Influence of the Charge Ratio of Guanine-Quadruplex Structure-Based CpG Oligodeoxynucleotides and Cationic DOTAP Liposomes on Cytokine Induction Profiles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Oligodeoxynucleotides
2.2. G4 Structure Formation
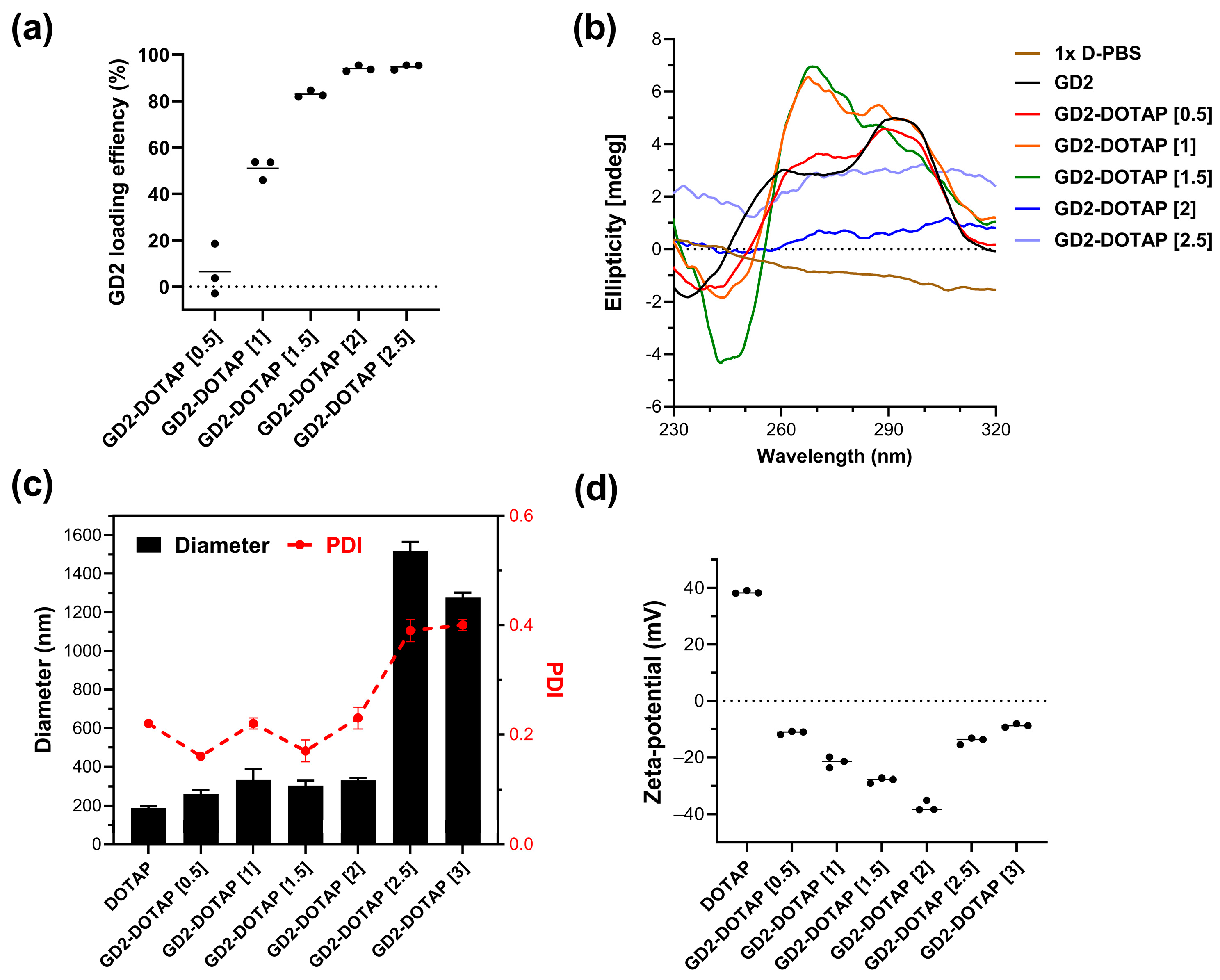
2.3. Preparation and Characterization of the G4-CpG ODN-DOTAP Liposome Complexes
2.4. Quantification of G4-CpG ODN Loading onto DOTAP Liposomes
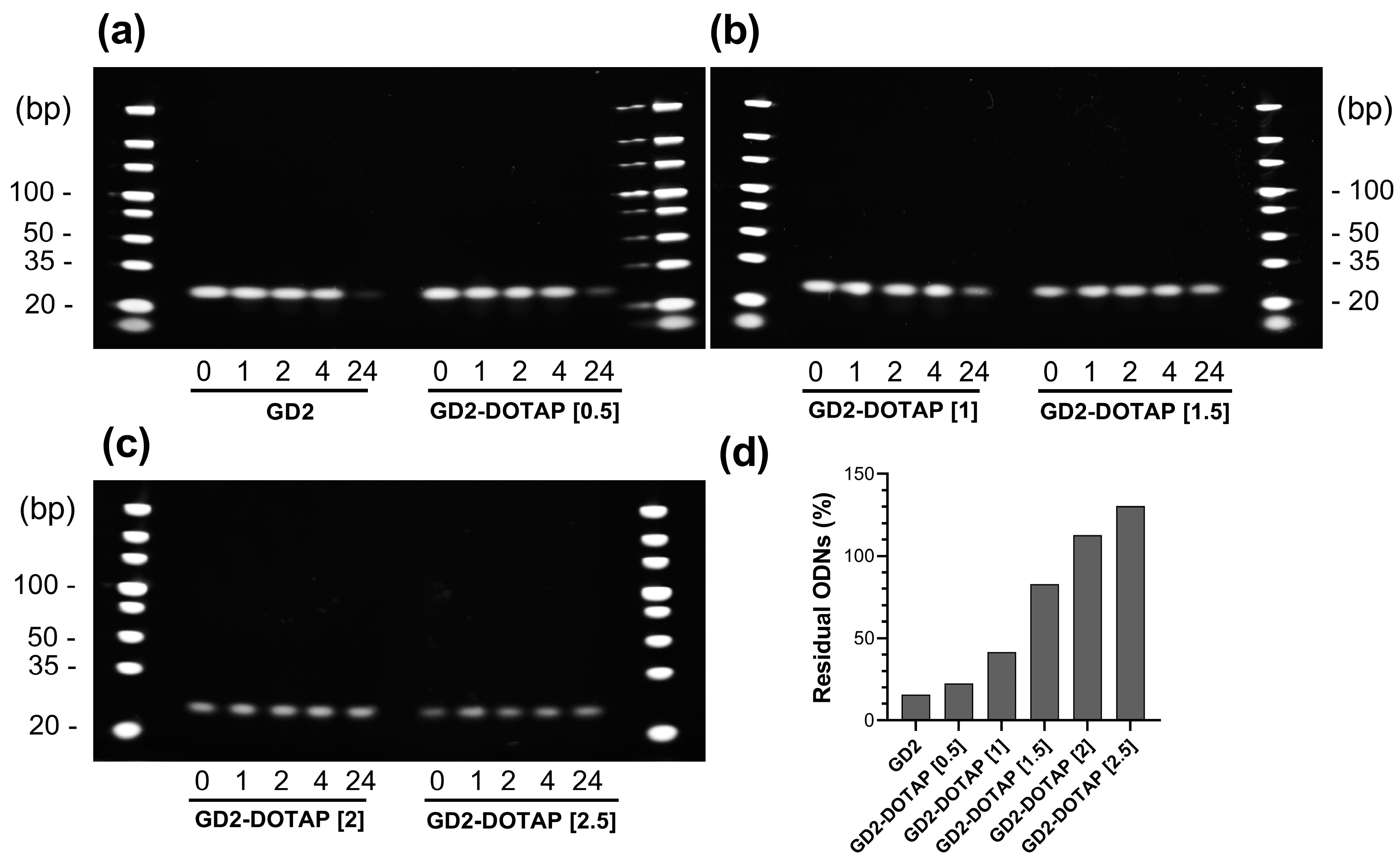
2.5. Serum Stability Assay
2.6. Cell Culture
2.7. Cell Viability Assay
2.8. Immunostimulatory Properties of the CpG ODN-DOTAP Liposome Complexes
2.9. Confocal Laser Scanning Microscopy (CLSM)
2.10. Statistical Analysis
3. Results
3.1. Preparation and Characterization of the G4-CpG ODN-DOTAP Liposome Complexes
3.2. Nuclease Resistance of G4-CpG ODNs in Complex
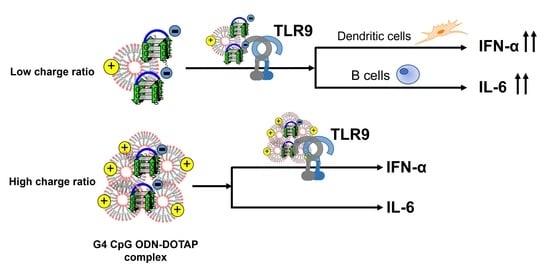
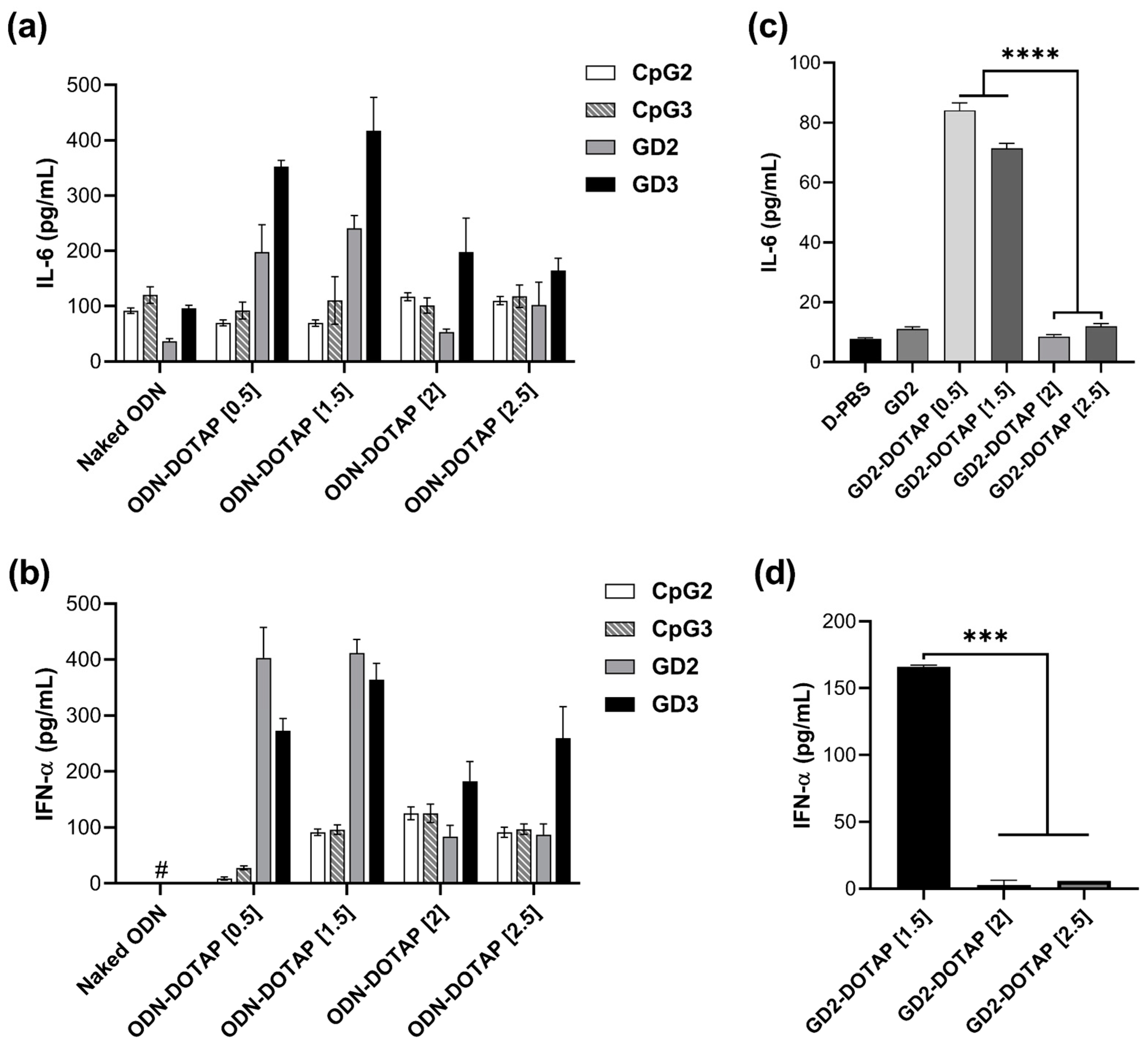
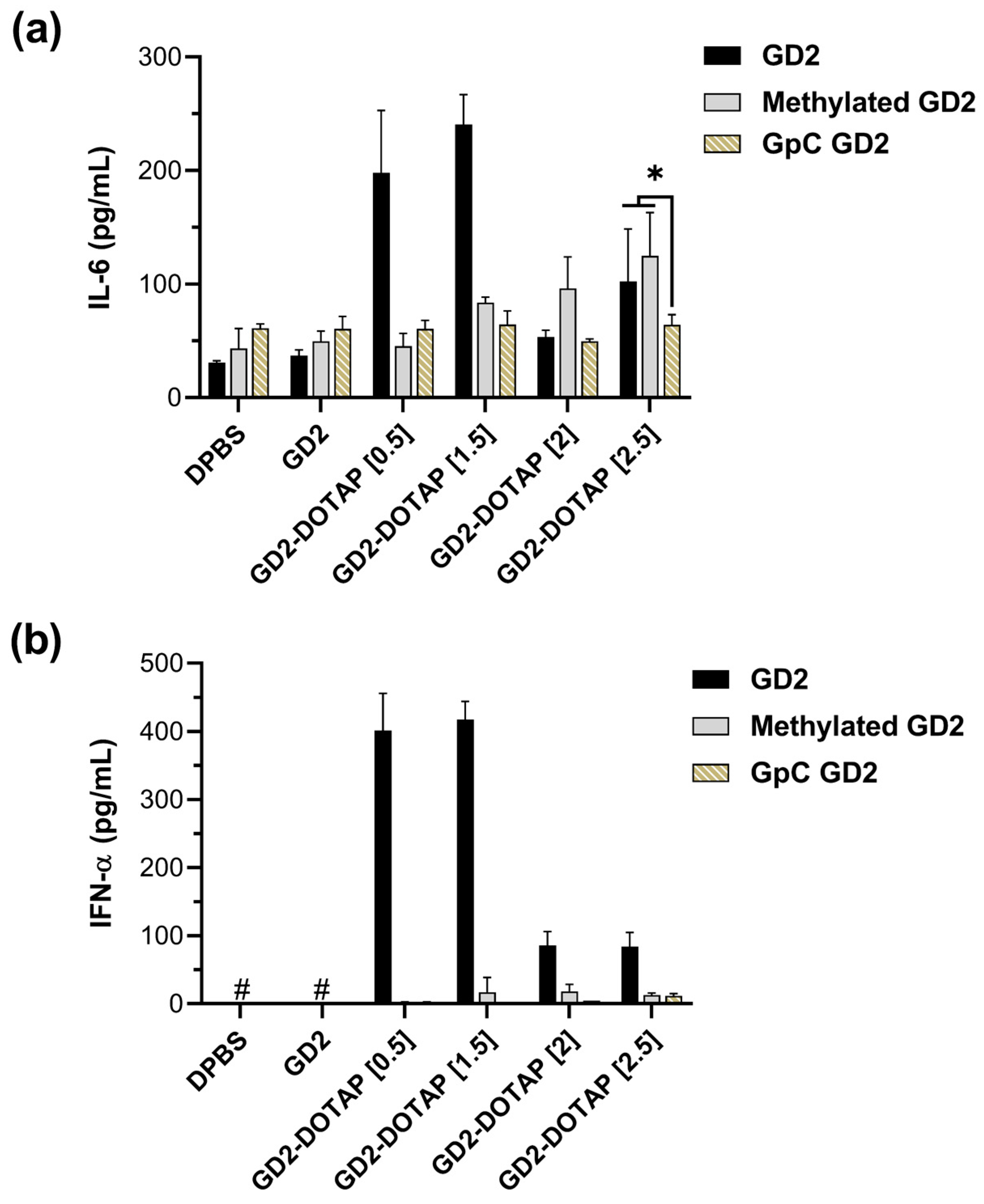
3.3. Cytokine Induction by the G4-CpG ODN-DOTAP Complexes of Various Charge Ratios
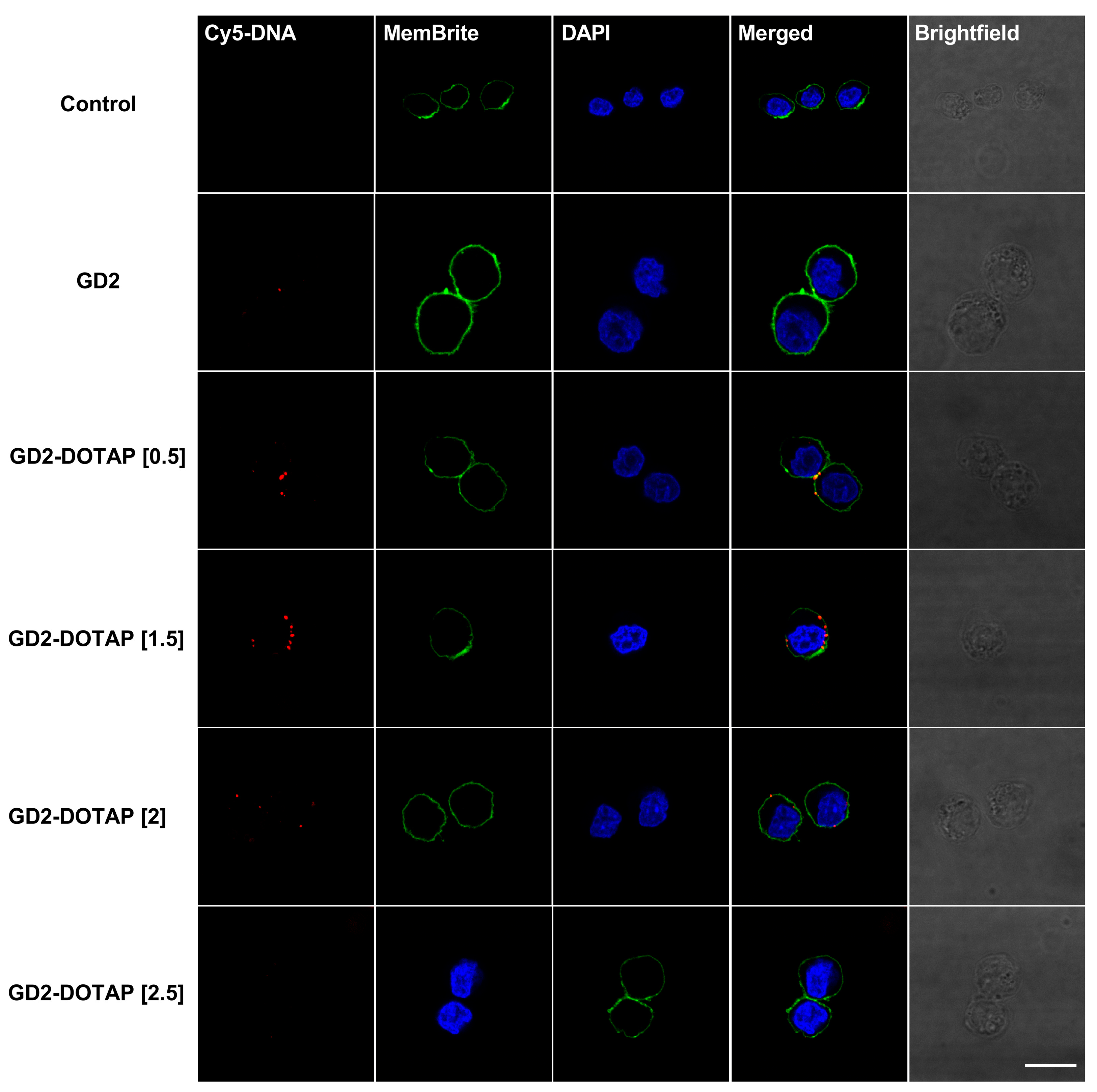
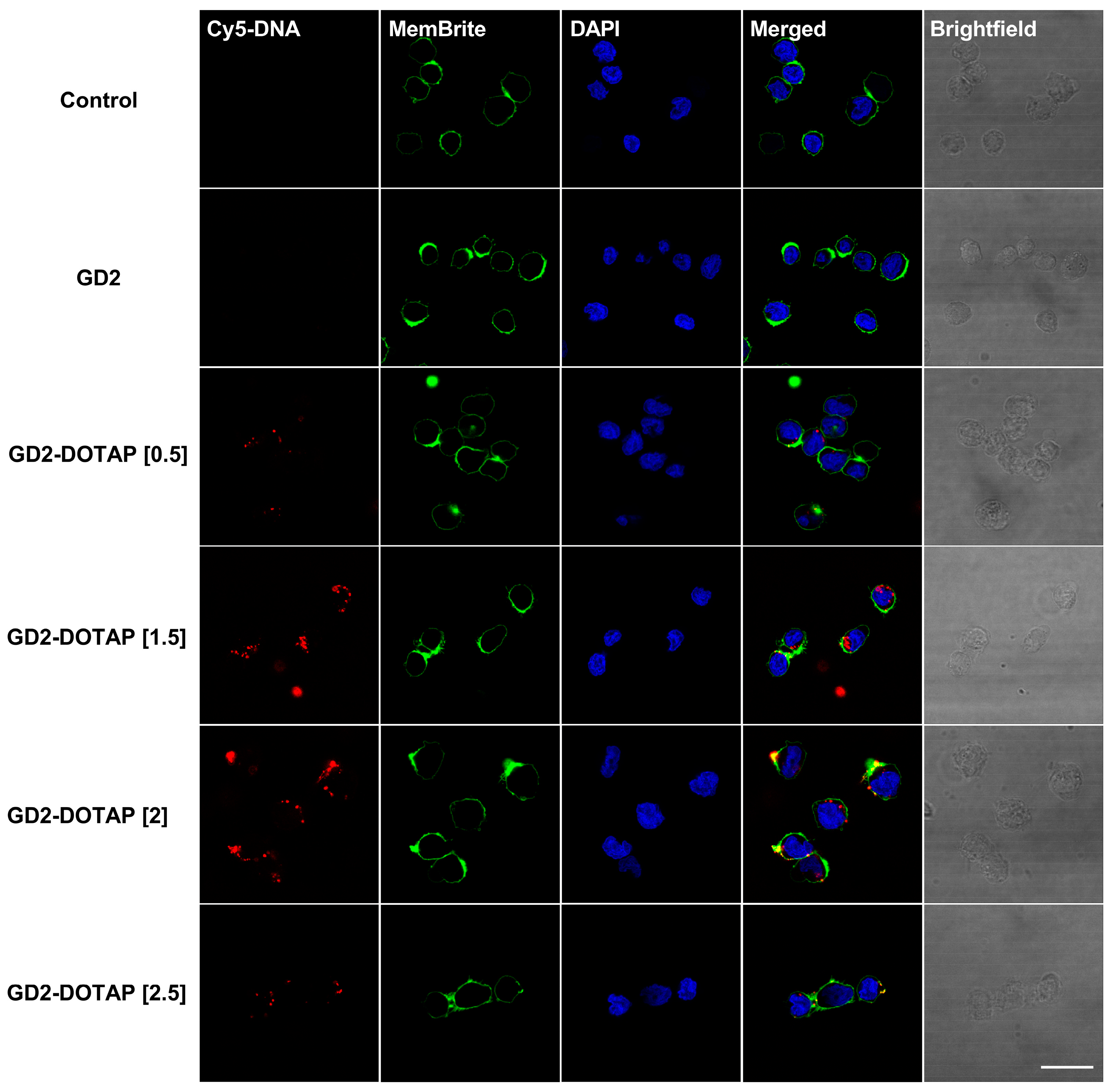
3.4. Cellular Uptake of G4-CpG ODN-DOTAP Complexes of Different Charge Ratios
3.5. Cytokine Induction by GpC ODN and Methylated CpG ODN-DOTAP Liposome Complexes in Human PBMCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A Toll-Like Receptor Recognizes Bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Yan, J.; Wu, W.; Tao, X.; Lu, X.; Liu, S.; Zhu, W. A CpG Oligodeoxynucleotide Enhances the Immune Response to Rabies Vaccination in Mice. Virol. J. 2018, 15, 174. [Google Scholar] [CrossRef]
