Assessing Genetic Algorithm-Based Docking Protocols for Prediction of Heparin Oligosaccharide Binding Geometries onto Proteins
Abstract
1. Introduction
2. Methods
2.1. Software
2.2. Structure Selection and Preparation
2.3. Hp/HS Sequence Preparation
2.4. Molecular Docking
3. Results
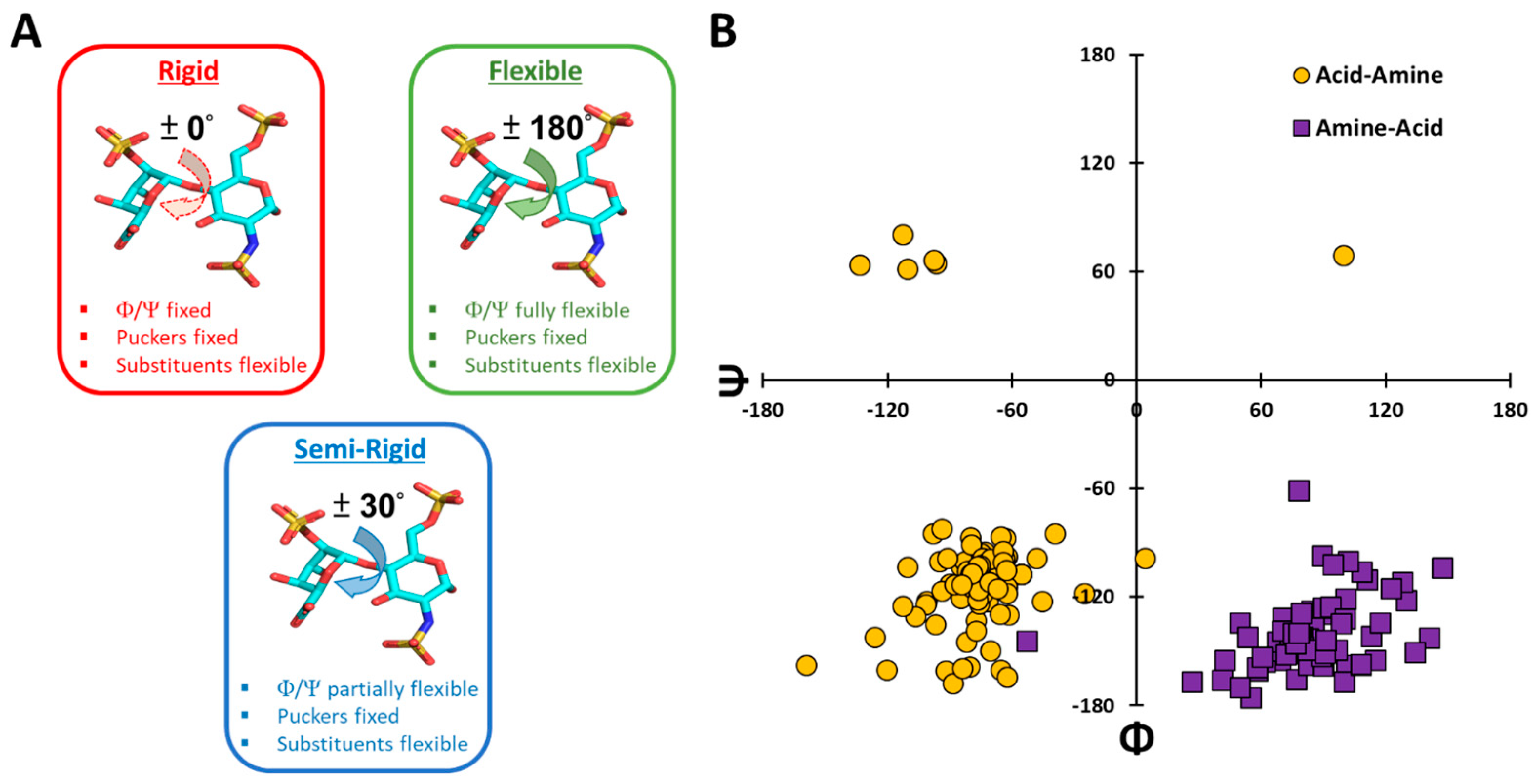
3.1. Basis Underlying the Semi-Rigid Docking Protocol
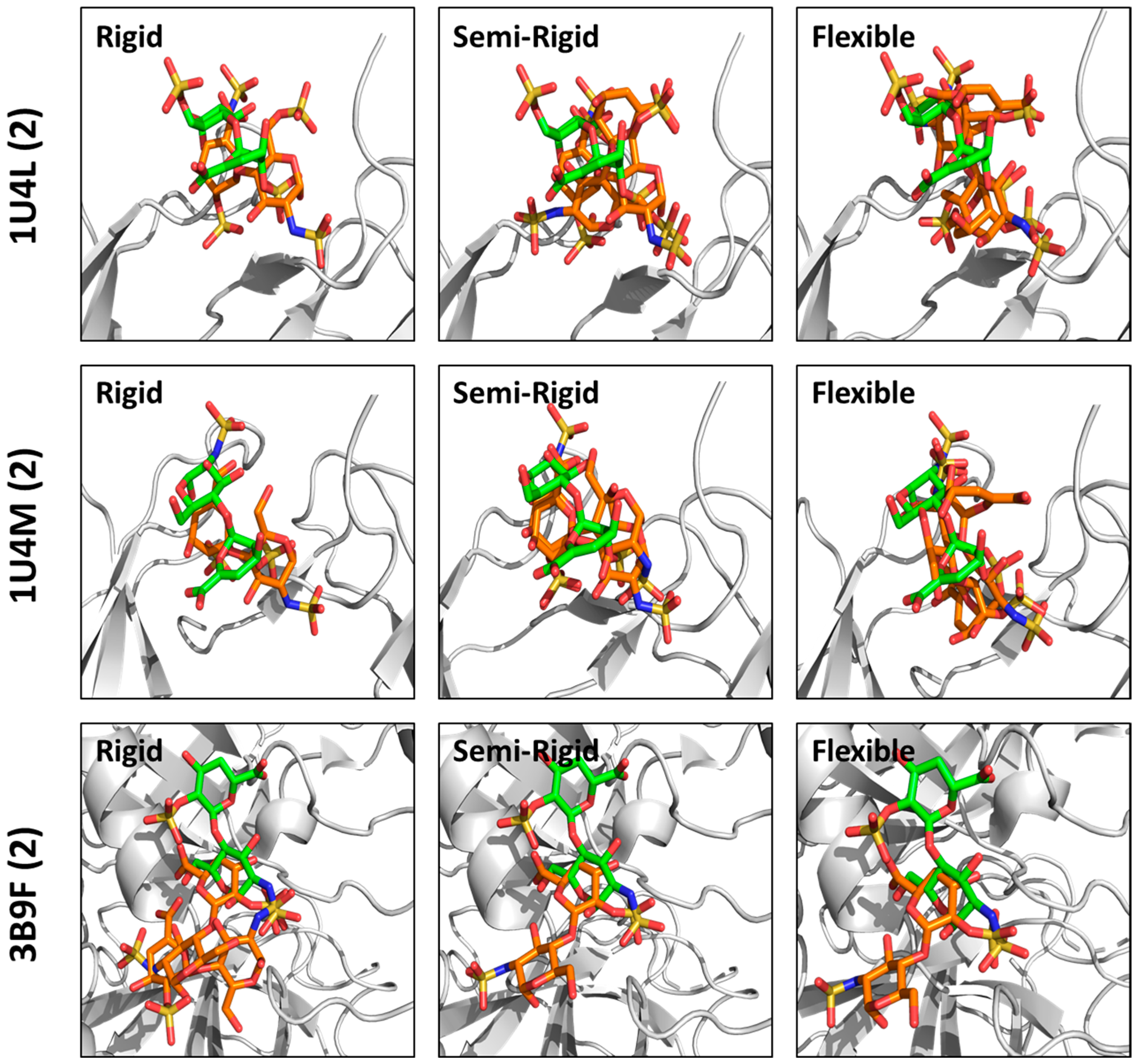
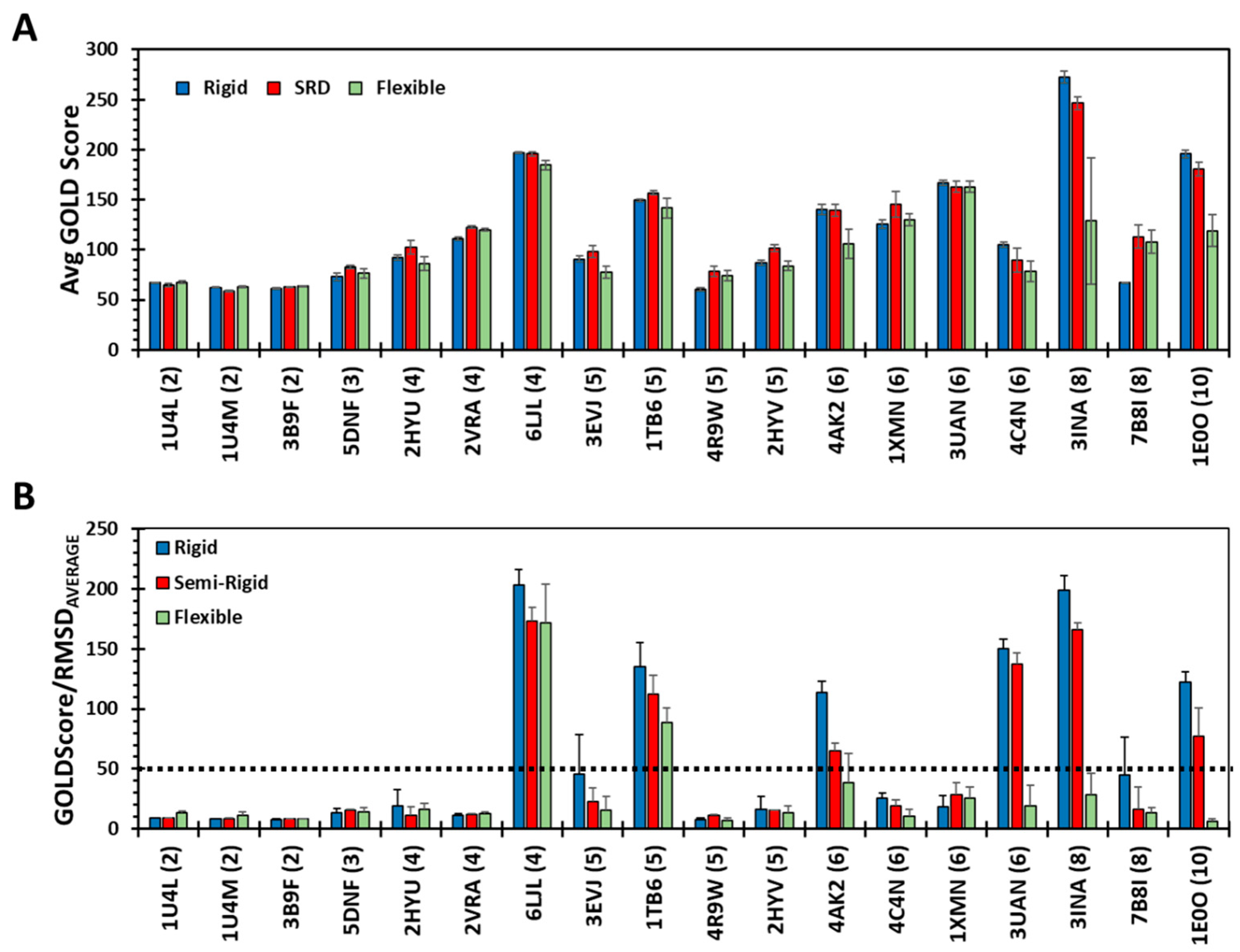
3.2. Does Rigid Docking Recapitulate the Native Pose?
3.3. How Does Flexible Docking Approach Compare to the Rigid Approach?
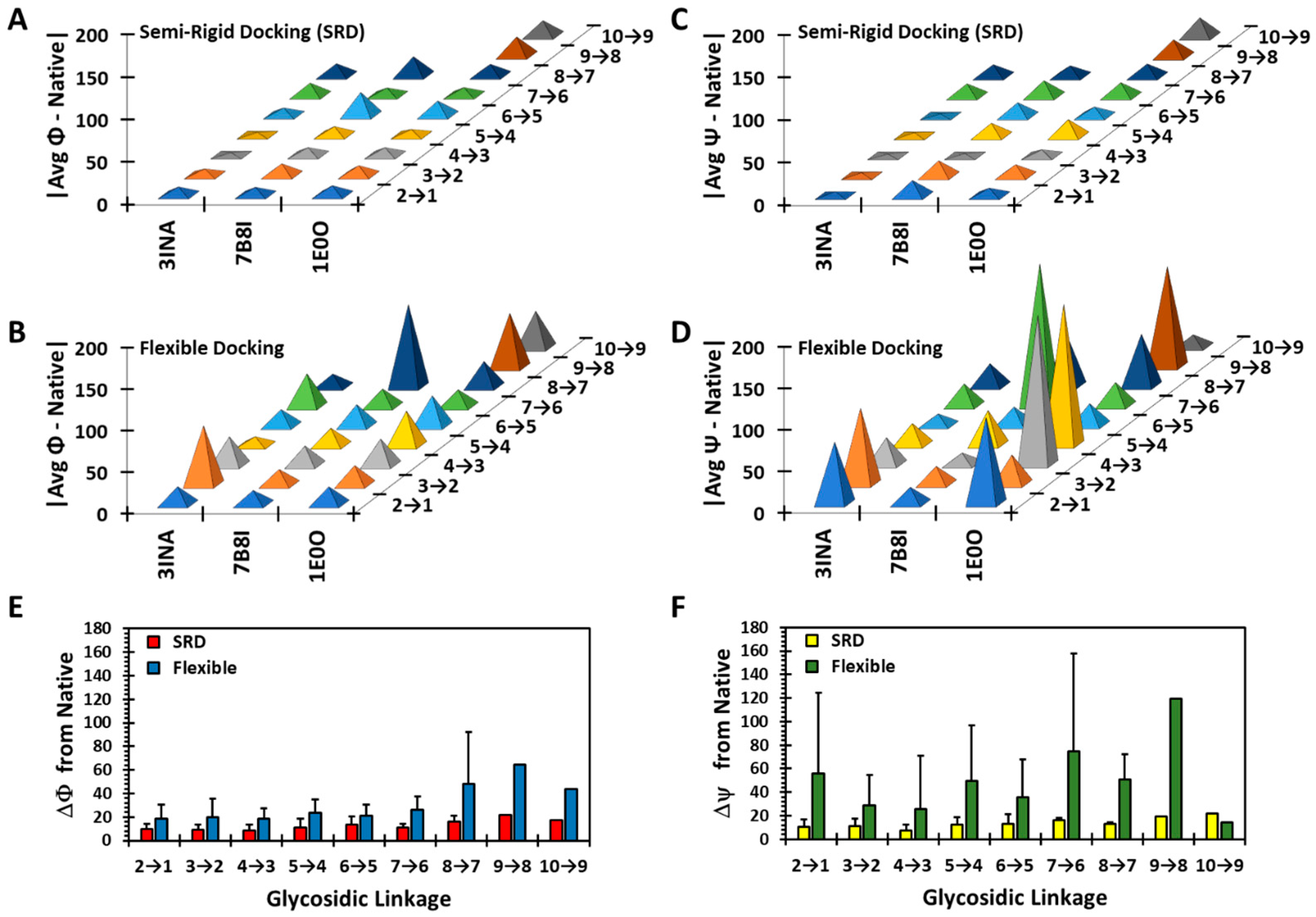
3.4. Can Semi-Rigid Docking Approach Offer a Better Alternative?
3.5. The Enigma of Disaccharides Finding the Native Pose?
3.6. A Parameter for Identifying Putative Drug-Like GAG Sequences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, I.D.; Blaney, J.M.; Oatley, S.J.; Langridge, R.; Ferrin, T.E. A geometric approach to macromolecule-ligand interactions. J. Mol. Biol. 1982, 161, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Software news and updates AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Kazmirchuk, T.D.D.; Bradbury-Jost, C.; Withey, T.A.; Gessese, T.; Azad, T.; Samanfar, B.; Dehne, F.; Golshani, A. Peptides of a feather: How computation Is taking peptide therapeutics under its wing. Genes 2023, 14, 1194. [Google Scholar] [CrossRef]
- Sherman, M.; Contreras, L. Computational approaches in design of nucleic acid-based therapeutics. Curr. Opin. Biotechnol. 2018, 53, 232–239. [Google Scholar] [CrossRef]
- Dagliyan, O.; Proctor, E.A.; D’Auria, K.M.; Ding, F.; Dokholyan, N.V. Structural and dynamic determinants of protein-peptide recognition. Structure 2011, 19, 1837–1845. [Google Scholar] [CrossRef]
- Huang, S.Y.; Zou, X. A knowledge-based scoring function for protein-RNA interactions derived from a statistical mechanics-based iterative method. Nucleic Acids Res. 2014, 42, e55. [Google Scholar] [CrossRef]
- Sun, L.; Fu, T.; Zhao, D.; Fan, H.; Zhong, S. Divide-and-link peptide docking: A fragment-based peptide docking protocol. Phys. Chem. Chem. Phys. 2021, 23, 22647–22660. [Google Scholar] [CrossRef]
- Tuszynska, I.; Magnus, M.; Jonak, K.; Dawson, W.; Bujnicki, J.M. NPDock: A web server for protein-nucleic acid docking. Nucleic Acids Res. 2015, 43, W425–W430. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, D.; Huang, S.Y. Efficient conformational ensemble generation of protein-bound peptides. J. Cheminform. 2017, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sanner, M.F. AutoDock CrankPep: Combining folding and docking to predict protein-peptide complexes. Bioinformatics 2019, 35, 5121–5127. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Jin, B.; Li, H.; Huang, S.Y. HPEPDOCK: A web server for blind peptide-protein docking based on a hierarchical algorithm. Nucleic Acids Res. 2018, 46, W443–W450. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, D.; Zhou, P.; Li, B.; Huang, S.Y. HDOCK: A web server for protein-protein and protein-DNA/RNA docking based on a hybrid strategy. Nucleic Acids Res. 2017, 45, W365–W373. [Google Scholar] [CrossRef] [PubMed]
- Hetényi, C.; van der Spoel, D. Efficient docking of peptides to proteins without prior knowledge of the binding site. Protein Sci. 2009, 11, 1729–1737. [Google Scholar] [CrossRef]
