Targeting of the Interleukin-13 Receptor (IL-13R)α2 Expressing Prostate Cancer by a Novel Hybrid Lytic Peptide
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies and Other Reagents
2.2. Cell Culture
2.3. Cell Transfection
2.4. Real-Time Polymerase Chain Reaction (RT-PCR)
2.5. Protein Extraction
2.6. Immunoblotting
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Crystal Violet Staining
2.9. Cell Viability Assay
2.10. Cytotoxicity Assay
2.11. ApoTox-GloTM Triplex Assay
2.12. TSA and 5-aza-dC Treatment
2.13. Three-Dimensional Culturing
2.14. Statistical Analysis
3. Results
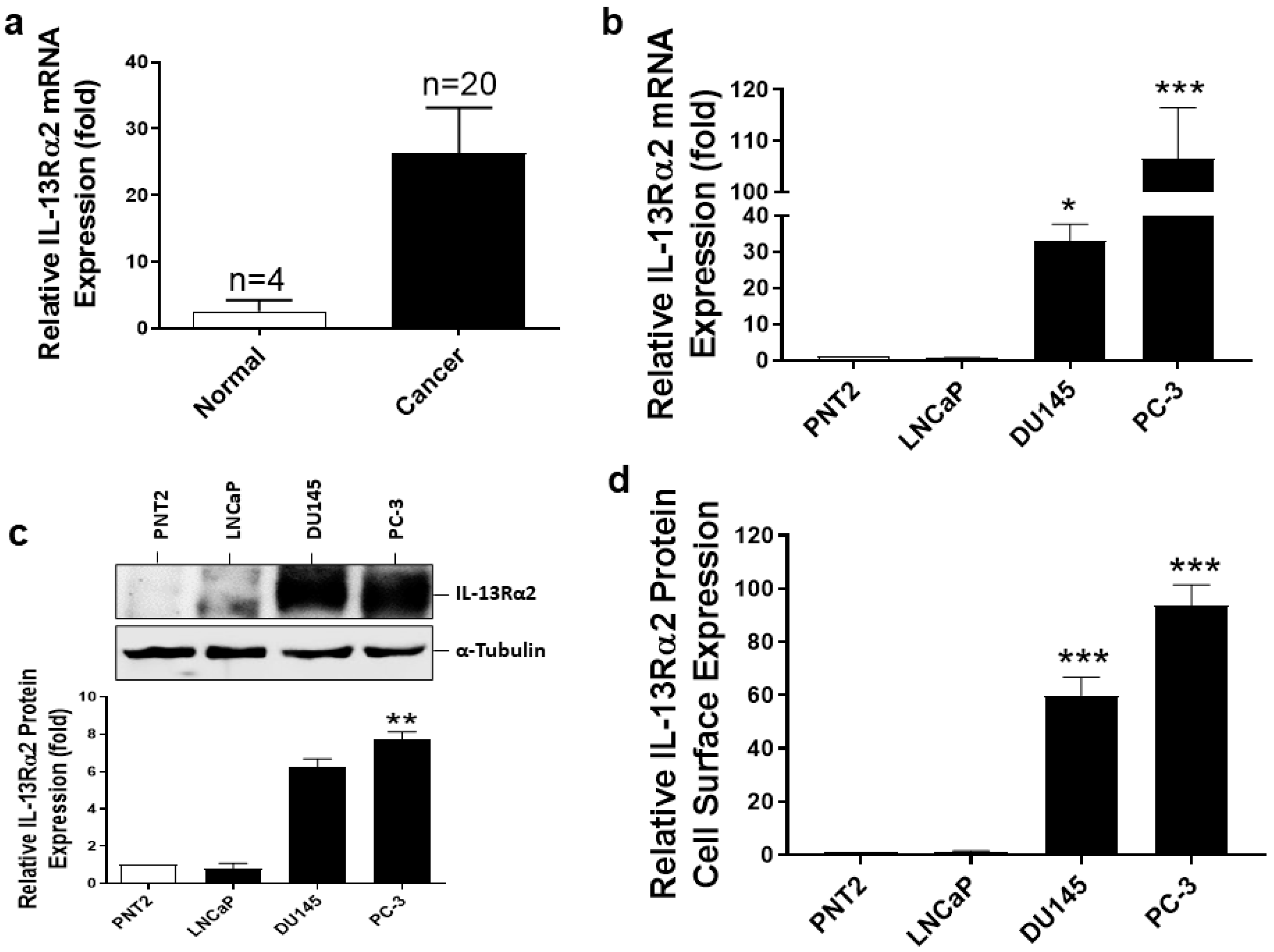
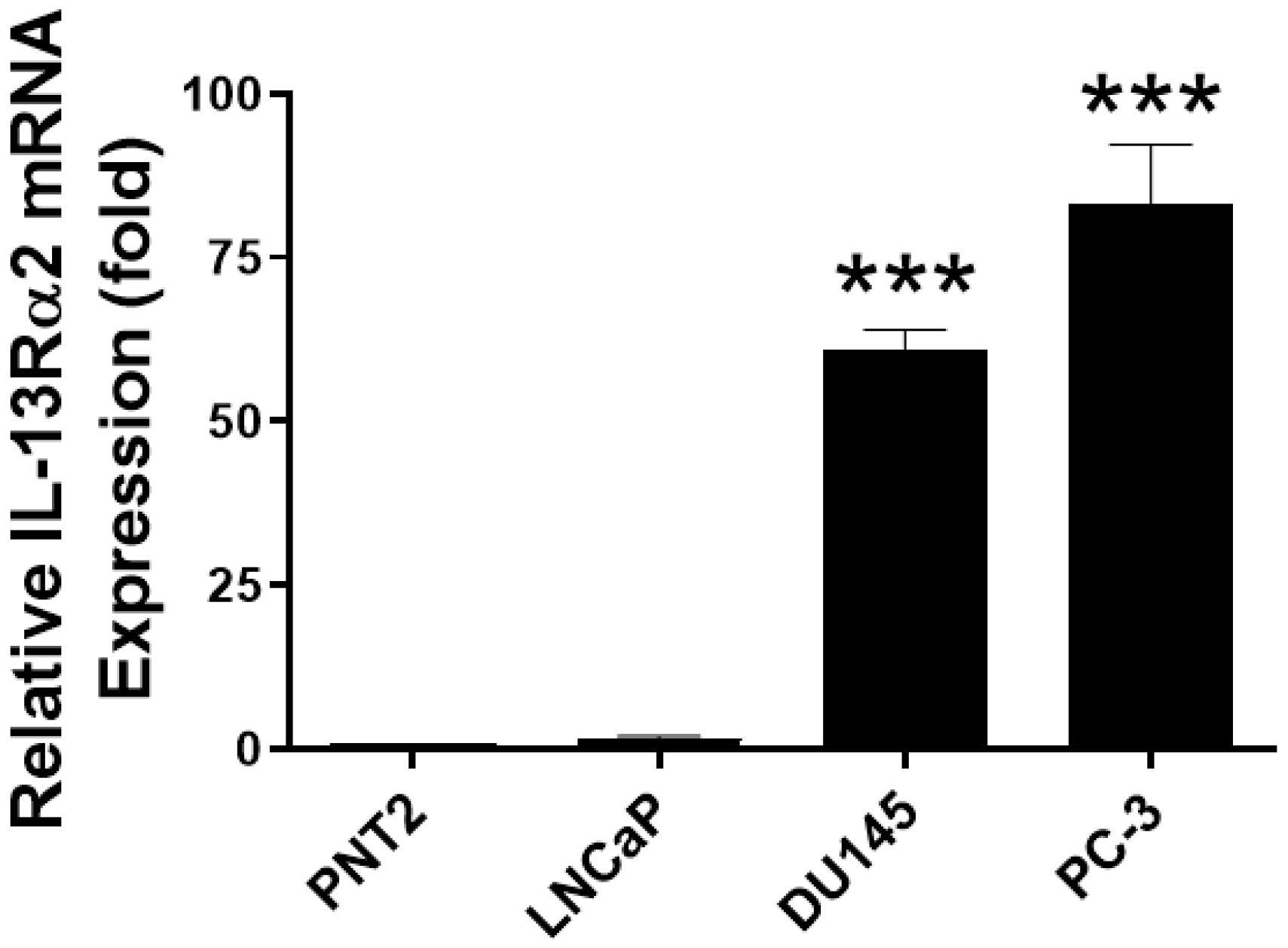
3.1. Expression of IL-13Rα2 in Prostate Cancer Tissues and Cell Lines
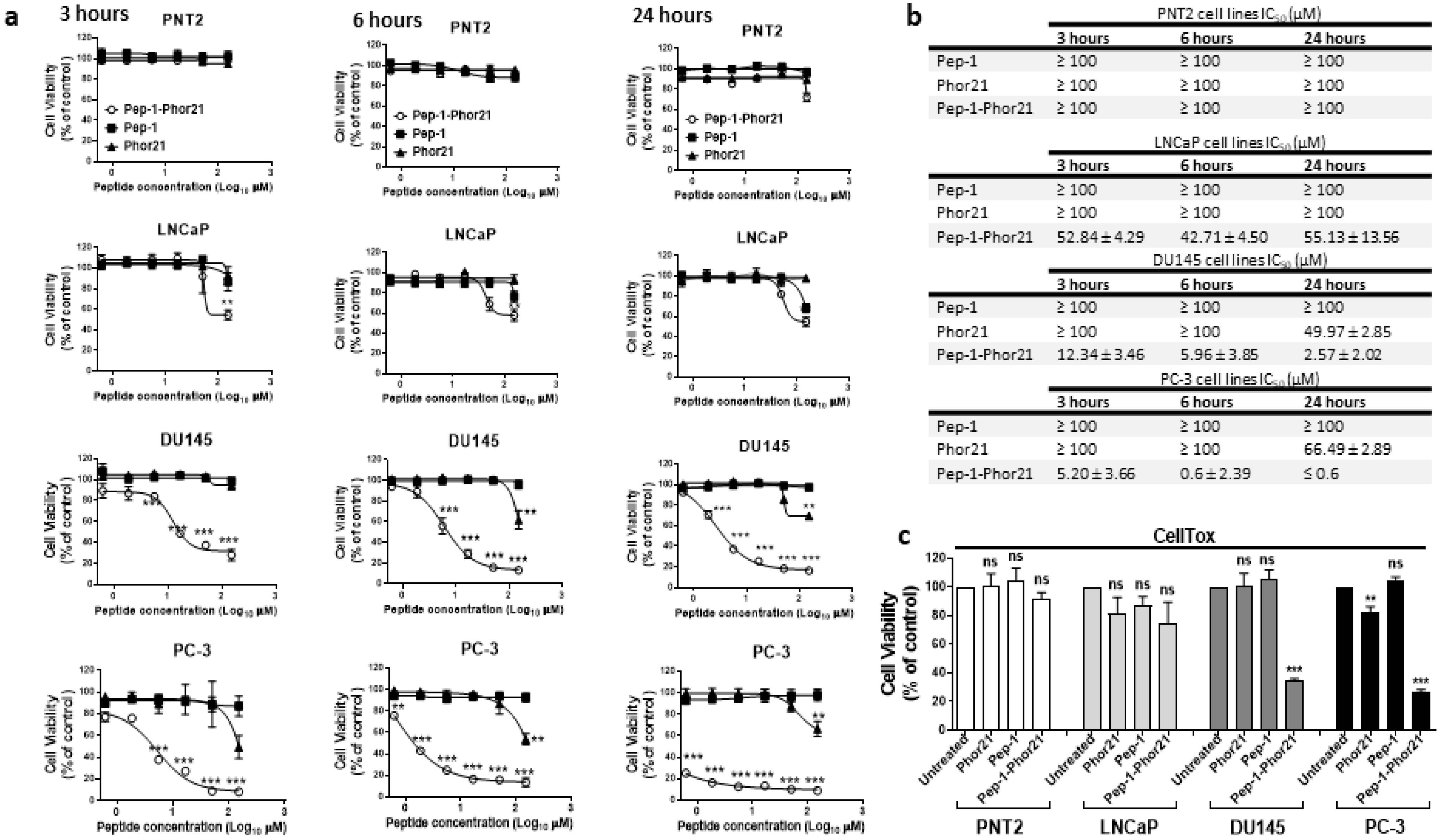
3.2. The Specificity of Pep-1-Phor21 in Targeting IL-13Rα2-Expressing Cells
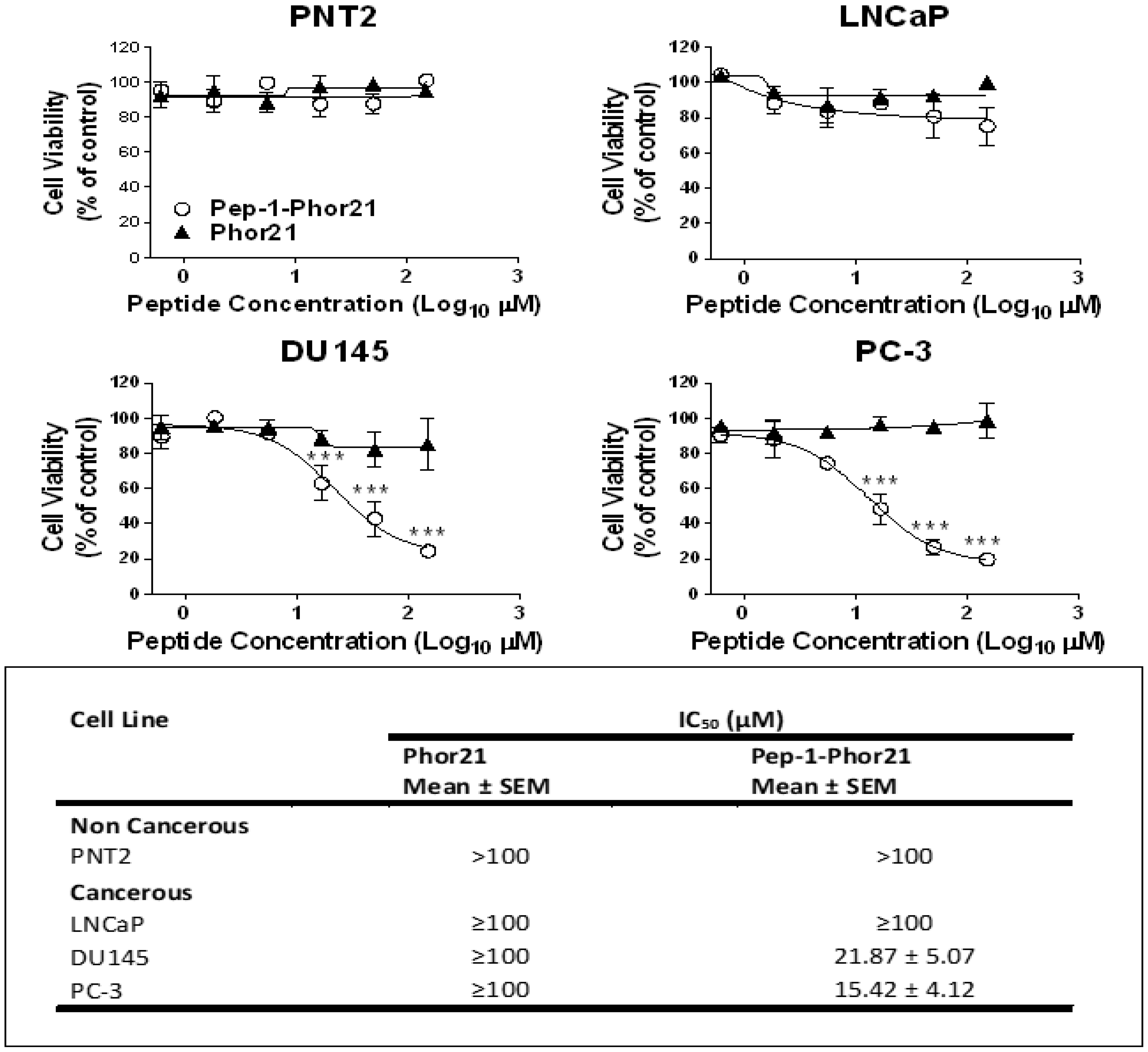
3.3. The Cytotoxic Effect of Pep-1-Phor21 Peptide on Prostate Cancer Cells
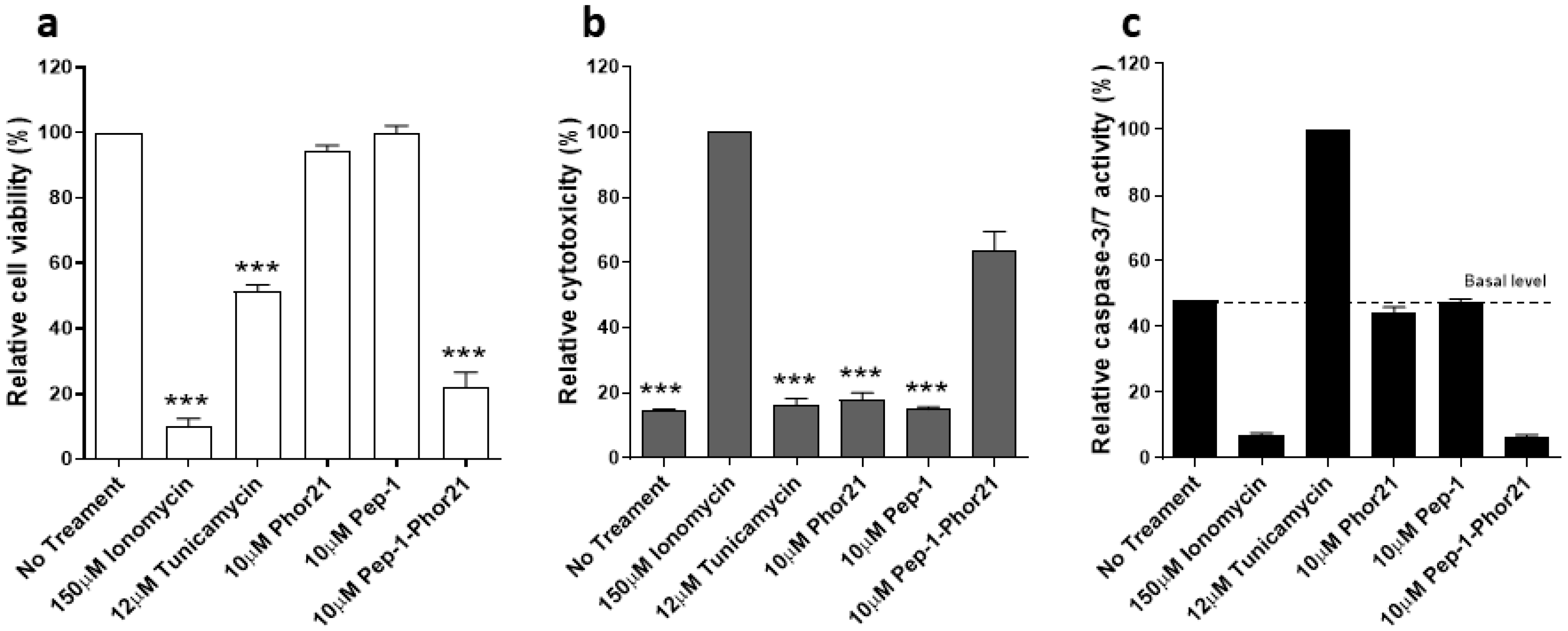
3.4. Characterisation of the Mode of Action of Pep-1-Phor21
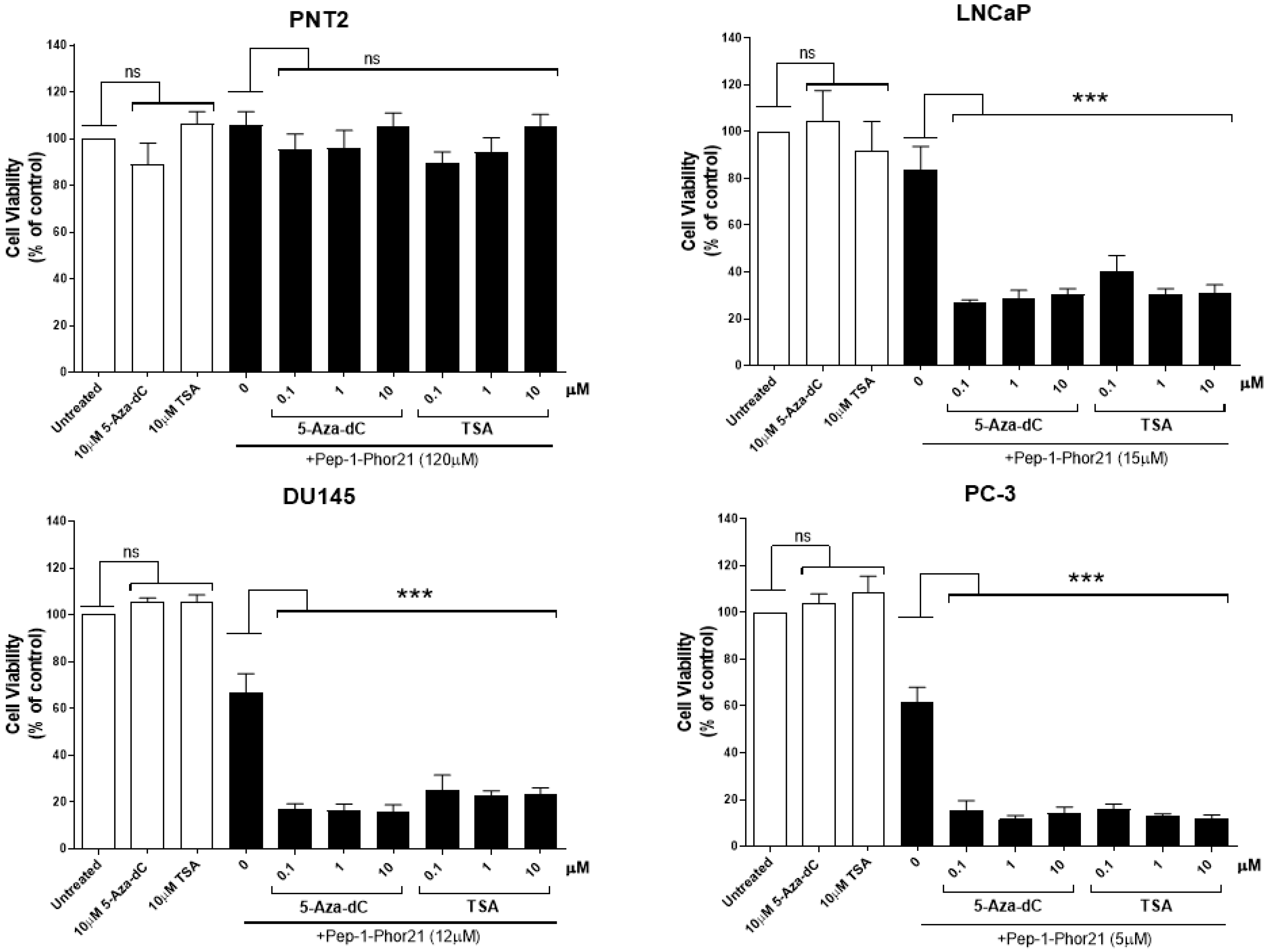
3.5. Analysis of IL-13Rα2 Expression in Prostate Cancer Cells Treated with TSA or 5-aza-dC
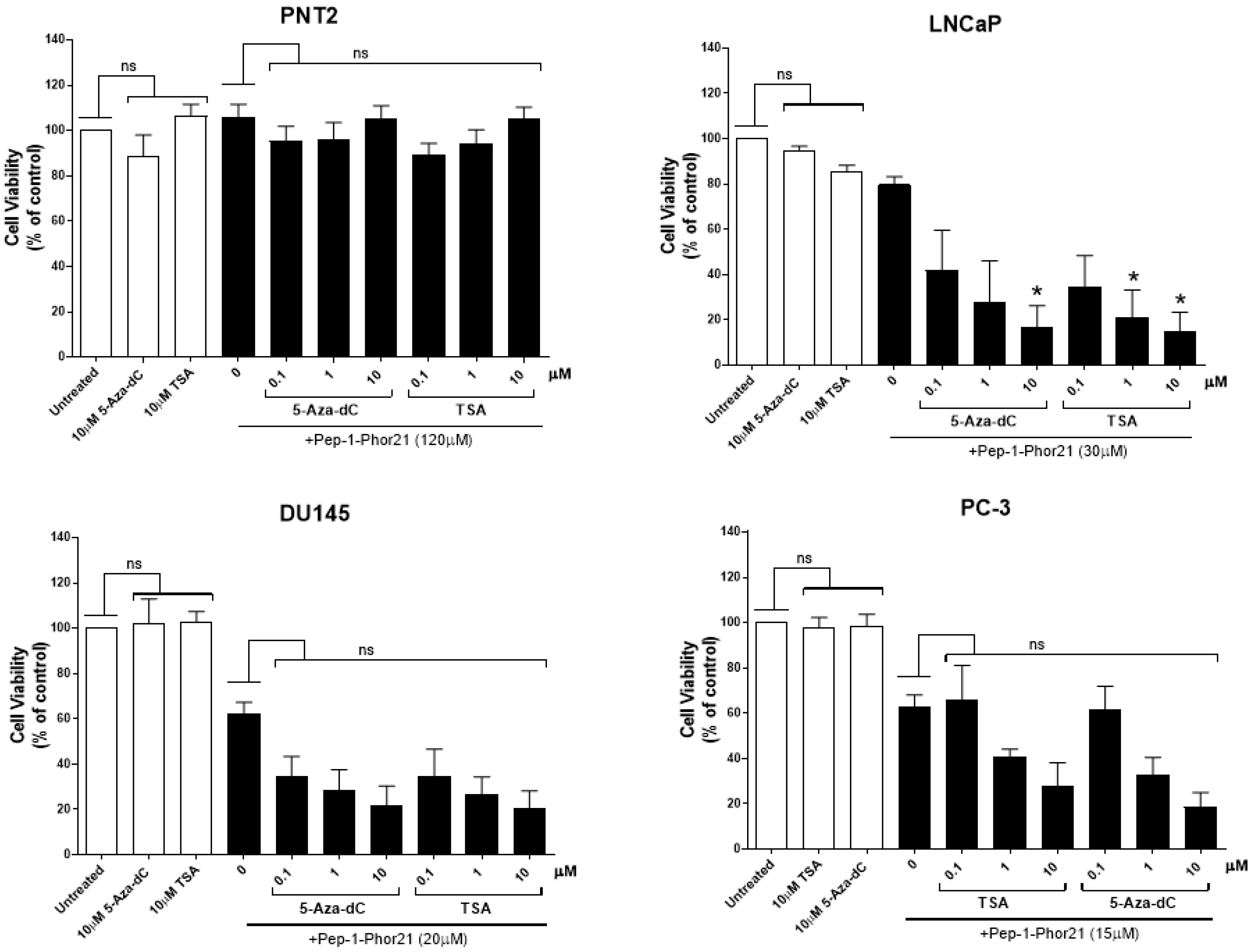
3.6. The Cytotoxic Effect of Pep-1-Phor21 on Prostate Cancer Cells Treated with TSA or 5-aza-dC
3.7. IL-13Rα2 mRNA Expression in 3D-Cultured Prostate Cancer Cells
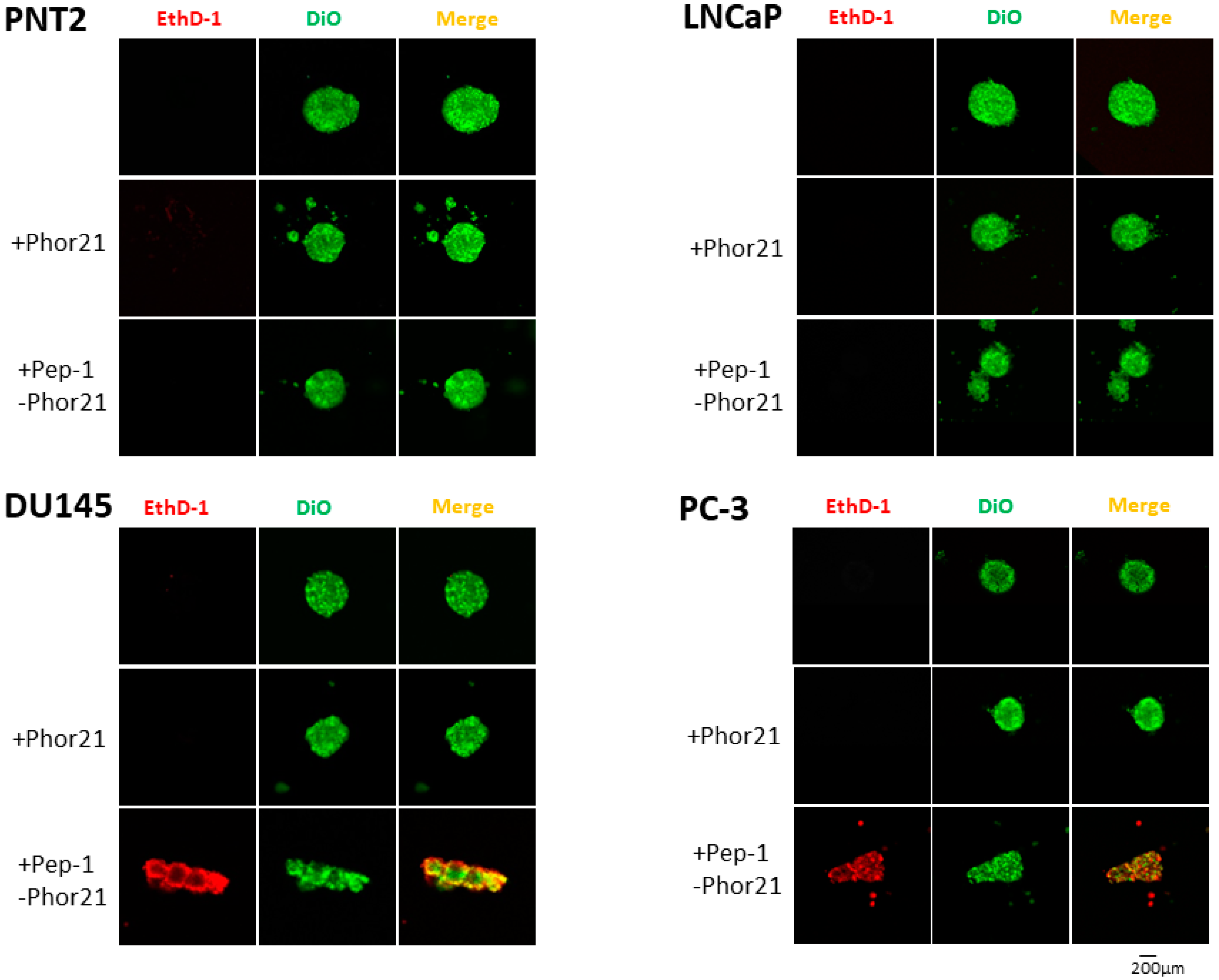
3.8. Effect of Pep-1-Phor21 on the Viability of 3D-Cultured Prostate Cancer Cells
3.9. IL-13Rα2 mRNA Expression in 3D-Cultured Prostate Cell Lines Treated with TSA and 5-aza-dC
3.10. The Cytotoxic Effect of Pep-1-Phor21 on Prostate Cell Spheroids Treated with TSA and 5-aza-dC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Harrington, S.E.; Smith, T.J. The Role of Chemotherapy at the End of Life: “When Is Enough, Enough?”. JAMA J. Am. Med. Assoc. 2008, 299, 2667–2678. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Wientjes, M.G.; Lu, D.; Au, J.L. Drug delivery and transport to solid tumors. Pharm. Res. 2003, 20, 1337–1350. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Otte, M. Occult micrometastasis: Enrichment, identification and characterization of single disseminated tumour cells. Semin. Cancer Biol. 2001, 11, 327–337. [Google Scholar] [CrossRef]
- Katragadda, S.; Budda, B.; Anand, B.S.; Mitra, A.K. Role of efflux pumps and metabolising enzymes in drug delivery. Expert Opin. Drug Deliv. 2005, 2, 683–705. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T. Endocrine treatment of prostate cancer. J. Steroid. Biochem. Mol. Biol. 2004, 92, 287–295. [Google Scholar] [CrossRef]