- Takeshita, F.; Leifer, C.A.; Gursel, I.; Ishii, K.J.; Takeshita, S.; Gursel, M.; Klinman, D.M. Cutting Edge: Role of Toll-like Receptor 9 in CpG DNA-Induced Activation of Human Cells. J. Immunol. 2001, 167, 3555–3558. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Kirschning, C.J.; Häcker, H.; Redecke, V.; Hausmann, S.; Akira, S.; Wagner, H.; Lipford, G.B. Human TLR9 Confers Responsiveness to Bacterial DNA via Species-Specific CpG Motif Recognition. Proc. Natl. Acad. Sci. USA 2001, 98, 9237–9242. [Google Scholar] [CrossRef]
- Hornung, V.; Rothenfusser, S.; Britsch, S.; Krug, A.; Jahrsdörfer, B.; Giese, T.; Endres, S.; Hartmann, G. Quantitative Expression of Toll-Like Receptor 1–10 MRNA in Cellular Subsets of Human Peripheral Blood Mononuclear Cells and Sensitivity to CpG Oligodeoxynucleotides. J. Immunol. 2002, 168, 4531–4537. [Google Scholar] [CrossRef] [PubMed]
- Klinman, D.M. Immunotherapeutic Uses of CpG Oligodeoxynucleotides. Nat. Rev. Immunol. 2004, 4, 249–258. [Google Scholar] [CrossRef]
- Hanagata, N. CpG Oligodeoxynucleotide Nanomedicines for the Prophylaxis or Treatment of Cancers, Infectious Diseases, and Allergies. Int. J. Nanomed. 2017, 12, 515–531. [Google Scholar] [CrossRef]
- Vollmer, J.; Krieg, A.M. Immunotherapeutic Applications of CpG Oligodeoxynucleotide TLR9 Agonists. Adv. Drug Deliv. Rev. 2009, 61, 195–204. [Google Scholar] [CrossRef]
- Mutwiri, G.K.; Nichani, A.K.; Babiuk, S.; Babiuk, L.A. Strategies for Enhancing the Immunostimulatory Effects of CpG Oligodeoxynucleotides. J. Control. Release 2004, 97, 1–17. [Google Scholar] [CrossRef]
- Levin, A.A. A Review of the Issues in the Pharmacokinetics and Toxicology of Phosphorothioate Antisense Oligonucleotides. Biochim. Biophys. Acta 1999, 1489, 69–84. [Google Scholar] [CrossRef]
- Hoshi, K.; Yamazaki, T.; Sugiyama, Y.; Tsukakoshi, K.; Tsugawa, W.; Sode, K.; Ikebukuro, K. G-Quadruplex Structure Improves the Immunostimulatory Effects of Cpg Oligonucleotides. Nucleic Acid Ther. 2019, 29, 224–229. [Google Scholar] [CrossRef]
