Tutorial for Stopped-Flow Water Flux Measurements: Why a Report about “Ultrafast Water Permeation through Nanochannels with a Densely Fluorous Interior Surface” Is Flawed
Abstract
1. Introduction
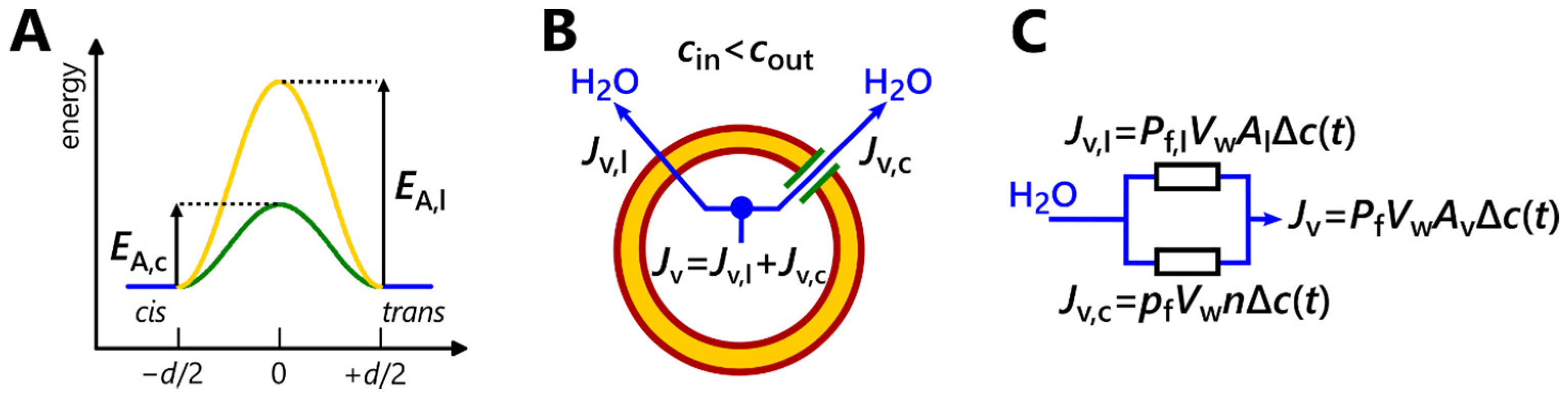
2. A Brief Tutorial on Water Flux Measurements Using Stopped-Flow (Materials and Methods)
2.1. Vesicles in an Osmotic Gradient
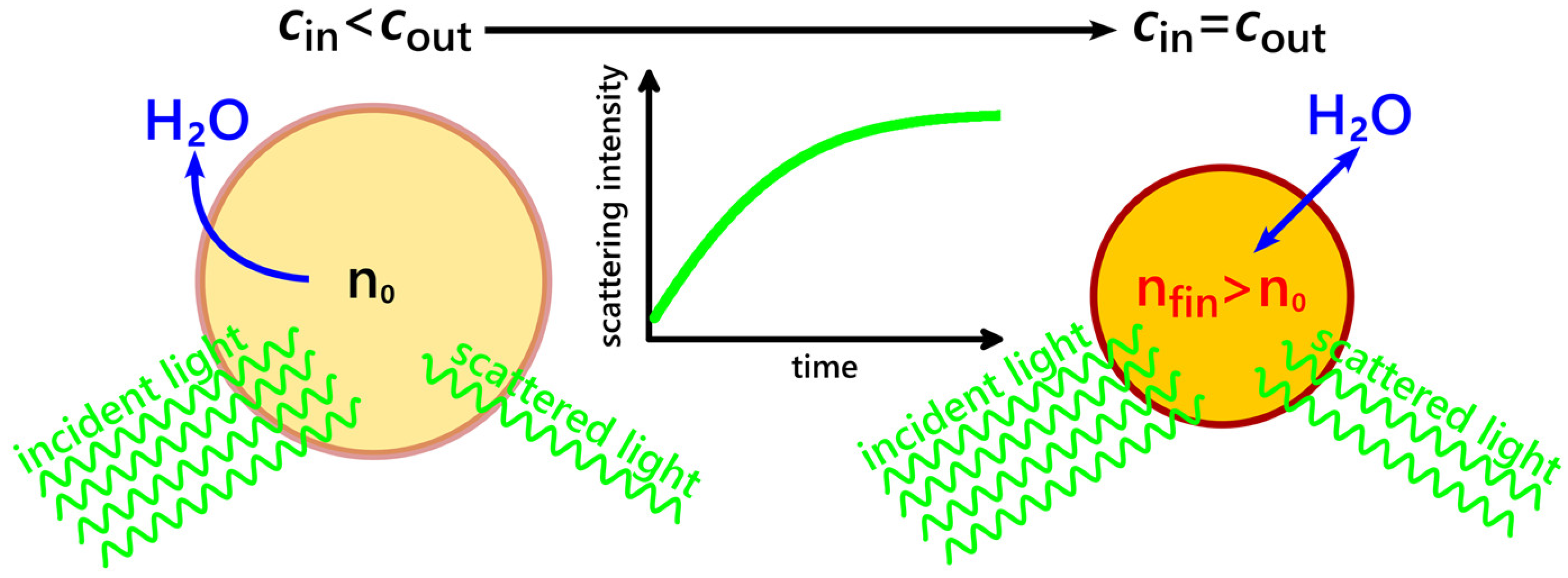
2.2. Assessing Vesicle Deflation Kinetics in a Liposome Suspension by Measuring the Intensity of Scattered Light
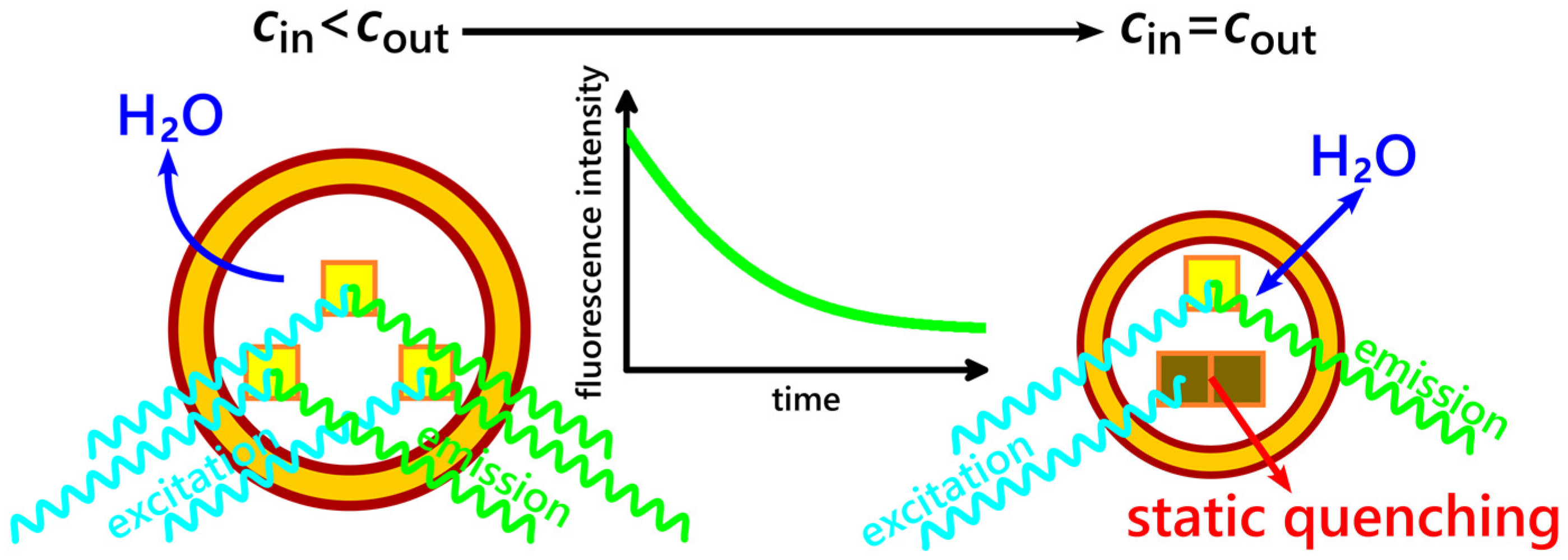
2.3. Evaluating Vesicle Deflation Kinetics in a Liposome Suspension by Measuring the Fluorescence Intensity of Encapsulated Aqueous Dyes
3. Results
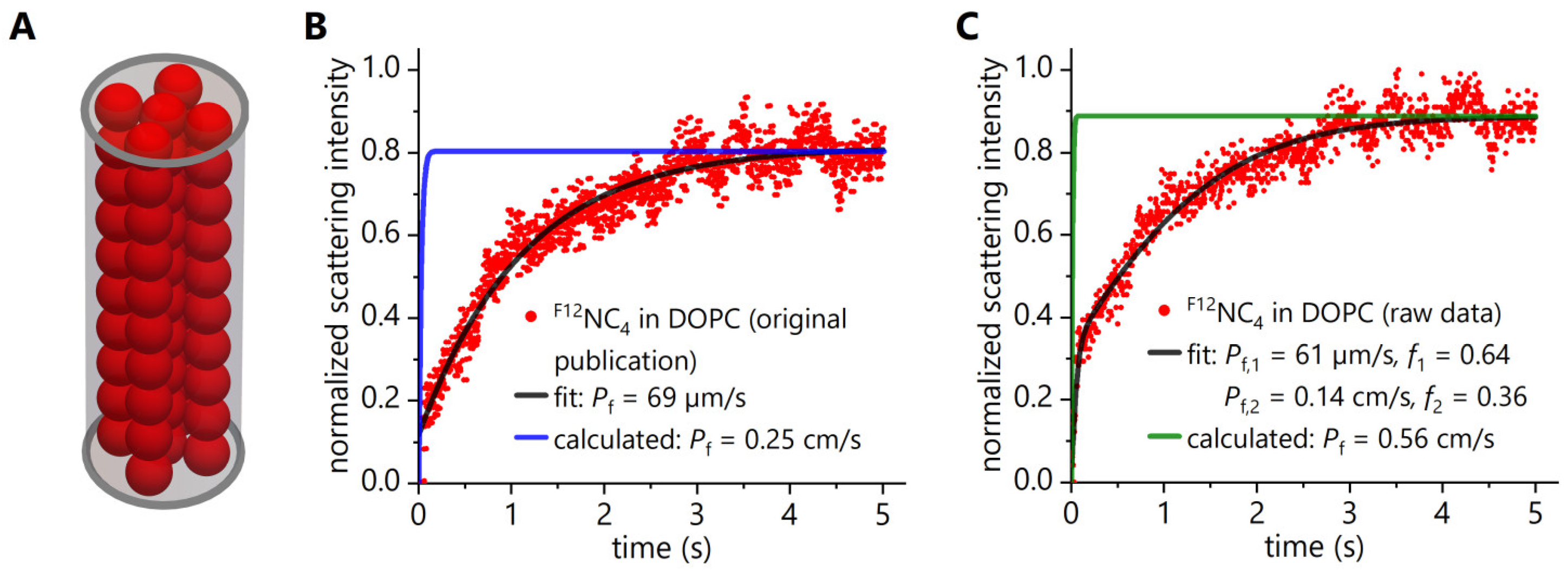
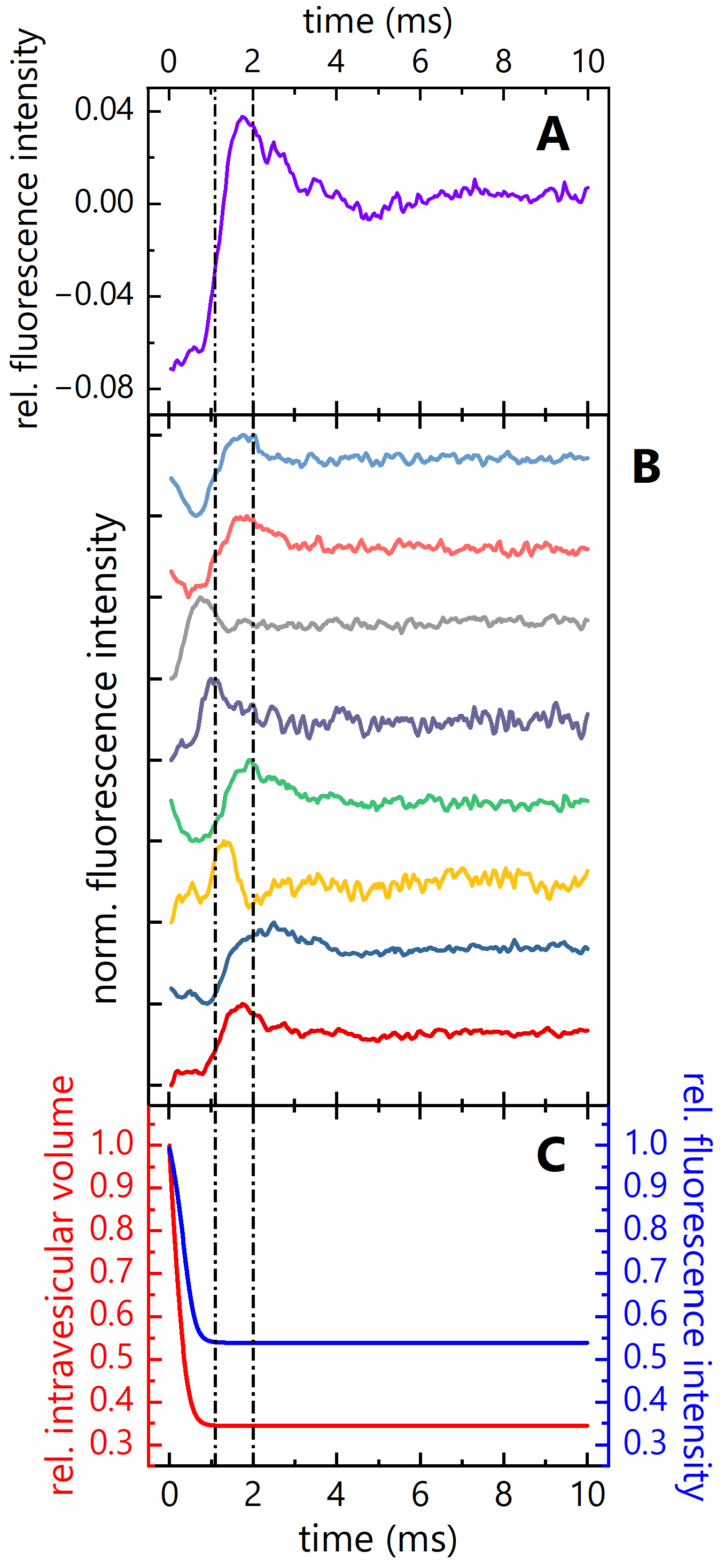
3.1. Reassessment of Experimental Data Obtained in Stopped-Flow Light Scattering Experiments with F12NC4
3.2. Reassessment of Experimental Data Obtained in Stopped-Flow Fluorescence Self-Quenching Experiments with F12NC4
4. Discussion
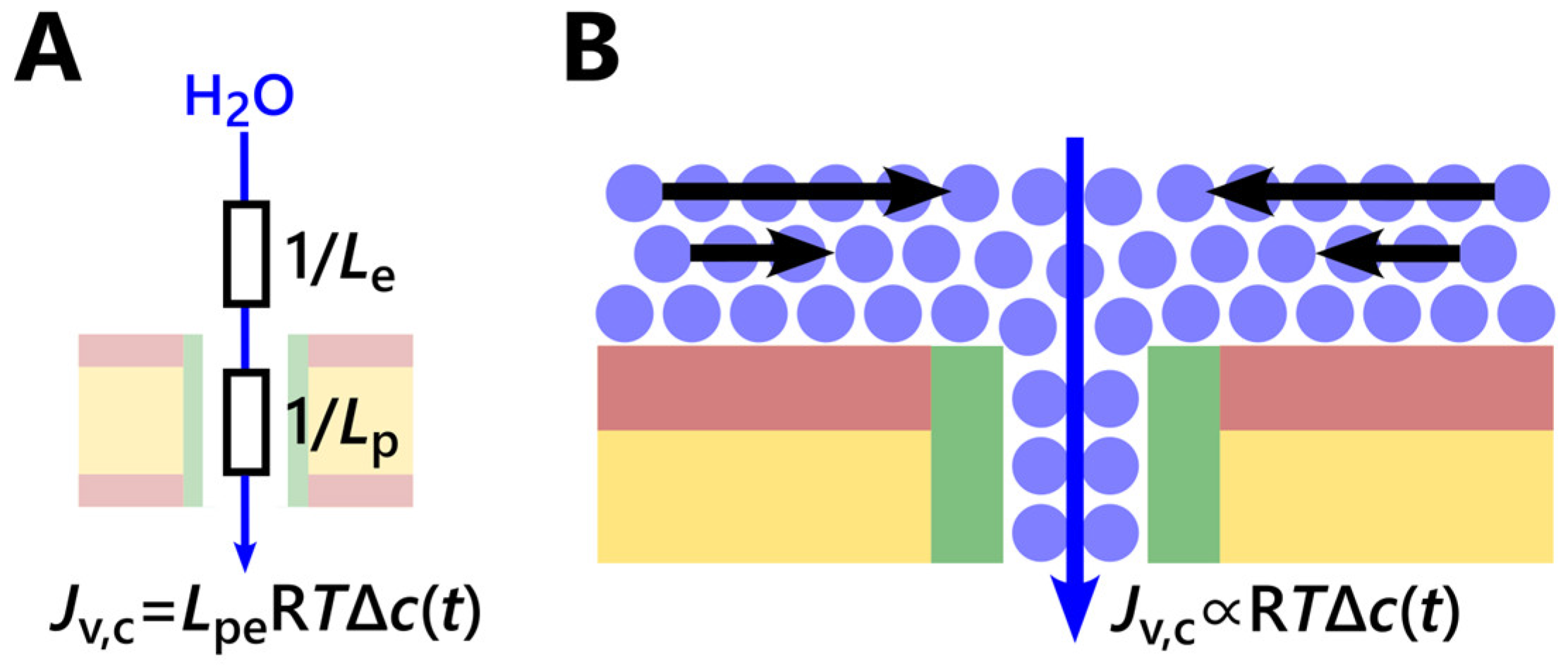
4.1. Criticism of the Stopped-Flow Water Flux Measurements with Fluorous Nanochannels
4.2. Estimation of the Permeability Limit for Narrow Water-Conducting Channels
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pohl, P.; Saparov, S.M.; Borgnia, M.J.; Agre, P. Highly selective water channel activity measured by voltage clamp: Analysis of planar lipid bilayers reconstituted with purified AqpZ. Proc. Natl. Acad. Sci. USA 2001, 98, 9624–9629. [Google Scholar] [CrossRef]
