Molecular Basis of Plant Profilins’ Cross-Reactivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Purification of nFra e 2.2 from Ash Pollen
2.2. Ash Profilin (rFra e 2) Expression and Purification
2.3. Recombinant Profilin Allergens Art v 4, Amb a 8, Phl p 12 and Bet v 2
2.4. mAbs (Anti-rHev b 8) Purification
2.5. Recognition of Plants Profilins (rArt v 4, rAmb a 8, rPhl p 12, rBet v 2, and rFra e 2) by mAbs 2D10 and 1B4 (Anti-rHev b 8)
2.6. Characterization of Recognition Sites of mAbs (1B4 and 2D10) with rZea m 12 Mutants
2.7. Evaluation of the Recognition of Sera from Maize and Latex Allergic Patients to rZea m 12 Mutants
2.8. Computational Analyses
2.9. Statistical Analyses
3. Results
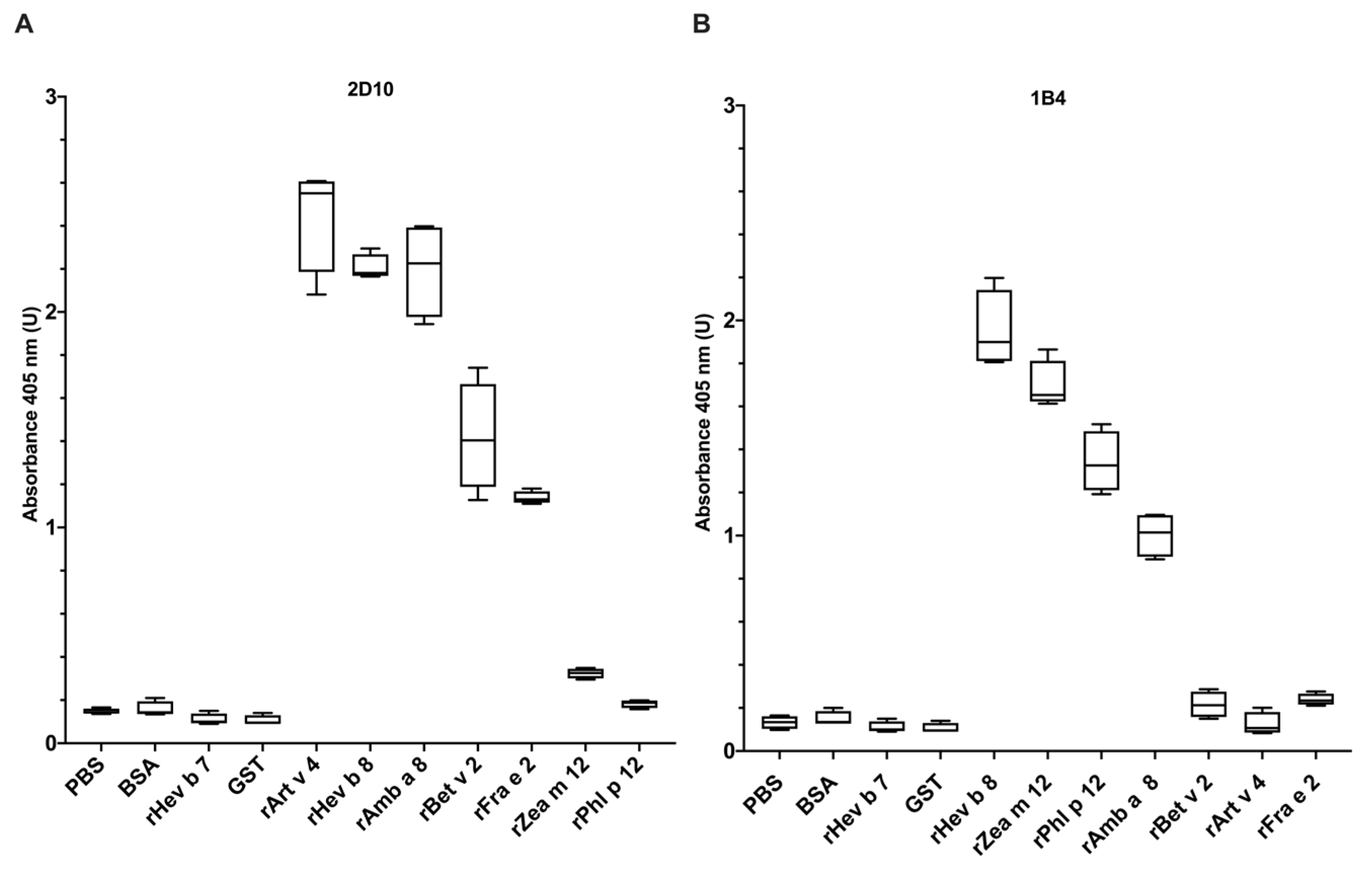
3.1. Evaluation of mAbs 2D10 and 1B4 (Anti-rHev b 8) Recognition towards Allergenic Profilins from Plants
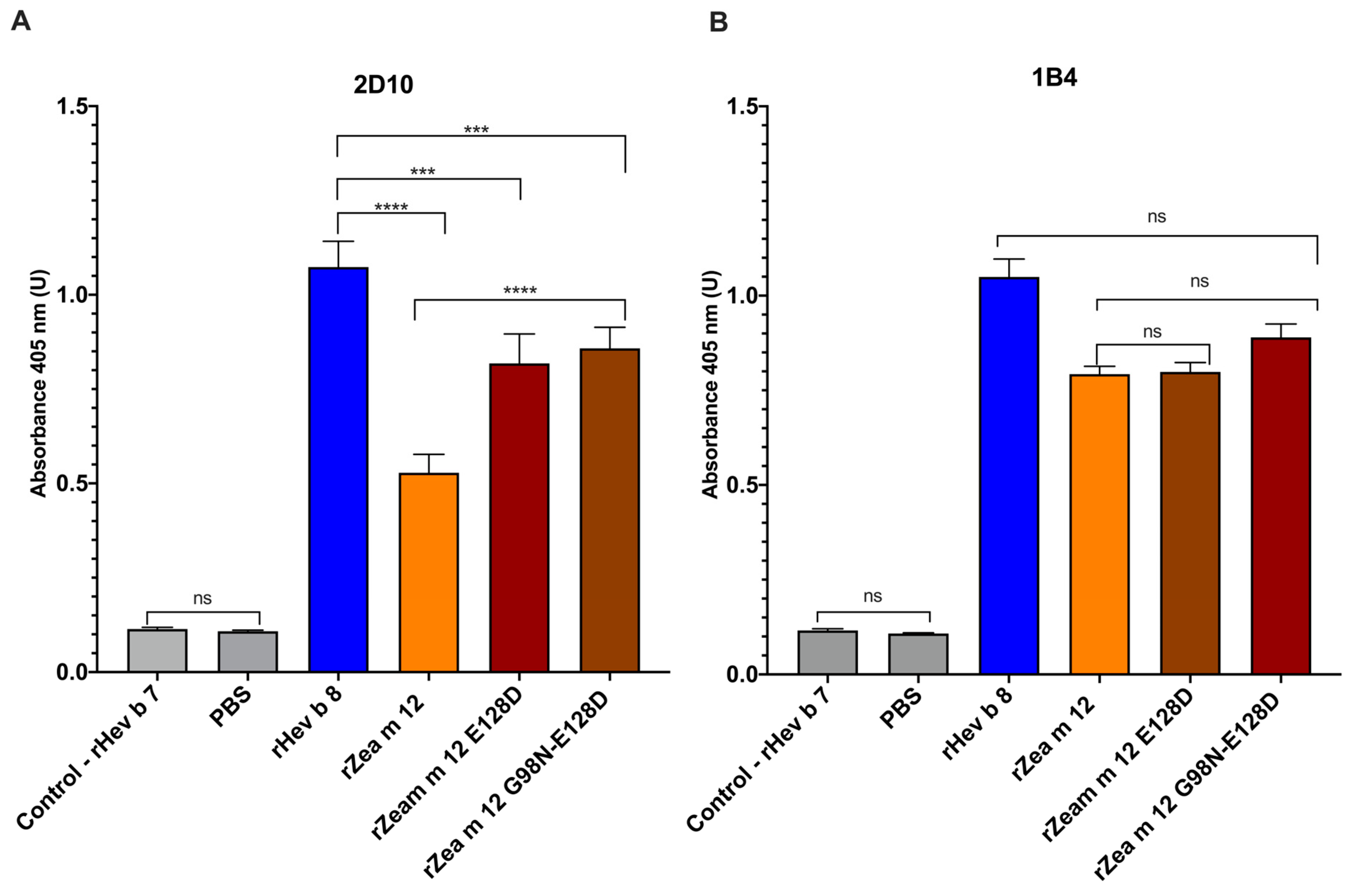
3.2. Characterization of Recognition Sites of IgG mAbs 1B4 and 2D10 (anti-rHev b 8) to the Two Maize Profilin Mutants (rZea m 12)
3.3. Increased Binding of IgE from a Pooled Sera from Maize and Latex Allergic Patients to the Double Mutant rZea m 12 G98N-E128D
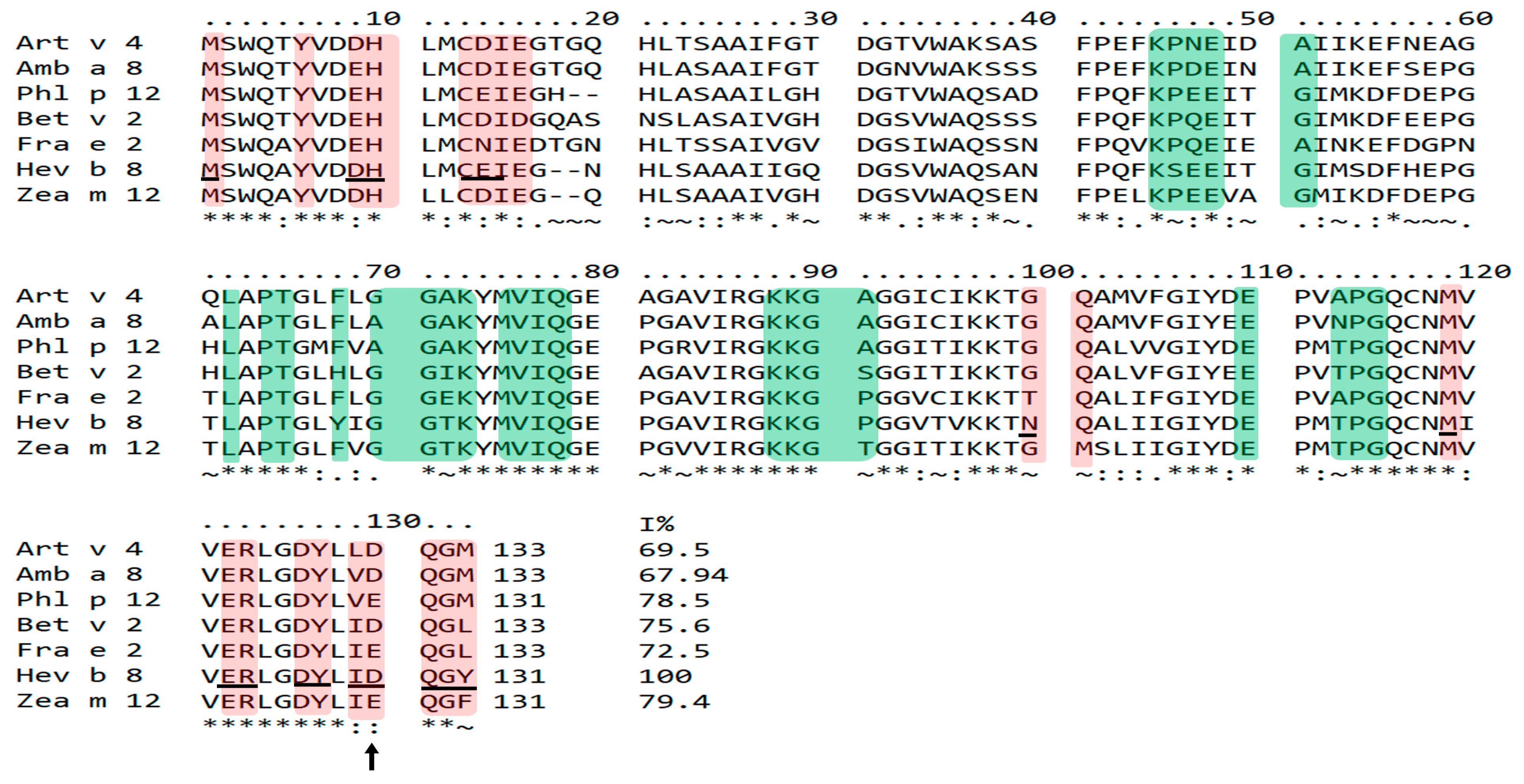
3.4. Identification of Potential Cross-Reactive Residues in Plant Profilin Sequences by Mapping Recognition Sites of mAbs 1B4 and 2D10 (Anti-rHev b 8)
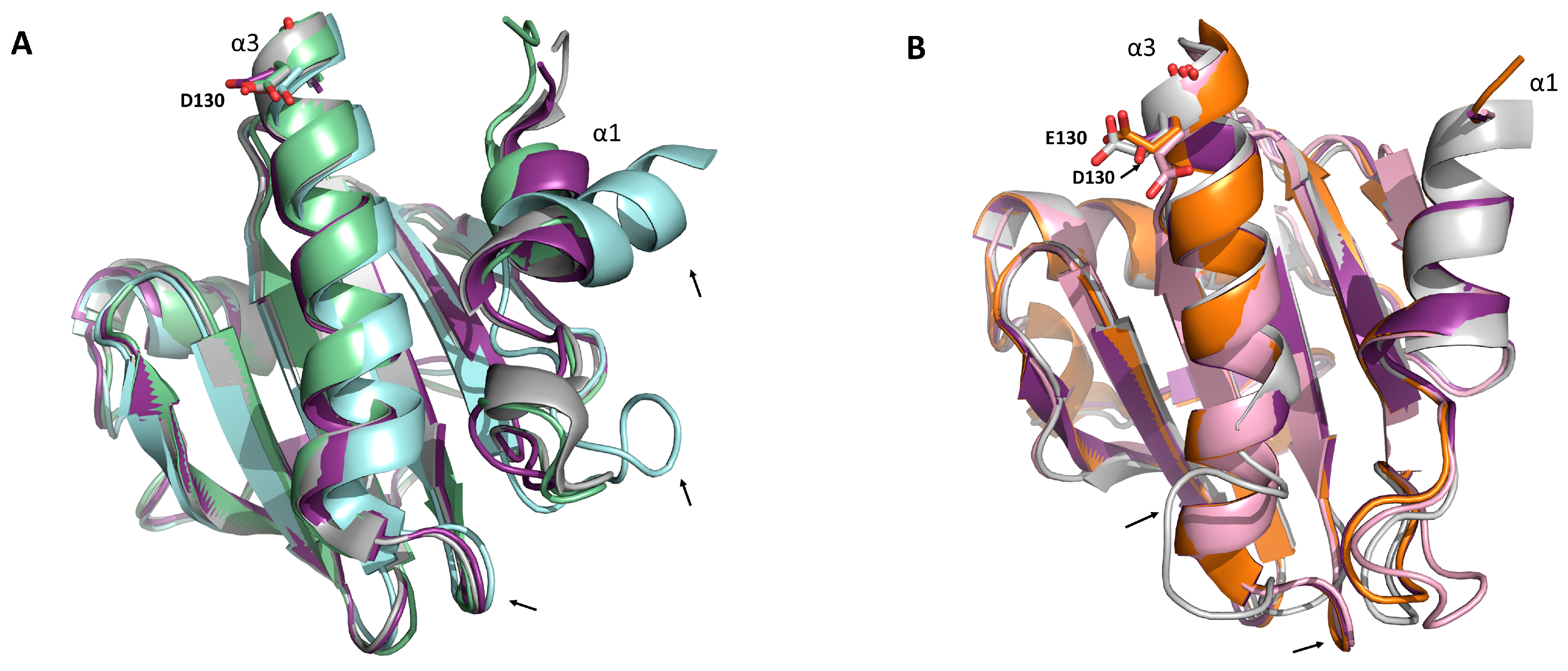
3.5. Identification of Relevant Structural Elements of Plant Profilins in mAb 2D10 (Anti-rHev b 8) Recognition
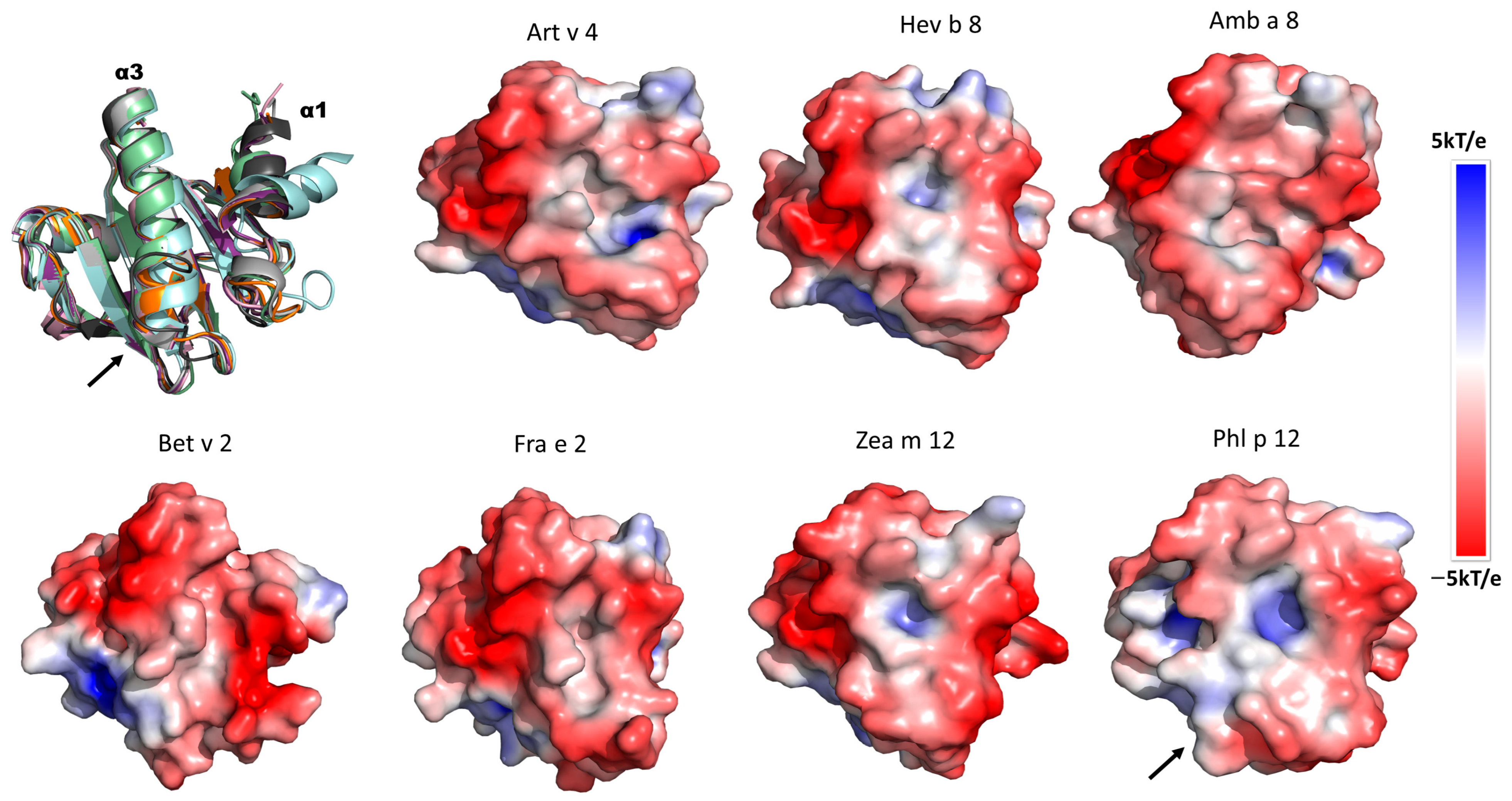