- Vallet, S.D.; Berthollier, C.; Ricard-Blum, S. The glycosaminoglycan interactome 2.0. Am. J. Physiol.-Cell Physiol. 2022, 322, C1271–C1278. [Google Scholar] [CrossRef]
- Cardin, A.D.; Weintraub, H.J. Molecular modeling of protein-glycosaminoglycan interactions. Arterioscler. Off. J. Am. Heart Assoc. Inc. 1989, 9, 21–32. [Google Scholar] [CrossRef]
- Rudd, T.R.; Preston, M.D.; Yates, E.A. The nature of the conserved basic amino acid sequences found among 437 heparin binding proteins determined by network analysis. Mol. BioSyst. 2017, 13, 852–865. [Google Scholar] [CrossRef]
- Kogut, M.M.; Marcisz, M.; Samsonov, S.A. Modeling glycosaminoglycan–protein complexes. Curr. Opin. Struct. Biol. 2022, 73, 102332. [Google Scholar] [CrossRef]
- Sankaranarayanan, N.V.; Nagarajan, B.; Desai, U.R. So you think computational approaches to understanding glycosaminoglycan–protein interactions are too dry and too rigid? Think again! Curr. Opin. Struct. Biol. 2018, 50, 91–100. [Google Scholar] [CrossRef]
- Marcisz, M.; Gaardløs, M.; Bojarski, K.K.; Siebenmorgen, T.; Zacharias, M.; Samsonov, S.A. Explicit solvent repulsive scaling replica exchange molecular dynamics (RS-REMD) in molecular modeling of protein-glycosaminoglycan complexes. J. Comput. Chem. 2022, 43, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Samsonov, S.A.; Gehrcke, J.-P.; Pisabarro, M.T. Flexibility and explicit solvent in molecular-dynamics-based docking of protein–glycosaminoglycan systems. J. Chem. Inf. Model. 2014, 54, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Boittier, E.D.; Burns, J.M.; Gandhi, N.S.; Ferro, V. GlycoTorch Vina: Docking Designed and Tested for Glycosaminoglycans. J. Chem. Inf. Model. 2020, 60, 6328–6343. [Google Scholar] [CrossRef] [PubMed]
- Nivedha, A.K.; Thieker, D.F.; Makeneni, S.; Hu, H.; Woods, R.J. Vina-carb: Improving glycosidic angles during carbohydrate docking. J. Chem. Theory Comput. 2016, 12, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Kozakov, D.; Hall, D.R.; Xia, B.; Porter, K.A.; Padhorny, D.; Yueh, C.; Beglov, D.; Vajda, S. The ClusPro web server for protein–protein docking. Nat. Protoc. 2017, 12, 255–278. [Google Scholar] [CrossRef]
- Mottarella, S.E.; Beglov, D.; Beglova, N.; Nugent, M.A.; Kozakov, D.; Vajda, S. Docking server for the identification of heparin binding sites on proteins. J. Chem. Inf. Model. 2014, 54, 2068–2078. [Google Scholar] [CrossRef]
- Boothello, R.S.; Sankaranarayanan, N.V.; Sistla, J.C.; Nagarajan, B.; Sharon, C.; Chittum, J.E.; Niyaz, R.Y.; Roy, S.; Nandi, A.; O’Hara, C.P.; et al. Glycan Modulation of Insulin-like Growth Factor-1 Receptor. Angew. Chem.-Int. Ed. 2022, 61, e202211320. [Google Scholar] [CrossRef]
- Chittum, J.E.; Sankaranarayanan, N.V.; O’Hara, C.P.; Desai, U.R. On the selectivity of heparan sulfate recognition by SARS-CoV-2 spike glycoprotein. ACS Med. Chem. Lett. 2021, 12, 1710–1717. [Google Scholar] [CrossRef]
- Raghuraman, A.; Mosier, P.D.; Desai, U.R. Understanding dermatan sulfate-heparin cofactor II interaction through virtual library screening. ACS Med. Chem. Lett. 2010, 1, 281–285. [Google Scholar] [CrossRef]
- Sankaranarayanan, N.V.; Desai, U.R. Toward a robust computational screening strategy for identifying glycosaminoglycan sequences that display high specificity for target proteins. Glycobiology 2014, 24, 1323–1333. [Google Scholar] [CrossRef]