- Murata, K.; Mitsuoka, K.; Hirai, T.; Walz, T.; Agre, P.; Heymann, J.B.; Engel, A.K.; Fujiyoshi, Y. Structural determinants of water permeation through aquaporin-1. Nature 2000, 407, 599–605. [Google Scholar] [CrossRef]
- Porter, C.J.; Werber, J.R.; Zhong, M.; Wilson, C.J.; Elimelech, M. Pathways and Challenges for Biomimetic Desalination Membranes with Sub-Nanometer Channels. ACS Nano 2020, 14, 10894–10916. [Google Scholar] [CrossRef]
- Hilf, R.J.C.; Dutzler, R. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature 2009, 457, 115–118. [Google Scholar] [CrossRef]
- Hilf, R.J.C.; Dutzler, R. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature 2008, 452, 375–379. [Google Scholar] [CrossRef]
- Hassaine, G.; Deluz, C.; Grasso, L.; Wyss, R.; Tol, M.B.; Hovius, R.; Graff, A.; Stahlberg, H.; Tomizaki, T.; Desmyter, A.; et al. X-ray structure of the mouse serotonin 5-HT3 receptor. Nature 2014, 512, 276–281. [Google Scholar] [CrossRef]
- Tunuguntla, R.H.; Henley, R.Y.; Yao, Y.-C.; Pham, T.A.; Wanunu, M.; Noy, A. Enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins. Science 2017, 357, 792–796. [Google Scholar] [CrossRef]
- Horner, A.; Pohl, P. Comment on “Enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins”. Science 2018, 359, eaap9173. [Google Scholar] [CrossRef]
- Pfeffermann, J.; Goessweiner-Mohr, N.; Pohl, P. The energetic barrier to single-file water flow through narrow channels. Biophys. Rev. 2021, 13, 913–923. [Google Scholar] [CrossRef]