- Tu, A.T.T.; Hoshi, K.; Ikebukuro, K.; Hanagata, N.; Yamazaki, T. Monomeric G-Quadruplex-Based CpG Oligodeoxynucleotides as Potent Toll-Like Receptor 9 Agonists. Biomacromolecules 2020, 21, 3644–3657. [Google Scholar] [CrossRef] [PubMed]
- Safitri, F.A.; Tu, A.T.T.; Hoshi, K.; Shobo, M.; Zhao, D.; Witarto, A.B.; Sumarsono, S.H.; Giri-Rachman, E.A.; Tsukakoshi, K.; Ikebukuro, K.; et al. Enhancement of the Immunostimulatory Effect of Phosphodiester CpG Oligodeoxynucleotides by an Antiparallel Guanine-Quadruplex Structural Scaffold. Biomolecules 2021, 11, 1617. [Google Scholar] [CrossRef]
- Spiegel, J.; Adhikari, S.; Balasubramanian, S. The Structure and Function of DNA G-Quadruplexes. Trends Chem. 2020, 2, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Granzhan, A.; Marquevielle, J.; Cucchiarini, A.; Lacroix, L.; Amrane, S.; Verga, D.; Mergny, J.L. Guidelines for G-Quadruplexes: I. In Vitro Characterization. Biochimie 2023, 214, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.; Lipps, H.J. G-Quadruplexes and Their Regulatory Roles in Biology. Nucleic Acids Res. 2015, 43, 8627–8637. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.F.; Wan, L.Y.; Peng, H.; Zhong, Y.M.; Cai, W.L.; Zhang, Y.Q.; Ai, W.B.; Wu, J.F. The Influencing Factors and Functions of DNA G-Quadruplexes. Cell Biochem. Funct. 2020, 38, 524–532. [Google Scholar] [CrossRef]
- Tu, A.T.T.; Hoshi, K.; Shobo, M.; Yamazaki, T. G-Quadruplex-Based CpG Oligodeoxynucleotide/DOTAP Complex Strongly Stimulates Immunity in CpG Motif-Specific and Loop-Length-Dependent Manners. Nanomedicine 2022, 40, 102508. [Google Scholar] [CrossRef]
- Zhao, D.; Tu, A.T.T.; Shobo, M.; Le, N.B.T.; Yoshikawa, C.; Sugai, K.; Hakamata, Y.; Yamazaki, T. Non-modified CpG Oligodeoxynucleotide Forming Guanine-Quadruplex Structure Complexes with ε-Poly-L-lysine Induce Antibody Production as Vaccine Adjuvants. Biomolecules 2022, 12, 1868. [Google Scholar] [CrossRef]