- Sankaranarayanan, N.V.; Nagarajan, B.; Desai, U.R. Combinatorial virtual library screening study of transforming growth factor-β2–chondroitin sulfate system. Int. J. Mol. Sci. 2021, 22, 7542. [Google Scholar] [CrossRef] [PubMed]
- Ballut, L.; Sapay, N.; Chautard, É.; Imberty, A.; Ricard-Blum, S. Mapping of heparin/heparan sulfate binding sites on αvβ3 integrin by molecular docking. J. Mol. Recognit. 2013, 26, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Bugatti, A.; Giagulli, C.; Urbinati, C.; Caccuris, F.; Chiodelli, P.; Oreste, P.; Fiorentini, S.; Orro, A.; Milanesi, L.; D’Ursi, P.; et al. Molecular interaction studies of HIV-1 matrix protein p17 and heparin: Identification of the heparin-binding motif of p17 as a target for the development of multitarget antagonists. J. Biol. Chem. 2013, 288, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.S.; Mancera, R.L. Prediction of heparin binding sites in bone morphogenetic proteins (BMPs). Biochim. Biophys. Acta-Proteins Proteom. 2012, 1824, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Panitz, N.; Theisgen, S.; Samsonov, S.A.; Gehrcke, J.P.; Baumann, L.; Bellmann-Sickert, K.; Köhling, S.; Teresa Pisabarro, M.; Rademann, J.; Huster, D.; et al. The structural investigation of glycosaminoglycan binding to CXCL12 displays distinct interaction sites. Glycobiology 2016, 26, 1209–1221. [Google Scholar] [CrossRef]
- Pichert, A.; Samsonov, S.A.; Theisgen, S.; Thomas, L.; Baumann, L.; Schiller, J.; Beck-Sickinger, A.G.; Huster, D.; Pisabarro, M.T. Characterization of the interaction of interleukin-8 with hyaluronan, chondroitin sulfate, dermatan sulfate and their sulfated derivatives by spectroscopy and molecular modeling. Glycobiology 2012, 22, 134–145. [Google Scholar] [CrossRef]
- Singh, A.; Kett, W.C.; Severin, I.C.; Agyekum, I.; Duan, J.; Amster, I.J.; Proudfoot, A.E.I.; Coombe, D.R.; Woods, R.J. The interaction of heparin tetrasaccharides with chemokine CCL5 is modulated by sulfation pattern and pH. J. Biol. Chem. 2015, 290, 15421–15436. [Google Scholar] [CrossRef]
- Agostino, M.; Gandhi, N.S.; Mancera, R.L. Development and application of site mapping methods for the design of glycosaminoglycans. Glycobiology 2014, 24, 840–851. [Google Scholar] [CrossRef]
- Marcisz, M.; Huard, B.; Lipska, A.G.; Samsonov, S.A. Further analyses of APRIL/APRIL-receptor/glycosaminoglycan interactions by biochemical assays linked to computational studies. Glycobiology 2021, 31, 772–786. [Google Scholar] [CrossRef]
- Winkler, S.; Derler, R.; Gesslbauer, B.; Krieger, E.; Kungl, A.J. Molecular dynamics simulations of the chemokine CCL2 in complex with pull down-derived heparan sulfate hexasaccharides. Biochim. Biophys. Acta-Gen. Subj. 2019, 1863, 528–533. [Google Scholar] [CrossRef]
- Marcisz, M.; Zacharias, M.; Samsonov, S.A. Modeling protein-glycosaminoglycan complexes: Does the size matter? J. Chem. Inf. Model. 2021, 61, 4475–4485. [Google Scholar] [CrossRef] [PubMed]
- Raghuraman, A.; Mosier, P.D.; Desai, U.R. Finding a needle in a haystack: Development of a combinatorial virtual screening approach for identifying high specificity heparin/heparan sulfate sequence(s). J. Med. Chem. 2006, 49, 3553–3562. [Google Scholar] [CrossRef] [PubMed]