- Itoh, Y.; Chen, S.; Hirahara, R.; Konda, T.; Aoki, T.; Ueda, T.; Shimada, I.; Cannon, J.J.; Shao, C.; Shiomi, J.; et al. Ultrafast water permeation through nanochannels with a densely fluorous interior surface. Science 2022, 376, 738–743. [Google Scholar] [CrossRef]
- Horner, A.; Zocher, F.; Preiner, J.; Ollinger, N.; Siligan, C.; Akimov, S.A.; Pohl, P. Mobility of Single-File Water Molecules in Aquaporins. Biophys. J. 2015, 108, 182A. [Google Scholar] [CrossRef]
- Nebel, S.; Ganz, P.; Seelig, J. Heat changes in lipid membranes under sudden osmotic stress. Biochemistry 1997, 36, 2853–2859. [Google Scholar] [CrossRef]
- Grossman, E.B.; Harris, H.W., Jr.; Star, R.A.; Zeidel, M.L. Water and nonelectrolyte permeabilities of apical membranes of toad urinary bladder granular cells. Am. J. Physiol.-Cell Physiol. 1992, 262, C1109–C1118. [Google Scholar] [CrossRef]
- Horner, A.; Zocher, F.; Preiner, J.; Ollinger, N.; Siligan, C.; Akimov, S.A.; Pohl, P. The mobility of single-file water molecules is governed by the number of H-bonds they may form with channel-lining residues. Sci. Adv. 2015, 1, e1400083. [Google Scholar] [CrossRef]
- Horner, A.; Pohl, P. Single-file transport of water through membrane channels. Faraday Discuss. 2018, 209, 9–33. [Google Scholar] [CrossRef]
- Rosenberg, P.A.; Finkelstein, A. Water permeability of gramicidin A-treated lipid bilayer membranes. J. Gen. Physiol. 1978, 72, 341–350. [Google Scholar] [CrossRef]
- Pohl, P.; Saparov, S.M. Solvent drag across gramicidin channels demonstrated by microelectrodes. Biophys. J. 2000, 78, 2426–2434. [Google Scholar] [CrossRef]
