Further Investigations of Nitroheterocyclic Compounds as Potential Antikinetoplastid Drug Candidates
Abstract
1. Introduction
1.1. Human African Trypanosomiasis (HAT)
1.2. American Trypanosomiasis (Chagas Disease)
1.3. Leishmaniasis
2. Current Treatments against Trypanosomatids
2.1. Current Drug Treatments against American Trypanosomiasis
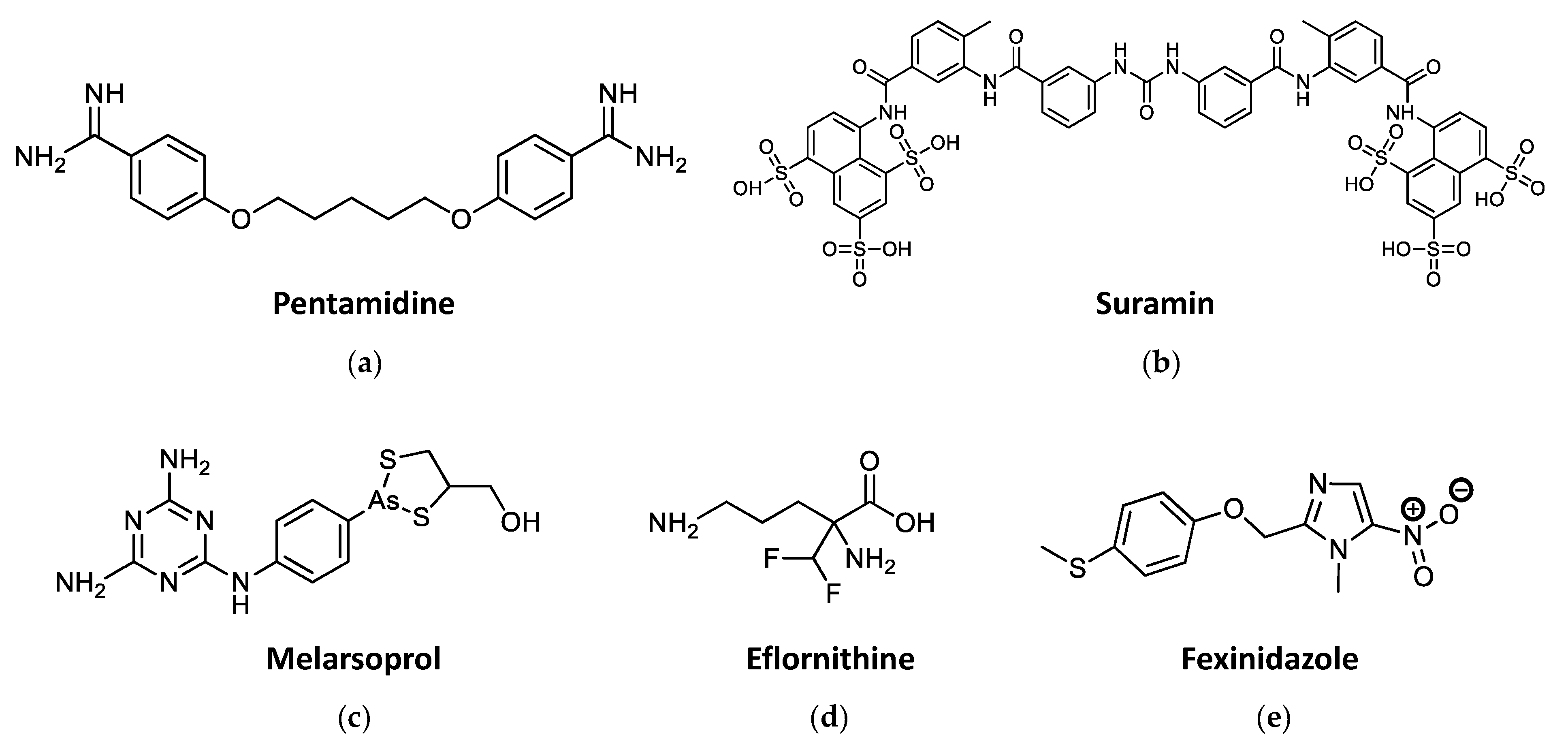
2.2. Current Drug Treatments against HAT
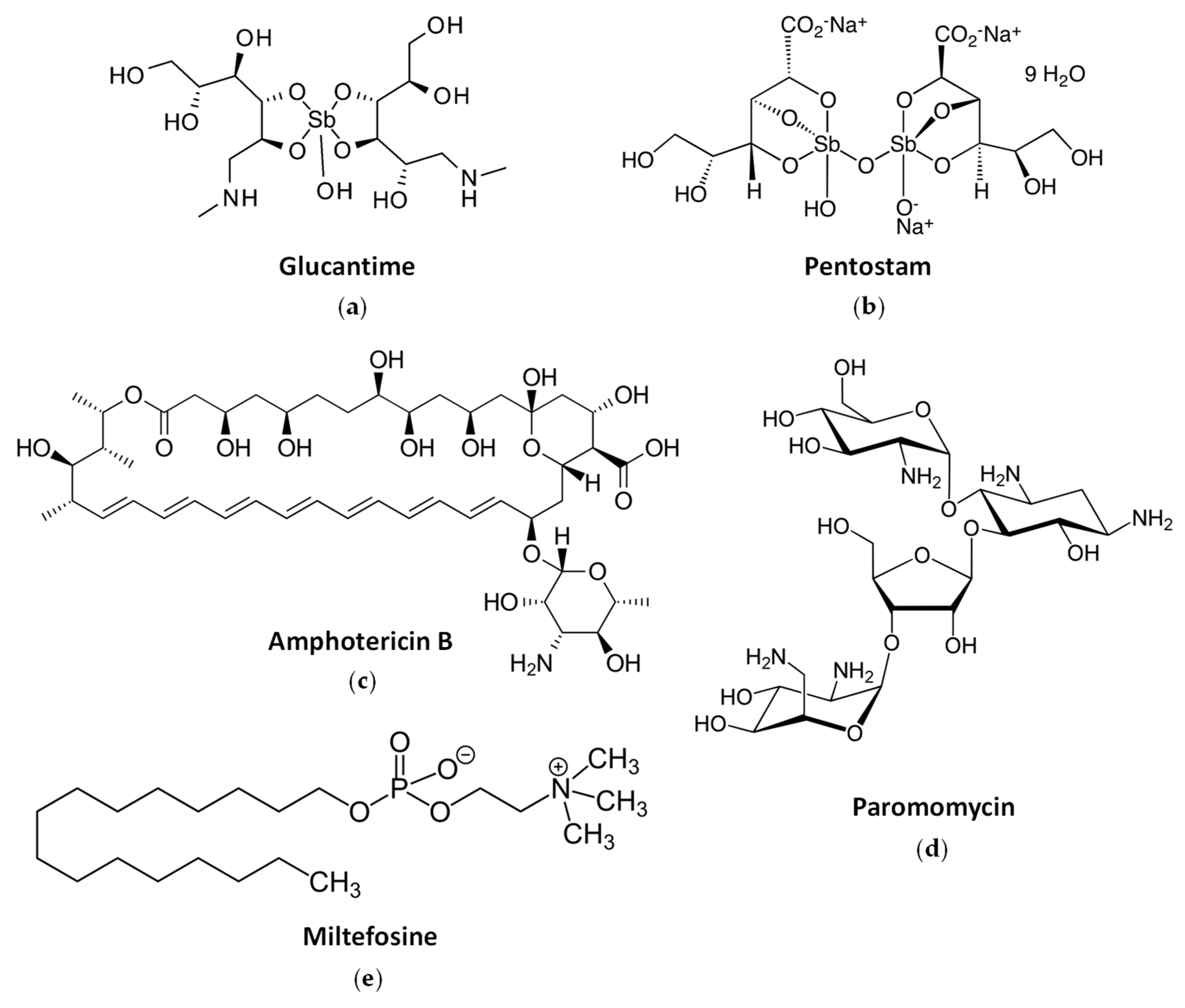
2.3. Current Drug Treatments against Leishmaniasis
2.4. Drug Resistance to Current Pharmacology
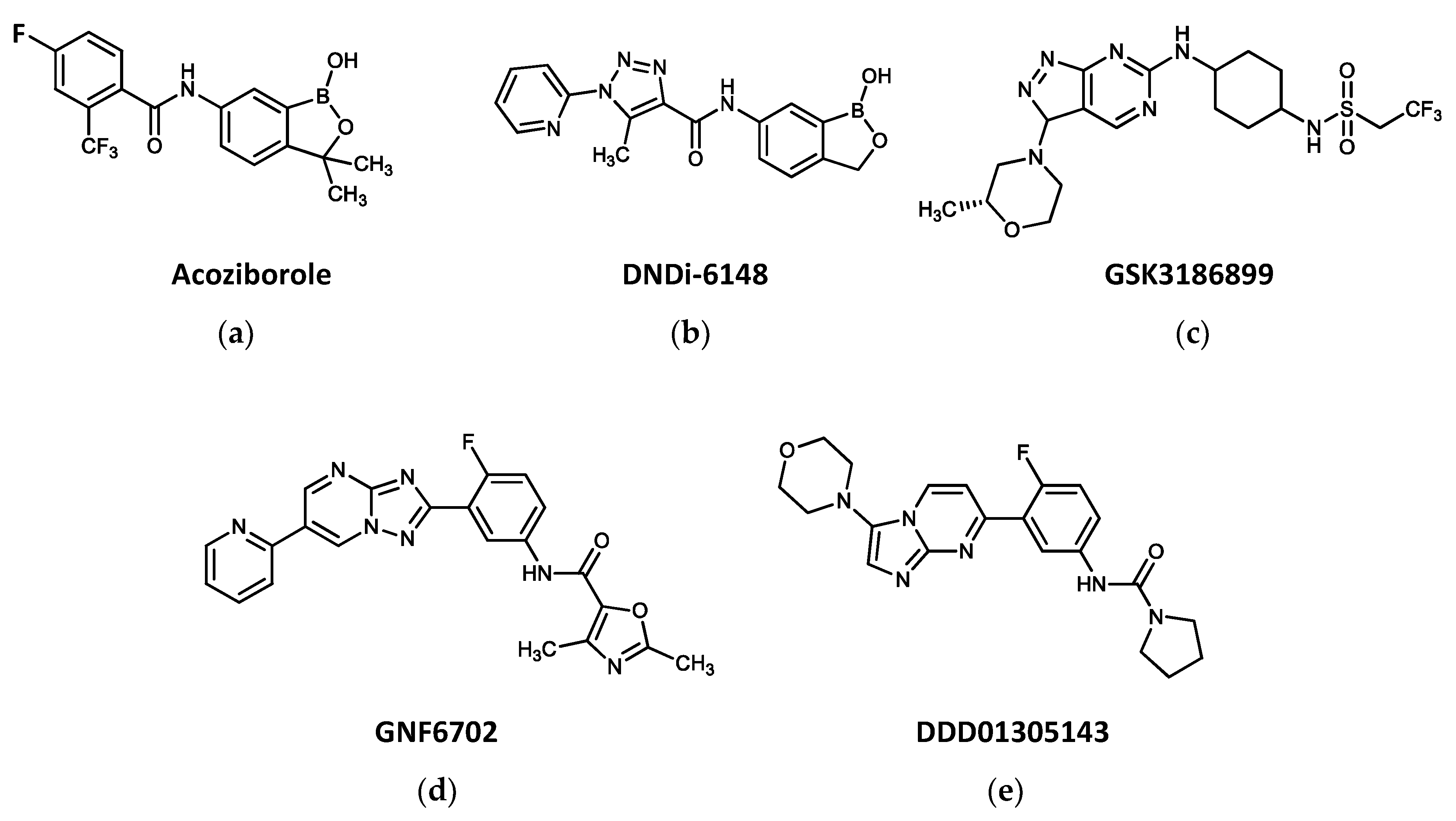
3. New Drugs Entering Clinical Trials against Trypanosomatid-Borne Diseases
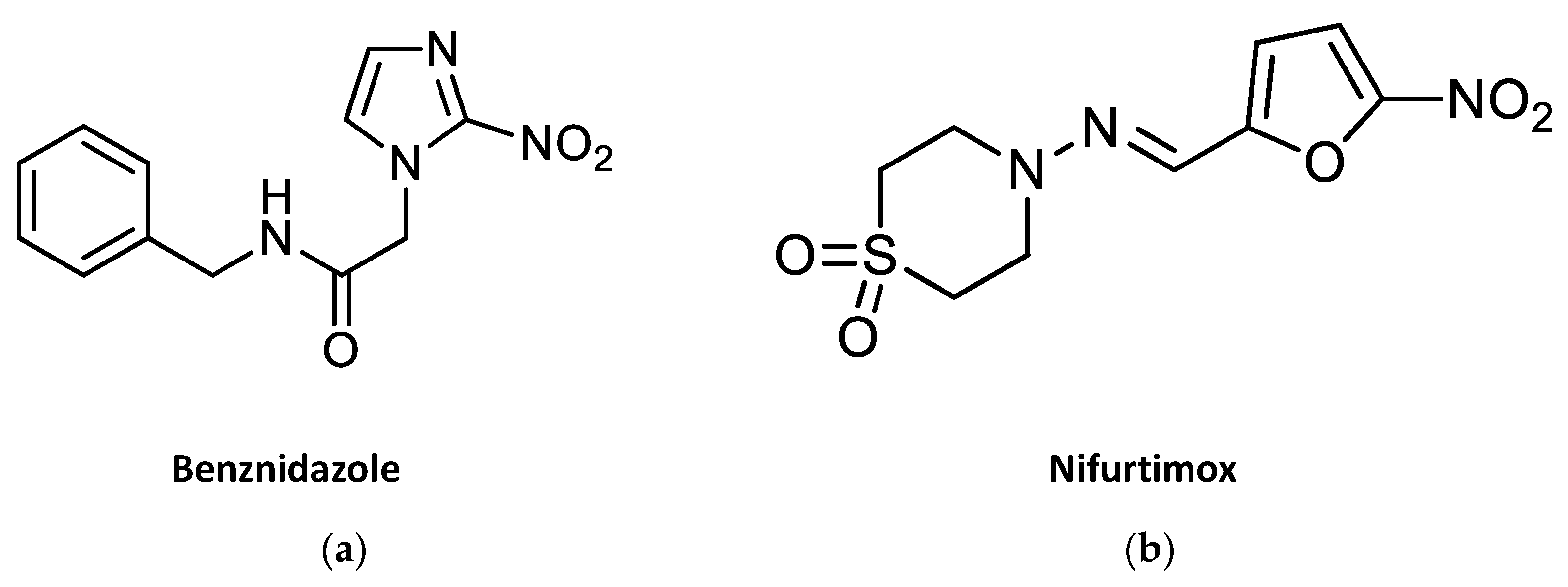
4. Development of Nitroheterocyclic Drugs against Trypanosomes
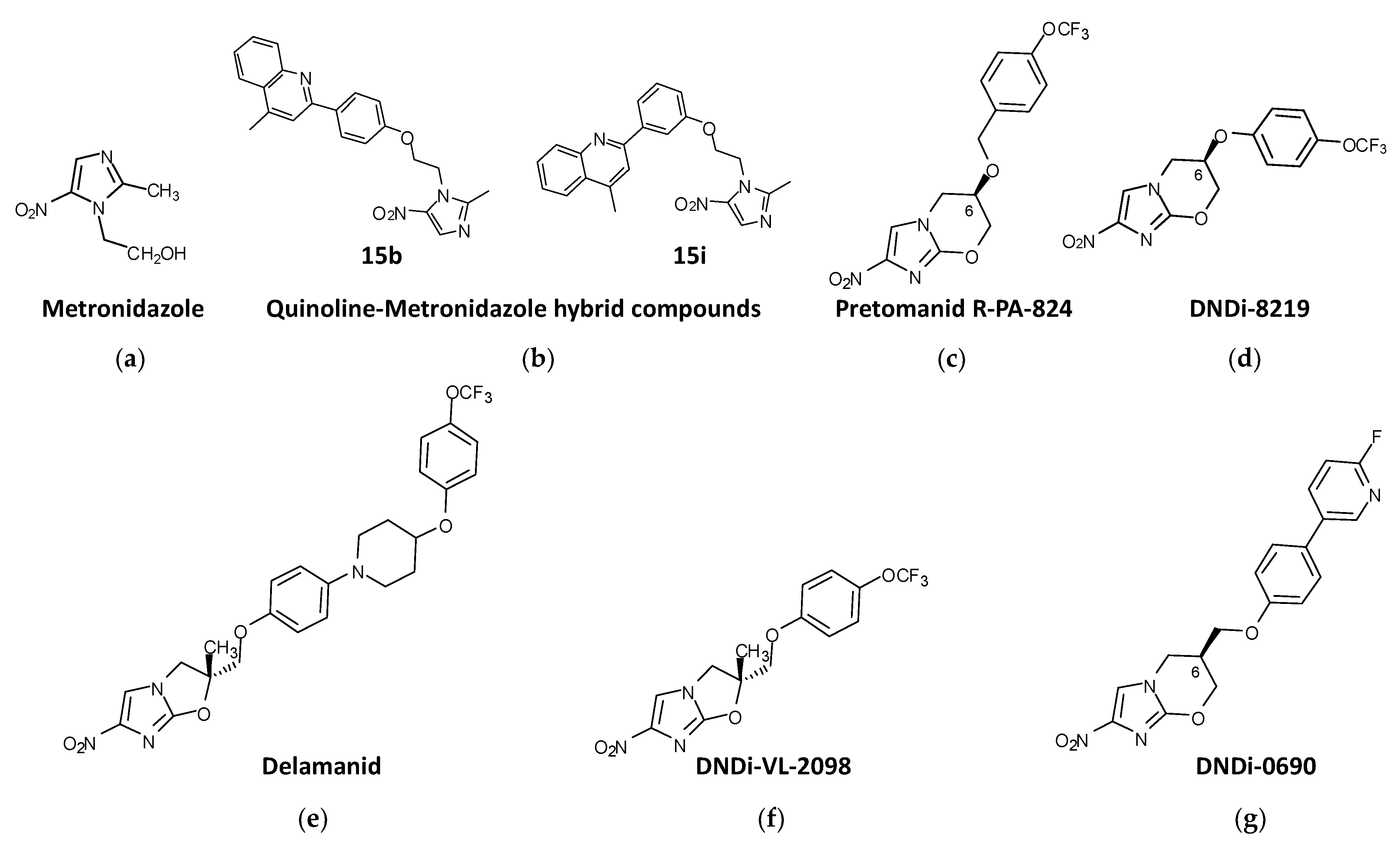
4.1. Nitroimidazoles
4.1.1. Fexinidazole
4.1.2. Metronidazole Derivatives
4.1.3. Nitroimidazo-Oxazines and Nitroimidazo-Oxazoles
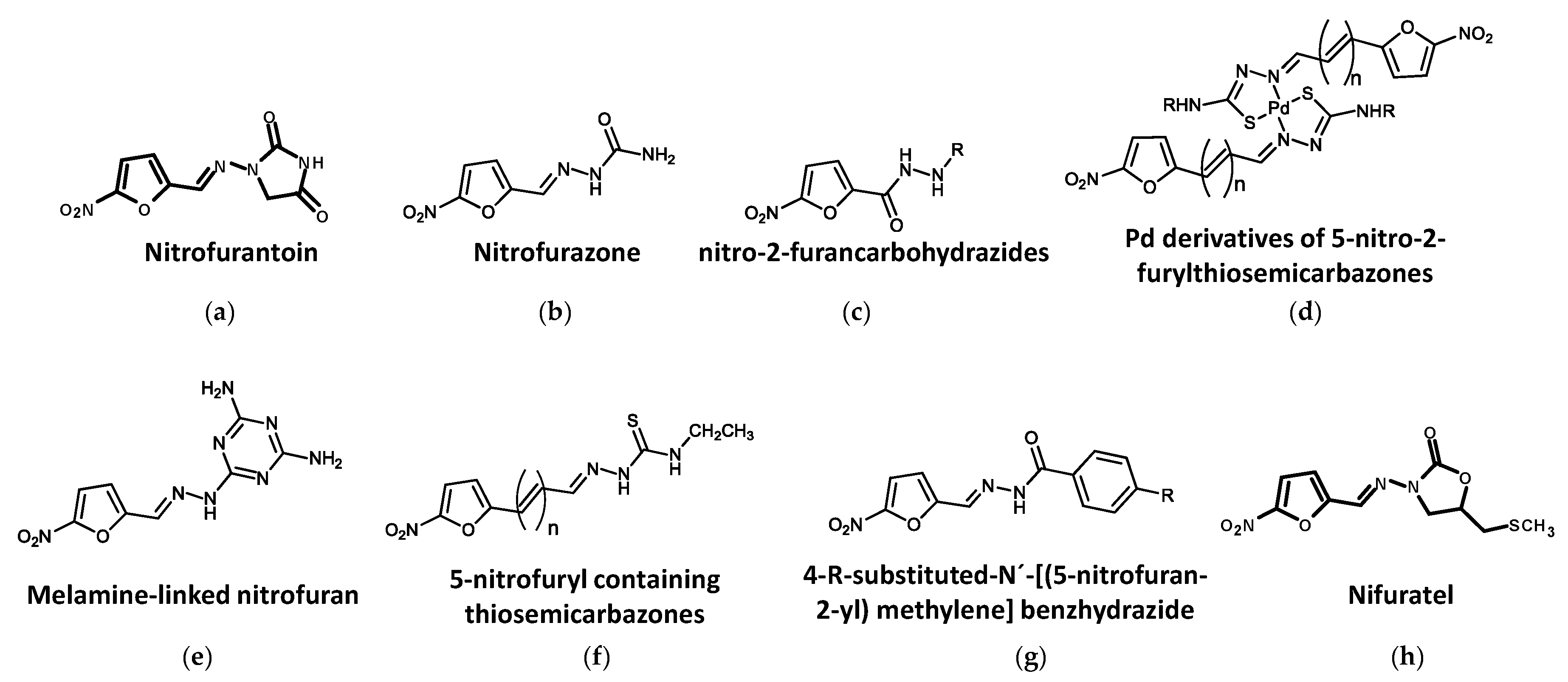
4.2. Nitrofurans
4.2.1. Nitrofurantoin
4.2.2. Nitrofurazone
4.2.3. Other Nitrofuran Derivatives
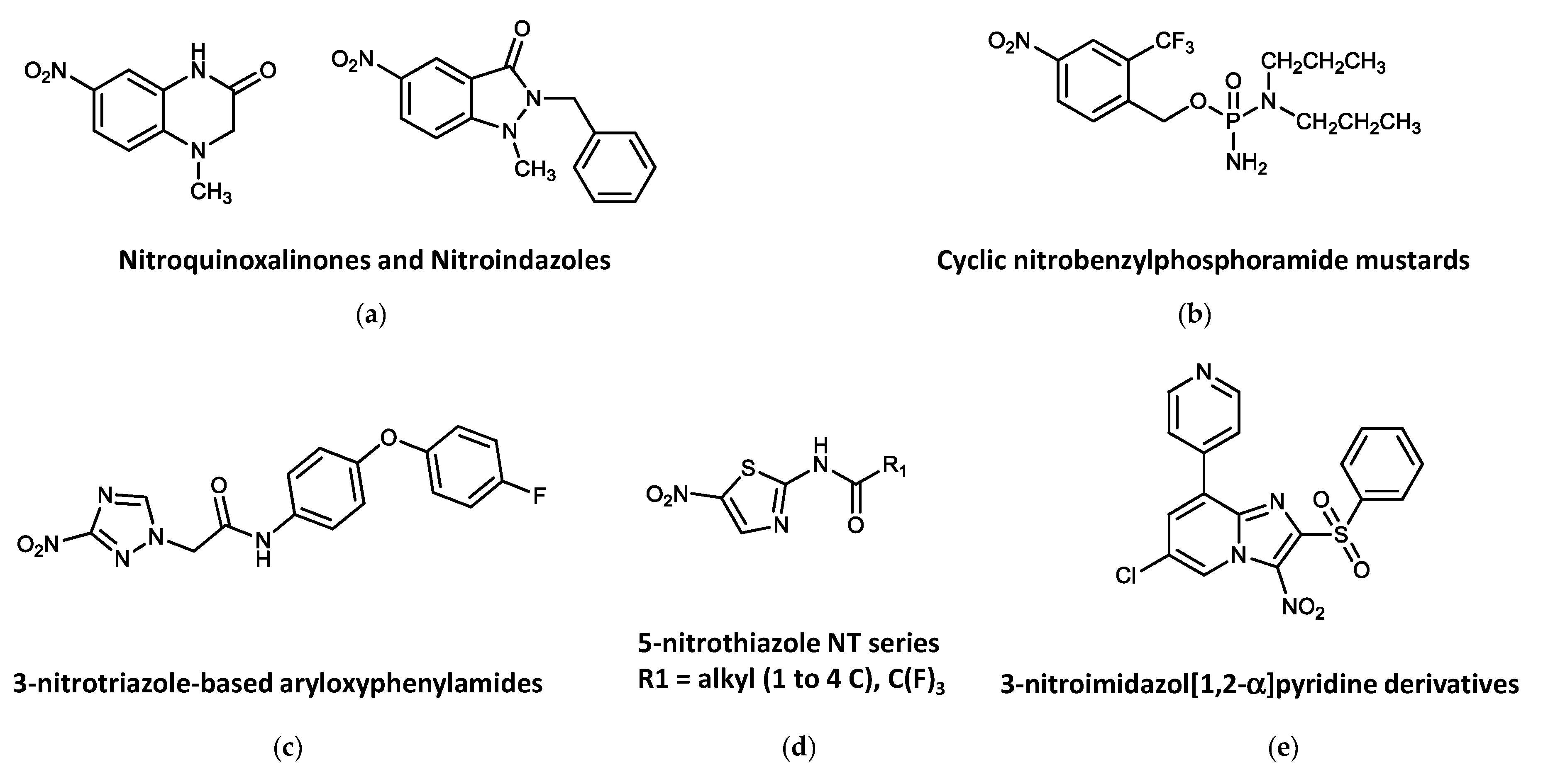
4.3. Other Nitroheterocycles
5. Activation of Nitroheterocyclic Compounds as a Key Factor in Their Use as Antitrypanosomatid Drugs
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global leishmaniasis surveillance, 2017–2018, and first report on 5 additional indicators. Wkly. Epidemiol. Rec. 2020, 95, 265–280. [Google Scholar]
- Field, M.C.; Horn, D.; Fairlamb, A.H.; Ferguson, M.A.; Gray, D.W.; Read, K.D.; De Rycker, M.; Torrie, L.S.; Wyatt, P.G.; Wyllie, S.; et al. Anti-trypanosomatid drug discovery: An ongoing challenge and a continuing need. Nat. Rev. Microbiol. 2017, 15, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Hedley, L.; Fink, D.; Sparkes, D.; Chiodini, P.L. African sleeping sickness. Br. J. Hosp. Med. 2016, 77, C157–C160. [Google Scholar] [CrossRef]
- Franco, J.R.; Cecchi, G.; Priotto, G.; Paone, M.; Diarra, A.; Grout, L.; Simarro, P.P.; Zhao, W.; Argaw, D. Monitoring the elimination of human African trypanosomiasis at continental and country level: Update to 2018. PLoS Negl. Trop. Dis. 2020, 14, e0008261. [Google Scholar] [CrossRef]
- Franco, J.R.; Cecchi, G.; Paone, M.; Diarra, A.; Grout, L.; Kadima Ebeja, A.; Simarro, P.P.; Zhao, W.; Argaw, D. The elimination of human African trypanosomiasis: Achievements in relation to WHO road map targets for 2020. PLoS Negl. Trop. Dis. 2022, 16, e0010047. [Google Scholar] [CrossRef]
- Hackett, F.; Berrang Ford, L.; Fèvre, E.; Simarro, P. Incorporating scale dependence in disease burden estimates: The case of human African trypanosomiasis in Uganda. PLoS Negl. Trop. Dis. 2014, 8, e2704. [Google Scholar] [CrossRef]
- GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef]
- Brun, R.; Blum, J.; Chappuis, F.; Burri, C. Human African trypanosomiasis. Lancet 2010, 375, 148–159. [Google Scholar] [CrossRef]
- Pérez-Molina, J.A.; Molina, I. Chagas disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef]
- Echevarría, L.E.; Morillo, C.A. American trypanosomiasis (Chagas Disease). Infect. Dis. Clin. N. Am. 2019, 33, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Noya, B.A.; Díaz-Bello, Z.; Colmenares, C.; Ruiz-Guevara, R.; Mauriello, L.; Muñoz-Calderón, A.; Noya, O. Update on oral Chagas disease outbreaks in Venezuela: Epidemiological, clinical and diagnostic approaches. Memórias Inst. Oswaldo Cruz 2011, 110, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Monge-Maillo, B.; López-Vélez, R. Challenges in the management of Chagas disease in Latin-American migrants in Europe. Clin. Microbiol. Infect. 2017, 23, 290–295. [Google Scholar] [CrossRef]
- WHO. Chagas disease in Latin America: An epidemiological update based on 2010 estimates. Wkly. Epidemiol. Rec. 2015, 6, 33–44. [Google Scholar]
- Chatelain, E. Chagas disease research and development: Is there light at the end of the tunnel? Comput. Struct. Biotechnol. J. 2016, 15, 98–103. [Google Scholar] [CrossRef]
- Chadalawada, S.; Sillau, S.; Archuleta, S.; Mundo, W.; Bandali, M.; Parra-Henao, G.; Rodríguez-Morales, A.J.; Villamil-Gómez, W.E.; Suárez, J.A.; Shapiro, L.; et al. Risk of chronic cardiomyopathy among patients with the acute phase or indeterminate form of Chagas disease: A systematic review and meta-analysis. JAMA Network Open 2020, 3, e2015072. [Google Scholar] [CrossRef] [PubMed]
- Jabari, S.; de Oliveira, E.C.; Brehmer, A.; da Silveira, A.B. Chagasic megacolon: Enteric neurons and related structures. Histochem. Cell Biol. 2014, 142, 235–244. [Google Scholar] [CrossRef]
- Abadías-Granado, I.; Diago, A.; Cerro, P.A.; Palma-Ruiz, A.M.; Gilaberte, Y. Cutaneous and mucocutaneous leishmaniasis. Actas Dermosifiliogr. 2021, 112, 601–618. [Google Scholar] [CrossRef]
- Caridha, D.; Vesely, B.; van Bocxlaer, K.; Arana, B.; Mowbray, C.E.; Rafati, S.; Uliana, S.; Reguera, R.; Kreishman-Deitrick, M.; Sciotti, R.; et al. Route map for the discovery and pre-clinical development of new drugs and treatments for cutaneous leishmaniasis. Int. J. Parasitol. Drugs Drug Resist. 2019, 11, 106–117. [Google Scholar] [CrossRef]
- Sarmento, V.A.; Falcão, G.G.V.S.C.; Lins-Kusterer, L.; Leite-Ribeiro, P.M. Orofacial manifestations of mucocutaneous leishmaniasis: A case series from Brazil. F1000Res 2020, 8, 756. [Google Scholar]