- Nivedha, A.K.; Makeneni, S.; Foley, B.L.; Tessier, M.B.; Woods, R.J. Importance of ligand conformational energies in carbohydrate docking: Sorting the wheat from the chaff. J. Comput. Chem. 2014, 35, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, M.; Guglieri, S.; Beccati, D.; Torri, G.; Viskov, C.; Mourier, P. Conformational transitions induced in heparin octasaccharides by binding with antithrombin III. Biochem. J. 2006, 399, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Hricovíni, M.; Guerrini, M.; Bisio, A.; Torri, G.; Naggi, A.; Casu, B. Active conformations of glycosaminoglycans. NMR determination of the conformation of heparin sequences complexed with antithrombin and fibroblast growth factors in solution. Semin. Thromb. Hemost. 2002, 28, 325–334. [Google Scholar] [CrossRef]
- Nagarajan, B.; Holmes, S.G.; Sankaranarayanan, N.V.; Desai, U.R. Molecular dynamics simulations to understand glycosaminoglycan interactions in the free- and protein-bound states. Curr. Opin. Struct. Biol. 2022, 74, 102356. [Google Scholar] [CrossRef]
- Nagarajan, B.; Sankaranarayanan, N.V.; Desai, U.R. Perspective on computational simulations of glycosaminoglycans. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2019, 9, e1388. [Google Scholar] [CrossRef]
- Samsonov, S.A.; Pisabarro, M.T. Importance of IdoA and IdoA(2S) ring conformations in computational studies of glycosaminoglycan-protein interactions. Carbohydr. Res. 2013, 381, 133–137. [Google Scholar] [CrossRef]
- Sankaranarayanan, N.V.; Bi, Y.; Kuberan, B.; Desai, U.R. Combinatorial virtual library screening analysis of antithrombin binding oligosaccharide motif generation by heparan sulfate 3-O-Sulfotransferase 1. Comput. Struct. Biotechnol. J. 2020, 18, 933–941. [Google Scholar] [CrossRef]
- Liebeschuetz, J.; Hennemann, J.; Olsson, T.; Groom, C.R. The good, the bad and the twisted: A survey of ligand geometry in protein crystal structures. J. Comput.-Aided Mol. Des. 2012, 26, 169–183. [Google Scholar] [CrossRef]
- Xu, D.; Esko, J.D. Demystifying heparan sulfate-protein interactions. Annu. Rev. Biochem. 2014, 83, 129–157. [Google Scholar] [CrossRef] [PubMed]
- Whalen, D.M.; Malinauskas, T.; Gilbert, R.J.C.; Siebold, C. Structural insights into proteoglycan-shaped Hedgehog signaling. Proc. Natl. Acad. Sci. USA 2013, 110, 16420–16425. [Google Scholar] [CrossRef] [PubMed]
- Nurisso, A.; Bravo, J.; Carrupt, P.-A.; Daina, A. Molecular docking using the molecular lipophilicity potential as hydrophobic descriptor: Impact on GOLD docking performance. J. Chem. Inf. Model. 2012, 52, 1319–1327. [Google Scholar] [CrossRef]
- Verdonk, M.L.; Cole, J.C.; Hartshorn, M.J.; Murray, C.W.; Taylor, R.D. Improved protein-ligand docking using GOLD. Proteins Struct. Funct. Bioinform. 2003, 52, 609–623. [Google Scholar] [CrossRef]
- Detering, C.; Varani, G. Validation of automated docking programs for docking and database screening against RNA drug targets. J. Med. Chem. 2004, 47, 4188–4201. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Seok, C. CSAlign and CSAlign-Dock: Structure alignment of ligands considering full flexibility and application to protein–ligand docking. Comput. Struct. Biotechnol. J. 2023, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Greenidge, P.A.; Lewis, R.A.; Ertl, P. Boosting pose ranking performance via rescoring with MM-GBSA. Chem. Biol. Drug Des. 2016, 88, 317–328. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Huey, R.; Olson, A.J. Distributed automated docking of flexible ligands to proteins: Parallel applications of AutoDock 2.4. J. Comput.-Aided Mol. Des. 1996, 10, 293–304. [Google Scholar] [CrossRef]