- Gössweiner-Mohr, N.; Siligan, C.; Pluhackova, K.; Umlandt, L.; Koefler, S.; Trajkovska, N.; Horner, A. The hidden intricacies of aquaporins: Remarkable details in a common structural scaffold. Small 2022, 18, 2202056. [Google Scholar] [CrossRef]
- Walz, T.; Smith, B.L.; Zeidel, M.L.; Engel, A.; Agre, P. Biologically active two-dimensional crystals of aquaporin chip. J. Biol. Chem. 1994, 269, 1583–1586. [Google Scholar] [CrossRef]
- Jahnke, N.; Krylova, O.O.; Hoomann, T.; Vargas, C.; Fiedler, S.; Pohl, P.; Keller, S. Real-time monitoring of membrane-protein reconstitution by isothermal titration calorimetry. Anal. Chem. 2014, 86, 920–927. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Murase, O.; Sugishita, K.; Yoneyama, S.; Akada, K.; Ueha, M.; Nakamura, A.; Kobayashi, S. Optical characterization of liposomes by right angle light scattering and turbidity measurement. Biochim. Biophys. Acta (BBA)—Biomembr. 2000, 1467, 219–226. [Google Scholar] [CrossRef]
- Wachlmayr, J.; Hannesschlaeger, C.; Speletz, A.; Barta, T.; Eckerstorfer, A.; Siligan, C.; Horner, A. Scattering versus fluorescence self-quenching: More than a question of faith for the quantification of water flux in large unilamellar vesicles? Nanoscale Adv. 2021, 4, 58–76. [Google Scholar] [CrossRef]
- Olbrich, K.; Rawicz, W.; Needham, D.; Evans, E. Water Permeability and Mechanical Strength of Polyunsaturated Lipid Bilayers. Biophys. J. 2000, 79, 321–327. [Google Scholar] [CrossRef]