- Van Griensven, J.; Diro, E. Visceral leishmaniasis. Infect. Di.s Clin. N. Am. 2012, 26, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Das, N.K.; Barbhuiya, J.N.; Chatterjee, M. Post-kala-azar dermal leishmaniasis-an overview. Int. J. Dermatol. 2010, 49, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Banjara, M.R.; Joshi, A.B. Evidence for visceral leishmaniasis elimination in Nepal. Lancet Glob. Health 2020, 8, e161–e162. [Google Scholar] [CrossRef]
- Villar, J.C.; Pérez, J.G.; Cortés, O.L.; Riarte, A.; Pepper, M.; Marin-Neto, J.A.; Guyatt, G.H. Trypanocidal drugs for chronic asymptomatic Trypanosoma cruzi infection. Cochrane Database Syst. Rev. 2014, 2014, CD003463. [Google Scholar] [CrossRef]
- Malone, C.J.; Nevis, I.; Fernández, E.; Sánchez, A. A rapid review on the efficacy and safety of pharmacological treatments for Chagas disease. Trop. Med. Infect. Dis. 2021, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Mansoldo, F.R.P.; Carta, F.; Angeli, A.; Cardoso, V.D.S.; Supuran, C.T.; Vermelho, A.B. Chagas Disease: Perspectives on the past and present and challenges in drug discovery. Molecules 2020, 25, 5483. [Google Scholar] [CrossRef]
- Ferreira, H.O. Ensaio terapêutico-clínico com benzonidazol na doenca de Chagas. Rev. Inst. Med. Trop. Sao Paulo 1976, 18, 357–364. [Google Scholar]
- Polak, A.; Richle, R. Mode of action of the 2-nitroimidazole derivative benznidazole. Ann. Trop. Med. Parasitol. 1978, 72, 45–54. [Google Scholar] [CrossRef]
- Canavaci, A.M.; Bustamante, J.M.; Padilla, A.M.; Pérez Brandan, C.M.; Simpson, L.J.; Xu, D.; Boehlke, C.L.; Tarleton, R.L. In vitro and in vivo high-throughput assays for the testing of anti-Trypanosoma cruzi compounds. PLoS Negl. Trop. Dis. 2010, 4, e740. [Google Scholar] [CrossRef]
- Moraes, C.B.; Giardini, M.A.; Kim, H.; Franco, C.H.; Araujo-Junior, A.M.; Schenkman, S.; Chatelain, E.; Freitas-Junior, L.H. Nitroheterocyclic compounds are more efficacious than CYP51 inhibitors against Trypanosoma cruzi: Implications for Chagas disease drug discovery and development. Sci. Rep. 2014, 4, 4703. [Google Scholar] [CrossRef]
- de Souza, C.C.; de Azevedo-França, J.A.; Barrias, E.; Cavalcante, S.C.F.; Vieira, E.G.; Ferreira, A.M.D.C.; de Souza, W.; Navarro, M. Silver and copper-benznidazole derivatives as potential antiparasitic metallodrugs: Synthesis, characterization, and biological evaluation. J. Inorg. Biochem. 2023, 239, 112047. [Google Scholar] [CrossRef]
- Molina, I.; Salvador, F.; Sánchez-Montalvá, A.; Artaza, M.A.; Moreno, R.; Perin, L.; Esquisabel, A.; Pinto, L.; Pedraz, J.L. Pharmacokinetics of benznidazole in healthy volunteers and implications in future clinical trials. Antimicrob. Agents Chemother. 2017, 61, e01912–e1916. [Google Scholar] [CrossRef] [PubMed]
- Sales Junior, P.A.; Molina, I.; Fonseca Murta, S.M.; Sánchez-Montalvá, A.; Salvador, F.; Corrêa-Oliveira, R.; Carneiro, C.M. Experimental and clinical treatment of Chagas disease: A review. Am. J. Trop. Med. Hyg. 2017, 97, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Kratz, J.M.; García Bournissen, F.; Forsyth, C.J.; Sosa-Estani, S. Clinical and pharmacological profile of benznidazole for treatment of Chagas disease. Expert Rev. Clin. Pharmacol. 2018, 11, 943–957. [Google Scholar] [CrossRef]
- Altcheh, J.; Moscatelli, G.; Mastrantonio, G.; Moroni, S.; Giglio, N.; Marson, M.E.; Ballering, G.; Bisio, M.; Koren, G.; García-Bournissen, F. Population pharmacokinetic study of benznidazole in pediatric Chagas disease suggests efficacy despite lower plasma concentrations than in adults. PLoS Negl. Trop. Dis. 2014, 8, e2907. [Google Scholar] [CrossRef]
- Lascano, F.; García Bournissen, F.; Altcheh, J. Review of pharmacological options for the treatment of Chagas disease. Br. J. Clin. Pharmacol. 2022, 88, 383–402. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, A.L.; Zicker, F.; de Oliveira, R.M.; Almeida Silva, S.; Luquetti, A.; Travassos, L.R.; Almeida, I.C.; de Andrade, S.S.; de Andrade, J.G.; Martelli, C.M. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet 1996, 348, 1407–1413. [Google Scholar] [CrossRef]
- Sosa Estani, S.; Segura, E.L.; Ruiz, A.M.; Velazquez, E.; Porcel, B.M.; Yampotis, C. Efficacy of chemotherapy with benznidazole in children in the indeterminate phase of Chagas. Am. J. Trop. Med. Hyg. 1998, 59, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Schijman, A.G.; Altcheh, J.; Burgos, J.M.; Biancardi, M.; Bisio, M.; Levin, M.J.; Freilij, H. Aetiological treatment of congenital Chagas’ disease diagnosed and monitored by the polymerase chain reaction. J. Antimicrob. Chemother. 2003, 52, 441–449. [Google Scholar] [CrossRef]
- Molina, I.; Gómez i Prat, J.; Salvador, F.; Treviño, B.; Sulleiro, E.; Serre, N.; Pou, D.; Roure, S.; Cabezos, J.; Valerio, L.; et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N. Engl. J. Med. 2014, 370, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Morillo, C.A.; Waskin, H.; Sosa-Estani, S.; Del Carmen Bangher, M.; Cuneo, C.; Milesi, R.; Mallagray, M.; Apt, W.; Beloscar, J.; Gascón, J.; et al. Benznidazole and posaconazole in eliminating parasites in asymptomatic T. cruzi carriers: The STOP-CHAGAS trial. J. Am. Coll. Cardiol. 2016, 9, 939–947. [Google Scholar]
- Torrico, F.; Gascon, J.; Ortiz, L.; Alonso-Vega, C.; Pinazo, M.J.; Schijman, A.; Almeida, I.C.; Alves, F.; Strub-Wourgaft, N.; Ribeiro, I.; et al. Treatment of adult chronic indeterminate Chagas disease with benznidazole and three E1224 dosing regimens: A proof-of-concept, randomised, placebo-controlled trial. Lancet Infect. Dis. 2018, 18, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, M.G.; Vigliano, C.; Lococo, B.; Bertocchi, G.; Viotti, R. Prevention of congenital Chagas disease by Benznidazole treatment in reproductive-age women. An observational study. Acta Trop. 2017, 174, 149–152. [Google Scholar] [CrossRef]