- Stefaniak, F.; Bujnicki, J.M. AnnapuRNA: A scoring function for predicting RNA-small molecule binding poses. PLoS Comput. Biol. 2021, 17, e1008309. [Google Scholar] [CrossRef]
- Li, W.; Johnson, D.J.D.; Esmon, C.T.; Huntington, J.A. Structure of the antithrombin–thrombin–heparin ternary complex reveals the antithrombotic mechanism of heparin. Nat. Struct. Mol. Biol. 2004, 11, 857–862. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, H.-Y.; Wei, L.; Lu, D.; Du, M.; Yuan, M.; Shi, D.; Chen, X.; Wang, P.; Chen, X.-L.; et al. Discovery of exolytic heparinases and their catalytic mechanism and potential application. Nat. Commun. 2021, 12, 1263. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.G.; Nagarajan, B.; Desai, U.R. 3-O-Sulfation induces sequence-specific compact topologies in heparan sulfate that encode a dynamic sulfation code. Comput. Struct. Biotechnol. J. 2022, 20, 3884–3898. [Google Scholar] [CrossRef] [PubMed]
- Janke, J.J.; Yu, Y.; Pomin, V.H.; Zhao, J.; Wang, C.; Linhardt, R.J.; Garciá, A.E. Characterization of heparin’s conformational ensemble by molecular dynamics simulations and nuclear magnetic resonance spectroscopy. J. Chem. Theory Comput. 2022, 18, 1894–1904. [Google Scholar] [CrossRef]
- Cilpa, G.; Hyvönen, M.T.; Koivuniemi, A.; Riekkola, M.L. Atomistic insight into chondroitin-6-sulfate glycosaminoglycan chain through quantum mechanics calculations and molecular dynamics simulation. J. Comput. Chem. 2010, 31, 1670–1680. [Google Scholar] [CrossRef]
- Hricovíni, M. Solution Structure of Heparin Pentasaccharide: NMR and DFT Analysis. J. Phys. Chem. B 2015, 119, 12397–12409. [Google Scholar] [CrossRef] [PubMed]
- Hricovíni, M.; Hricovíni, M. Solution conformation of heparin tetrasaccharide. DFT analysis of structure and spin–spin coupling constants. Molecules 2018, 23, 3042. [Google Scholar] [CrossRef]
- Pągielska, M.; Samsonov, S.A. Molecular dynamics-based comparative analysis of chondroitin and dermatan sulfates. Biomolecules 2023, 13, 247. [Google Scholar] [CrossRef]
- Gesteira, T.F.; Pol-Fachin, L.; Coulson-Thomas, V.J.; Lima, M.A.; Verli, H.; Nader, H.B. Insights into the N-sulfation mechanism: Molecular dynamics simulations of the N-sulfotransferase domain of Ndst1 and mutants. PLoS ONE 2013, 8, e70880. [Google Scholar] [CrossRef]
- Joseph, P.R.B.; Mosier, P.D.; Desai, U.R.; Rajarathnam, K. Solution NMR characterization of chemokine CXCL8/IL-8 monomer and dimer binding to glycosaminoglycans: Structural plasticity mediates differential binding interactions. Biochem. J. 2015, 472, 121–133. [Google Scholar] [CrossRef]
- Nakamichi, Y.; Mikami, B.; Murata, K.; Hashimoto, W. Crystal structure of a bacterial unsaturated glucuronyl hydrolase with specificity for heparin. J. Biol. Chem. 2014, 289, 4787–4797. [Google Scholar] [CrossRef]
