisms of action, and roles in innate immunity. Microbes Infect. 2002, 4, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.; Rudolph, T.; Pietzsch, J.; Meurer, M. Conversion of vitamin D3 to 1alpha,25-dihydroxyvitamin D3 in human skin equivalents. Exp. Derm. 2000, 9, 97–103. [Google Scholar] [CrossRef]
- Lehmann, B.; Tiebel, O.; Meurer, M. Expression of vitamin D3 25-hydroxylase (CYP27) mRNA after induction by vitamin D3 or UVB radiation in keratinocytes of human skin equivalents--a preliminary study. Arch. Derm. Res. 1999, 291, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Korf, H.; Wenes, M.; Stijlemans, B.; Takiishi, T.; Robert, S.; Miani, M.; Eizirik, D.L.; Gysemans, C.; Mathieu, C. 1,25-Dihydroxyvitamin D3 curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology 2012, 217, 1292–1300. [Google Scholar] [CrossRef]
- Stoffels, K.; Overbergh, L.; Giulietti, A.; Verlinden, L.; Bouillon, R.; Mathieu, C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J. Bone Min. Res. 2006, 21, 37–47. [Google Scholar] [CrossRef]
- Weber, G.; Heilborn, J.D.; Chamorro Jimenez, C.I.; Hammarsjo, A.; Torma, H.; Stahle, M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J. Invest. Dermatol. 2005, 124, 1080–1082. [Google Scholar] [CrossRef]
- Takahashi, K.; Nakayama, Y.; Horiuchi, H.; Ohta, T.; Komoriya, K.; Ohmori, H.; Kamimura, T. Human neutrophils express messenger RNA of vitamin D receptor and respond to 1alpha,25-dihydroxyvitamin D3. Immunopharmacol. Immunotoxicol. 2002, 24, 335–347. [Google Scholar] [CrossRef]
- Hirsch, D.; Archer, F.E.; Joshi-Kale, M.; Vetrano, A.M.; Weinberger, B. Decreased anti-inflammatory responses to vitamin D in neonatal neutrophils. Mediat. Inflamm. 2011, 2011, 598345. [Google Scholar] [CrossRef] [PubMed]
- Bishop, E.L.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory. JBMR Plus 2021, 5, e10405. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M. Some interfaces of dendritic cell biology. Apmis 2003, 111, 675–697. [Google Scholar] [CrossRef]
- Adorini, L.; Penna, G. Induction of tolerogenic dendritic cells by vitamin D receptor agonists. Handb. Exp. Pharm. 2009, 188, 251–273. [Google Scholar] [CrossRef]
- Adorini, L.; Penna, G.; Giarratana, N.; Roncari, A.; Amuchastegui, S.; Daniel, K.C.; Uskokovic, M. Dendritic cells as key targets for immunomodulation by Vitamin D receptor ligands. J. Steroid. Biochem. Mol. Biol. 2004, 89–90, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, F.A.; Oettgen, H.C. Adaptive immunity. J. Allergy Clin. Immunol. 2010, 125, S33–S40. [Google Scholar] [CrossRef] [PubMed]
- Lovely, G.A.; Sen, R. Evolving adaptive immunity. Genes Dev. 2016, 30, 873–875. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S. Taking regulatory T cells into medicine. J. Exp. Med. 2021, 218, e20210831. [Google Scholar] [CrossRef]
- Peelen, E.; Knippenberg, S.; Muris, A.H.; Thewissen, M.; Smolders, J.; Tervaert, J.W.; Hupperts, R.; Damoiseaux, J. Effects of vitamin D on the peripheral adaptive immune system: A review. Autoimmun. Rev. 2011, 10, 733–743. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Snyder, L.; Lin, Y.D.; Yang, L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef]
- Veldman, C.M.; Cantorna, M.T.; DeLuca, H.F. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch. Biochem. Biophys. 2000, 374, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bruce, D.; Cantorna, M.T. Vitamin D receptor expression controls proliferation of naïve CD8+ T cells and development of CD8 mediated gastrointestinal inflammation. BMC Immunol. 2014, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Mahon, B.D.; Wittke, A.; Weaver, V.; Cantorna, M.T. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J. Cell. Biochem. 2003, 89, 922–932. [Google Scholar] [CrossRef]
- Provvedini, D.M.; Tsoukas, C.D.; Deftos, L.J.; Manolagas, S.C. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science 1983, 221, 1181–1183. [Google Scholar] [CrossRef]
- Manolagas, S.C.; Provvedini, D.M.; Tsoukas, C.D. Interactions of 1,25-dihydroxyvitamin D3 and the immune system. Mol. Cell. Endocrinol. 1985, 43, 113–122. [Google Scholar] [CrossRef]
- Ooi, J.H.; McDaniel, K.L.; Weaver, V.; Cantorna, M.T. Murine CD8+ T cells but not macrophages express the vitamin D 1α-hydroxylase. J. Nutr. Biochem. 2014, 25, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Ooi, J.H.; Chen, J.; Cantorna, M.T. Vitamin D regulation of immune function in the gut: Why do T cells have vitamin D receptors? Mol. Asp. Med. 2012, 33, 77–82. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Waddell, A. The vitamin D receptor turns off chronically activated T cells. Ann. N. Y. Acad. Sci. 2014, 1317, 70–75. [Google Scholar] [CrossRef]
- Rafique, A.; Rejnmark, L.; Heickendorff, L.; Møller, H.J. 25(OH)D3 and 1.25(OH)2D3 inhibits TNF-α expression in human monocyte derived macrophages. PLoS ONE 2019, 14, e0215383. [Google Scholar] [CrossRef]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Lipsky, P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef]
- Lemire, J.M.; Adams, J.S.; Sakai, R.; Jordan, S.C. 1 alpha,25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J. Clin. Invest. 1984, 74, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Paolino, S.; Sulli, A.; Smith, V.; Pizzorni, C.; Seriolo, B. Vitamin D, steroid hormones, and autoimmunity. Ann. N. Y. Acad. Sci. 2014, 1317, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Barbour, G.L.; Coburn, J.W.; Slatopolsky, E.; Norman, A.W.; Horst, R.L. Hypercalcemia in an anephric patient with sarcoidosis: Evidence for extrarenal generation of 1,25-dihydroxyvitamin D. N. Engl. J. Med. 1981, 305, 440–443. [Google Scholar] [CrossRef]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef]
- Bikle, D.D. Extraskeletal actions of vitamin D. Ann. N. Y. Acad. Sci. 2016, 1376, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Haas, J. Vigantol—Adolf Windaus and the history of vitamin D. Wurzbg Med. Mitt. 2007, 26, 144–181. [Google Scholar]