- Pinazo, M.J.; Guerrero, L.; Posada, E.; Rodríguez, E.; Soy, D.; Gascón, J. Benznidazole-related adverse drug reactions and their relationship to serum drug concentrations in patients with chronic Chagas disease. Antimicrob. Agents Chemother. 2013, 57, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Torrico, F.; Gascón, J.; Barreira, F.; Blum, B.; Almeida, I.C.; Alonso-Vega, C.; Barboza, T.; Bilbe, G.; Correia, E.; García, W.; et al. New regimens of benznidazole monotherapy and in combination with fosravuconazole for treatment of Chagas disease (BENDITA): A phase 2, double-blind, randomised trial. Lancet Infect. Dis. 2021, 21, 1129–1140. [Google Scholar] [CrossRef]
- Molina-Morant, D.; Fernández, M.L.; Bosch-Nicolau, P.; Sulleiro, E.; Bangher, M.; Salvador, F.; Sánchez-Montalvá, A.; Ribeiro, A.L.P.; de Paula, A.M.B.; Eloi, S.; et al. Efficacy and safety assessment of different dosage of benznidazol for the treatment of Chagas disease in chronic phase in adults (MULTIBENZ study): Study protocol for a multicenter randomized phase II non- inferiority clinical trial. Trials 2020, 21, 328. [Google Scholar] [CrossRef]
- Cafferata, M.L.; Toscani, M.A.; Althabe, F.; Belizán, J.M.; Bergel, E.; Berrueta, M.; Capparelli, E.V.; Ciganda, Á.; Danesi, E.; Dumonteil, E.; et al. Short-course benznidazole treatment to reduce Trypanosoma cruzi parasitic load in women of reproductive age (BETTY): A non-inferiority randomized controlled trial study protocol. Reprod. Health 2020, 17, 128. [Google Scholar] [CrossRef]
- Montalto de Mecca, M.; Diaz, E.G.; Castro, J.A. Nifurtimox biotransformation to reactive metabolites or nitrite in liver subcellular fractions and model systems. Toxicol Lett 2002, 136, 1–8. [Google Scholar] [CrossRef]
- Pérez-Molina, J.A.; Crespillo-Andújar, C.; Bosch-Nicolau, P.; Molina, I. Trypanocidal treatment of Chagas disease. Enferm. Infecc. Microbiol. Clin. 2021, 39, 458–470. [Google Scholar] [CrossRef]
- Castro, J.A.; De Mecca, M.M.; Bartel, L.C. Toxic side effects of drugs used to treat CD (American trypanosomiasis). Hum. Exp. Toxicol. 2006, 25, 471–479. [Google Scholar] [CrossRef]
- Paulos, C.; Paredes, J.; Vasquez, I.; Thambo, S.; Arancibia, A.; González-Martín, G. Pharmacokinetics of a nitrofuran compound, nifurtimox, in healthy volunteers. Int. J. Clin. Pharmacol. Ther. Toxicol. 1989, 27, 454–457. [Google Scholar]
- Moroni, S.; Marson, M.E.; Moscatelli, G.; Mastrantonio, G.; Bisio, M.; González, N.; Ballering, G.; Altcheh, J.; García-Bournissen, F. Negligible exposure to nifurtimox through breast milk during maternal treatment for Chagas Disease. PLoS Negl. Trop. Dis. 2019, 13, e0007647. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.C.; Herrera, V.M.; Pérez Carreño, J.G.; Váquiro Herrera, E.; Castellanos Domínguez, Y.Z.; Vásquez, S.M.; Cucunubá, Z.M.; Prado, N.G.; Hernández, Y. Nifurtimox versus benznidazole or placebo for asymptomatic Trypanosoma cruzi infection (Equivalence of Usual Interventions for Trypanosomiasis—EQUITY): Study protocol for a randomised controlled trial. Trials 2019, 20, 431. [Google Scholar] [CrossRef]
- Altcheh, J.; Castro, L.; Dib, J.C.; Grossmann, U.; Huang, E.; Moscatelli, G.; Pinto Rocha, J.J.; Ramírez, T.E.; CHICO Study Group. Prospective, historically controlled study to evaluate the efficacy and safety of a new paediatric formulation of nifurtimox in children aged 0 to 17 years with Chagas disease one year after treatment (CHICO). PLoS Negl. Trop. Dis. 2021, 15, e0008912. [Google Scholar] [CrossRef] [PubMed]
- Doua, F.; Miezan, T.W.; Sanon Singaro, J.R.; Boa Yapo, F.; Baltz, T. The efficacy of pentamidine in the treatment of early-late stage Trypanosoma brucei gambiense trypanosomiasis. Am. J. Trop. Med. Hyg. 1996, 55, 586–588. [Google Scholar] [CrossRef] [PubMed]
- Dorlo, T.P.; Kager, P.A. Pentamidine dosage: A base/salt confusion. PLoS Negl. Trop. Dis. 2008, 2, e225. [Google Scholar] [CrossRef]
- Burri, C. Chemotherapy against human African trypanosomiasis: Is there a road of success? Parasitology 2010, 137, 1987–1994. [Google Scholar] [CrossRef]
- Wiedemar, N.; Hauser, D.A.; Mäser, P. 100 years of suramin. Antimicrob. Agents Chemother. 2020, 64, e01168-19. [Google Scholar] [CrossRef] [PubMed]
- Burri, C.; Brun, R. Chapter 76: Human African trypanosomiasis. In Manson’s Tropical Diseases; Cook, G., Zumla, A., Eds.; W.B. Saunders: London, UK, 2008; pp. 1307–1325. [Google Scholar]
- Kuepfer, I.; Schmid, C.; Allan, M.; Edielu, A.; Haary, E.P.; Kakembo, A.; Kibona, S.; Blum, J.; Burri, C. Safety and efficacy of the 10-day melarsoprol schedule for the treatment of second stage Rhodesiense sleeping sickness. PLoS Negl. Trop. Dis. 2012, 6, e1695. [Google Scholar] [CrossRef]
- Schmid, C.; Richer, M.; Bilenge, C.M.; Josenando, T.; Chappuis, F.; Manthelot, C.R.; Nangouma, A.; Doua, F.; Asumu, P.N.; Simarro, P.P.; et al. Effectiveness of a 10-day melarsoprol schedule for the treatment of late-stage human African trypanosomiasis: Confirmation from a multinational study (Impamel II). J. Infect. Dis. 2005, 191, 1922–1931. [Google Scholar] [CrossRef]
