Periostin Augments Vascular Smooth Muscle Cell Calcification via β-Catenin Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. RNA Isolation and RT-PCR
- ALPL fw: GGGACTGGTACTCAGACAACG;
- ALPL rev: GTAGGCGATGTCCTTACAGCC;
- CBFA1 fw: GCCTTCCACTCTCAGTAAGAAGA;
- CBFA1 rev: GCCTGGGGTCTGAAAAAGGG;
- GAPDH fw: GAGTCAACGGATTTGGTCGT;
- GAPDH rev: GACAAGCTTCCCGTTCTCAG;
- MMP2 fw: TACAGGATCATTGGCTACACACC;
- MMP2 rev: GGTCACATCGCTCCAGACT;
- MSX2 fw: TGCAGAGCGTGCAGAGTTC;
- MSX2 rev: GGCAGCATAGGTTTTGCAGC;
- PIT1 fw: GGAAGGGCTTGATTGACGTG;
- PIT1 rev: CAGAACCAAACATAGCACTGACT;
- POSTN fw: GCTATTCTGACGCCTCAAAACT;
- POSTN rev: AGCCTCATTACTCGGTGCAAA;
- WNT3A fw: AGCTACCCGATCTGGTGGTC;
- WNT3A rev: CAAACTCGATGTCCTCGCTAC;
- WNT7A fw: CTGTGGCTGCGACAAAGAGAA;
- WNT7A rev: GCCGTGGCACTTACATTCC.
2.3. Protein Isolation and Western Blotting
2.4. ALP Activity Assay
2.5. Determination of Calcification
2.6. Human Samples