3.6. Electrostatic Potential Analysis of Plant Profilins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wopfner, N.; Gruber, P.; Wallner, M.; Briza, P.; Ebner, C.; Mari, A.; Richter, K.; Vogel, L.; Ferreira, F. Molecular and Immunological Characterization of Novel Weed Pollen Pan-Allergens. Allergy Eur. J. Allergy Clin. Immunol. 2008, 63, 872–881. [Google Scholar] [CrossRef] [PubMed]
- López-Torrejón, G.; Díaz-Perales, A.; Rodríguez, J.; Sánchez-Monge, R.; Crespo, J.F.; Salcedo, G.; Pacios, L.F. An Experimental and Modeling-Based Approach to Locate IgE Epitopes of Plant Profilin Allergens. J. Allergy Clin. Immunol. 2007, 119, 1481–1488. [Google Scholar] [CrossRef]
- Valenta, R.; Duchene, M.; Ebner, C.; Valent, P.; Sillaber, C.; Deviller, P.; Ferreira, F.; Tejkl, M.; Edelmann, H.; Kraft, D.; et al. Profilins Constitute a Novel Family of Functional Plant Pan-Allergens. J. Exp. Med 1992, 175, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Ciprandi, G.; Comite, P.; Mussap, M.; de Amici, M.; Quaglini, S.; Barocci, F.; Marseglia, G.L.; Scala, E. Profiles of Birch Sensitization (Bet v 1, Bet v 2, and Bet v 4) and Oral Allergy Syndrome across Italy. J. Investig. Allergol. Clin. Immunol. 2016, 26, 244–248. [Google Scholar] [CrossRef]
- Ganglberger, E.; Wagner, S. Hev b 8, the Hevea brasiliensis Latex Profilin, Is a Cross-Reactive Allergen of Latex, Plant Foods and Pollen. Int. Arch. Allergy Immunol. 