- del Villar-Guerra, R.; Trent, J.O.; Chaires, J.B. G-Quadruplex Secondary Structure Obtained from Circular Dichroism Spectroscopy. Angew. Chem. Int. Ed. Engl. 2018, 57, 7171–7175. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular Uptake of Nanoparticles: Journey inside the Cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Wiethoff, C.M.; Middaugh, C.R. Synthetic Gene Therapy Vectors. Methods Mol. Biol. 2014, 65, 349–376. [Google Scholar]
- Hardy, C.L.; LeMasurier, J.S.; Mohamud, R.; Yao, J.; Xiang, S.D.; Rolland, J.M.; O’Hehir, R.E.; Plebanski, M. Differential Uptake of Nanoparticles and Microparticles by Pulmonary APC Subsets Induces Discrete Immunological Imprints. J. Immunol. 2013, 191, 5278–5290. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Jennings, G.T. Vaccine Delivery: A Matter of Size, Geometry, Kinetics and Molecular Patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, F.; Inoue, R.; Nishino, Y.; Okuda, A.; Matsumoto, O.; Taga, T.; Yamashita, F.; Takakura, Y.; Hashida, M. Effect of DNA/Liposome Mixing Ratio on the Physicochemical Characteristics, Cellular Uptake and Intracellular Trafficking of Plasmid DNA/Cationic Liposome Complexes and Subsequent Gene Expression. J. Control. Release 2000, 66, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Sun, B.; Tran, K.K.; Shen, H. Effects of Particle Size on Toll-like 9-Mediated Cytokine Profiles. Biomaterials 2012, 32, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Inoh, Y.; Nagai, M.; Matsushita, K.; Nakanishi, M.; Furuno, T. Gene Transfection Efficiency into Dendritic Cells Is Influenced by the Size of Cationic Liposomes/DNA Complexes. Eur. J. Pharm. Sci. 2017, 102, 230–236. [Google Scholar] [CrossRef]