- Huster, D.; Jin, A.J.; Arnold, K.; Gawrisch, K. Water permeability of polyunsaturated lipid membranes measured by 17O NMR. Biophys. J. 1997, 73, 855–864. [Google Scholar] [CrossRef]
- Mathai, J.C.; Tristram-Nagle, S.; Nagle, J.F.; Zeidel, M.L. Structural determinants of water permeability through the lipid membrane. J. Gen. Physiol. 2008, 131, 69–76. [Google Scholar] [CrossRef]
- Jansen, M.; Blume, A. A comparative study of diffusive and osmotic water permeation across bilayers composed of phospholipids with different head groups and fatty acyl chains. Biophys. J. 1995, 68, 997–1008. [Google Scholar] [CrossRef]
- Nagle, J.F.; Tristram-Nagle, S. Structure of lipid bilayers. Biochim. Biophys. Acta (BBA)—Biomembr. 2000, 1469, 159–195. [Google Scholar] [CrossRef]
- Finkelstein, A. Water Movement through Lipid Bilayers, Pores, and Plasma Membranes; Wiley & Sons: New York, NY, USA, 1987. [Google Scholar]
- Kavokine, N.; Netz, R.R.; Bocquet, L. Fluids at the Nanoscale: From Continuum to Subcontinuum Transport. Annu. Rev. Fluid Mech. 2021, 53, 377–410. [Google Scholar] [CrossRef]
- Eliseev, A.A.; Poyarkov, A.A.; Chernova, E.A.; Eliseev, A.A.; Chumakov, A.P.; Konovalov, O.V.; Petukhov, D.I. Operando study of water vapor transport through ultra-thin graphene oxide membranes. 2d Mater. 2019, 6, 035039. [Google Scholar] [CrossRef]
- Saparov, S.M.; Pfeifer, J.R.; Al-Momani, L.; Portella, G.; de Groot, B.L.; Koert, U.; Pohl, P. Mobility of a one-dimensional confined file of water molecules as a function of file length. Phys. Rev. Lett. 2006, 96, 148101. [Google Scholar] [CrossRef]