- Blum, J.; Nkunku, S.; Burri, C. Clinical description of encephalopathic syndromes and risk factors for their occurrence and outcome during melarsoprol treatment of human African trypanosomiasis. Trop. Med. Int. Health 2001, 6, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Burri, C.; Brun, R. Eflornithine for the treatment of human African trypanosomiasis. Parasitol. Res. 2003, 90, S49–S52. [Google Scholar] [CrossRef] [PubMed]
- Reguera, R.M.; Balaña-Fouce, R.; Cubría, J.C.; Alvarez Bujidos, M.L.; Ordóñez, D. Fluorinated analogues of L-ornithine are powerful inhibitors of ornithine decarboxylase and cell growth of Leishmania infantum promastigotes. Life Sci. 1994, 56, 223–230. [Google Scholar] [CrossRef]
- Lutje, V.; Probyn, K.; Seixas, J.; Bergman, H.; Villanueva, G. Chemotherapy for second- stage human African trypanosomiasis: Drugs in use. Cochrane Database Syst. Rev. 2021, 12, CD015374. [Google Scholar] [PubMed]
- Milord, F.; Pépin, J.; Loko, L.; Ethier, L.; Mpia, B. Efficacy and toxicity of eflornithine for treatment of Trypanosoma brucei gambiense sleeping sickness. Lancet 1992, 340, 652–655. [Google Scholar] [CrossRef]
- Imran, M.; Khan, S.A.; Alshammari, M.K.; Alqahtani, A.M.; Alanazi, T.A.; Kamal, M.; Jawaid, T.; Ghoneim, M.M.; Alshehri, S.; Shakeel, F. Discovery, development, inventions and patent review of fexinidazole: The first all-oral therapy for human African trypanosomiasis. Pharmaceuticals 2022, 15, 128. [Google Scholar] [CrossRef]
- Winkelmann, E.R.W. New chemotherapeutically active nitroimidazoles. Curr. Chemother. Infect. Dis. 1980, 2, 969–970. [Google Scholar]
- Raether, W.; Seidenath, H. The activity of fexinidazole (HOE 239) against experimental infections with Trypanosoma cruzi, trichomonads and Entamoeba histolytica. Ann. Trop. Med. Parasitol. 1983, 77, 13–26. [Google Scholar] [CrossRef]
- Jennings, F.W.; Urquhart, G.M. The use of the 2 substituted 5-nitroimidazole, Fexinidazole (Hoe 239) in the treatment of chronic T. brucei infections in mice. Z. Parasitenkd. 1983, 69, 577–581. [Google Scholar] [CrossRef]
- Torreele, E.; Bourdin Trunz, B.; Tweats, D.; Kaiser, M.; Brun, R.; Mazué, G.; Bray, M.A.; Pécoul, B. Fexinidazole—A new oral nitroimidazole drug candidate entering clinical development for the treatment of sleeping sickness. PLoS Negl. Trop. Dis. 2010, 4, e923. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J. An update on pharmacotherapy for leishmaniasis. Expert Opin. Pharmacother. 2015, 16, 237–252. [Google Scholar] [CrossRef]
- Deeks, E.D. Fexinidazole: First global approval. Drugs 2019, 79, 215–220. [Google Scholar] [CrossRef]
- Tarral, A.; Blesson, S.; Mordt, O.V.; Torreele, E.; Sassella, D.; Bray, M.A.; Hovsepian, L.; Evène, E.; Gualano, V.; Felices, M.; et al. Determination of an optimal dosing regimen for fexinidazole, a novel oral drug for the treatment of human African trypanosomiasis: First-in-human studies. Clin. Pharmacokinet. 2014, 53, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.A.; Strub-Wourgraft, N.; Tarral, A.; Ribeiro, I.; Tarning, J.; White, N.J. Pharmacokinetic-pharmacodynamic assessment of the hepatic and bone marrow toxicities of the new trypanoside fexinidazole. Antimicrob. Agents Chemother. 2019, 63, e02515–e02518. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Bray, M.A.; Cal, M.; Bourdin Trunz, B.; Torreele, E.; Brun, R. Antitrypanosomal activity of fexinidazole, a new oral nitroimidazole drug candidate for treatment of sleeping sickness. Antimicrob. Agents Chemother. 2011, 55, 5602–5608. [Google Scholar] [CrossRef]
- Burrell-Saward, H.; Harris, A.J.; de LaFlor, R.; Sallam, H.; Alavijeh, M.S.; Ward, T.H.; Croft, S.L. Dose-dependent effect and pharmacokinetics of fexinidazole and its metabolites in a mouse model of human African trypanosomiasis. Int. J. Antimicrob. Agents 2017, 50, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Mesu, V.K.B.K.; Kalonji, W.M.; Bardonneau, C.; Mordt, O.V.; Blesson, S.; Simon, F.; Delhomme, S.; Bernhard, S.; Kuziena, W.; Lubaki, J.F.; et al. Oral fexinidazole for late-stage African Trypanosoma brucei gambiense trypanosomiasis: A pivotal multicentre, randomised, non-inferiority trial. Lancet 2018, 391, 144–154. [Google Scholar] [CrossRef]
- Mesu, V.K.B.K.; Kalonji, W.M.; Bardonneau, C.; Mordt, O.V.; Blesson, S.; Simon, F.; Delhomme, S.; Bernhard, S.; Mbembo, H.M.; Moke, C.M.; et al. Oral fexinidazole for stage 1 or early stage 2 African Trypanosoma brucei gambiense trypanosomiasis: A prospective, multicentre, open-label, cohort study. Lancet Glob. Health 2021, 9, e999–e1008. [Google Scholar] [CrossRef]
- Neau, P.; Hänel, H.; Lameyre, V.; Strub-Wourgaft, N.; Kuykens, L. Innovative partnerships for the elimination of Human African Trypanosomiasis and the development of fexinidazole. Trop. Med. Infect. Dis. 2020, 5, 17. [Google Scholar] [CrossRef]
- Lindner, A.K.; Lejon, V.; Chappuis, F.; Seixas, J.; Kazumba, L.; Barrett, M.P.; Mwamba, E.; Erphas, O.; Akl, E.A.; Villanueva, G.; et al. New WHO guidelines for treatment of gambiense human African trypanosomiasis including fexinidazole: Substantial changes for clinical practice. Lancet Infect. Dis. 2020, 20, e38–e46. [Google Scholar] [CrossRef]
- Yun, O.; Priotto, G.; Tong, J.; Flevaud, L.; Chappuis, F. NECT is next: Implementing the new drug combination therapy for Trypanosoma brucei gambiense sleeping sickness. PLoS Negl. Trop. Dis. 2010, 4, e720. [Google Scholar] [CrossRef]
- WHO. Model Lists of Essential Medicines, 16th List. 2010. Available online: http://apps.who.int/iris/bitstream/handle/10665/70643/a95060_eng.pdf?sequence=1 (accessed on 3 March 2023).