- Ma, Y.; Zhuang, Y.; Xie, X.; Wang, C.; Wang, F.; Zhou, D.; Zeng, J.; Cai, L. The Role of Surface Charge Density in Cationic Liposome-Promoted Dendritic Cell Maturation and Vaccine-Induced Immune Responses. Nanoscale 2011, 3, 2307–2314. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Ohba, Y.; Yanai, H.; Negishi, H.; Mizutani, T.; Takaoka, A.; Taya, C.; Taniguchi, T. Spatiotemporal Regulation of MyD88-IRF-7 Signalling for Robust Type-I Interferon Induction. Nature 2005, 434, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Verthelyi, D.; Ishii, K.J.; Gursel, M.; Takeshita, F.; Klinman, D.M. Human Peripheral Blood Cells Differentially Recognize and Respond to Two Distinct CpG Motifs. J. Immunol. 2001, 166, 2372–2377. [Google Scholar] [CrossRef]
- Krug, A.; Rothenfusser, S.; Hornung, V.; Jahrsdörfer, B.; Blackwell, S.; Ballas, Z.K.; Endres, S.; Krieg, A.M.; Hartmann, G. Identification of CpG Oligonucleotide Sequences with High Induction of IFN-α/β in Plasmacytoid Dendritic Cells. Eur. J. Immunol. 2001, 31, 2154–2163. [Google Scholar] [CrossRef]
- Hartmann, G.; Weeratna, R.D.; Ballas, Z.K.; Payette, P.; Blackwell, S.; Suparto, I.; Rasmussen, W.L.; Waldschmidt, M.; Sajuthi, D.; Purcell, R.H.; et al. Delineation of a CpG Phosphorothioate Oligodeoxynucleotide for Activating Primate Immune Responses In Vitro and In Vivo. J. Immunol. 2000, 164, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, M.; Akkaya, B.; Miozzo, P.; Rawat, M.; Pena, M.; Sheehan, P.W.; Kim, A.S.; Kamenyeva, O.; Kabat, J.; Bolland, S.; et al. B Cells Produce Type 1 IFNs in Response to the TLR9 Agonist CpG-A Conjugated to Cationic Lipids. J. Immunol. 2017, 199, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Van Snick, J. Interleukin-6: An Overview. Annu. Rev. Immunol. 1990, 8, 253–278. [Google Scholar] [CrossRef]