- Zurawski, S.M.; Vega, F.; Huyghe, B.; Zurawski, G. Receptors for interleukin-13 and interleukin-4 are complex and share a novel component that functions in signal transduction. EMBO J. 1993, 12, 2663–2670. [Google Scholar] [CrossRef]
- Andrews, A.-L.; Nasir, T.; Bucchieri, F.; Holloway, J.W.; Holgate, S.T.; Davies, D.E. IL-13 receptor α 2: A regulator of IL-13 and IL-4 signal transduction in primary human fibroblasts. J. Allergy Clin. Immunol. 2006, 118, 858–865. [Google Scholar] [CrossRef]
- Kawakami, K.; Takeshita, F.; Puri, R.K. Identification of Distinct Roles for a Dileucine and a Tyrosine Internalization Motif in the Interleukin (IL)-13 Binding Component IL-13 Receptor α2 Chain. J. Biol. Chem. 2001, 276, 25114–25120. [Google Scholar] [CrossRef]
- Hershey, G.K.K. IL-13 receptors and signaling pathways: An evolving web. J. Allergy Clin. Immunol. 2003, 111, 677–690. [Google Scholar] [CrossRef]
- Debinski, W.; Gibo, D.M. Molecular expression analysis of restrictive receptor for interleukin 13, a brain tumor-associated cancer/testis antigen. Mol. Med. 2000, 6, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Moreno, O.; Calvo, A.; Joshi, B.H.; Abasolo, I.; Leland, P.; Wang, Z.; Montuenga, L.; Puri, R.K.; Green, J.E. Gene expression profiling identifies IL-13 receptor alpha 2 chain as a therapeutic target in prostate tumor cells overexpressing adrenomedullin. Int. J. Cancer. J. Int. Du. Cancer 2005, 114, 870–878. [Google Scholar] [CrossRef]
- He, H.; Xu, J.; Nelson, P.S.; Marshall, F.F.; Chung, L.W.K.; Zhau, H.E.; He, D.; Wang, R. Differential expression of the α2 chain of the interleukin-13 receptor in metastatic human prostate cancer ARCaPM cells. Prostate 2010, 70, 993–1001. [Google Scholar] [CrossRef]
- Kioi, M.; Kawakami, M.; Shimamura, T.; Husain, S.R.; Puri, R.K. Interleukin-13 receptor α2 chain. Cancer 2006, 107, 1407–1418. [Google Scholar] [CrossRef]
- Kawakami, K.; Terabe, M.; Kawakami, M.; Berzofsky, J.A.; Puri, R.K. Characterization of a novel human tumor antigen interleukin-13 receptor alpha2 chain. Cancer Res. 2006, 66, 4434–4442. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, S.O.; Sharma, P.; Harbor, P.C.; Aman, M.J.; Vogelbaum, M.A.; Haque, S.J. IL-13Rα2, a Decoy Receptor for IL-13 Acts As an Inhibitor of IL-4-dependent Signal Transduction in Glioblastoma Cells. Cancer Res. 2002, 62, 1103–1109. [Google Scholar] [PubMed]