- Aaron, L.; Patricia, J.; Torsten, M. The world incidence and prevalence of autoimmune diseases is increasing. Int. J. Celiac Dis. 2015, 3, 151–155. [Google Scholar]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Badenhoop, K.; Kahles, H.; Penna-Martinez, M. Vitamin D, immune tolerance, and prevention of type 1 diabetes. Curr. Diab. Rep. 2012, 12, 635–642. [Google Scholar] [CrossRef]
- Cyprian, F.; Lefkou, E.; Varoudi, K.; Girardi, G. Immunomodulatory Effects of Vitamin D in Pregnancy and Beyond. Front. Immunol. 2019, 10, 2739. [Google Scholar] [CrossRef]
- Booth, D.R.; Ding, N.; Parnell, G.P.; Shahijanian, F.; Coulter, S.; Schibeci, S.D.; Atkins, A.R.; Stewart, G.J.; Evans, R.M.; Downes, M.; et al. Cistromic and genetic evidence that the vitamin D receptor mediates susceptibility to latitude-dependent autoimmune diseases. Genes Immun. 2016, 17, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Illescas-Montes, R.; Melguizo-Rodríguez, L.; Ruiz, C.; Costela-Ruiz, V.J. Vitamin D and autoimmune diseases. Life Sci. 2019, 233, 116744. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Tonacci, A.; Negrini, S.; Greco, M.; Borro, M.; Puppo, F.; Gangemi, S. Emerging role of vitamin D in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun. Rev. 2019, 18, 102350. [Google Scholar] [CrossRef] [PubMed]
- Bellan, M.; Sainaghi, P.P.; Pirisi, M. Role of Vitamin D in Rheumatoid Arthritis. Adv. Exp. Med. Biol. 2017, 996, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Bragazzi, N.L.; Watad, A.; Neumann, S.G.; Simon, M.; Brown, S.B.; Abu Much, A.; Harari, A.; Tiosano, S.; Amital, H.; Shoenfeld, Y. Vitamin D and rheumatoid arthritis: An ongoing mystery. Curr. Opin. Rheumatol. 2017, 29, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Buondonno, I.; Rovera, G.; Sassi, F.; Rigoni, M.M.; Lomater, C.; Parisi, S.; Pellerito, R.; Isaia, G.C.; D’Amelio, P. Vitamin D and immunomodulation in early rheumatoid arthritis: A randomized double-blind placebo-controlled study. PLoS ONE 2017, 12, e0178463. [Google Scholar] [CrossRef]
- Ishikawa, L.L.W.; Colavite, P.M.; Fraga-Silva, T.F.C.; Mimura, L.A.N.; França, T.G.D.; Zorzella-Pezavento, S.F.G.; Chiuso-Minicucci, F.; Marcolino, L.D.; Penitenti, M.; Ikoma, M.R.V.; et al. Vitamin D Deficiency and Rheumatoid Arthritis. Clin. Rev. Allergy Immunol. 2017, 52, 373–388. [Google Scholar] [CrossRef]
- Kostoglou-Athanassiou, I.; Athanassiou, P.; Lyraki, A.; Raftakis, I.; Antoniadis, C. Vitamin D and rheumatoid arthritis. Adv. Endocrinol. Metab. 2012, 3, 181–187. [Google Scholar] [CrossRef]
- Song, G.G.; Bae, S.C.; Lee, Y.H. Association between vitamin D intake and the risk of rheumatoid arthritis: A meta-analysis. Clin. Rheumatol. 2012, 31, 1733–1739. [Google Scholar] [CrossRef]
- Dupuis, M.L.; Pagano, M.T.; Pierdominici, M.; Ortona, E. The role of vitamin D in autoimmune diseases: Could sex make the difference? Biol. Sex. Differ. 2021, 12, 12. [Google Scholar] [CrossRef]
- Watad, A.; Neumann, S.G.; Soriano, A.; Amital, H.; Shoenfeld, Y. Vitamin D and Systemic Lupus Erythematosus: Myth or Reality? Isr. Med. Assoc. J. 2016, 18, 177–182. [Google Scholar] [PubMed]
- Guan, S.Y.; Cai, H.Y.; Wang, P.; Lv, T.T.; Liu, L.N.; Mao, Y.M.; Zhao, C.N.; Wu, Q.; Dan, Y.L.; Sam, N.B.; et al. Association between circulating 25-hydroxyvitamin D and systemic lupus erythematosus: A systematic review and meta-analysis. Int. J. Rheum. Dis. 2019, 22, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.C.; Birmingham, D.J.; Leung, H.W.; Hebert, L.A.; Song, H.; Rovin, B.H. Vitamin D levels in Chinese patients with systemic lupus erythematosus: Relationship with disease activity, vascular risk factors and atherosclerosis. Rheumatology 2012, 51, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.C.; Lee, Y.H. Vitamin D level and risk of systemic lupus erythematosus and rheumatoid arthritis: A Mendelian randomization. Clin. Rheumatol. 2018, 37, 2415–2421. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T. Vitamin D and its role in immunology: Multiple sclerosis, and inflammatory bowel disease. Prog. Biophys. Mol. Biol. 2006, 92, 60–64. [Google Scholar] [CrossRef]
- Cantorna, M.T. Vitamin D, multiple sclerosis and inflammatory bowel disease. Arch. Biochem. Biophys. 2012, 523, 103–106. [Google Scholar] [CrossRef]
- Correale, J.; Ysrraelit, M.C.; Gaitan, M.I. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain 2009, 132, 1146–1160. [Google Scholar] [CrossRef]
- Gianfrancesco, M.A.; Stridh, P.; Rhead, B.; Shao, X.; Xu, E.; Graves, J.S.; Chitnis, T.; Waldman, A.; Lotze, T.; Schreiner, T.; et al. Evidence for a causal relationship between low vitamin D, high BMI, and pediatric-onset MS. Neurology 2017, 88, 1623–1629. [Google Scholar] [CrossRef]
- Kragt, J.; van Amerongen, B.; Killestein, J.; Dijkstra, C.; Uitdehaag, B.; Polman, C.; Lips, P. Higher levels of 25-hydroxyvitamin D are associated with a lower incidence of multiple sclerosis only in women. Mult. Scler. 2009, 15, 9–15. [Google Scholar] [CrossRef]
- Hausmann, J.; Kubesch, A.; Amiri, M.; Filmann, N.; Blumenstein, I. Vitamin D Deficiency is Associated with Increased Disease Activity in Patients with Inflammatory Bowel Disease. J. Clin. Med. 2019, 8, 1319. [Google Scholar] [CrossRef]
- Sairenji, T.; Collins, K.L.; Evans, D.V. An Update on Inflammatory Bowel Disease. Prim. Care 2017, 44, 673–692. [Google Scholar] [CrossRef] [PubMed]
- de Souza, H.S.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.; Cooper, S.C.; Ghosh, S.; Hewison, M. The Role of Vitamin D in Inflammatory Bowel Disease: Mechanism to Management. Nutrients 2019, 11, 1019. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cui, X.; Li, J.; Wang, H.; Li, Y.; Chen, Y.; Zhang, H. Clinical evaluation of vitamin D status and its relationship with disease activity and changes of intestinal immune function in patients with Crohn’s disease in the Chinese population. Scand. J. Gastroenterol. 2021, 56, 20–29. [Google Scholar] [CrossRef]
- Yang, L.; Weaver, V.; Smith, J.P.; Bingaman, S.; Hartman, T.J.; Cantorna, M.T. Therapeutic effect of vitamin d supplementation in a pilot study of Crohn’s patients. Clin. Transl. Gastroenterol. 2013, 4, e33. [Google Scholar] [CrossRef] [PubMed]