2001, 125, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hornillos, J.; López-Matas, M.A.; Berges Jimeno, P.; Henríquez, A.; Blanco, S.; Seoane-Rodríguez, M.; Mahíllo, I.; Carnés, J. Profilin Is a Marker of Severity in Allergic Respiratory Diseases. Allergy 2020, 75, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Landolina, N.; Levi-Schaffer, F. Monoclonal Antibodies: The New Magic Bullets for Allergy: IUPHAR Review 17. Br. J. Pharmacol. 2016, 173, 793–803. [Google Scholar] [CrossRef]
- Ecker, D.M.; Jones, S.D.; Levine, H.L. The Therapeutic Monoclonal Antibody Market. mAbs 2015, 7, 9–14. [Google Scholar] [CrossRef]
- Sankian, M.; Shirazi, F.G.; Arafi, M.; Moghadam, M. Production and Characterization of Monoclonal Antibody against Saffron Pollen Profilin, Cro s 2. Iran. J. Immunol. 2008, 5, 156–162. [Google Scholar]
- Abedini, S.; Sankian, M.; Falak, R.; Tehrani, M.; Talebi, F.; Shirazi, F.G.; Varasteh, A.R. An Approach for Detection and Quantification of Fruits Natural Profilin: Natural Melon Profilin as a Model. Food Agric. Immunol. 2011, 22, 47–55. [Google Scholar] [CrossRef]
- Asturias, J.A.; Gomez-Bayon, N.; Arilla, M.C.; Sánchez-Pulido, L.; Valencia, A.; Martinez, A. Molecular and Structural Analysis of the Panallergen Profilin B Cell Epitopes Defined by Monoclonal Antibodies. Int. Immunol. 2002, 14, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- García-Ramírez, B.; Mares-Mejía, I.; Rodríguez-Hernández, A.; Cano-Sánchez, P.; Torres-Larios, A.; Ortega, E.; Rodríguez-Romero, A. A Native IgE in Complex with Profilin Provides Insights into Allergen Recognition and Cross-Reactivity. Commun. Biol. 2022, 5, 748. [Google Scholar] [CrossRef] [PubMed]