- WHO. Model Lists of Essential Medicines for Children, 4th List. 2013. Available online: https://apps.who.int/iris/bitstream/handle/10665/93143/EMLc_4_eng.pdf;jsessionid=5BAB0668B5399535021865B2635D8883?sequence=1 (accessed on 3 March 2023).
- Priotto, G.; Kasparian, S.; Mutombo, W.; Ngouama, D.; Ghorashian, S.; Arnold, U.; Ghabri, S.; Baudin, E.; Buard, V.; Kazadi-Kyanza, S.; et al. Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: A multicentre, randomised, phase III, non-inferiority trial. Lancet 2009, 374, 56–64. [Google Scholar] [CrossRef]
- Kansiime, F.; Adibaku, S.; Wamboga, C.; Idi, F.; Kato, C.D.; Yamuah, L.; Vaillant, M.; Kioy, D.; Olliaro, P.; Matovu, E. A multicentre, randomised, non-inferiority clinical trial comparing a nifurtimox-eflornithine combination to standard eflornithine monotherapy for late-stage Trypanosoma brucei gambiense human African trypanosomiasis in Uganda. Parasites Vectors 2018, 11, 105. [Google Scholar] [CrossRef]
- WHO. Model List of Essential Medicines: 21st List 2019; World Health Organization: Geneva, Switzerland, 2019; Available online: https://www.who.int/publications/i/item/WHOMVPEMPIAU2019.06 (accessed on 3 March 2023).
- Jeganathan, S.; Sanderson, L.; Dogruel, M.; Rodgers, J.; Croft, S.; Thomas, S.A. The distribution of nifurtimox across the healthy and trypanosome-infected murine blood-brain and blood-cerebrospinal fluid barriers. J. Pharmacol. Exp. Ther. 2011, 336, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Babokhov, P.; Sanyaolu, A.O.; Oyibo, W.A.; Fagbenro-Beyioku, A.F.; Iriemenam, N.C. A current analysis of chemotherapy strategies for the treatment of human African trypanosomiasis. Pathog. Glob. Health 2013, 107, 242–252. [Google Scholar] [CrossRef]
- Schmid, C.; Kuemmerle, A.; Blum, J.; Ghabri, S.; Kande, V.; Mutombo, W.; Ilunga, M.; Lumpungu, I.; Mutanda, S.; Nganzobo, P.; et al. In-hospital safety in field conditions of nifurtimox eflornithine combination therapy (NECT) for T. b. gambiense sleeping sickness. PLoS Negl. Trop. Dis. 2012, 6, e1920. [Google Scholar] [CrossRef] [PubMed]
- Kuemmerle, A.; Schmid, C.; Bernhard, S.; Kande, V.; Mutombo, W.; Ilunga, M.; Lumpungu, I.; Mutanda, S.; Nganzobo, P.; Tete, D.N.; et al. Effectiveness of Nifurtimox Eflornithine Combination Therapy (NECT) in T. b. gambiense second stage sleeping sickness patients in the Democratic Republic of Congo: Report from a field study. PLoS Negl. Trop. Dis. 2021, 15, e0009903. [Google Scholar] [CrossRef] [PubMed]
- Frézard, F.; Demicheli, C.; Ribeiro, R.R. Pentavalent antimonials: New perspectives for old drugs. Molecules 2009, 14, 2317–2336. [Google Scholar] [CrossRef]
- Shaked-Mishan, P.; Ulrich, N.; Ephros, M.; Zilberstein, D. Novel intracellular SbV reducing activity correlates with antimony susceptibility in Leishmania donovani. J. Biol. Chem. 2001, 276, 3971–3976. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J. Leishmaniasis: An update of current pharmacotherapy. Expert Opin. Pharmacother. 2013, 14, 53–63. [Google Scholar] [CrossRef]
- Zijlstra, E.E.; Alves, F.; Rijal, S.; Arana, B.; Alvar, J. Post-kala-azar dermal leishmaniasis in the Indian subcontinent: A threat to the South-East Asia Region Kala-azar Elimination Programme. PLoS Negl. Trop. Dis. 2017, 11, e0005877. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J. Antimony toxicity. Int. J. Environ. Res. Public Health 2010, 7, 4267–4277. [Google Scholar] [CrossRef]
- Chappuis, F.; Alirol, E.; Worku, D.T.; Mueller, Y.; Ritmeijer, K. High mortality among older patients treated with pentavalent antimonials for visceral leishmaniasis in East Africa and rationale for switch to liposomal amphotericin B. Antimicrob. Agents Chemother. 2011, 55, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Chakravarty, J.; Agarwal, D.; Rai, M.; Murray, H.W. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N. Engl. J. Med. 2010, 362, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Singh, A.; Agrawal, N.; Chakravarty, J. Effectiveness of single-dose liposomal amphotericin b in visceral leishmaniasis in Bihar. Am. J. Trop. Med. Hyg. 2019, 101, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.T.; Lam, F.C.; Perrier, D.G.; Souter, A. A stability study of amphotericin B in aqueous media using factorial design. Int. J. Pharm. 1988, 44, 117–123. [Google Scholar] [CrossRef]
- Sundar, S.; Singh, A. Chemotherapeutics of visceral leishmaniasis: Present and future developments. Parasitology 2018, 145, 481–489. [Google Scholar] [CrossRef]
- Palić, S.; Beijnen, J.H.; Dorlo, T.P. An update on the clinical pharmacology of miltefosine in the treatment of leishmaniasis. Int. J. Antimicrob. Agents 2021, 22, 106459. [Google Scholar] [CrossRef]
- Berman, J. Miltefosine to treat leishmaniasis. Expert Opin. Pharmacother. 2005, 6, 1381–1388. [Google Scholar] [CrossRef]
- Dorlo, T.P.; Huitema, A.D.; Beijnen, J.H.; de Vries, P.J. Optimal dosing of miltefosine in children and adults with visceral leishmaniasis. Antimicrob Agents Chemother. 2012, 56, 3864–3872. [Google Scholar] [CrossRef]
- Sindermann, H.; Engel, J. Development of miltefosine as an oral treatment for leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, S17–S20. [Google Scholar] [CrossRef] [PubMed]
- Hailu, A.; Musa, A.; Wasunna, M.; Balasegaram, M.; Yifru, S.; Mengistu, G.; Hurissa, Z.; Hailu, W.; Weldegebreal, T.; Tesfaye, S.; et al. Geographical variation in the response of visceral leishmaniasis to paromomycin in East Africa: A multicentre, open-label, randomized trial. PLoS Negl. Trop. Dis. 2010, 4, e709. [Google Scholar] [CrossRef]
- Musa, A.; Khalil, E.; Hailu, A.; Olobo, J.; Balasegaram, M.; Omollo, R.; Edwards, T.; Rashid, J.; Mbui, J.; Musa, B.; et al. Sodium stibogluconate (SSG) & paromomycin combination compared to SSG for visceral leishmaniasis in East Africa: A randomised controlled trial. PLos Negl. Trop. Dis. 2012, 6, e1674. [Google Scholar]
- Caljon, G.; De Muylder, G.; Durnez, L.; Jennes, W.; Vanaerschot, M.; Dujardin, J.C. Alice in microbes’ land: Adaptations and counter-adaptations of vector-borne parasitic protozoa and their hosts. FEMS Microbiol. Rev. 2016, 40, 664–685. [Google Scholar] [CrossRef]
- Barrett, M.P.; Kyle, D.E.; Sibley, L.D.; Radke, J.B.; Tarleton, R.L. Protozoan persister-like cells and drug treatment failure. Nat. Rev. Microbiol. 2019, 17, 607–620. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Corbeil, A.; do Monte-Neto, R.L.; Fernandez-Prada, C. Of drugs and trypanosomatids: New tools and knowledge to reduce bottlenecks in drug discovery. Genes 2020, 11, 722. [Google Scholar] [CrossRef]
- Perry, M.R.; Wyllie, S.; Prajapati, V.K.; Feldmann, J.; Sundar, S.; Boelaert, M.; Fairlamb, A.H. Visceral leishmaniasis and arsenic: An ancient poison contributing to antimonial treatment failure in the Indian subcontinent? PLoS Negl. Trop. Dis. 2011, 5, e1227. [Google Scholar] [CrossRef] [PubMed]
- Mandal, G.; Mandal, S.; Sharma, M.; Charret, K.S.; Papadopoulou, B.; Bhattacharjee, H.; Mukhopadhyay, R. Species-specific antimonial sensitivity in Leishmania is driven by post-transcriptional regulation of AQP1. PLoS Negl. Trop. Dis. 2015, 9, e0003500. [Google Scholar] [CrossRef] [PubMed]
- Fairlamb, A.H.; Horn, D. Melarsoprol Resistance in African Trypanosomiasis. Trends Parasitol. 2018, 34, 481–492. [Google Scholar] [CrossRef]
- Perez-Victoria, F.J.; Gamarro, F.; Ouellette, M.; Castanys, S. Functional cloning of the miltefosine transporter. A novel P-type phospholipid translocase from Leishmania involved in drug resistance. J. Biol. Chem. 2003, 278, 49965–49971. [Google Scholar]
- De Koning, P.H. The drugs of sleeping sickness: Their mechanisms of action and resistance, and a brief history. Trop. Med. Infect. Dis. 2020, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Bacchi, C.J.; Garofalo, J.; Ciminelli, M.; Rattendi, D.; Goldberg, B.; McCann, P.P.; Yarlett, N. Resistance to DL-α-difluoromethylornithine by clinical isolates of Trypanosoma brucei rhodesiense. Role of S-adenosylmethionine. Biochem. Pharmacol. 1993, 46, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Reguera, R.M.; Tekwani, B.L.; Balaña-Fouce, R. Polyamine transport in parasites: A potential target for new antiparasitic drug development. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2005, 140, 151–164. [Google Scholar] [CrossRef]
- Hall, B.S.; Bot, C.; Wilkinson, S.R. Nifurtimox activation by trypanosomal type I nitroreductases generates cytotoxic nitrile metabolites. J. Biol. Chem. 2011, 286, 13088–13095. [Google Scholar] [CrossRef]
- Sokolova, A.Y.; Wyllie, S.; Patterson, S.; Oza, S.L.; Read, K.D.; Fairlamb, A.H. Cross-resistance to nitro drugs and implications for treatment of human African trypanosomiasis. Antimicrob. Agents Chemother. 2010, 54, 2893–2900. [Google Scholar] [CrossRef] [PubMed]
- Alpizar-Sosa, E.A.; Ithnin, N.R.B.; Wei, W.; Pountain, A.W.; Weidt, S.K.; Donachie, A.M.; Ritchie, R.; Dickie, E.A.; Burchmore, R.J.S.; Denny, P.W.; et al. Amphotericin B resistance in Leishmania mexicana: Alterations to sterol metabolism and oxidative stress response. PLoS Negl. Trop. Dis. 2022, 16, e0010779. [Google Scholar] [CrossRef]
- Francisco, A.F.; Jayawardhana, S.; Lewis, M.D.; Taylor, M.C.; Kelly, J.M. Biological factors that impinge on Chagas disease drug development. Parasitology 2017, 144, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, D.; Dalton, J.E.; Kaye, P.M.; Chatterjee, M. Post kala-azar dermal leishmaniasis: An unresolved mystery. Trends Parasitol. 2014, 30, 65–74. [Google Scholar] [CrossRef]