- Lemke, D.; Klement, R.J.; Schweiger, F.; Schweiger, B.; Spitz, J. Vitamin D Resistance as a Possible Cause of Autoimmune Diseases: A Hypothesis Confirmed by a Therapeutic High-Dose Vitamin D Protocol. Front. Immunol. 2021, 12, 655739. [Google Scholar] [CrossRef]
- Merlino, L.A.; Curtis, J.; Mikuls, T.R.; Cerhan, J.R.; Criswell, L.A.; Saag, K.G. Vitamin D intake is inversely associated with rheumatoid arthritis: Results from the Iowa Women’s Health Study. Arthritis Rheum. 2004, 50, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Costenbader, K.H.; Feskanich, D.; Holmes, M.; Karlson, E.W.; Benito-Garcia, E. Vitamin D intake and risks of systemic lupus erythematosus and rheumatoid arthritis in women. Ann. Rheum. Dis. 2008, 67, 530–535. [Google Scholar] [CrossRef]
- Hiraki, L.T.; Arkema, E.V.; Cui, J.; Malspeis, S.; Costenbader, K.H.; Karlson, E.W. Circulating 25-hydroxyvitamin D level and risk of developing rheumatoid arthritis. Rheumatology 2014, 53, 2243–2248. [Google Scholar] [CrossRef]
- Cutolo, M.; Otsa, K.; Laas, K.; Yprus, M.; Lehtme, R.; Secchi, M.E.; Sulli, A.; Paolino, S.; Seriolo, B. Circannual vitamin d serum levels and disease activity in rheumatoid arthritis: Northern versus Southern Europe. Clin. Exp. Rheumatol. 2006, 24, 702–704. [Google Scholar]
- Cutolo, M.; Otsa, K.; Uprus, M.; Paolino, S.; Seriolo, B. Vitamin D in rheumatoid arthritis. Autoimmun. Rev. 2007, 7, 59–64. [Google Scholar] [CrossRef]
- Attar, S.M. Vitamin D deficiency in rheumatoid arthritis. Prevalence and association with disease activity in Western Saudi Arabia. Saudi Med. J. 2012, 33, 520–525. [Google Scholar] [PubMed]
- Baykal, T.; Senel, K.; Alp, F.; Erdal, A.; Ugur, M. Is there an association between serum 25-hydroxyvitamin D concentrations and disease activity in rheumatoid arthritis? Bratisl. Lek. Listy 2012, 113, 610–611. [Google Scholar] [CrossRef] [PubMed]
- Yazmalar, L.; Ediz, L.; Alpayci, M.; Hiz, O.; Toprak, M.; Tekeoglu, I. Seasonal disease activity and serum vitamin D levels in rheumatoid arthritis, ankylosing spondylitis and osteoarthritis. Afr. Health Sci. 2013, 13, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Atwa, M.A.; Balata, M.G.; Hussein, A.M.; Abdelrahman, N.I.; Elminshawy, H.H. Serum 25-hydroxyvitamin D concentration in patients with psoriasis and rheumatoid arthritis and its association with disease activity and serum tumor necrosis factor-alpha. Saudi Med. J. 2013, 34, 806–813. [Google Scholar]
- Sahebari, M.; Mirfeizi, Z.; Rezaieyazdi, Z.; Rafatpanah, H.; Goshyeshi, L. 25(OH) vitamin D serum values and rheumatoid arthritis disease activity (DA S28 ESR). Casp. J. Intern. Med. 2014, 5, 148–155. [Google Scholar]
- Gheita, T.A.; Sayed, S.; Gheita, H.A.; Kenawy, S.A. Vitamin D status in rheumatoid arthritis patients: Relation to clinical manifestations, disease activity, quality of life and fibromyalgia syndrome. Int. J. Rheum. Dis. 2016, 19, 294–299. [Google Scholar] [CrossRef]
- Chen, J.; Liu, W.; Lin, Q.; Chen, L.; Yin, J.; Huang, H. Vitamin D deficiency and low bone mineral density in native Chinese rheumatoid arthritis patients. Int. J. Rheum. Dis. 2014, 17, 66–70. [Google Scholar] [CrossRef]
- Hong, Q.; Xu, J.; Xu, S.; Lian, L.; Zhang, M.; Ding, C. Associations between serum 25-hydroxyvitamin D and disease activity, inflammatory cytokines and bone loss in patients with rheumatoid arthritis. Rheumatology 2014, 53, 1994–2001. [Google Scholar] [CrossRef]
- Sharma, R.; Saigal, R.; Goyal, L.; Mital, P.; Yadav, R.N.; Meena, P.D.; Agrawal, A. Estimation of vitamin D levels in rheumatoid arthritis patients and its correlation with the disease activity. J. Assoc. Physicians. India 2014, 62, 678–681. [Google Scholar]
- Brance, M.L.; Brun, L.R.; Lioi, S.; Sánchez, A.; Abdala, M.; Oliveri, B. Vitamin D levels and bone mass in rheumatoid arthritis. Rheumatol. Int. 2015, 35, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Grazio, S.; Naglić, Đ.; Anić, B.; Grubišić, F.; Bobek, D.; Bakula, M.; Kavanagh, H.S.; Kuna, A.T.; Cvijetić, S. Vitamin D serum level, disease activity and functional ability in different rheumatic patients. Am. J. Med. Sci. 2015, 349, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.Y.; Luo, J.; Li, X.F. Vitamin D Levels and Associations With Disease Activity in Chinese Han Patients with Early Rheumatoid Arthritis. J. Clin. Rheumatol. 2015, 21, 276–277. [Google Scholar] [CrossRef]
- Senosi, M.R.; Fathi, H.M.; Baki, N.M.A.; Zaki, O.; Magdy, A.M.; Gheita, T.A. Bone mineral density, vitamin D receptor (VDR) gene polymorphisms, fracture risk assessment (FRAX), and trabecular bone score (TBS) in rheumatoid arthritis patients: Connecting pieces of the puzzle. Clin. Rheumatol. 2022, 41, 1333–1342. [Google Scholar] [CrossRef]
- Lin, J.; Liu, J.; Davies, M.L.; Chen, W. Serum Vitamin D Level and Rheumatoid Arthritis Disease Activity: Review and Meta-Analysis. PLoS ONE 2016, 11, e0146351. [Google Scholar] [CrossRef] [PubMed]
- Giannini, D.; Antonucci, M.; Petrelli, F.; Bilia, S.; Alunno, A.; Puxeddu, I. One year in review 2020: Pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 2020, 38, 387–397. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 2017, 389, 2328–2337. [Google Scholar] [CrossRef]
- Rao, D.A.; Gurish, M.F.; Marshall, J.L.; Slowikowski, K.; Fonseka, C.Y.; Liu, Y.; Donlin, L.T.; Henderson, L.A.; Wei, K.; Mizoguchi, F.; et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 2017, 542, 110–114. [Google Scholar] [CrossRef]
- Wehr, P.; Purvis, H.; Law, S.C.; Thomas, R. Dendritic cells, T cells and their interaction in rheumatoid arthritis. Clin. Exp. Immunol. 2019, 196, 12–27. [Google Scholar] [CrossRef]
- Volkov, M.; van Schie, K.A.; van der Woude, D. Autoantibodies and B Cells: The ABC of rheumatoid arthritis pathophysiology. Immunol. Rev. 2020, 294, 148–163. [Google Scholar] [CrossRef]