- Stetson, D.B.; Medzhitov, R. Type I Interferons in Host Defense. Immunity 2006, 25, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Chinnathambi, S.; Chen, S.; Ganesan, S.; Hanagata, N. Binding Mode of CpG Oligodeoxynucleotides to Nanoparticles Regulates Bifurcated Cytokine Induction via Toll-Like Receptor 9. Sci. Rep. 2012, 2, 534. [Google Scholar] [CrossRef]
- Chinnathambi, S.; Pi, X.; Xu, M.; Hanagata, N. Regulation of Bifurcated Cytokine Induction by Surface Charge of Nanoparticles During Interaction between CpG Oligodeoxynucleotides and Toll-Like Receptor 9. J. Drug Deliv. Sci. Technol. 2015, 29, 251–260. [Google Scholar] [CrossRef]
- Schäfer, J.; Höbel, S.; Bakowsky, U.; Aigner, A. Liposome-Polyethylenimine Complexes for Enhanced DNA and SiRNA Delivery. Biomaterials 2010, 31, 6892–6900. [Google Scholar] [CrossRef] [PubMed]
- Rädler, J.O.; Koltover, I.; Salditt, T.; Safinya, C.R. Structure of DNA-Cationic Liposome Complexes: DNA Intercalation in Multilamellar Membranes in Distinct Interhelical Packing Regimes. Science 1997, 275, 810–814. [Google Scholar] [CrossRef]
- Birchall, J.C.; Kellaway, I.W.; Mills, S.N. Physico-chemical Characterisation and Transfection Efficiency of Lipid-Based Gene Delivery Complexes. Int. J. Pharm. 1999, 183, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hui, S.W.; Frederik, P.; Szoka, F.C. Physicochemical Characterization and Purification of Cationic Lipoplexes. Biophys. J. 1999, 77, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Digiacomo, L.; Giulimondi, F.; Mahmoudi, M.; Caracciolo, G. Effect of Molecular Crowding on the Biological Identity of Liposomes: An Overlooked Factor at the Bio-Nano Interface. Nanoscale Adv. 2019, 1, 2518–2522. [Google Scholar] [CrossRef] [PubMed]
- Brgles, M.; Šantak, M.; Halassy, B.; Forcic, D.; Tomašić, J. Influence of Charge Ratio of Liposome/DNA Complexes on Their Size After Extrusion and Transfection Efficiency. Int. J. Nanomed. 2012, 7, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Hanagata, N. Structure-Dependent Immunostimulatory Effect of CpG Oligodeoxynucleotides and Their Delivery System. Int. J. Nanomed. 2012, 7, 2181–2195. [Google Scholar] [CrossRef] [PubMed]