- Dirkx, L.; Hendrickx, S.; Merlot, M.; Bulté, D.; Starick, M.; Elst, J.; Bafica, A.; Ebo, D.G.; Maes, L.; Van Weyenbergh, J.; et al. Long-term hematopoietic stem cells as a parasite niche during treatment failure in visceral leishmaniasis. Commun. Biol. 2022, 5, 626. [Google Scholar] [CrossRef]
- Hill, J.A.; Cowen, L.E. Using combination therapy to thwart drug resistance. Future Microbiol. 2015, 10, 1719–1726. [Google Scholar] [CrossRef]
- Alves, F.; Bilbe, G.; Blesson, S.; Goyal, V.; Monnerat, S.; Mowbray, C.; Muthoni Ouattara, G.; Pécoul, B.; Rijal, S.; Rode, J.; et al. Recent development of visceral leishmaniasis treatments: Successes, pitfalls, and perspectives. Clin. Microbiol. Rev. 2018, 31, e00048-18. [Google Scholar] [CrossRef] [PubMed]
- Balaña-Fouce, R.; Pérez Pertejo, M.Y.; Domínguez-Asenjo, B.; Gutiérrez-Corbo, C.; Reguera, R.M. Walking a tightrope: Drug discovery in visceral leishmaniasis. Drug Discov. Today 2019, 24, 1209–1216. [Google Scholar] [CrossRef]
- Jinna, S.; Finch, J. Spotlight on tavaborole for the treatment of onychomycosis. Drug Des. Devel. Ther. 2015, 9, 6185–6190. [Google Scholar] [PubMed]
- Dickie, E.A.; Giordani, F.; Gould, M.K.; Mäser, P.; Burri, C.; Mottram, J.C.; Rao, S.P.S.; Barrett, M.P. New drugs for human African trypanosomiasis: A twenty first century success story. Trop. Med. Infect. Dis. 2020, 5, 29. [Google Scholar] [CrossRef]
- Van Bocxlaer, K.; Caridha, D.; Black, C.; Vesely, B.; Leed, S.; Sciotti, R.J.; Wijnant, G.J.; Yardley, V.; Braillard, S.; Mowbray, C.E.; et al. Novel benzoxaborole, nitroimidazole and aminopyrazoles with activity against experimental cutaneous leishmaniasis. Int. J. Parasitol. Drugs Drug Resist. 2019, 11, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Mowbray, C.E.; Braillard, S.; Glossop, P.A.; Whitlock, G.A.; Jacobs, R.T.; Speake, J.; Pandi, B.; Nare, B.; Maes, L.; Yardley, V.; et al. DNDI-6148: A novel benzoxaborole preclinical candidate for the treatment of visceral leishmaniasis. J. Med. Chem. 2021, 64, 16159–16176. [Google Scholar] [CrossRef] [PubMed]
- Vermelho, A.B.; Rodrigues, G.C.; Supuran, C.T. Why hasn’t there been more progress in new Chagas disease drug discovery? Expert Opin. Drug Discov. 2020, 15, 145–158. [Google Scholar] [CrossRef]
- Padilla, A.M.; Wang, W.; Akama, T.; Carter, D.S.; Easom, E.; Freund, Y.; Halladay, J.S.; Liu, Y.; Hamer, S.A.; Hodo, C.L.; et al. Discovery of an orally active benzoxaborole prodrug effective in the treatment of Chagas disease in non-human primates. Nat. Microbiol. 2022, 7, 1536–1546. [Google Scholar] [CrossRef]
- Woodland, A.; Grimaldi, R.; Luksch, T.; Cleghorn, L.A.; Ojo, K.K.; Van Voorhis, W.C.; Brenk, R.; Frearson, J.A.; Gilbert, I.H.; Wyatt, P.G. From on-target to off-target activity: Identification and optimisation of Trypanosoma brucei GSK3 inhibitors and their characterisation as anti-Trypanosoma brucei drug discovery lead molecules. ChemMedChem 2013, 8, 1127–1137. [Google Scholar] [CrossRef]
- Wyllie, S.; Thomas, M.; Patterson, S.; Crouch, S.; De Rycker, M.; Lowe, R.; Gresham, S.; Urbaniak, M.D.; Otto, T.D.; Stojanovski, L.; et al. Cyclin-dependent kinase 12 is a drug target for visceral leishmaniasis. Nature 2018, 560, 192–197. [Google Scholar] [CrossRef]
- Thomas, M.G.; De Rycker, M.; Ajakane, M.; Albrecht, S.; Álvarez-Pedraglio, A.I.; Boesche, M.; Brand, S.; Campbell, L.; Cantizani-Pérez, J.; Cleghorn, L.A.T.; et al. Identification of GSK3186899/DDD853651 as a preclinical development candidate for the treatment of visceral leishmaniasis. J. Med. Chem. 2019, 62, 1180–1202. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.; Nagle, A.S.; Biggart, A.; Lai, Y.H.; Liang, F.; Davis, L.C.; Barnes, S.W.; Mathison, C.J.; Myburgh, E.; Gao, M.Y.; et al. Proteasome inhibition for treatment of leishmaniasis, Chagas disease and sleeping sickness. Nature 2016, 537, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Nagle, A.; Biggart, A.; Be, C.; Srinivas, H.; Hein, A.; Caridha, D.; Sciotti, R.J.; Pybus, B.; Kreishman-Deitrick, M.; Bursulaya, B.; et al. Discovery and characterization of clinical candidate LXE408 as a kinetoplastid-selective proteasome inhibitor for the treatment of leishmaniases. J. Med. Chem. 2020, 63, 10773–10781. [Google Scholar] [CrossRef]
- Wyllie, S.; Brand, S.; Thomas, M.; De Rycker, M.; Chung, C.W.; Pena, I.; Bingham, R.P.; Bueren-Calabuig, J.A.; Cantizani, J.; Cebrián, D.; et al. Preclinical candidate for the treatment of visceral leishmaniasis that acts through proteasome inhibition. Proc. Natl. Acad. Sci. USA 2019, 116, 9318–9323. [Google Scholar] [CrossRef] [PubMed]
- Gursel, M.; Verthelyi, D.; Klinman, D.M. CpG oligodeoxynucleotides induce human monocytes to mature into functional dendritic cells. Eur. J. Immunol. 2002, 32, 2617–2622. [Google Scholar] [CrossRef]
- Thacker, S.G.; McWilliams, I.L.; Bonnet, B.; Halie, L.; Beaucage, S.; Rachuri, S.; Dey, R.; Duncan, R.; Modabber, F.; Robinson, S.; et al. CpG ODN D35 improves the response to abbreviated low-dose pentavalent antimonial treatment in non-human primate model of cutaneous leishmaniasis. PLoS Negl. Trop. Dis. 2020, 14, e0008050. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.; Wyllie, S. Nitro drugs for the treatment of trypanosomatid diseases: Past, present, and future prospects. Trends Parasitol. 2014, 30, 289–298. [Google Scholar] [CrossRef]
- Brenk, R. Lessons learnt from assembling screening libraries for drug discovery for neglected diseases. ChemMedChem 2008, 3, 435–444. [Google Scholar] [CrossRef]
- Domínguez-Asenjo, B.; Gutiérrez-Corbo, C.; Álvarez-Bardón, M.; Pérez-Pertejo, Y.; Balaña-Fouce, R.; Reguera, R.M. Ex vivo phenotypic screening of two small repurposing drug collections identifies nifuratel as a potential new treatment against visceral and cutaneous leishmaniasis. ACS Infect. Dis. 2021, 7, 2390–2401. [Google Scholar] [CrossRef]
- Fernando da Silva Santos-Júnior, P.; Rocha Silva, L.; José Quintans-Júnior, L.; Ferreira da Silva-Júnior, E. Nitro compounds against trypanosomatidae parasites: Heroes or villains? Bioorg. Med. Chem. Lett. 2022, 75, 128930. [Google Scholar] [CrossRef]
- Thomas, C.; Gwenin, C.D. The role of nitroreductases in resistance to nitroimidazoles. Biology 2021, 10, 388. [Google Scholar] [CrossRef] [PubMed]
- Zuma, A.A.; de Souza, W. Fexinidazole interferes with the growth and structural organization of Trypanosoma cruzi. Sci. Rep. 2022, 12, 20388. [Google Scholar] [CrossRef]
- Bahia, M.T.; de Andrade, I.M.; Martins, T.A.; do Nascimento, Á.F.; Diniz Lde, F.; Caldas, I.S.; Talvani, A.; Trunz, B.B.; Torreele, E.; Ribeiro, I. Fexinidazole: A potential new drug candidate for Chagas disease. PLoS Negl. Trop. Dis. 2012, 6, e1870. [Google Scholar] [CrossRef] [PubMed]
- Bahia, M.T.; Nascimento, A.F.; Mazzeti, A.L.; Marques, L.F.; Gonçalves, K.R.; Mota, L.W.; Diniz, L.d.F.; Caldasm, I.S.; Talvani, A.; Shackleford, D.M.; et al. Antitrypanosomal activity of fexinidazole metabolites, potential new drug candidates for Chagas disease. Antimicrob. Agents Chemother. 2014, 58, 4362–4370. [Google Scholar] [CrossRef] [PubMed]
- Francisco, A.F.; Jayawardhana, S.; Lewis, M.D.; White, K.L.; Shackleford, D.M.; Chen, G.; Saunders, J.; Osuna-Cabello, M.; Read, K.D.; Charman, S.A.; et al. Nitroheterocyclic drugs cure experimental Trypanosoma cruzi infections more effectively in the chronic stage than in the acute stage. Sci. Rep. 2016, 6, 35351. [Google Scholar] [CrossRef]
- Wyllie, S.; Patterson, S.; Stojanovski, L.; Simeons, F.R.; Norval, S.; Kime, R.; Read, K.D.; Fairlamb, A.H. The anti-trypanosome drug fexinidazole shows potential for treating visceral leishmaniasis. Sci. Transl. Med. 2012, 4, 119re1. [Google Scholar] [CrossRef]
- de Morais-Teixeira, E.; Rabello, A.; Aguiar, M.M.G. In vitro activity and in vivo efficacy of fexinidazole against New World Leishmania species. J. Antimicrob. Chemother. 2019, 74, 2318–2325. [Google Scholar] [CrossRef]
- Levi, G.C.; Neto, V.A. Tratamento, pelo metronidazol, de pacientes com a forma crônica da doença de Chagas. Rev. Soc. Bras. Med. Trop. 1970, 4, 173–175. [Google Scholar] [CrossRef]
- Laínez, H.N.; Fernández, E.S. Forma aguda de la enfermedad de Chagas, importancia semiológica del Signo de Romana—Informe de los dos primeros casos en Honduras. Med. Hondur. 1971, 39, 5–15. [Google Scholar]
- Simões-Silva, M.R.; De Araújo, J.S.; Oliveira, G.M.; Demarque, K.C.; Peres, R.B.; D’Almeida-Melo, I.; Batista, D.G.J.; Da Silva, C.F.; Cardoso-Santos, C.; Da Silva, P.B.; et al. Drug repurposing strategy against Trypanosoma cruzi infection: In vitro and in vivo assessment of the activity of metronidazole in mono- and combined therapy. Biochem. Pharmacol. 2017, 145, 46–53. [Google Scholar] [CrossRef]
- Al-Waiz, M.; Sharquie, K.E.; Al-Assir, M. Treatment of cutaneous leishmaniasis by intralesional metronidazole. Saudi Med. J. 2004, 25, 1512–1513. [Google Scholar] [PubMed]
- Bahman, H.B.; Shabu, S.H.; Sieman, S.A. Intralesional pentostam versus intralesional metronidazole in treating cutaneous leishmaniasis: A comparison study. Zanco J. Med. Sci. 2019, 23, 257–263. [Google Scholar]
- Somaratne, V.N.; Ranawaka, R.R.; Jayaruwan, H.M.; Wipuladasa, D.M.; de Silva, S.H.P. Randomized, double-blind study on intralesional metronidazole versus intralesional sodium stibogluconate in Leishmania donovani cutaneous leishmaniasis. J. Dermatolog. Treat. 2019, 30, 87–91. [Google Scholar] [CrossRef]