- Higgins, M.J.; Mackie, S.L.; Thalayasingam, N.; Bingham, S.J.; Hamilton, J.; Kelly, C.A. The effect of vitamin D levels on the assessment of disease activity in rheumatoid arthritis. Clin. Rheumatol. 2013, 32, 863–867. [Google Scholar] [CrossRef]
- Dennis, G., Jr.; Holweg, C.T.; Kummerfeld, S.K.; Choy, D.F.; Setiadi, A.F.; Hackney, J.A.; Haverty, P.M.; Gilbert, H.; Lin, W.Y.; Diehl, L.; et al. Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. Arthritis Res. 2014, 16, R90. [Google Scholar] [CrossRef] [PubMed]
- Shoenfeld, Y.; Giacomelli, R.; Azrielant, S.; Berardicurti, O.; Reynolds, J.A.; Bruce, I.N. Vitamin D and systemic lupus erythematosus—The hype and the hope. Autoimmun. Rev. 2018, 17, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Perricone, C.; Agmon-Levin, N.; Colafrancesco, S.; Shoenfeld, Y. Vitamins and systemic lupus erythematosus: To D or not to D. Expert. Rev. Clin. Immunol. 2013, 9, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Khandker, S.S.; Alam, S.S.; Kotyla, P.; Hassan, R. Vitamin D status in patients with systemic lupus erythematosus (SLE): A systematic review and meta-analysis. Autoimmun. Rev. 2019, 18, 102392. [Google Scholar] [CrossRef] [PubMed]
- Mak, A. The Impact of Vitamin D on the Immunopathophysiology, Disease Activity, and Extra-Musculoskeletal Manifestations of Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2018, 19, 2355. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.C. Vitamin D and systemic lupus erythematosus: An update. Expert. Rev. Clin. Immunol. 2013, 9, 453–463. [Google Scholar] [CrossRef]
- Stagi, S.; Rigante, D. Vitamin D and juvenile systemic lupus erythematosus: Lights, shadows and still unresolved issues. Autoimmun. Rev. 2018, 17, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Yap, K.S.; Morand, E.F. Vitamin D and systemic lupus erythematosus: Continued evolution. Int. J. Rheum. Dis. 2015, 18, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Irastorza, G.; Egurbide, M.V.; Olivares, N.; Martinez-Berriotxoa, A.; Aguirre, C. Vitamin D deficiency in systemic lupus erythematosus: Prevalence, predictors and clinical consequences. Rheumatology 2008, 47, 920–923. [Google Scholar] [CrossRef]
- Shahin, D.; El-Farahaty, R.M.; Houssen, M.E.; Machaly, S.A.; Sallam, M.; ElSaid, T.O.; Neseem, N.O. Serum 25-OH vitamin D level in treatment-naïve systemic lupus erythematosus patients: Relation to disease activity, IL-23 and IL-17. Lupus 2017, 26, 917–926. [Google Scholar] [CrossRef]
- Yao, H.H.; Tang, S.M.; Wang, Z.M.; Zhang, X.; Chen, X.Y.; Gao, L.; Liu, J.; Dai, Y.J.; Hu, Z.H.; Zhang, X.W.; et al. Study of bone mineral density and serum bone turnover markers in newly diagnosed systemic lupus erythematosus patients. Beijing Da Xue Xue Bao Yi Xue Ban 2018, 50, 998–1003. [Google Scholar] [PubMed]
- Kamen, D.L.; Cooper, G.S.; Bouali, H.; Shaftman, S.R.; Hollis, B.W.; Gilkeson, G.S. Vitamin D deficiency in systemic lupus erythematosus. Autoimmun. Rev. 2006, 5, 114–117. [Google Scholar] [CrossRef]
- Bogaczewicz, J.; Sysa-Jedrzejowska, A.; Arkuszewska, C.; Zabek, J.; Kontny, E.; McCauliffe, D.; Wozniacka, A. Vitamin D status in systemic lupus erythematosus patients and its association with selected clinical and laboratory parameters. Lupus 2012, 21, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.C.; Lee, Y.H. Association between Vitamin D level and/or deficiency, and systemic lupus erythematosus: A meta-analysis. Cell. Mol. Biol. 2018, 64, 7–13. [Google Scholar] [CrossRef]
- Sarkar, M.K.; Hile, G.A.; Tsoi, L.C.; Xing, X.; Liu, J.; Liang, Y.; Berthier, C.C.; Swindell, W.R.; Patrick, M.T.; Shao, S.; et al. Photosensitivity and type I IFN responses in cutaneous lupus are driven by epidermal-derived interferon kappa. Ann. Rheum. Dis. 2018, 77, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, J.; Marsch, A. Photosensitivity and photoprotection in patients with lupus erythematosus. Lupus 2019, 28, 697–702. [Google Scholar] [CrossRef]
- Klein, R.G.; Arnaud, S.B.; Gallagher, J.C.; Deluca, H.F.; Riggs, B.L. Intestinal calcium absorption in exogenous hypercortisonism. Role of 25-hydroxyvitamin D and corticosteroid dose. J. Clin. Invest. 1977, 60, 253–259. [Google Scholar] [CrossRef]
- O’Leary, T.J.; Jones, G.; Yip, A.; Lohnes, D.; Cohanim, M.; Yendt, E.R. The effects of chloroquine on serum 1,25-dihydroxyvitamin D and calcium metabolism in sarcoidosis. N. Engl. J. Med. 1986, 315, 727–730. [Google Scholar] [CrossRef]
- Carvalho, J.F.; Blank, M.; Kiss, E.; Tarr, T.; Amital, H.; Shoenfeld, Y. Anti-vitamin D, vitamin D in SLE: Preliminary results. Ann. N. Y. Acad. Sci. 2007, 1109, 550–557. [Google Scholar] [CrossRef]
- Julkunen, H.; Ekblom-Kullberg, S.; Miettinen, A. Nonrenal and renal activity of systemic lupus erythematosus: A comparison of two anti-C1q and five anti-dsDNA assays and complement C3 and C4. Rheumatol. Int. 2012, 32, 2445–2451. [Google Scholar] [CrossRef]
- Athanassiou, L.; Kostoglou-Athanassiou, I.; Tsakiridis, P.; Devetzi, E.; Mavroudi, M.; Fytas, P.; Koutsilieris, M.; Athanassiou, P. Vitamin D levels in Greek patients with systemic lupus erythematosus. Lupus 2022, 31, 125–132. [Google Scholar] [CrossRef]
- Abediazar, S.; Jafari-Nakhjavani, M.; Ghorbanihaghjo, A.; Shekarchi, M.; Zununi Vahed, S. Serum Levels of CXCL10 and Vitamin D in Patients with Lupus Nephritis. Iran J. Kidney Dis. 2019, 13, 389–397. [Google Scholar] [PubMed]
- Sun, J.; Zhang, S.; Liu, J.S.; Gui, M.; Zhang, H. Expression of vitamin D receptor in renal tissue of lupus nephritis and its association with renal injury activity. Lupus 2019, 28, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Ye, M.; Zhao, L.; Zou, W.; Shen, W.; Zhang, H.; Gong, J.; He, Q. The novel involvement of podocyte autophagic activity in the pathogenesis of lupus nephritis. Histol. Histopathol. 2018, 33, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Qiao, Y.; Liu, D.; Liu, F.; Gao, C.; Duan, J.; Liang, L.; Di, X.; Yuan, Y.; Gao, Y.; et al. Vitamin D protects podocytes from autoantibodies induced injury in lupus nephritis by reducing aberrant autophagy. Arthritis Res. Ther. 2019, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Monticielo, O.A.; Teixeira Tde, M.; Chies, J.A.; Brenol, J.C.; Xavier, R.M. Vitamin D and polymorphisms of VDR gene in patients with systemic lupus erythematosus. Clin. Rheumatol. 2012, 31, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- de Azevêdo Silva, J.; Monteiro Fernandes, K.; Trés Pancotto, J.A.; Sotero Fragoso, T.; Donadi, E.A.; Crovella, S.; Sandrin-Garcia, P. Vitamin D receptor (VDR) gene polymorphisms and susceptibility to systemic lupus erythematosus clinical manifestations. Lupus 2013, 22, 1110–1117. [Google Scholar] [CrossRef]