- Tretiakova, D.S.; Vodovozova, E.L. Liposomes as Adjuvants and Vaccine Delivery Systems. Biochem. Suppl. Ser. A Membr. Cell Biol. 2022, 16, 1–20. [Google Scholar] [CrossRef]
- Yasuda, K.; Yu, P.; Kirschning, C.J.; Schlatter, B.; Schmitz, F.; Heit, A.; Bauer, S.; Hochrein, H.; Wagner, H. Endosomal Translocation of Vertebrate DNA Activates Dendritic Cells via TLR9-Dependent and -Independent Pathways. J. Immunol. 2005, 174, 6129–6136. [Google Scholar] [CrossRef]
- Sanjuan, M.A.; Rao, N.; Lai, K.T.A.; Gu, Y.; Sun, S.; Fuchs, A.; Fung-Leung, W.P.; Colonna, M.; Karlsson, L. CpG-Induced Tyrosine Phosphorylation Occurs via a TLR9-Independent Mechanism and Is Required for Cytokine Secretion. J. Cell Biol. 2006, 172, 1057–1068. [Google Scholar] [CrossRef]
- Bode, C.; Poth, J.M.; Fox, M.; Schulz, S.; Klinman, D.M.; Latz, E.; Steinhagen, F. Cytosolic D-Type CpG-Oligonucleotides Induce a Type I Interferon Response by Activating the CGAS-STING Signaling Pathway. Eur. J. Immunol. 2021, 51, 1686–1697. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the Mechanism of IL-1β Secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Tahtinen, S.; Tong, A.J.; Himmels, P.; Oh, J.; Paler-Martinez, A.; Kim, L.; Wichner, S.; Oei, Y.; McCarron, M.J.; Freund, E.C.; et al. IL-1 and IL-1RA Are Key Regulators of the Inflammatory Response to RNA Vaccines. Nat. Immunol. 2022, 23, 532–542. [Google Scholar] [CrossRef]
| Name | Sequence from 5′ to 3′ | Length (mer) | Structure |
|---|---|---|---|
| GD2 | GGGTTGGGGTCGTTTTGTCGTTGGGTTGGG | 30 | G4 |
| GD3 | GGGTTGGGGTCGTTTTGTCGTTTTGTCGTTGGGTTGGG | 38 | G4 |
| CpG2 | GTGTTGTGGTCGTTTTGTCGTTGTGTTGTG | 30 | Linear |