- Upadhyay, A.; Chandrakar, P.; Gupta, S.; Parmar, N.; Singh, S.K.; Rashid, M.; Kushwaha, P.; Wahajuddin, M.; Sashidhara, K.V.; Kar, S. Synthesis, biological evaluation, structure- activity relationship, and mechanism of action studies of quinoline-metronidazole derivatives against experimental visceral leishmaniasis. J. Med. Chem. 2019, 62, 5655–5671. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Pretomanid: First approval. Drugs 2019, 79, 1797–1803. [Google Scholar] [CrossRef]
- Patterson, S.; Wyllie, S.; Stojanovski, L.; Perry, M.R.; Simeons, F.R.; Norval, S.; Osuna-Cabello, M.; De Rycker, M.; Read, K.D.; Fairlamb, A.H. The R enantiomer of the antitubercular drug PA-824 as a potential oral treatment for visceral leishmaniasis. Antimicrob. Agents Chemother. 2013, 57, 4699–4706. [Google Scholar] [CrossRef]
- Van den Kerkhof, M.; Mabille, D.; Chatelain, E.; Mowbray, C.E.; Braillard, S.; Hendrickx, S.; Maes, L.; Caljon, G. In vitro and in vivo pharmacodynamics of three novel antileishmanial lead series. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 81–86. [Google Scholar] [CrossRef]
- Thompson, A.M.; O’Connor, P.D.; Marshall, A.J.; Blaser, A.; Yardley, V.; Maes, L.; Gupta, S.; Launay, D.; Braillard, S.; Chatelain, E.; et al. Development of (6 R)-2-nitro-6-[4-(trifluoromethoxy)-phenoxy]-6,7-dihydro-5H-imidazo-[2,1-b][1,3]-oxazine (DNDI-8219): A new lead for visceral leishmaniasis. J. Med. Chem. 2018, 61, 2329–2352. [Google Scholar] [CrossRef]
- Thompson, A.M.; O’Connor, P.D.; Marshall, A.J.; Francisco, A.F.; Kelly, J.M.; Riley, J.; Read, K.D.; Pérez, C.J.; Cornwall, S.; Thompson, R.C.A.; et al. Re-evaluating pretomanid analogues for Chagas disease: Hit-to-lead studies reveal both in vitro and in vivo trypanocidal efficacy. Eur. J. Med. Chem. 2020, 207, 112849. [Google Scholar] [CrossRef]
- Ryan, N.J.; Lo, J.H. Delamanid: First global approval. Drugs 2014, 74, 1041–1045. [Google Scholar] [CrossRef]
- Patterson, S.; Wyllie, S.; Norval, S.; Stojanovski, L.; Simeons, F.R.; Auer, J.L.; Osuna-Cabello, M.; Read, K.D.; Fairlamb, A.H. The anti-tubercular drug delamanid as a potential oral treatment for visceral leishmaniasis. Elife 2016, 5, e09744. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Yardley, V.; Vishwakarma, P.; Shivahare, R.; Sharma, B.; Launay, D.; Martin, D.; Puri, S.K. Nitroimidazo-oxazole compound DNDI-VL-2098: An orally effective preclinical drug candidate for the treatment of visceral leishmaniasis. J. Antimicrob. Chemother. 2015, 70, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.M.; O’Connor, P.D.; Blaser, A.; Yardley, V.; Maes, L.; Gupta, S.; Launay, D.; Martin, D.; Franzblau, S.G.; Wan, B.; et al. Repositioning antitubercular 6-nitro-2,3-dihydroimidazo-[2,1-b][1,3]-oxazoles for Neglected Tropical Diseases: Structure-activity studies on a preclinical candidate for visceral leishmaniasis. J. Med. Chem. 2016, 59, 2530–2550. [Google Scholar] [CrossRef] [PubMed]
- Wijnant, G.J.; Croft, S.L.; de la Flor, R.; Alavijeh, M.; Yardley, V.; Braillard, S.; Mowbray, C.; Van Bocxlaer, K. Pharmacokinetics and pharmacodynamics of the nitroimidazole DNDi-0690 in mouse models of cutaneous leishmaniasis. Antimicrob. Agents Chemother. 2019, 63, e00829-19. [Google Scholar] [CrossRef]
- Thompson, A.M.; O’Connor, P.D.; Marshall, A.J.; Yardley, V.; Maes, L.; Gupta, S.; Launay, D.; Braillard, S.; Chatelain, E.; Franzblau, S.G.; et al. 7-substituted-2-nitro-5,6-dihydroimidazo-[2,1-b][1,3]-oxazines: Novel antitubercular agents lead to a new preclinical candidate for visceral leishmaniasis. J. Med. Chem. 2017, 60, 4212–4233. [Google Scholar] [CrossRef]
- Van Bocxlaer, K.; McArthur, K.N.; Harris, A.; Alavijeh, M.; Braillard, S.; Mowbray, C.E.; Croft, S.L. Film-forming systems for the delivery of DNDI-0690 to treat cutaneous leishmaniasis. Pharmaceutics 2021, 13, 516. [Google Scholar] [CrossRef]
- Thompson, A.M.; O’Connor, P.D.; Marshall, A.J.; Yardley, V.; Maes, L.; Gupta, S.; Launay, D.; Braillard, S.; Chatelain, E.; Wan, B.; et al. Heteroaryl ether analogues of an antileishmanial 7-substituted-2-nitroimidazo oxazine lead afford attenuated hERG risk: In vitro and in vivo appraisal. Eur. J. Med. Chem. 2021, 209, 112914. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gray, J.P.; Mishin, V.; Heck, D.E.; Laskin, D.L.; Laskin, J.D. Role of cytochrome P450 reductase in nitrofurantoin-induced redox cycling and cytotoxicity. Free Radic. Biol. Med. 2008, 44, 1169–1179. [Google Scholar] [CrossRef]
- Hall, B.S.; Wu, X.; Hu, L.; Wilkinson, S.R. Exploiting the drug-activating properties of a novel trypanosomal nitroreductase. Antimicrob. Agents Chemother. 2010, 54, 1193–1199. [Google Scholar] [CrossRef]
- Zuma, N.H.; Aucamp, J.; N’Da, D.D. An update on derivatisation and repurposing of clinical nitrofuran drugs. Eur. J. Pharm. Sci. 2019, 140, 105092. [Google Scholar] [CrossRef]
- Spain, J.C. Biodegradation of nitroaromatic compounds. Annu. Rev. Microbiol. 1995, 49, 523–555. [Google Scholar] [CrossRef]
- Trukhacheva, L.A.; Grigorev, N.B.; Arzamastsev, A.P.; Granik, V.G. Hydrolytic and reductive transformations of nifuroxazide. Pharm. Chem. J. 2005, 39, 381–384. [Google Scholar] [CrossRef]
- Horton, J.M. Urinary tract agents: Nitrofurantoin, fosfomycin, and methenamine. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed.; Bennett, J.E., Blaser, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 447–451.e1. [Google Scholar]
- Freeman, F.; Wilson, P.L.; Kazan, B.H. Trypanosoma cruzi: Antimicrobial activity and strain differentiating properties of some five- and six-membered heterocyclic compounds on trypomastigotes. Exp. Parasitol. 1975, 38, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Munsimbwe, L.; Seetsi, A.; Namangala, B.; N’Da, D.D.; Inoue, N.; Suganuma, K. In vitro and in vivo trypanocidal efficacy of synthesized nitrofurantoin analogs. Molecules 2021, 26, 3372. [Google Scholar] [CrossRef]
- Suganuma, K.; N’Da, D.D.; Watanabe, K.I.; Tanaka, Y.; Mossaad, E.; Elata, A.; Inoue, N.; Kawazu, S.I. Therapeutic efficacy of orally administered nitrofurantoin against animal African trypanosomosis caused by Trypanosoma congolense infection. Pathogens 2022, 11, 331. [Google Scholar] [CrossRef] [PubMed]
- Boyce, S.T.; Warden, G.D.; Holder, I.A. Cytotoxicity testing of topical antimicrobial agents on human keratinocytes and fibroblasts for cultured skin grafts. J. Burn Care Rehabil. 1995, 16, 97–103. [Google Scholar] [CrossRef]
- Ryan, A.; Kaplan, E.; Laurieri, N.; Lowe, E.; Sim, E. Activation of nitrofurazone by azoreductases: Multiple activities in one enzyme. Sci. Rep. 2011, 1, 63. [Google Scholar] [CrossRef]
- Andrade, Z.A.; Brener, Z. Action of nitrofurazone (5-nitro-2-furaldehyde-semicarbazone) on the intracellular forms of Trypanosoma intracellular forms of Trypanosoma cruzi in experimental Chagas’ disease. Rev. Inst. Med. Trop. Sao Paulo 1969, 11, 222–228. [Google Scholar] [PubMed]
- Chung, M.C.; Güido, R.V.; Martinelli, T.F.; Gonçalves, M.F.; Polli, M.C.; Botelho, K.C.; Varanda, E.A.; Colli, W.; Miranda, M.T.; Ferreira, E.I. Synthesis and in vitro evaluation of potential antichagasic hydroxymethylnitrofurazone (NFOH-121): A new nitrofurazone prodrug. Bioorg. Med. Chem. 2003, 11, 4779–4783. [Google Scholar] [CrossRef]
- Millet, R.; Maes, L.; Landry, V.; Sergheraert, C.; Davioud-Charvet, E. Antitrypanosomal activities and cytotoxicity of 5-nitro-2-furancarbohydrazides. Bioorg. Med. Chem. Lett. 2002, 12, 3601–3604. [Google Scholar] [CrossRef]
- Otero, L.; Vieites, M.; Boiani, L.; Denicola, A.; Rigol, C.; Opazo, L.; Olea-Azar, C.; Maya, J.D.; Morello, A.; Krauth-Siegel, R.L.; et al. Novel antitrypanosomal agents based on palladium nitrofurylthiosemicarbazone complexes: DNA and redox metabolism as potential therapeutic targets. J. Med. Chem. 2006, 49, 3322–3331. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.L.; Bueno, G.J.; Baliani, A.; Klenke, B.; Brun, R.; Brock, J.M.; Gilbert, I.H.; Barrett, M.P. Trypanocidal activity of melamine-based nitroheterocycles. Antimicrob. Agents Chemother. 2004, 48, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Baliani, A.; Bueno, G.J.; Stewart, M.L.; Yardley, V.; Brun, R.; Barrett, M.P.; Gilbert, I.H. Design and synthesis of a series of melamine-based nitroheterocycles with activity against trypanosomatid parasites. J. Med. Chem. 2005, 48, 5570–5579. [Google Scholar] [CrossRef]
- Giordani, F.; Buschini, A.; Baliani, A.; Kaiser, M.; Brun, R.; Barrett, M.P.; Pellacani, C.; Poli, P.; Gilbert, I.H. Characterization of a melamino nitroheterocycle as a potential lead for the treatment of human African trypanosomiasis. Antimicrob. Agents Chemother. 2014, 58, 5747–5757. [Google Scholar] [CrossRef]
- Aguirre, G.; Boiani, L.; Cerecetto, H.; Fernández, M.; González, M.; Denicola, A.; Otero, L.; Gambino, D.; Rigol, C.; Olea-Azar, C.; et al. In vitro activity and mechanism of action against the protozoan parasite Trypanosoma cruzi of 5-nitrofuryl containing thiosemicarbazones. Bioorg. Med. Chem. 2004, 12, 4885–4893. [Google Scholar] [CrossRef]
- Bot, C.; Hall, B.S.; Alvarez, G.; Di Maio, R.; González, M.; Cerecetto, H.; Wilkinson, S.R. Evaluating 5-nitrofurans as trypanocidal agents. Antimicrob. Agents Chemother. 2013, 57, 1638–1647. [Google Scholar] [CrossRef]