- Andreoli, L.; Dall’Ara, F.; Piantoni, S.; Zanola, A.; Piva, N.; Cutolo, M.; Tincani, A. A 24-month prospective study on the efficacy and safety of two different monthly regimens of vitamin D supplementation in pre-menopausal women with systemic lupus erythematosus. Lupus 2015, 24, 499–506. [Google Scholar] [CrossRef]
- Aranow, C.; Kamen, D.L.; Dall’Era, M.; Massarotti, E.M.; Mackay, M.C.; Koumpouras, F.; Coca, A.; Chatham, W.W.; Clowse, M.E.; Criscione-Schreiber, L.G.; et al. Randomized, Double-Blind, Placebo-Controlled Trial of the Effect of Vitamin D3 on the Interferon Signature in Patients With Systemic Lupus Erythematosus. Arthritis Rheumatol. 2015, 67, 1848–1857. [Google Scholar] [CrossRef]
- Karimzadeh, H.; Shirzadi, M.; Karimifar, M. The effect of Vitamin D supplementation in disease activity of systemic lupus erythematosus patients with Vitamin D deficiency: A randomized clinical trial. J. Res. Med. Sci. 2017, 22, 4. [Google Scholar] [CrossRef] [PubMed]
- Marinho, A.; Carvalho, C.; Boleixa, D.; Bettencourt, A.; Leal, B.; Guimarães, J.; Neves, E.; Oliveira, J.C.; Almeida, I.; Farinha, F.; et al. Vitamin D supplementation effects on FoxP3 expression in T cells and FoxP3(+)/IL-17A ratio and clinical course in systemic lupus erythematosus patients: A study in a Portuguese cohort. Immunol. Res. 2017, 65, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Al-Kushi, A.G.; Azzeh, F.S.; Header, E.A.; ElSawy, N.A.; Hijazi, H.H.; Jazar, A.S.; Ghaith, M.M.; Alarjah, M.A. Effect of Vitamin D and Calcium Supplementation in Patients with Systemic Lupus Erythematosus. Saudi J. Med. Med. Sci. 2018, 6, 137–142. [Google Scholar] [CrossRef]
- Magro, R.; Saliba, C.; Camilleri, L.; Scerri, C.; Borg, A.A. Vitamin D supplementation in systemic lupus erythematosus: Relationship to disease activity, fatigue and the interferon signature gene expression. BMC Rheumatol. 2021, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Kavadichanda, C.; Singh, P.; Maurya, S.; Tota, S.; Kiroubagarin, A.; Kounassegarane, D.; Anand, S.; Negi, V.S.; Aggarwal, A. Clinical and serological association of plasma 25-hydroxyvitamin D (25(OH)D) levels in lupus and the short-term effects of oral vitamin D supplementation. Arthritis Res. 2023, 25, 2. [Google Scholar] [CrossRef]
- Kong, W.; Tang, Y.; Tang, K.; Yan, Z.; Liu, T.; Tao, Q.; Wang, J.; Liu, J.; Yan, X. Leukemia inhibitory factor is dysregulated in ankylosing spondylitis and contributes to bone formation. Int. J. Rheum. Dis. 2022, 25, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shabat, N.; Watad, A.; Shabat, A.; Bragazzi, N.L.; Comaneshter, D.; Cohen, A.D.; Amital, H. Low Vitamin D Levels Predict Mortality in Ankylosing Spondylitis Patients: A Nationwide Population-Based Cohort Study. Nutrients 2020, 12, 1400. [Google Scholar] [CrossRef]
- Durmus, B.; Altay, Z.; Baysal, O.; Ersoy, Y. Does vitamin D affect disease severity in patients with ankylosing spondylitis? Chin. Med. J. 2012, 125, 2511–2515. [Google Scholar]
- Lange, U.; Teichmann, J.; Strunk, J.; Müller-Ladner, U.; Schmidt, K.L. Association of 1.25 vitamin D3 deficiency, disease activity and low bone mass in ankylosing spondylitis. Osteoporos. Int. 2005, 16, 1999–2004. [Google Scholar] [CrossRef]
- Cai, G.; Wang, L.; Fan, D.; Xin, L.; Liu, L.; Hu, Y.; Ding, N.; Xu, S.; Xia, G.; Jin, X.; et al. Vitamin D in ankylosing spondylitis: Review and meta-analysis. Clin. Chim. Acta 2015, 438, 316–322. [Google Scholar] [CrossRef]
- Jiang, J.; Shao, M.; Wu, X. Vitamin D and risk of ankylosing spondylitis: A two-sample mendelian randomization study. Hum. Immunol. 2022, 83, 81–85. [Google Scholar] [CrossRef]
- Touma, Z.; Eder, L.; Zisman, D.; Feld, J.; Chandran, V.; Rosen, C.F.; Shen, H.; Cook, R.J.; Gladman, D.D. Seasonal variation in vitamin D levels in psoriatic arthritis patients from different latitudes and its association with clinical outcomes. Arthritis Care Res. 2011, 63, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Kincse, G.; Bhattoa, P.H.; Herédi, E.; Varga, J.; Szegedi, A.; Kéri, J.; Gaál, J. Vitamin D3 levels and bone mineral density in patients with psoriasis and/or psoriatic arthritis. J. Derm. 2015, 42, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Gamonal, S.B.L.; Gamonal, A.C.C.; Marques, N.C.V.; Brandão, M.A.F.; Raposo, N.R.B. Is vitamin D status relevant to psoriasis and psoriatic arthritis? A retrospective cross-sectional study. Sao Paulo Med. J. 2022, 141, e2022216. [Google Scholar] [CrossRef]
- Theodoridis, X.; Grammatikopoulou, M.G.; Stamouli, E.M.; Talimtzi, P.; Pagkalidou, E.; Zafiriou, E.; Haidich, A.B.; Bogdanos, D.P. Effectiveness of oral vitamin D supplementation in lessening disease severity among patients with psoriasis: A systematic review and meta-analysis of randomized controlled trials. Nutrition 2021, 82, 111024. [Google Scholar] [CrossRef] [PubMed]
- Baldini, C.; Delle Sedie, A.; Luciano, N.; Pepe, P.; Ferro, F.; Talarico, R.; Tani, C.; Mosca, M. Vitamin D in “early” primary Sjögren’s syndrome: Does it play a role in influencing disease phenotypes? Rheumatol. Int. 2014, 34, 1159–1164. [Google Scholar] [CrossRef]
- Erten, Ş.; Şahin, A.; Altunoğlu, A.; Gemcioğlu, E.; Koca, C. Comparison of plasma vitamin D levels in patients with Sjögren’s syndrome and healthy subjects. Int. J. Rheum. Dis. 2015, 18, 70–75. [Google Scholar] [CrossRef]