- Zhao, B.; LiWang, P.J. Characterization of the interactions of vMIP-II, and a dimeric variant of vMIP-II, with glycosaminoglycans. Biochemistry 2010, 49, 7012–7022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, L.; Huang, H.; Linhardt, R.J. Chemoenzymatic synthesis of glycosaminoglycans. Acc. Chem. Res. 2020, 53, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Zulueta, M.M.L.; Lin, S.Y.; Hu, Y.P.; Hung, S.C. Synthesis of glycosaminoglycans. In Glycochemical Synthesis: Strategies and Applications; Wiley Blackwell: Hoboken, NJ, USA, 2015; pp. 235–261. [Google Scholar] [CrossRef]
- Balius, T.E.; Fischer, M.; Stein, R.M.; Adler, T.B.; Nguyen, C.N.; Cruz, A.; Gilson, M.K.; Kurtzman, T.; Shoichet, B.K. Testing inhomogeneous solvation theory in structure-based ligand discovery. Proc. Natl. Acad. Sci. USA 2017, 114, E6839–E6846. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Coleman, R.G.; Fraser, J.S.; Shoichet, B.K. Incorporation of protein flexibility and conformational energy penalties in docking screens to improve ligand discovery. Nat. Chem. 2014, 6, 575–583. [Google Scholar] [CrossRef]
- Sarkar, A.; Yu, W.; Desai, U.R.; Mackerell, A.D.; Mosier, P.D. Estimating glycosaminoglycan-protein interaction affinity: Water dominates the specific antithrombin-heparin interaction. Glycobiology 2016, 26, 1041–1047. [Google Scholar] [CrossRef]
- Liang, W.G.; Triandafillou, C.G.; Huang, T.Y.; Zulueta, M.M.L.; Banerjee, S.; Dinner, A.R.; Hung, S.C.; Tang, W.J. Structural basis for oligomerization and glycosaminoglycan binding of CCL5 and CCL3. Proc. Natl. Acad. Sci. USA 2016, 113, 5000–5005. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.P.; Johnson, Z.; Borlat, F.; Zwahlen, C.; Kungl, A.; Roulin, K.; Harrenga, A.; Wells, T.N.C.; Proudfoot, A.E.I. The X-ray structure of RANTES: Heparin-derived disaccharides allows the rational design of chemokine inhibitors. Structure 2004, 12, 2081–2093. [Google Scholar] [CrossRef]
- Carter, W.J.; Cama, E.; Huntington, J.A. Crystal structure of thrombin bound to heparin. J. Biol. Chem. 2005, 280, 2745–2749. [Google Scholar] [CrossRef]
- Koo, B.H.; Han, J.H.; Yeom, Y.I.I.; Kim, D.S. Thrombin-dependent MMP-2 activity is regulated by heparan sulfate. J. Biol. Chem. 2010, 285, 41270–41279. [Google Scholar] [CrossRef]
- Wang, C.; Jin, Y.; Desai, U.R.; Yadavalli, V.K. Investigation of the heparin-thrombin interaction by dynamic force spectroscopy. Biochim. Biophys. Acta-Gen. Subj. 2015, 1850, 1099–1106. [Google Scholar] [CrossRef]
- Cai, Z.; Yarovoi, S.V.; Zhu, Z.; Rauova, L.; Hayes, V.; Lebedeva, T.; Liu, Q.; Poncz, M.; Arepally, G.; Cines, D.B.; et al. Atomic description of the immune complex involved in heparin-induced thrombocytopenia. Nat. Commun. 2015, 6, 8277. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.; Zhang, F.; Dordick, J.S.; Linhardt, R.J. Platelet factor 4 polyanion immune complexes: Heparin induced thrombocytopenia and vaccine-induced immune thrombotic thrombocytopenia. Thromb. J. 2021, 19, 66. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Greinacher, A.; Delcea, M. Quantitative description of thermodynamic and kinetic properties of the platelet factor 4/heparin bonds. Nanoscale 2015, 7, 10130–10139. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Yang, Y.; Huynh, A.; Nazy, I.; Kaltashov, I.A. Platelet factor 4 interactions with short heparin oligomers: Implications for folding and assembly. Biophys. J. 2020, 119, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Belvedere, R.; Morretta, E.; Pessolano, E.; Novizio, N.; Tosco, A.; Porta, A.; Whiteford, J.; Perretti, M.; Filippelli, A.; Monti, M.C.; et al. Mesoglycan exerts its fibrinolytic effect through the activation of annexin A2. J. Cell. Physiol. 2021, 236, 4926–4943. [Google Scholar] [CrossRef] [PubMed]