- Petri e Silva, S.C.; Palace-Berl, F.; Tavares, L.C.; Soares, S.R.; Lindoso, J.A. Effects of nitro-heterocyclic derivatives against Leishmania (Leishmania) infantum promastigotes and intracellular amastigotes. Exp. Parasitol. 2016, 163, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Álvarez, E.; Stamatakis, K.; Punzón, C.; Álvarez-Velilla, R.; Tejería, A.; Escudero-Martínez, J.M.; Pérez-Pertejo, Y.; Fresno, M.; Balaña-Fouce, R.; Reguera, R.M. Infrared fluorescent imaging as a potent tool for in vitro, ex vivo and in vivo models of visceral leishmaniasis. PLoS Negl. Trop. Dis. 2015, 9, e0003666. [Google Scholar] [CrossRef]
- Fowler, W.; Hussain, M. Nifuratel (Magmilor) in trichomonal vaginitis. Br. J. Vener. Dis. 1968, 44, 331–333. [Google Scholar] [CrossRef]
- Melcón-Fernandez, E.; Galli, G.; García-Estrada, C.; Balaña-Fouce, R.; Reguera, R.M.; Pérez-Pertejo, Y. Miltefosine and Nifuratel Combination: A Promising Therapy for the Treatment of Leishmania donovani Visceral Leishmaniasis. Int. J. Mol. Sci. 2023, 24, 1635. [Google Scholar] [CrossRef]
- Arán, V.J.; Kaiser, M.; Dardonville, C. Discovery of nitroheterocycles active against African trypanosomes. In vitro screening and prelimiary SAR studies. Bioorg. Med. Chem. Lett. 2012, 22, 4506–4516. [Google Scholar] [CrossRef]
- Voak, A.A.; Gobalakrishnapillai, V.; Seifert, K.; Balczo, E.; Hu, L.; Hall, B.S.; Wilkinson, S.R. An essential type I nitroreductase from Leishmania major can be used to activate leishmanicidal prodrugs. J. Biol. Chem. 2013, 288, 28466–28476. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, M.V.; Bloomer, W.D.; Rosenzweig, H.S.; O’Shea, I.P.; Wilkinson, S.R.; Kaiser, M.; Chatelain, E.; Ioset, J.R. Discovery of potent nitrotriazole-based antitrypanosomal agents: In vitro and in vivo evaluation. Bioorg. Med. Chem. 2015, 23, 6467–6476. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, I.P.; Shahed, M.; Aguilera-Venegas, B.; Wilkinson, S.R. Evaluating 5-nitrothiazoles as trypanocidal agents. Antimicrob. Agents Chemother. 2015, 60, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Fersing, C.; Boudot, C.; Paoli-Lombardo, R.; Primas, N.; Pinault, E.; Hutter, S.; Castera-Ducros, C.; Kabri, Y.; Pedron, J.; Bourgeade-Delmas, S.; et al. Antikinetoplastid SAR study in 3-nitroimidazopyridine series: Identification of a novel non-genotoxic and potent anti-T. b. brucei hit-compound with improved pharmacokinetic properties. Eur. J. Med. Chem. 2020, 206, 112668. [Google Scholar] [CrossRef]
- Hall, B.S.; Wilkinson, S.R. Activation of benznidazole by trypanosomal type I nitroreductases results in glyoxal formation. Antimicrob. Agents Chemother. 2012, 5, 115–123. [Google Scholar] [CrossRef]
- Streeter, A.J.; Hoener, B.A. Evidence for the involvement of a nitrenium ion in the covalent binding of nitrofurazone to DNA. Pharm. Res. 1988, 5, 434–436. [Google Scholar] [CrossRef]
- Amslinger, A. The tunable functionality of α,β-unsaturated carbonyl compounds enables their differential application in biological systems. ChemMedChem 2010, 5, 351–356. [Google Scholar] [CrossRef]
- Wyllie, S.; Patterson, S.; Fairlamb, A.H. Assessing the essentiality of Leishmania donovani nitroreductase and its role in nitro drug activation. Antimicrob. Agents Chemother. 2013, 57, 901–906. [Google Scholar] [CrossRef]
- Arias, D.G.; Herrera, F.E.; Garay, A.S.; Rodrigues, D.; Forastieri, P.S.; Luna, L.E.; Bürgi, M.D.; Prieto, C.; Iglesias, A.A.; Cravero, R.M.; et al. Rational design of nitrofuran derivatives: Synthesis and valuation as inhibitors of Trypanosoma cruzi trypanothione reductase. Eur. J. Med. Chem. 2017, 125, 1088–1097. [Google Scholar] [CrossRef]
- Peterson, F.J.; Mason, R.P.; Hovsepian, J.; Holtzman, J.L. Oxygen-sensitive and—Insensitive nitroreduction by Escherichia coli and rat hepatic microsomes. J. Biol. Chem. 1979, 254, 4009–4014. [Google Scholar] [CrossRef] [PubMed]
- Olender, D.; Żwawiak, J.; Zaprutko, L. Multidirectional efficacy of biologically active nitro compounds included in medicines. Pharmaceuticals 2018, 11, 54. [Google Scholar] [CrossRef]
- Maya, J.D.; Bollo, S.; Nuñez-Vergara, L.J.; Squella, J.A.; Repetto, Y.; Morello, A.; Périé, J.; Chauvière, G. Trypanosoma cruzi: Effect and mode of action of nitroimidazole and nitrofuran derivatives. Biochem. Pharmacol. 2003, 65, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.R.; Taylor, M.C.; Horn, D.; Kelly, J.M.; Cheeseman, I. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc. Natl. Acad. Sci. USA 2008, 105, 5022–5027. [Google Scholar] [CrossRef]
- Boiani, M.; Piacenza, L.; Hernández, P.; Boiani, L.; Cerecetto, H.; González, M.; Denicola, A. Mode of action of nifurtimox and N-oxide-containing heterocycles against Trypanosoma cruzi: Is oxidative stress involved? Biochem. Pharmacol. 2010, 79, 1736–1745. [Google Scholar] [CrossRef]
- Bernardes, L.S.; Zani, C.L.; Carvalho, I. Trypanosomatidae diseases: From the current therapy to the efficacious role of trypanothione reductase in drug discovery. Curr. Med. Chem. 2013, 20, 2673–2696. [Google Scholar] [CrossRef]
- Wyllie, S.; Roberts, A.J.; Norval, S.; Patterson, S.; Foth, B.J.; Berriman, M.; Read, K.D.; Fairlamb, A.H. Activation of bicyclic nitro-drugs by a novel nitroreductase (NTR2) in Leishmania. PLoS Pathog. 2016, 12, e1005971. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.E.; Bruce, N.C. ‘New uses for an Old Enzyme’—The Old Yellow Enzyme family of flavoenzymes. Microbiology 2002, 148, 1607–1614. [Google Scholar] [CrossRef]
- Stuermer, R.; Hauer, B.; Hall, M.; Faber, K. Asymmetric bioreduction of activated C=C bonds using enoate reductases from the old yellow enzyme family. Curr. Opin. Chem. Biol. 2007, 11, 203–213. [Google Scholar] [CrossRef]
- Toogood, H.S.; Scrutton, N.S. New developments in ‘ene’-reductase catalysed biological hydrogenations. Curr. Opin. Chem. Biol. 2014, 19, 107–115. [Google Scholar] [CrossRef]
- Díaz-Viraqué, F.; Chiribao, M.L.; Trochine, A.; González-Herrera, F.; Castillo, C.; Liempi, A.; Kemmerling, U.; Maya, J.D.; Robello, C. Old yellow enzyme from Trypanosoma cruzi exhibits in vivo prostaglandin F2α synthase activity and has a key role in parasite infection and drug susceptibility. Front. Immunol. 2018, 9, 456. [Google Scholar] [CrossRef]
- García-Huertas, P.; Mejía-Jaramillo, A.M.; Machado, C.R.; Guimarães, A.C.; Triana-Chávez, O. Prostaglandin F2α synthase in Trypanosoma cruzi plays critical roles in oxidative stress and susceptibility to benznidazole. R. Soc. Open Sci. 2017, 4, 170773. [Google Scholar] [CrossRef] [PubMed]
- Dattani, A.; Drammeh, I.; Mahmood, A.; Rahman, M.; Szular, J.; Wilkinson, S.R. Unraveling the antitrypanosomal mechanism of benznidazole and related 2-nitroimidazoles: From prodrug activation to DNA damage. Mol. Microbiol. 2021, 116, 674–689. [Google Scholar] [CrossRef]
- Aguilera-Venegas, B.; Olea-Azar, C.; Norambuena, E.; Arán, V.J.; Mendizábal, F.; Lapier, M.; Maya, J.D.; Kemmerling, U.; López-Muñoz, R. ESR, electrochemical, molecular modeling and biological evaluation of 4-substituted and 1,4-disubstituted 7-nitroquinoxalin-2-ones as potential anti-Trypanosoma cruzi agents. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 78, 1004–1012. [Google Scholar] [CrossRef]
- Henderson, G.B.; Ulrich, P.; Fairlamb, A.H.; Rosenberg, I.; Pereira, M.; Sela, M.; Cerami, A. “Subversive” substrates for the enzyme trypanothione disulfide reductase: Alternative approach to chemotherapy of Chagas disease. Proc. Natl. Acad. Sci. USA 1988, 85, 5374–5378. [Google Scholar] [CrossRef]
- Muro, B.; Reviriego, F.; Navarro, P.; Marín, C.; Ramírez-Macías, I.; Rosales, M.J.; Sánchez-Moreno, M.; Arán, V.J. New perspectives on the synthesis and antichagasic activity of 3-alkoxy-1-alkyl-5-nitroindazoles. Eur. J. Med. Chem. 2014, 74, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Alsford, S.; Eckert, S.; Baker, N.; Glover, L.; Sánchez-Flores, A.; Leung, K.F.; Turner, D.J.; Field, M.C.; Berriman, M.; Horn, D. High-throughput decoding of antitrypanosomal drug efficacy and resistance. Nature 2012, 482, 232–236. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Estrada, C.; Pérez-Pertejo, Y.; Domínguez-Asenjo, B.; Holanda, V.N.; Murugesan, S.; Martínez-Valladares, M.; Balaña-Fouce, R.; Reguera, R.M. Further Investigations of Nitroheterocyclic Compounds as Potential Antikinetoplastid Drug Candidates. Biomolecules 2023, 13, 637. https://doi.org/10.3390/biom13040637
García-Estrada C, Pérez-Pertejo Y, Domínguez-Asenjo B, Holanda VN, Murugesan S, Martínez-Valladares M, Balaña-Fouce R, Reguera RM. Further Investigations of Nitroheterocyclic Compounds as Potential Antikinetoplastid Drug Candidates. Biomolecules. 2023; 13(4):637. https://doi.org/10.3390/biom13040637
Chicago/Turabian StyleGarcía-Estrada, Carlos, Yolanda Pérez-Pertejo, Bárbara Domínguez-Asenjo, Vanderlan Nogueira Holanda, Sankaranarayanan Murugesan, María Martínez-Valladares, Rafael Balaña-Fouce, and Rosa M. Reguera. 2023. "Further Investigations of Nitroheterocyclic Compounds as Potential Antikinetoplastid Drug Candidates" Biomolecules 13, no. 4: 637. https://doi.org/10.3390/biom13040637
APA StyleGarcía-Estrada, C., Pérez-Pertejo, Y., Domínguez-Asenjo, B., Holanda, V. N., Murugesan, S., Martínez-Valladares, M., Balaña-Fouce, R., & Reguera, R. M. (2023). Further Investigations of Nitroheterocyclic Compounds as Potential Antikinetoplastid Drug Candidates. Biomolecules, 13(4), 637. https://doi.org/10.3390/biom13040637