- Cho, W.K.; Lee, C.M.; Kang, M.J.; Huang, Y.; Giordano, F.J.; Lee, P.J.; Trow, T.K.; Homer, R.J.; Sessa, W.C.; Elias, J.A.; et al. IL-13 receptor alpha2-arginase 2 pathway mediates IL-13-induced pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 304, L112–L124. [Google Scholar] [CrossRef]
- Fichtner-Feigl, S.; Strober, W.; Kawakami, K.; Puri, R.K.; Kitani, A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat. Med. 2006, 12, 99–106. [Google Scholar] [CrossRef]
- Lumsden, R.V.; Worrell, J.C.; Boylan, D.; Walsh, S.M.; Cramton, J.; Counihan, I.; O’Beirne, S.; Medina, M.F.; Gauldie, J.; Fabre, A.; et al. Modulation of pulmonary fibrosis by IL-13Ralpha2. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L710–L718. [Google Scholar] [CrossRef]
- Xie, M.; Wu, X.J.; Zhang, J.J.; He, C.S. IL-13 receptor alpha2 is a negative prognostic factor in human lung cancer and stimulates lung cancer growth in mice. Oncotarget 2015, 6, 32902–32913. [Google Scholar] [CrossRef]
- Tu, M.; Wange, W.; Cai, L.; Zhu, P.; Gao, Z.; Zheng, W. IL-13 receptor alpha2 stimulates human glioma cell growth and metastasis through the Src/PI3K/Akt/mTOR signaling pathway. Tumour Biol. 2016, 37, 14701–14709. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Suzuki, A.; Leland, P.; Joshi, B.H.; Puri, R.K. Identification of a novel role of IL-13Ralpha2 in human Glioblastoma multiforme: Interleukin-13 mediates signal transduction through AP-1 pathway. J. Transl. Med. 2018, 16, 369. [Google Scholar] [CrossRef]
- Newman, J.P.; Wang, G.Y.; Arima, K.; Guan, S.P.; Waters, M.R.; Cavenee, W.K.; Pan, E.; Aliwarga, E.; Chong, S.T.; Kok, C.Y.L.; et al. Interleukin-13 receptor alpha 2 cooperates with EGFRvIII signaling to promote glioblastoma multiforme. Nat. Commun. 2017, 8, 1913. [Google Scholar] [CrossRef]
- Fujisawa, T.; Joshi, B.; Nakajima, A.; Puri, R.K. A novel role of interleukin-13 receptor alpha2 in pancreatic cancer invasion and metastasis. Cancer Res. 2009, 69, 8678–8685. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Choi, J.E.; Bae, Y.K. Interleukin-13 receptor alpha 2 expression in tumor cells is associated with reduced disease-free survival in patients with luminal subtype invasive breast cancer. Tumour Biol. 2018, 40, 1010428318783657. [Google Scholar] [CrossRef] [PubMed]
- Knudson, K.M.; Hwang, S.; McCann, M.S.; Joshi, B.H.; Husain, S.R.; Puri, R.K. Recent Advances in IL-13Ralpha2-Directed Cancer Immunotherapy. Front. Immunol. 2022, 13, 878365. [Google Scholar] [CrossRef] [PubMed]