- Zardi, E.M.; Basta, F.; Afeltra, A. Levels of Vitamin D, Disease Activity and Subclinical Atherosclerosis in Post-menopausal Women with Sjögren’s Syndrome: Does a Link Exist? Vivo 2016, 30, 721–725. [Google Scholar]
- Li, L.; Chen, J.; Jiang, Y. The association between vitamin D level and Sjögren’s syndrome: A meta-analysis. Int. J. Rheum. Dis. 2019, 22, 532–533. [Google Scholar] [CrossRef]
- Agmon-Levin, N.; Kivity, S.; Tzioufas, A.G.; López Hoyos, M.; Rozman, B.; Efes, I.; Shapira, Y.; Shamis, A.; Amital, H.; Youinou, P.; et al. Low levels of vitamin-D are associated with neuropathy and lymphoma among patients with Sjögren’s syndrome. J. Autoimmun. 2012, 39, 234–239. [Google Scholar] [CrossRef]
- Piera-Velazquez, S.; Wermuth, P.J.; Gomez-Reino, J.J.; Varga, J.; Jimenez, S.A. Chemical exposure-induced systemic fibrosing disorders: Novel insights into systemic sclerosis etiology and pathogenesis. Semin. Arthritis Rheum. 2020, 50, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Schinke, S.; Riemekasten, G. Systemic sclerosis. Internist 2019, 60, 1251–1269. [Google Scholar] [CrossRef] [PubMed]
- Denton, C.P. Advances in pathogenesis and treatment of systemic sclerosis. Clin. Med. 2015, 15 (Suppl. S6), s58–s63. [Google Scholar] [CrossRef] [PubMed]
- Elhai, M.; Avouac, J.; Kahan, A.; Allanore, Y. Systemic sclerosis: Recent insights. Jt. Bone Spine 2015, 82, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Pearson, D.R.; Werth, V.P.; Pappas-Taffer, L. Systemic sclerosis: Current concepts of skin and systemic manifestations. Clin. Derm. 2018, 36, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, E.; Codullo, V.; Avouac, J.; Allanore, Y. Systemic sclerosis: Recent insight in clinical management. Jt. Bone Spine 2020, 87, 293–299. [Google Scholar] [CrossRef]
- Groseanu, L.; Bojinca, V.; Gudu, T.; Saulescu, I.; Predeteanu, D.; Balanescu, A.; Berghea, F.; Opris, D.; Borangiu, A.; Constantinescu, C.; et al. Low vitamin D status in systemic sclerosis and the impact on disease phenotype. Eur. J. Rheumatol. 2016, 3, 50–55. [Google Scholar] [CrossRef]
- Runowska, M.; Majewski, D.; Majewska, K.; Puszczewicz, M. Vitamin D supply in patients with rheumatic diseases in Poland—A pilot study. Reumatologia 2021, 59, 146–152. [Google Scholar] [CrossRef]
- Arnson, Y.; Amital, H.; Agmon-Levin, N.; Alon, D.; Sánchez-Castañón, M.; López-Hoyos, M.; Matucci-Cerinic, M.; Szücs, G.; Shapira, Y.; Szekanecz, Z.; et al. Serum 25-OH vitamin D concentrations are linked with various clinical aspects in patients with systemic sclerosis: A retrospective cohort study and review of the literature. Autoimmun. Rev. 2011, 10, 490–494. [Google Scholar] [CrossRef]
- Perazzi, M.; Gallina, E.; Manfredi, G.F.; Patrucco, F.; Acquaviva, A.; Colangelo, D.; Pirisi, M.; Bellan, M. Vitamin D in Systemic Sclerosis: A Review. Nutrients 2022, 14, 3908. [Google Scholar] [CrossRef]
- Slominski, A.T.; Li, W.; Bhattacharya, S.K.; Smith, R.A.; Johnson, P.L.; Chen, J.; Nelson, K.E.; Tuckey, R.C.; Miller, D.; Jiao, Y.; et al. Vitamin D analogs 17,20S(OH)2pD and 17,20R(OH)2pD are noncalcemic and exhibit antifibrotic activity. J. Invest. Dermatol. 2011, 131, 1167–1169. [Google Scholar] [CrossRef] [PubMed]
- Zerr, P.; Vollath, S.; Palumbo-Zerr, K.; Tomcik, M.; Huang, J.; Distler, A.; Beyer, C.; Dees, C.; Gela, K.; Distler, O.; et al. Vitamin D receptor regulates TGF-β signalling in systemic sclerosis. Ann. Rheum. Dis. 2015, 74, e20. [Google Scholar] [CrossRef] [PubMed]
- Di Liberto, D.; Scazzone, C.; La Rocca, G.; Cipriani, P.; Lo Pizzo, M.; Ruscitti, P.; Agnello, L.; Ciaccio, M.; Dieli, F.; Giacomelli, R.; et al. Vitamin D increases the production of IL-10 by regulatory T cells in patients with systemic sclerosis. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S119), 76–81. [Google Scholar]
- Atteritano, M.; Sorbara, S.; Bagnato, G.; Miceli, G.; Sangari, D.; Morgante, S.; Visalli, E. Bone mineral density, bone turnover markers and fractures in patients with systemic sclerosis: A case control study. PLoS ONE 2013, 8, e66991. [Google Scholar] [CrossRef]
- Atteritano, M.; Santoro, D.; Corallo, G.; Visalli, E.; Buemi, M.; Catalano, A.; Lasco, A.; Bitto, A.; Squadrito, F. Skin Involvement and Pulmonary Hypertension Are Associated with Vitamin D Insufficiency in Scleroderma. Int. J. Mol. Sci. 2016, 17, 2103. [Google Scholar] [CrossRef]
- Corrado, A.; Colia, R.; Mele, A.; Di Bello, V.; Trotta, A.; Neve, A.; Cantatore, F.P. Relationship between Body Mass Composition, Bone Mineral Density, Skin Fibrosis and 25(OH) Vitamin D Serum Levels in Systemic Sclerosis. PLoS ONE 2015, 10, e0137912. [Google Scholar] [CrossRef]
- Zhang, L.; Duan, Y.; Zhang, T.P.; Huang, X.L.; Li, B.Z.; Ye, D.Q.; Wang, J. Association between the serum level of vitamin D and systemic sclerosis in a Chinese population: A case control study. Int. J. Rheum. Dis. 2017, 20, 1002–1008. [Google Scholar] [CrossRef]
- Ahmadi, R.; Hajialilo, M.; Ghorbanihaghjo, A.; Mota, A.; Raeisi, S.; Bargahi, N.; Valilo, M.; Askarian, F. FGF-23, Klotho and Vitamin D Levels in Scleroderma. Iran J. Public Health 2017, 46, 530–536. [Google Scholar]
- Hajialilo, M.; Noorabadi, P.; Tahsini Tekantapeh, S.; Malek Mahdavi, A. Endothelin-1, α-Klotho, 25(OH) Vit D levels and severity of disease in scleroderma patients. Rheumatol. Int. 2017, 37, 1651–1657. [Google Scholar] [CrossRef]
- Gupta, S.; Mahajan, V.K.; Yadav, R.S.; Mehta, K.S.; Bhushan, S.; Chauhan, P.S.; Rawat, R.; Sharma, V. Evaluation of Serum Vitamin D Levels in Patients with Systemic Sclerosis and Healthy Controls: Results of a Pilot Study. Indian Derm. Online J. 2018, 9, 250–255. [Google Scholar] [CrossRef]
- Hax, V.; Gasparin, A.A.; Schneider, L.; Monticielo, O.A.; Soares, H.M.F.; Streit, M.D.A.; Pfaffenseller, B.; Xavier, R.M.; Chakr, R. Vitamin D and Cytokine Profiles in Patients with Systemic Sclerosis. J. Clin. Rheumatol. 2020, 26, 289–294. [Google Scholar] [CrossRef]
- Horváth, Á.; Végh, E.; Pusztai, A.; Pethő, Z.; Hamar, A.; Czókolyová, M.; Bhattoa, H.P.; Nagy, G.; Juhász, B.; Hodosi, K.; et al. Complex assessment of bone mineral density, fracture risk, vitamin D status, and bone metabolism in Hungarian systemic sclerosis patients. Arthritis Res. 2019, 21, 274. [Google Scholar] [CrossRef] [PubMed]