- Kassam, G.; Manro, A.; Braat, C.E.; Louie, P.; Fitzpatrick, S.L.; Waisman, D.M. Characterization of the heparin binding properties of annexin II tetramer. J. Biol. Chem. 1997, 272, 15093–15100. [Google Scholar] [CrossRef]
- Shao, C.; Zhang, F.; Kemp, M.M.; Linhardt, R.J.; Waisman, D.M.; Head, J.F.; Seaton, B.A. Crystallographic analysis of calcium-dependent heparin binding to annexin A2. J. Biol. Chem. 2006, 281, 31689–31695. [Google Scholar] [CrossRef]
- Ahmed, Y.A.; Yates, E.A.; Moss, D.J.; Loeven, M.A.; Hussain, S.A.; Hohenester, E.; Turnbull, J.E.; Powell, A.K. Panels of chemically-modified heparin polysaccharides and natural heparan sulfate saccharides both exhibit differences in binding to Slit and Robo, as well as variation between protein binding and cellular activity. Mol. BioSyst. 2016, 12, 3166–3175. [Google Scholar] [CrossRef]
- Fukuhara, N.; Howitt, J.A.; Hussain, S.A.; Hohenester, E. Structural and functional analysis of slit and heparin binding to immunoglobulin-like domains 1 and 2 of Drosophila robo. J. Biol. Chem. 2008, 283, 16226–16234. [Google Scholar] [CrossRef]
- Li, Z.; Moniz, H.; Wang, S.; Ramiah, A.; Zhang, F.; Moremen, K.W.; Linhardt, R.J.; Sharp, J.S. High structural resolution hydroxyl radical protein footprinting reveals an extended Robo1-heparin binding interface. J. Biol. Chem. 2015, 290, 10729–10740. [Google Scholar] [CrossRef]
- Williams, R.V.; Huang, C.; Moremen, K.W.; Amster, I.J.; Prestegard, J.H. NMR analysis suggests the terminal domains of Robo1 remain extended but are rigidified in the presence of heparan sulfate. Sci. Rep. 2022, 12, 14769. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.C.; Mulloy, B.; Magee, A.I.; Couchman, J.R. Two distinct sites in sonic hedgehog combine for heparan sulfate interactions and cell signaling functions. J. Biol. Chem. 2011, 286, 44391–44402. [Google Scholar] [CrossRef] [PubMed]
- Ortmann, C.; Pickhinke, U.; Exner, S.; Ohlig, S.; Lawrence, R.; Jboor, H.; Dreier, R.; Grobe, K. Correction to Sonic hedgehog processing and release are regulated by glypican heparan sulfate proteoglycans. J. Cell Sci. 2015, 128, 2374–2385. [Google Scholar] [CrossRef] [PubMed]
- Deshauer, C.; Morgan, A.M.; Ryan, E.O.; Handel, T.M.; Prestegard, J.H.; Wang, X. Interactions of the chemokine CCL5/RANTES with medium-sized chondroitin sulfate ligands. Structure 2015, 23, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Dyer, D.P.; Salanga, C.L.; Volkman, B.F.; Kawamura, T.; Handel, T.M. The dependence of chemokine-glycosaminoglycan interactions on chemokine oligomerization. Glycobiology 2015, 26, 312–326. [Google Scholar] [CrossRef]
- Ofosu, F.A.; Modi, G.J.; Smith, L.M.; Cerskus, A.L.; Hirsh, J.; Blajchman, M.A. Heparan sulfate and dermatan sulfate inhibit the generation of thrombin activity in plasma by complementary pathways. Blood 1984, 64, 742–747. [Google Scholar] [CrossRef]