- Kioi, M.; Kawakami, K.; Puri, R.K. Analysis of antitumor activity of an interleukin-13 (IL-13) receptor-targeted cytotoxin composed of IL-13 antagonist and Pseudomonas exotoxin. Clin. Cancer Res. 2004, 10, 6231–6238. [Google Scholar] [CrossRef]
- Kunwar, S.; Prados, M.D.; Chang, S.M.; Berger, M.S.; Lang, F.F.; Piepmeier, J.M.; Sampson, J.H.; Ram, Z.; Gutin, P.H.; Gibbons, R.D.; et al. Direct Intracerebral Delivery of Cintredekin Besudotox (IL13-PE38QQR) in Recurrent Malignant Glioma: A Report by the Cintredekin Besudotox Intraparenchymal Study Group. J. Clin. Oncol. 2007, 25, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Debinski, W.; Obiri, N.I.; Powers, S.K.; Pastan, I.; Puri, R.K. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and pseudomonas exotoxin. Clin. Cancer Res. 1995, 1, 1253–1258. [Google Scholar]
- Kim, J.W.; Young, J.S.; Solomaha, E.; Kanojia, D.; Lesniak, M.S.; Balyasnikova, I.V. A novel single-chain antibody redirects adenovirus to IL13Ralpha2-expressing brain tumors. Sci. Rep. 2015, 5, 18133. [Google Scholar] [CrossRef] [PubMed]
- Smith, Z.D.; Meissner, A. DNA methylation: Roles in mammalian development. Nat. Rev. Genet. 2013, 14, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Hartono, S.R.; Korf, I.F.; Chédin, F. GC skew is a conserved property of unmethylated CpG island promoters across vertebrates. Nucleic Acids Res. 2015, 43, 9729–9741. [Google Scholar] [CrossRef]
- Jones, P.A.; Laird, P.W. Cancer epigenetics comes of age. Nat. Genet. 1999, 21, 163–167. [Google Scholar] [CrossRef]
- Momparler, R.L.; Bovenzi, V. DNA methylation and cancer. J. Cell Physiol. 2000, 183, 145–154. [Google Scholar] [CrossRef]
- Hebbes, T.R.; Thorne, A.W.; Crane-Robinson, C. A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J. 1988, 7, 1395–1402. [Google Scholar] [CrossRef]
- Monneret, C. Histone deacetylase inhibitors for epigenetic therapy of cancer. Anticancer. Drugs 2007, 18, 363–370. [Google Scholar] [CrossRef]
- Wu, A.H.; Low, W.C. Molecular cloning and identification of the human interleukin 13 alpha 2 receptor (IL-13Ra2) promoter. Neuro. Oncol. 2003, 5, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Joshi, B.H.; Puri, R.K. Histone modification enhances the effectiveness of IL-13 receptor targeted immunotoxin in murine models of human pancreatic cancer. J. Transl. Med. 2011, 9, 37. [Google Scholar] [CrossRef]
- Griffiths, E.A.; Gore, S.D. DNA methyltransferase and histone deacetylase inhibitors in the treatment of myelodysplastic syndromes. Semin. Hematol. 2008, 45, 23–30. [Google Scholar] [CrossRef]