- Mares-Mejía, I.; García-Ramírez, B.; Torres-Larios, A.; Rodríguez-Hernández, A.; Osornio-Hernández, A.; Terán-Olvera, G.; Ortega, E.; Rodríguez-Romero, A. Novel Murine MAbs Define Specific and Cross-Reactive Epitopes on the Latex Profilin Panallergen Hev b 8. Mol. Immunol. 2020, 128, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Mas, S.; Garrido-Arandia, M.; Batanero, E.; Purohit, A.; Pauli, G.; Rodríguez, R.; Barderas, R.; Villalba, M. Characterization of Profilin and Polcalcin Panallergens from Ash Pollen. J. Investig. Allergol. Clin. Immunol. 2014, 24, 257–266. [Google Scholar] [PubMed]
- Cudowska, B.; Kapingidza, A.B.; Pawłowicz, M.; Pampuch, A.; Hyduke, N.; Pote, S.; Schlachter, C.R.; Lebensztejn, D.M.; Chruszcz, M.; Kowal, K. Production and Use of Recombinant Profilins Amb a 8, Art v 4, Bet v 2, and Phl p 12 for Allergenic Sensitization Studies. Molecules 2020, 25, 369. [Google Scholar] [CrossRef] [PubMed]
- Malley, A.O.; Kapingidza, A.B.; Hyduke, N.; Dolamore, C.; Kowa, K.; Chruszcz, M. Crystal Structure of Timothy Grass Allergen Phl p 12.0101 Reveals an Unusual Profilin Dimer. Acta Biochim. Pol. 2021, 68, 15–22. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Jurrus, E.; Engel, D.; Star, K.; Monson, K.; Brandi, J.; Felberg, L.E.; Brookes, D.H.; Wilson, L.; Chen, J.; Liles, K.; et al. Improvements to the APBS Biomolecular Solvation Software Suite. Protein Sci. 2018, 27, 112–128. [Google Scholar] [CrossRef]
- Abdi, H. (Ed.) Encyclopedia of Measurement and Statistics; Sage: Thousand Oaks, CA, USA, 2007; pp. 103–107. [Google Scholar]