- Zhu, F.; Tajkhorshid, E.; Schulten, K. Collective diffusion model for water permeation through microscopic channels. Phys. Rev. Lett. 2004, 93, 224501. [Google Scholar] [CrossRef] [PubMed]
- Lauga, E.; Brenner, M.; Stone, H. Microfluidics: The No-Slip Boundary Condition. In Springer Handbook of Experimental Fluid Mechanics; Tropea, C., Yarin, A.L., Foss, J.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1219–1240. [Google Scholar] [CrossRef]
- Secchi, E.; Marbach, S.; Niguès, A.; Stein, D.; Siria, A.; Bocquet, L. Massive radius-dependent flow slippage in carbon nanotubes. Nature 2016, 537, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Hille, B. Ion Channels of Excitable Membranes, 3rd ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2001. [Google Scholar]
- Gravelle, S.; Joly, L.; Detcheverry, F.; Ybert, C.; Cottin-Bizonne, C.; Bocquet, L. Optimizing water permeability through the hourglass shape of aquaporins. Proc. Natl. Acad. Sci. USA 2013, 110, 16367–16372. [Google Scholar] [CrossRef]
- Portella, G.; de Groot, B.L. Determinants of water permeability through nanoscopic hydrophilic channels. Biophys. J. 2009, 96, 925–938. [Google Scholar] [CrossRef] [PubMed]
- Bellissent-Funel, M.-C.; Hassanali, A.; Havenith, M.; Henchman, R.; Pohl, P.; Sterpone, F.; van der Spoel, D.; Xu, Y.; Garcia, A.E. Water Determines the Structure and Dynamics of Proteins. Chem. Rev. 2016, 116, 7673–7697. [Google Scholar] [CrossRef] [PubMed]