- Pandya, H.; Gibo, D.M.; Garg, S.; Kridel, S.; Debinski, W. An interleukin 13 receptor α 2–specific peptide homes to human Glioblastoma multiforme xenografts. Neuro.-Oncol. 2012, 14, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Muller, A.; Vuorenoja, S.; Tuominen, M.; Waclawik, A.; Brokken, L.J.S.; Ziecik, A.J.; Huhtaniemi, I.; Rahman, N.A. Use of hecate-chorionic gonadotropin beta conjugate in therapy of lutenizing hormone receptor expressing gonadal somatic cell tumors. Mol. Cell Endocrinol. 2007, 269, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Maini, A.; Hillman, G.; Haas, G.P.; Wang, C.Y.; Montecillo, E.; Hamzavi, F.; Pontes, J.E.; Leland, P.; Pastan, I.; Debinski, W.; et al. Interleukin-13 receptors on human prostate carcinoma cell lines represent a novel target for a chimeric protein composed of IL-13 and a mutated form of Pseudomonas exotoxin. J. Urol. 1997, 158, 948–953. [Google Scholar] [CrossRef]
- Wang, B.; Lv, L.; Wang, Z.; Zhao, Y.; Wu, L.; Fang, X.; Xu, Q.; Xin, H. Nanoparticles functionalized with Pep-1 as potential glioma targeting delivery system via interleukin 13 receptor alpha2-mediated endocytosis. Biomaterials 2014, 35, 5897–5907. [Google Scholar] [CrossRef] [PubMed]
- Sattiraju, A.; Solingapuram Sai, K.K.; Xuan, A.; Pandya, D.N.; Almaguel, F.G.; Wadas, T.J.; Herpai, D.M.; Debinski, W.; Mintz, A. IL13RA2 targeted alpha particle therapy against glioblastomas. Oncotarget 2017, 8, 42997–43007. [Google Scholar] [CrossRef]
- Ziecik, A.J.; Kaczmarek, M.M.; Blitek, A.; Kowalczyk, A.E.; Li, X.; Rahman, N.A. Novel biological and possible applicable roles of LH/hCG receptor. Mol. Cell Endocrinol. 2007, 269, 51–60. [Google Scholar] [CrossRef]
- Leuschner, C.; Hansel, W. Membrane disrupting lytic peptides for cancer treatments. Curr. Pharm. Des. 2004, 10, 2299–2310. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.; Veiga, A.S.; Castanho, M.A. From antimicrobial to anticancer peptides. A review. Front. Microbiol. 2013, 4, 294. [Google Scholar] [CrossRef]
- Zhao, H.; Qin, X.; Yang, D.; Jiang, Y.; Zheng, W.; Wang, D.; Tian, Y.; Liu, Q.; Xu, N.; Li, Z. The development of activatable lytic peptides for targeting triple negative breast cancer. Cell Death Discov. 2017, 3, 17037. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hansel, W.; Enright, F.; Leuschner, C. Destruction of breast cancers and their metastases by lytic peptide conjugates in vitro and in vivo. Mol. Cell Endocrinol. 2007, 260–262, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Noker, P.E.; Piazza, G.A.; Leuschner, C.; Hansel, W.; Gorman, G.S.; Coward, L.U.; Tomaszewski, J. Pharmacokinetics and pharmacodynamics of Phor21-betaCG(ala), a lytic peptide conjugate. J. Pharm. Pharm. 2008, 60, 1441–1448. [Google Scholar] [CrossRef]