- Sližienė, A.; Plečkaitytė, M.; Zaveckas, M.; Juškaitė, K.; Rudokas, V.; Žvirblis, G.; Žvirblienė, A. Monoclonal Antibodies against the Newly Identified Allergen β-Enolase from Common Carp (Cyprinus Carpio). Food Agric. Immunol. 2022, 33, 129–149. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, N.; Yang, Y.; Hong, S.; Bachert, C. Local Immunoglobulin E in Nasal Polyps: Role and Modulation. Front. Immunol. 2022, 13, 961503. [Google Scholar] [CrossRef]
- Zhang, N.; Holtappels, G.; Gevaert, P.; Patou, J.; Dhaliwal, B.; Gould, H.; Bachert, C. Mucosal Tissue Polyclonal IgE Is Functional in Response to Allergen and SEB. Allergy 2011, 66, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Sankian, M.; Varasteh, A.; Pazouki, N.; Mahmoudi, M. Sequence Homology: A Poor Predictive Value for Profilins. Clin. Mol. Allergy 2005, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Radauer, C.; Willerroider, M.; Fuchs, H.; Hoffmann-Sommergruber, K.; Thalhamer, J.; Ferreira, F.; Scheiner, O.; Breiteneder, H. Cross-Reactive and Species-Specific Immunoglobulin E Epitopes of Plant Profilins: An Experimental and Structure-Based Analysis. Clin. Exp. Allergy 2006, 36, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Kapingidza, A.B.; Pye, S.E.; Hyduke, N.; Dolamore, C.; Schlachter, C.R.; Commins, S.P.; Kowal, K. Comparative Structural and Thermal Stability Studies of Cuc m 2.0101, Art v 4.0101 and Other Allergenic Profilins Allergic Profilins. Mol. Immunol. 2019, 114, 19–29. [Google Scholar] [CrossRef]