- de Groot, B.L.; Tieleman, D.P.; Pohl, P.; Grubmüller, H. Water permeation through gramicidin A: Desformylation and the double helix: A molecular dynamics study. Biophys. J. 2002, 82, 2934–2942. [Google Scholar] [CrossRef]
- Hummer, G.; Rasaiah, J.C.; Noworyta, J.P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature 2001, 414, 188–190. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfeffermann, J.; Pohl, P. Tutorial for Stopped-Flow Water Flux Measurements: Why a Report about “Ultrafast Water Permeation through Nanochannels with a Densely Fluorous Interior Surface” Is Flawed. Biomolecules 2023, 13, 431. https://doi.org/10.3390/biom13030431
Pfeffermann J, Pohl P. Tutorial for Stopped-Flow Water Flux Measurements: Why a Report about “Ultrafast Water Permeation through Nanochannels with a Densely Fluorous Interior Surface” Is Flawed. Biomolecules. 2023; 13(3):431. https://doi.org/10.3390/biom13030431
Chicago/Turabian StylePfeffermann, Juergen, and Peter Pohl. 2023. "Tutorial for Stopped-Flow Water Flux Measurements: Why a Report about “Ultrafast Water Permeation through Nanochannels with a Densely Fluorous Interior Surface” Is Flawed" Biomolecules 13, no. 3: 431. https://doi.org/10.3390/biom13030431
APA StylePfeffermann, J., & Pohl, P. (2023). Tutorial for Stopped-Flow Water Flux Measurements: Why a Report about “Ultrafast Water Permeation through Nanochannels with a Densely Fluorous Interior Surface” Is Flawed. Biomolecules, 13(3), 431. https://doi.org/10.3390/biom13030431