- Kurihara, R.; Horibe, T.; Shimizu, E.; Torisawa, A.; Gaowa, A.; Kohno, M.; Kawakami, K. A novel interleukin-13 receptor alpha 2-targeted hybrid peptide for effective glioblastoma therapy. Chem. Biol. Drug Des. 2019, 94, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Bartolome, R.A.; Jaen, M.; Casal, J.I. An IL13Ralpha2 peptide exhibits therapeutic activity against metastatic colorectal cancer. Br. J. Cancer 2018, 119, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Daines, M.O.; Tabata, Y.; Walker, B.A.; Chen, W.G.; Warrier, M.R.; Basu, S.; Hershey, G.K.K. Level of expression of IL-13R alpha 2 impacts receptor distribution and IL-13 signaling. J. Immunol. 2006, 176, 7495–7501. [Google Scholar] [CrossRef] [PubMed]
- Kanamarlapudi, V.; Thompson, A.; Kelly, E.; Bernal, A.L. ARF6 Activated by the LHCG Receptor through the Cytohesin Family of Guanine Nucleotide Exchange Factors Mediates the Receptor Internalization and Signaling. J. Biol. Chem. 2012, 287, 20443–20455. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kanamarlapudi, V.; Tamaddon-Jahromi, S.; Murphy, K. ADP-ribosylation factor 6 expression increase in oesophageal adenocarcinoma suggests a potential biomarker role for it. PLoS ONE 2022, 17, e0263845. [Google Scholar] [CrossRef]
- Kanamarlapudi, V.; Owens, S.E.; Lartey, J.; Lopez Bernal, A. ADP-ribosylation factor 6 expression and activation are reduced in myometrium in complicated pregnancies. PLoS ONE 2012, 7, e37954. [Google Scholar] [CrossRef]
- Davies, J.C.B.; Tamaddon-Jahromi, S.; Jannoo, R.; Kanamarlapudi, V. Cytohesin 2/ARF6 regulates preadipocyte migration through the activation of ERK1/2. Biochem. Pharmacol. 2014, 92, 651–660. [Google Scholar] [CrossRef][Green Version]
- Thompson, A.; Kanamarlapudi, V. The regions within the N-terminus critical for human glucagon like peptide-1 receptor (hGLP-1R) cell Surface expression. Sci. Rep. 2014, 4, 7410. [Google Scholar] [CrossRef]
- Takenouchi, M.; Hirai, S.; Sakuragi, N.; Yagita, H.; Hamada, H.; Kato, K. Epigenetic modulation enhances the therapeutic effect of anti-IL-13R(alpha)2 antibody in human mesothelioma xenografts. Clin. Cancer Res. 2011, 17, 2819–2829. [Google Scholar] [CrossRef]