- Kotyla, P.J.; Kruszec-Zytniewska, A.; Owczarek, A.J.; Olszanecka-Glinianowicz, M.; Chudek, J. Fibroblast Growth Factor 23 to Alpha-Klotho Index Correlates with Systemic Sclerosis Activity: A Proposal for Novel Disease Activity Marker. J. Clin. Med. 2018, 7, 558. [Google Scholar] [CrossRef] [PubMed]
- Seriolo, B.; Molfetta, L.; Cutolo, M. Seasonal variations in serum levels of 25-hydroxyvitamin D in patients with systemic sclerosis. Clin. Rheumatol. 2011, 30, 445–446. [Google Scholar] [CrossRef] [PubMed]
- Vacca, A.; Cormier, C.; Piras, M.; Mathieu, A.; Kahan, A.; Allanore, Y. Vitamin D deficiency and insufficiency in 2 independent cohorts of patients with systemic sclerosis. J. Rheumatol. 2009, 36, 1924–1929. [Google Scholar] [CrossRef]
- Carmel, N.N.; Rotman-Pikielny, P.; Lavrov, A.; Levy, Y. Vitamin D Antibodies in Systemic Sclerosis Patients: Findings and Clinical Correlations. Isr. Med. Assoc. J. 2015, 17, 80–84. [Google Scholar]
- An, L.; Sun, M.H.; Chen, F.; Li, J.R. Vitamin D levels in systemic sclerosis patients: A meta-analysis. Drug Des. Devel. 2017, 11, 3119–3125. [Google Scholar] [CrossRef]
- Diaconu, A.D.; Ostafie, I.; Ceasovschih, A.; Șorodoc, V.; Lionte, C.; Ancuța, C.; Șorodoc, L. Role of Vitamin D in Systemic Sclerosis: A Systematic Literature Review. J. Immunol. Res. 2021, 2021, 9782994. [Google Scholar] [CrossRef]
- Caimmi, C.; Bertoldo, E.; Pozza, A.; Caramaschi, P.; Orsolini, G.; Gatti, D.; Rossini, M.; Viapiana, O. Vitamin D serum levels and the risk of digital ulcers in systemic sclerosis: A longitudinal study. Int. J. Rheum. Dis. 2019, 22, 1041–1045. [Google Scholar] [CrossRef]
- Park, E.K.; Park, J.H.; Kweon, S.M.; Kim, G.T.; Lee, S.G. Vitamin D deficiency is associated with digital ulcer but not with atherosclerosis or arterial stiffness in patients with systemic sclerosis: A pilot study. Clin. Rheumatol. 2017, 36, 1325–1333. [Google Scholar] [CrossRef]
- Bose, N.; Chiesa-Vottero, A.; Chatterjee, S. Scleroderma renal crisis. Semin. Arthritis Rheum. 2015, 44, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Rios-Fernández, R.; Callejas-Rubio, J.L.; Fernández-Roldán, C.; Simeón-Aznar, C.P.; García-Hernández, F.; Castillo-García, M.J.; Fonollosa Pla, V.; Barnosi Marín, A.C.; González-Gay, M.; Ortego-Centeno, N. Bone mass and vitamin D in patients with systemic sclerosis from two Spanish regions. Clin. Exp. Rheumatol. 2012, 30, 905–911. [Google Scholar] [PubMed]
- Ibn Yacoub, Y.; Amine, B.; Laatiris, A.; Wafki, F.; Znat, F.; Hajjaj-Hassouni, N. Bone density in Moroccan women with systemic scleroderma and its relationships with disease-related parameters and vitamin D status. Rheumatol. Int. 2012, 32, 3143–3148. [Google Scholar] [CrossRef] [PubMed]
- Giuggioli, D.; Colaci, M.; Cassone, G.; Fallahi, P.; Lumetti, F.; Spinella, A.; Campomori, F.; Manfredi, A.; Manzini, C.U.; Antonelli, A.; et al. Serum 25-OH vitamin D levels in systemic sclerosis: Analysis of 140 patients and review of the literature. Clin. Rheumatol. 2017, 36, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Azali, P.; Barbasso Helmers, S.; Kockum, I.; Olsson, T.; Alfredsson, L.; Charles, P.J.; Piehl Aulin, K.; Lundberg, I.E. Low serum levels of vitamin D in idiopathic inflammatory myopathies. Ann. Rheum. Dis. 2013, 72, 512–516. [Google Scholar] [CrossRef]
- Cox, M.; Sandler, R.D.; Matucci-Cerinic, M.; Hughes, M. Bone health in idiopathic inflammatory myopathies. Autoimmun. Rev. 2021, 20, 102782. [Google Scholar] [CrossRef] [PubMed]
- Piantoni, S.; Andreoli, L.; Scarsi, M.; Zanola, A.; Dall’Ara, F.; Pizzorni, C.; Cutolo, M.; Airò, P.; Tincani, A. Phenotype modifications of T-cells and their shift toward a Th2 response in patients with systemic lupus erythematosus supplemented with different monthly regimens of vitamin D. Lupus 2015, 24, 490–498. [Google Scholar] [CrossRef]
- Andjelkovic, Z.; Vojinovic, J.; Pejnovic, N.; Popovic, M.; Dujic, A.; Mitrovic, D.; Pavlica, L.; Stefanovic, D. Disease modifying and immunomodulatory effects of high dose 1 alpha (OH) D3 in rheumatoid arthritis patients. Clin. Exp. Rheumatol. 1999, 17, 453–456. [Google Scholar]
- Gopinath, K.; Danda, D. Supplementation of 1,25 dihydroxy vitamin D3 in patients with treatment naive early rheumatoid arthritis: A randomised controlled trial. Int. J. Rheum. Dis. 2011, 14, 332–339. [Google Scholar] [CrossRef]
- Salesi, M.; Farajzadegan, Z. Efficacy of vitamin D in patients with active rheumatoid arthritis receiving methotrexate therapy. Rheumatol. Int. 2012, 32, 2129–2133. [Google Scholar] [CrossRef]
- Dehghan, A.; Rahimpour, S.; Soleymani-Salehabadi, H.; Owlia, M.B. Role of vitamin D in flare ups of rheumatoid arthritis. Z Rheumatol. 2014, 73, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.E.; Bartels, C.M.; Gangnon, R.E.; Jones, A.N.; Gogineni, J. An evaluation of high-dose vitamin D for rheumatoid arthritis. J. Clin. Rheumatol. 2014, 20, 112–114. [Google Scholar] [CrossRef]
- Chandrashekara, S.; Patted, A. Role of vitamin D supplementation in improving disease activity in rheumatoid arthritis: An exploratory study. Int. J. Rheum. Dis. 2017, 20, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Lahiry, S.; Thakur, S.; Chakraborty, D.S. Effect of 1,25 dihydroxy vitamin D3 supplementation on pain relief in early rheumatoid arthritis. J. Fam. Med. Prim. Care 2019, 8, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.C.; Oh, J.S.; Park, M.C.; Kim, Y.G. Effect of Vitamin D Supplementation on Bone Mineral Density in Rheumatoid Arthritis Patients With Osteoporosis. Front. Med. 2020, 7, 443. [Google Scholar] [CrossRef] [PubMed]
- Di Franco, M.; Barchetta, I.; Iannuccelli, C.; Gerardi, M.C.; Frisenda, S.; Ceccarelli, F.; Valesini, G.; Cavallo, M.G. Hypovitaminosis D in recent onset rheumatoid arthritis is predictive of reduced response to treatment and increased disease activity: A 12 month follow-up study. BMC Musculoskelet. Disord. 2015, 16, 53. [Google Scholar] [CrossRef]