- Fedorov, A.A.; Ball, T.; Mahoney, N.M.; Valenta, R.; Almo, S.C. The Molecular Basis for Allergen Cross-Reactivity: Crystal Structure and IgE-Epitope Mapping of Birch Pollen Profilin. Structure 1997, 5, 33–45. [Google Scholar] [CrossRef]
- Offermann, L.R.; Schlachter, C.R.; Perdue, M.L.; Majorek, K.A.; He, J.Z.; Booth, W.T.; Garrett, J.; Kowal, K.; Chruszcz, M. Structural, Functional, and Immunological Characterization of Profilin Panallergens Amb a 8, Art v 4, and Bet v 2. J. Biol. Chem. 2016, 291, 15447–15459. [Google Scholar] [CrossRef]
- Fuchs, T.; Spitzaue, S.; Vente, C.; Hevler, J.; Kapiotis, S.; Rumpold, H.; Kraft, D.; Valenta, R. Natural Latex, Grass Pollen, and Weed Pollen Share IgE Epitopes. J. Allergy Clin. Immunol. 1997, 100, 356–364. [Google Scholar] [CrossRef]
- Soh, W.T.; Briza, P.; Dall, E.; Asam, C.; Schubert, M.; Huber, S.; Aglas, L.; Bohle, B.; Ferreira, F.; Brandstetter, H. Two Distinct Conformations in Bet v 2 Determine Its Proteolytic Resistance to Cathepsin S. Int. J. Mol. Sci. 2017, 18, 2156. [Google Scholar] [CrossRef]
- Westritschnig, K.; Linhart, B.; Focke-Tejkl, M.; Pavkov, T.; Keller, W.; Ball, T.; Mari, A.; Hartl, A.; Stöcklinger, A.; Scheiblhofer, S.; et al. A Hypoallergenic Vaccine Obtained by Tail-to-Head Restructuring of Timothy Grass Pollen Profilin, Phl p 12, for the Treatment of Cross-Sensitization to Profilin. J. Immunol. 2007, 179, 7624–7634. [Google Scholar] [CrossRef]
- Pomés, A.; Mueller, G.A.; Chruszcz, M. Structural Aspects of the Allergen-Antibody Interaction. Front. Immunol. 2020, 11, 1–19. [Google Scholar] [CrossRef]