- Hsiao, A.Y.; Torisawa, Y.S.; Tung, Y.C.; Sud, S.; Taichman, R.S.; Pienta, K.J.; Takayama, S. Microfluidic system for formation of PC-3 prostate cancer co-culture spheroids. Biomaterials 2009, 30, 3020–3027. [Google Scholar] [CrossRef] [PubMed]
- Candolfi, M.; Xiong, W.D.; Yagiz, K.; Liu, C.Y.; Muhammad, A.K.M.G.; Puntel, M.; Foulad, D.; Zadmehr, A.; Ahlzadeh, G.E.; Kroeger, K.M.; et al. Gene therapy-mediated delivery of targeted cytotoxins for glioma therapeutics. Proc. Natl. Acad. Sci. USA 2010, 107, 20021–20026. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Thaci, B.; Crawford, A.C.; Sampath, P. Interleukin-13 Receptor Alpha 2-Targeted Glioblastoma Immunotherapy. Biomed. Res. Int. 2014, 2014, 952128. [Google Scholar] [CrossRef] [PubMed]
- Minn, A.J.; Gupta, G.P.; Siegel, P.M.; Bos, P.D.; Shu, W.; Giri, D.D.; Viale, A.; Olshen, A.B.; Gerald, W.L.; Massague, J. Genes that mediate breast cancer metastasis to lung. Nature 2005, 436, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Ericson, K.; Chao, W.; Low, W.C. NFAT and AP1 are essential for the expression of a glioblastoma multiforme related IL-13Ra2 transcript. Cell Oncol. 2010, 32, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Shaulian, E.; Karin, M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002, 4, E131–E136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, L.; Yang, H.; Huang, X.; Otu, H.; Libermann, T.A.; DeWolf, W.C.; Khosravi-Far, R.; Olumi, A.F. c-Fos as a Proapoptotic Agent in TRAIL-Induced Apoptosis in Prostate Cancer Cells. Cancer Res. 2007, 67, 9425–9434. [Google Scholar] [CrossRef]
- Chen, S.Y.; Cai, C.; Fisher, C.J.; Zheng, Z.; Omwancha, J.; Hsieh, C.L.; Shemshedini, L. c-Jun enhancement of androgen receptor transactivation is associated with prostate cancer cell proliferation. Oncogene 2006, 25, 7212–7223. [Google Scholar] [CrossRef] [PubMed]
- le Roux, L.; Volgin, A.; Maxwell, D.; Ishihara, K.; Gelovani, J.; Schellingerhout, D. Optimizing Imaging of Three-Dimensional Multicellular Tumor Spheroids with Fluorescent Reporter Proteins Using Confocal Microscopy. Mol. Imaging 2008, 7, 214–221. [Google Scholar] [CrossRef]
- Rofstad, E.K.; Eide, K.; Skoyum, R.; Hystad, M.E.; Lyng, H. Apoptosis, energy metabolism, and fraction of radiobiologically hypoxic cells: A study of human melanoma multicellular spheroids. Int. J. Radiat. Biol. 1996, 70, 241–249. [Google Scholar] [CrossRef]