- Cao, L.; Coventry, B.; Goreshnik, I.; Huang, B.; Sheffler, W.; Park, J.S.; Jude, K.M.; Marković, I.; Kadam, R.U.; Verschueren, K.H.G.; et al. Design of protein-binding proteins from the target structure alone. Nature 2022, 605, 551–560. [Google Scholar] [CrossRef]
- Huang, S.Y.; Zou, X. An iterative knowledge-based scoring function for protein-protein recognition. Proteins Struct. Funct. Genet. 2008, 72, 557–579. [Google Scholar] [CrossRef]
- Kaufmann, K.W.; Lemmon, G.H.; Deluca, S.L.; Sheehan, J.H.; Meiler, J. Practically useful: What the R osetta protein modeling suite can do for you. Biochemistry 2010, 49, 2987–2998. [Google Scholar] [CrossRef]
- Sankarayanarayanan, N.V.; Strebel, T.R.; Boothello, R.S.; Sheerin, K.; Raghuraman, A.; Sallas, F.; Mosier, P.D.; Watermeyer, N.D.; Oscarson, S.; Desai, U.R. A hexasaccharide containing rare 2-O-sulfate-glucuronic acid residues selectively activates heparin cofactor II. Angew. Chem.-Int. Ed. 2017, 56, 2312–2317. [Google Scholar] [CrossRef]
- Samsonov, S.A.; Teyra, J.; Pisabarro, M.T. Docking glycosaminoglycans to proteins: Analysis of solvent inclusion. J. Comput.-Aided Mol. Des. 2011, 25, 477–489. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Uciechowska-Kaczmarzyk, U.; Chauvot de Beauchene, I.; Samsonov, S. Docking software performance in protein-glycosaminoglycan systems. J. Mol. Graph. Model. 2019, 90, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Griffith, A.R.; Rogers, C.J.; Miller, G.M.; Abrol, R.; Hsieh-Wilson, L.C.; Goddard, W.A. Predicting glycosaminoglycan surface protein interactions and implications for studying axonal growth. Proc. Natl. Acad. Sci. USA 2017, 114, 13697–13702. [Google Scholar] [CrossRef] [PubMed]
- Chopra, P.; Joshi, A.; Wu, J.; Lu, W.; Yadavalli, T.; Wolfert, M.A.; Shukla, D.; Zaia, J.; Boons, G.-J. The 3-O-sulfation of heparan sulfate modulates protein binding and lyase degradation. Proc. Natl. Acad. Sci. USA 2021, 118, e2012935118. [Google Scholar] [CrossRef]
- Liu, J.; Shriver, Z.; Marshall Pope, R.; Thorp, S.C.; Duncan, M.B.; Copeland, R.J.; Raska, C.S.; Yoshida, K.; Eisenberg, R.J.; Cohen, G.; et al. Characterization of a heparan sulfate octasaccharide that binds to herpes simplex virus type 1 glycoprotein D. J. Biol. Chem. 2002, 277, 33456–33467. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holmes, S.G.; Desai, U.R. Assessing Genetic Algorithm-Based Docking Protocols for Prediction of Heparin Oligosaccharide Binding Geometries onto Proteins. Biomolecules 2023, 13, 1633. https://doi.org/10.3390/biom13111633
Holmes SG, Desai UR. Assessing Genetic Algorithm-Based Docking Protocols for Prediction of Heparin Oligosaccharide Binding Geometries onto Proteins. Biomolecules. 2023; 13(11):1633. https://doi.org/10.3390/biom13111633
Chicago/Turabian StyleHolmes, Samuel G., and Umesh R. Desai. 2023. "Assessing Genetic Algorithm-Based Docking Protocols for Prediction of Heparin Oligosaccharide Binding Geometries onto Proteins" Biomolecules 13, no. 11: 1633. https://doi.org/10.3390/biom13111633
APA StyleHolmes, S. G., & Desai, U. R. (2023). Assessing Genetic Algorithm-Based Docking Protocols for Prediction of Heparin Oligosaccharide Binding Geometries onto Proteins. Biomolecules, 13(11), 1633. https://doi.org/10.3390/biom13111633