- Guan, Y.; Hao, Y.; Bu, H.; Wang, H. The Effect of Vitamin D Supplementation on Rheumatoid Arthritis Patients: A Systematic Review and Meta-Analysis. Front. Med. 2020, 7, 596007. [Google Scholar] [CrossRef]
- Franco, A.S.; Freitas, T.Q.; Bernardo, W.M.; Pereira, R.M.R. Vitamin D supplementation and disease activity in patients with immune-mediated rheumatic diseases: A systematic review and meta-analysis. Medicine 2017, 96, e7024. [Google Scholar] [CrossRef]
- Singgih Wahono, C.; Diah Setyorini, C.; Kalim, H.; Nurdiana, N.; Handono, K. Effect of Curcuma xanthorrhiza Supplementation on Systemic Lupus Erythematosus Patients with Hypovitamin D Which Were Given Vitamin D(3) towards Disease Activity (SLEDAI), IL-6, and TGF-β1 Serum. Int. J. Rheumatol. 2017, 2017, 7687053. [Google Scholar] [CrossRef] [PubMed]
- Piemonti, L.; Monti, P.; Sironi, M.; Fraticelli, P.; Leone, B.E.; Dal Cin, E.; Allavena, P.; Di Carlo, V. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J. Immunol. 2000, 164, 4443–4451. [Google Scholar] [CrossRef]
- Weeres, M.A.; Robien, K.; Ahn, Y.O.; Neulen, M.L.; Bergerson, R.; Miller, J.S.; Verneris, M.R. The effects of 1,25-dihydroxyvitamin D3 on in vitro human NK cell development from hematopoietic stem cells. J. Immunol. 2014, 193, 3456–3462. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and immune function: Autocrine, paracrine or endocrine? Scand. J. Clin. Lab. Invest. Suppl. 2012, 243, 92–102. [Google Scholar] [PubMed]
- Lemire, J.M.; Archer, D.C.; Beck, L.; Spiegelberg, H.L. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: Preferential inhibition of Th1 functions. J. Nutr. 1995, 125, 1704s–1708s. [Google Scholar]
- Mocanu, V.; Oboroceanu, T.; Zugun-Eloae, F. Current status in vitamin D and regulatory T cells—Immunological implications. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2013, 117, 965–973. [Google Scholar] [PubMed]
- Yamamoto, E.A.; Jørgensen, T.N. Relationships Between Vitamin D, Gut Microbiome, and Systemic Autoimmunity. Front. Immunol. 2019, 10, 3141. [Google Scholar] [CrossRef]
- Mangin, M.; Sinha, R.; Fincher, K. Inflammation and vitamin D: The infection connection. Inflamm. Res. 2014, 63, 803–819. [Google Scholar] [CrossRef] [PubMed]
- Querfeld, U. Vitamin D and inflammation. Pediatr. Nephrol. 2013, 28, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D for skeletal and non-skeletal health: What we should know. J. Clin. Orthop. Trauma 2019, 10, 1082–1093. [Google Scholar] [CrossRef]
- Islam, M.Z.; Bhuiyan, N.H.; Akhtaruzzaman, M.; Allardt, C.L.; Fogelholm, M. Vitamin D deficiency in Bangladesh: A review of prevalence, causes and recommendations for mitigation. Asia Pac. J. Clin. Nutr. 2022, 31, 167–180. [Google Scholar] [CrossRef]
- Carlberg, C. Vitamin D and Pigmented Skin. Nutrients 2022, 14, 325. [Google Scholar] [CrossRef]
- Alfredsson, L.; Armstrong, B.K.; Butterfield, D.A.; Chowdhury, R.; de Gruijl, F.R.; Feelisch, M.; Garland, C.F.; Hart, P.H.; Hoel, D.G.; Jacobsen, R.; et al. Insufficient Sun Exposure Has Become a Real Public Health Problem. Int. J. Env. Res. Public Health 2020, 17, 5014. [Google Scholar] [CrossRef] [PubMed]
- Luxwolda, M.F.; Kuipers, R.S.; Kema, I.P.; van der Veer, E.; Dijck-Brouwer, D.A.; Muskiet, F.A. Vitamin D status indicators in indigenous populations in East Africa. Eur. J. Nutr. 2013, 52, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Nutrigenomics of Vitamin D. Nutrients 2019, 11, 676. [Google Scholar] [CrossRef] [PubMed]
| Authors (Date) | Type of Study | Study Population | Results |
|---|---|---|---|
| Andjelkovic et al. (1999) [218] | Open label | RA = 19 | Improvement of clinical indices |
| Gopinath and Danda (2011) [219] | Open label | RA = 121 | Improved pain relief |
| Salesi et al. (2012) [220] | Double-blind | RA = 117 | Improved DAS28 |
| Dheghan et al. (2014) [221] | RCT | RA = 80 | No improvement in relapse rate |
| Hansen et al. (2014) [222] | RCT | RA = 22 | Improved bone formation |
| Buondonno et al. (2017) [86] | RCT | RA = 70 | Reduced inflammatory cytokines |
| Chandrashekara and Patted (2017) [223] | Open label | RA = 73 | Improved DAS28, CRP |
| Mukherjee et al. (2019) [224] | Open label | RA = 25 | Improved pain |
| Kwon et al. (2020) [225] | Open label | RA with osteoporosis = 187 | Improved bone mineral density |
| Authors (Date) | Type of Study | Study Population | Results |
|---|---|---|---|
| Andreoli et al. (2015) [159] | Randomized prospective study | SLE = 34 female | No significant effect on clinical outcomes |
| Aranow et al. (2015) [160] | Double-blind placebo controlled | SLE = 54 | No significant effects |
| Karimzadeh et al. (2017) [161] | Double-blind placebo controlled | SLE = 90 | No significant effects |
| Marinho et al. (2017) [162] | Prospective cross-sectional study | SLE = 24 | Decreased SLEDAI, immunologic effects |
| Al-Kushi et al. (2018) [163] | RCT | SLE = 81 | Improved osteopenia osteoporosis |
| Magro et al. (2021) [164] | Open label | SLE = 31 | Improved disease activity, fatique |
| Kavadichanda et al. (2023) [165] | Cross sectional study | SLE = 702 | No significant effects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athanassiou, L.; Kostoglou-Athanassiou, I.; Koutsilieris, M.; Shoenfeld, Y. Vitamin D and Autoimmune Rheumatic Diseases. Biomolecules 2023, 13, 709. https://doi.org/10.3390/biom13040709
Athanassiou L, Kostoglou-Athanassiou I, Koutsilieris M, Shoenfeld Y. Vitamin D and Autoimmune Rheumatic Diseases. Biomolecules. 2023; 13(4):709. https://doi.org/10.3390/biom13040709
Chicago/Turabian StyleAthanassiou, Lambros, Ifigenia Kostoglou-Athanassiou, Michael Koutsilieris, and Yehuda Shoenfeld. 2023. "Vitamin D and Autoimmune Rheumatic Diseases" Biomolecules 13, no. 4: 709. https://doi.org/10.3390/biom13040709
APA StyleAthanassiou, L., Kostoglou-Athanassiou, I., Koutsilieris, M., & Shoenfeld, Y. (2023). Vitamin D and Autoimmune Rheumatic Diseases. Biomolecules, 13(4), 709. https://doi.org/10.3390/biom13040709