| CpG3 | GTGTTGTGGTCGTTTTGTCGTTTTGTCGTTGTGTTGTG | 38 | Linear |
| Methylated GD2 | GGGTTGGGGTCMetGTTTTGTCMetGTTGGGTTGGG | 30 | G4 |
| GpC GD2 | GGGTTGGGGTGCTTTTGTGCTTGGGTTGGG | 30 | G4 |
| ssODN | GTCGTTTTGTCGTTTTGTCGTTTTGTCGTT | 30 | Linear |
| Charge Ratio | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 |
|---|---|---|---|---|---|---|
| The concentration of ODNs in the complex solution (μM) | 10 | |||||
| The concentration of DOTAP in the complex solution (μM) | 0.15 | 0.3 | 0.45 | 0.6 | 0.75 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, N.B.T.; Tu, A.T.T.; Zhao, D.; Yoshikawa, C.; Kawakami, K.; Kaizuka, Y.; Yamazaki, T. Influence of the Charge Ratio of Guanine-Quadruplex Structure-Based CpG Oligodeoxynucleotides and Cationic DOTAP Liposomes on Cytokine Induction Profiles. Biomolecules 2023, 13, 1639. https://doi.org/10.3390/biom13111639
Le NBT, Tu ATT, Zhao D, Yoshikawa C, Kawakami K, Kaizuka Y, Yamazaki T. Influence of the Charge Ratio of Guanine-Quadruplex Structure-Based CpG Oligodeoxynucleotides and Cationic DOTAP Liposomes on Cytokine Induction Profiles. Biomolecules. 2023; 13(11):1639. https://doi.org/10.3390/biom13111639
Chicago/Turabian StyleLe, Nguyen Bui Thao, Anh Thi Tram Tu, Dandan Zhao, Chiaki Yoshikawa, Kohsaku Kawakami, Yoshihisa Kaizuka, and Tomohiko Yamazaki. 2023. "Influence of the Charge Ratio of Guanine-Quadruplex Structure-Based CpG Oligodeoxynucleotides and Cationic DOTAP Liposomes on Cytokine Induction Profiles" Biomolecules 13, no. 11: 1639. https://doi.org/10.3390/biom13111639
APA StyleLe, N. B. T., Tu, A. T. T., Zhao, D., Yoshikawa, C., Kawakami, K., Kaizuka, Y., & Yamazaki, T. (2023). Influence of the Charge Ratio of Guanine-Quadruplex Structure-Based CpG Oligodeoxynucleotides and Cationic DOTAP Liposomes on Cytokine Induction Profiles. Biomolecules, 13(11), 1639. https://doi.org/10.3390/biom13111639