- Padavattan, S.; Schirmer, T.; Schmidt, M.; Akdis, C.; Valenta, R.; Mittermann, I.; Soldatova, L.; Slater, J.; Mueller, U.; Markovic-Housley, Z. Identification of a B-Cell Epitope of Hyaluronidase, a Major Bee Venom Allergen, from Its Crystal Structure in Complex with a Specific Fab. J. Mol. Biol. 2007, 368, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Mirza, O.; Henriksen, A.; Ipsen, H.; Larsen, J.N.; Wissenbach, M.; Spangfort, M.D.; Gajhede, M. Dominant Epitopes and Allergic Cross-Reactivity: Complex Formation Between a Fab Fragment of a Monoclonal Murine IgG Antibody and the Major Allergen from Birch Pollen Bet v 1. J. Immunol. 2000, 165, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Chruszcz, M.; Pomés, A.; Glesner, J.; Vailes, L.D.; Osinski, T.; Porebski, P.J.; Majorek, K.A.; Heymann, P.W.; Platts-Mills, T.A.E.; Minor, W.; et al. Molecular Determinants for Antibody Binding on Group 1 House Dust Mite Allergens. J. Biol. Chem. 2012, 287, 7388–7398. [Google Scholar] [CrossRef]
- Orengo, J.M.; Radin, A.R.; Kamat, V.; Badithe, A.; Ben, L.H.; Bennett, B.L.; Zhong, S.; Birchard, D.; Limnander, A.; Rafique, A.; et al. Treating Cat Allergy with Monoclonal IgG Antibodies That Bind Allergen and Prevent IgE Engagement. Nat. Commun. 2018, 9, 1421. [Google Scholar] [CrossRef] [PubMed]
- Atanasio, A.; Franklin, M.C.; Kamat, V.; Hernandez, A.R.; Badithe, A.; Ben, L.H.; Jones, J.; Bautista, J.; Yancopoulos, G.D.; Olson, W.; et al. Targeting Immunodominant Bet v 1 Epitopes with Monoclonal Antibodies Prevents the Birch Allergic Response. JACI 2022, 149, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Sae-Wong, C.; Mizutani, N.; Kangsanant, S.; Yoshino, S. Topical Skin Treatment with Fab Fragments of an Allergen-Specific IgG1 Monoclonal Antibody Suppresses Allergen-Induced Atopic Dermatitis-like Skin Lesions in Mice. Eur. J. Pharmacol. 2016, 779, 131–137. [Google Scholar] [CrossRef]
- Yoshino, S.; Mizutani, N. Intranasal Exposure to Monoclonal Antibody Fab Fragments to Japanese Cedar Pollen Cry J1 Suppresses Japanese Cedar Pollen-Induced Allergic Rhinitis. Br. J. Pharmacol. 2016, 173, 1629–1638. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terán, M.G.; García-Ramírez, B.; Mares-Mejía, I.; Ortega, E.; O’Malley, A.; Chruszcz, M.; Rodríguez-Romero, A. Molecular Basis of Plant Profilins’ Cross-Reactivity. Biomolecules 2023, 13, 608. https://doi.org/10.3390/biom13040608
Terán MG, García-Ramírez B, Mares-Mejía I, Ortega E, O’Malley A, Chruszcz M, Rodríguez-Romero A. Molecular Basis of Plant Profilins’ Cross-Reactivity. Biomolecules. 2023; 13(4):608. https://doi.org/10.3390/biom13040608
Chicago/Turabian StyleTerán, María G., Benjamín García-Ramírez, Israel Mares-Mejía, Enrique Ortega, Andrea O’Malley, Maksymilian Chruszcz, and Adela Rodríguez-Romero. 2023. "Molecular Basis of Plant Profilins’ Cross-Reactivity" Biomolecules 13, no. 4: 608. https://doi.org/10.3390/biom13040608
APA StyleTerán, M. G., García-Ramírez, B., Mares-Mejía, I., Ortega, E., O’Malley, A., Chruszcz, M., & Rodríguez-Romero, A. (2023). Molecular Basis of Plant Profilins’ Cross-Reactivity. Biomolecules, 13(4), 608. https://doi.org/10.3390/biom13040608