- Liu, T.F.; Cai, J.Z.; Gibo, D.M.; Debinski, W. Reoxygenation of Hypoxic Glioblastoma Multiforme Cells Potentiates the Killing Effect of an Interleukin-13-Based Cytotoxin. Clin. Cancer Res. 2009, 15, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Doillon, C.J.; Gagnon, E.; Paradis, R.; Koutsilieris, M. Three-dimensional culture system as a model for studying cancer cell invasion capacity and anticancer drug sensitivity. Anticancer Res. 2004, 24, 2169–2177. [Google Scholar] [PubMed]
- Dalton, W.S. The tumor microenvironment as a determinant of drug response and resistance. Drug Resist. Updat. 1999, 2, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Condello, S.; Morgan, C.A.; Nagdas, S.; Cao, L.; Turek, J.; Hurley, T.D.; Matei, D. beta-Catenin-regulated ALDH1A1 is a target in ovarian cancer spheroids. Oncogene 2015, 34, 2297–2308. [Google Scholar] [CrossRef] [PubMed]
- Szczepanski, C.; Tenstad, O.; Baumann, A.; Martinez, A.; Myklebust, R.; Bjerkvig, R.; Prestegarden, L. Identification of a novel lytic peptide for the treatment of solid tumours. Genes Cancer 2014, 5, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.H.; Leland, P.; Calvo, A.; Green, J.E.; Puri, R.K. Human Adrenomedullin Up-Regulates Interleukin-13 Receptor Alpha2 Chain in Prostate Cancer In Vitro and In Vivo: A Novel Approach to Sensitize Prostate Cancer to Anticancer Therapy Comment. J. Urol. 2009, 181, 2824–2825. [Google Scholar]
- Nakashima, H.; Terabe, M.; Berzofsky, J.A.; Husain, S.R.; Puri, R.K. A novel combination immunotherapy for cancer by IL-13Ralpha2-targeted DNA vaccine and immunotoxin in murine tumor models. J. Immunol. 2011, 187, 4935–4946. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jannoo, R.; Xia, Z.; Row, P.E.; Kanamarlapudi, V. Targeting of the Interleukin-13 Receptor (IL-13R)α2 Expressing Prostate Cancer by a Novel Hybrid Lytic Peptide. Biomolecules 2023, 13, 356. https://doi.org/10.3390/biom13020356
Jannoo R, Xia Z, Row PE, Kanamarlapudi V. Targeting of the Interleukin-13 Receptor (IL-13R)α2 Expressing Prostate Cancer by a Novel Hybrid Lytic Peptide. Biomolecules. 2023; 13(2):356. https://doi.org/10.3390/biom13020356
Chicago/Turabian StyleJannoo, Riaz, Zhidao Xia, Paula E. Row, and Venkateswarlu Kanamarlapudi. 2023. "Targeting of the Interleukin-13 Receptor (IL-13R)α2 Expressing Prostate Cancer by a Novel Hybrid Lytic Peptide" Biomolecules 13, no. 2: 356. https://doi.org/10.3390/biom13020356
APA StyleJannoo, R., Xia, Z., Row, P. E., & Kanamarlapudi, V. (2023). Targeting of the Interleukin-13 Receptor (IL-13R)α2 Expressing Prostate Cancer by a Novel Hybrid Lytic Peptide. Biomolecules, 13(2), 356. https://doi.org/10.3390/biom13020356