2.7. Statistics
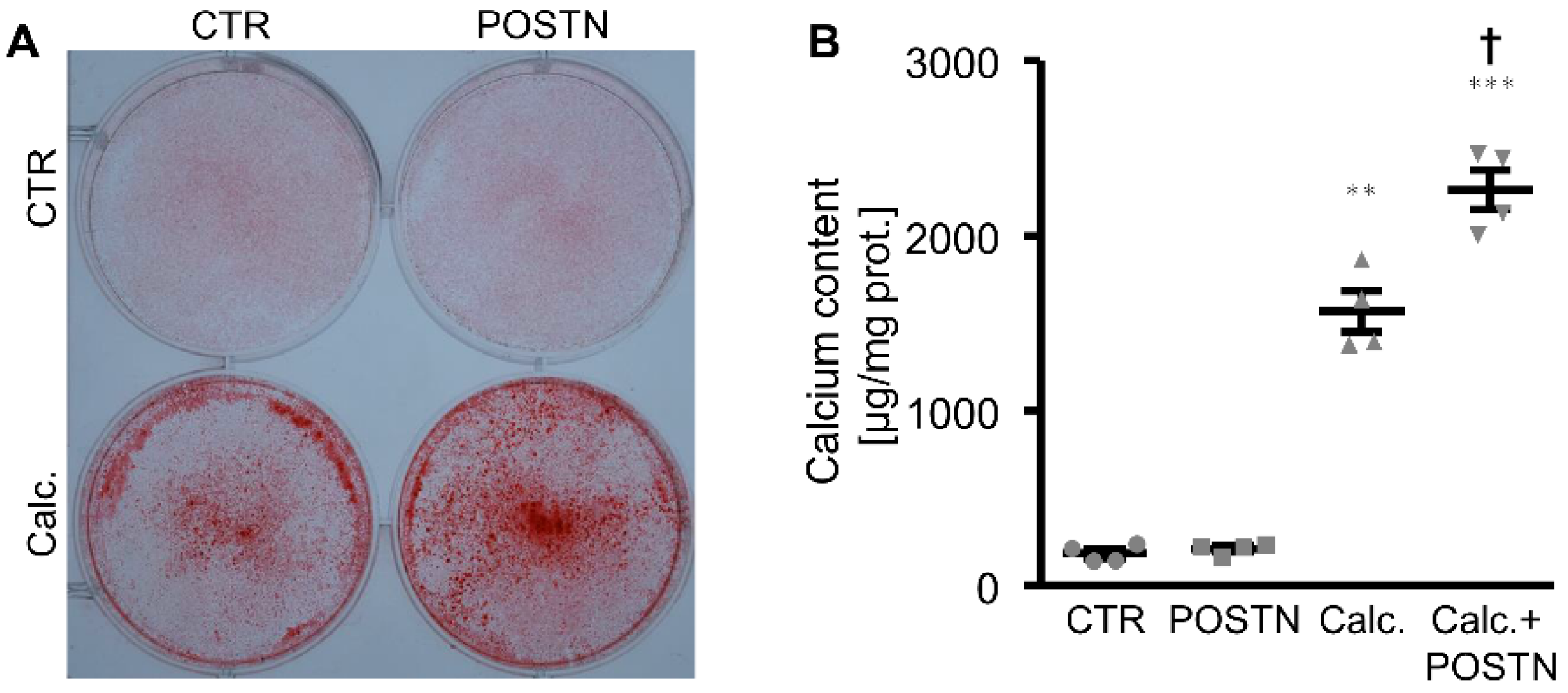
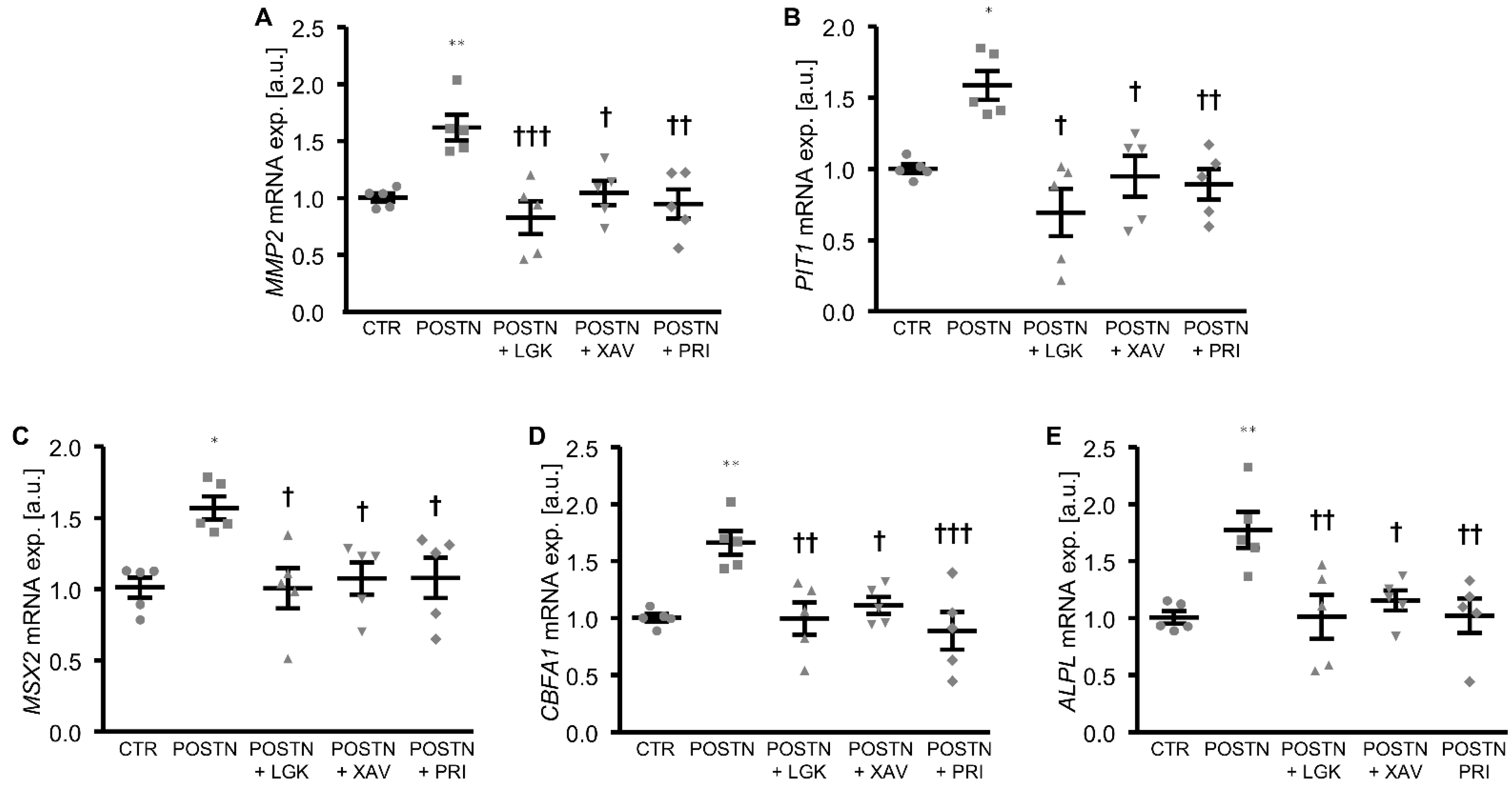
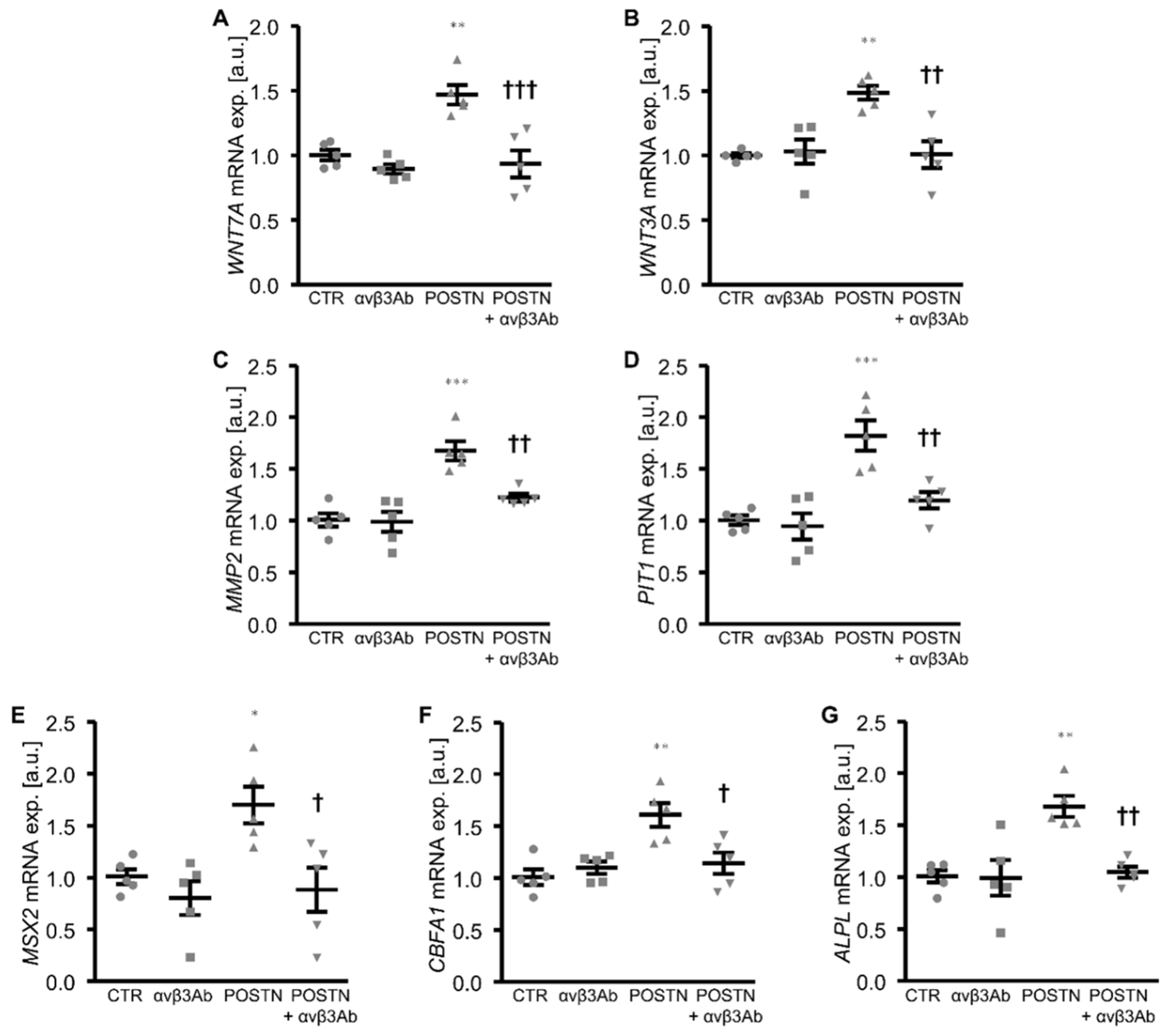
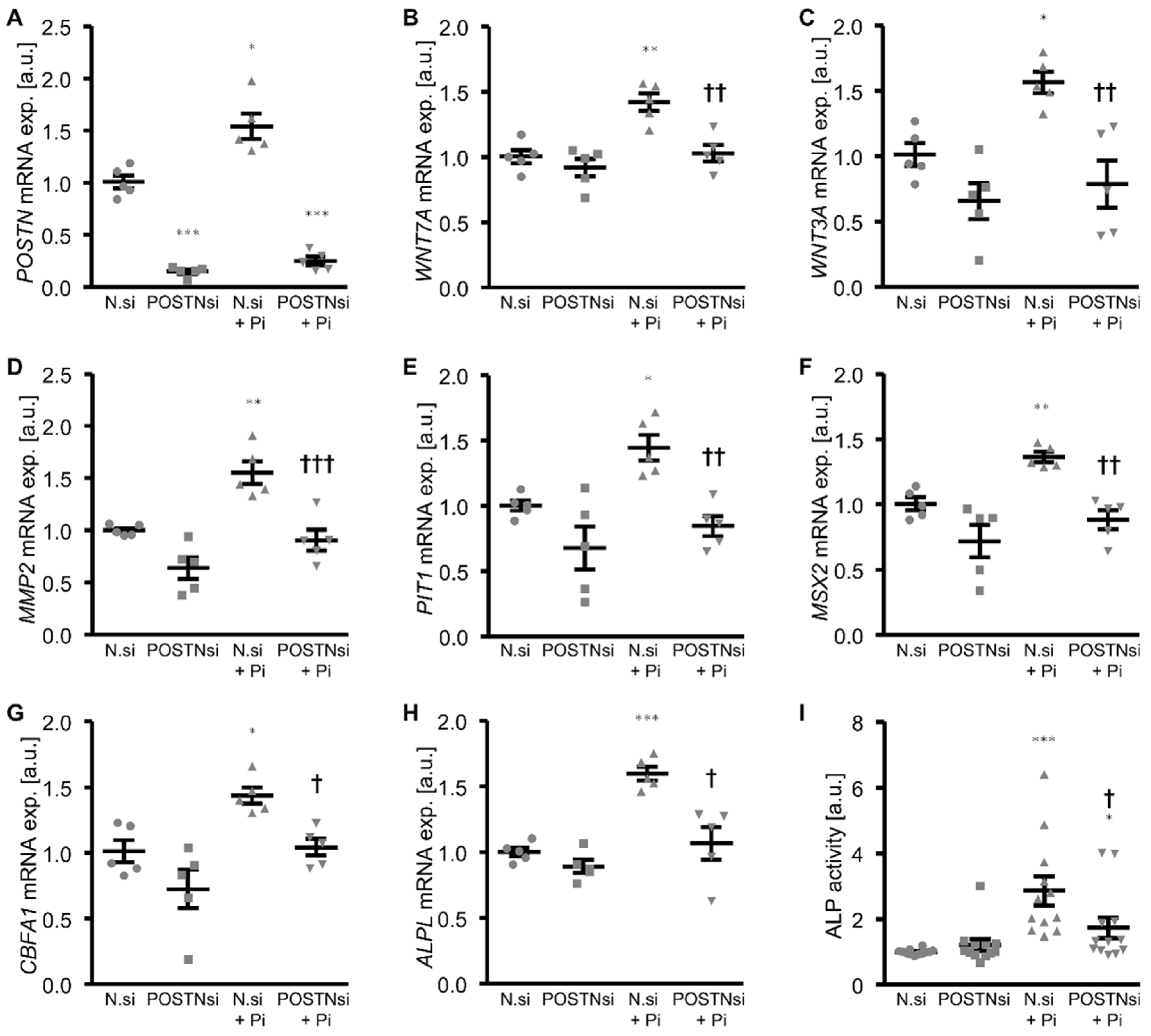
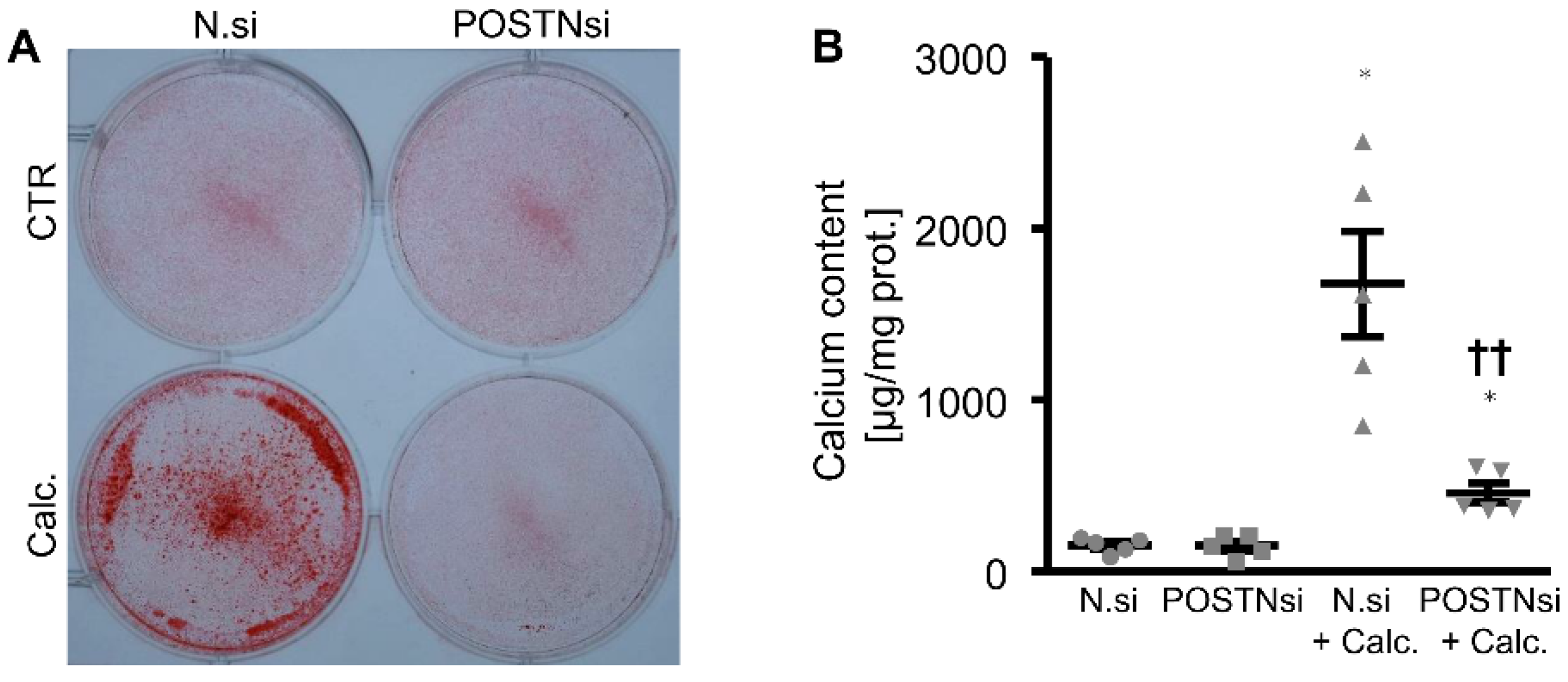
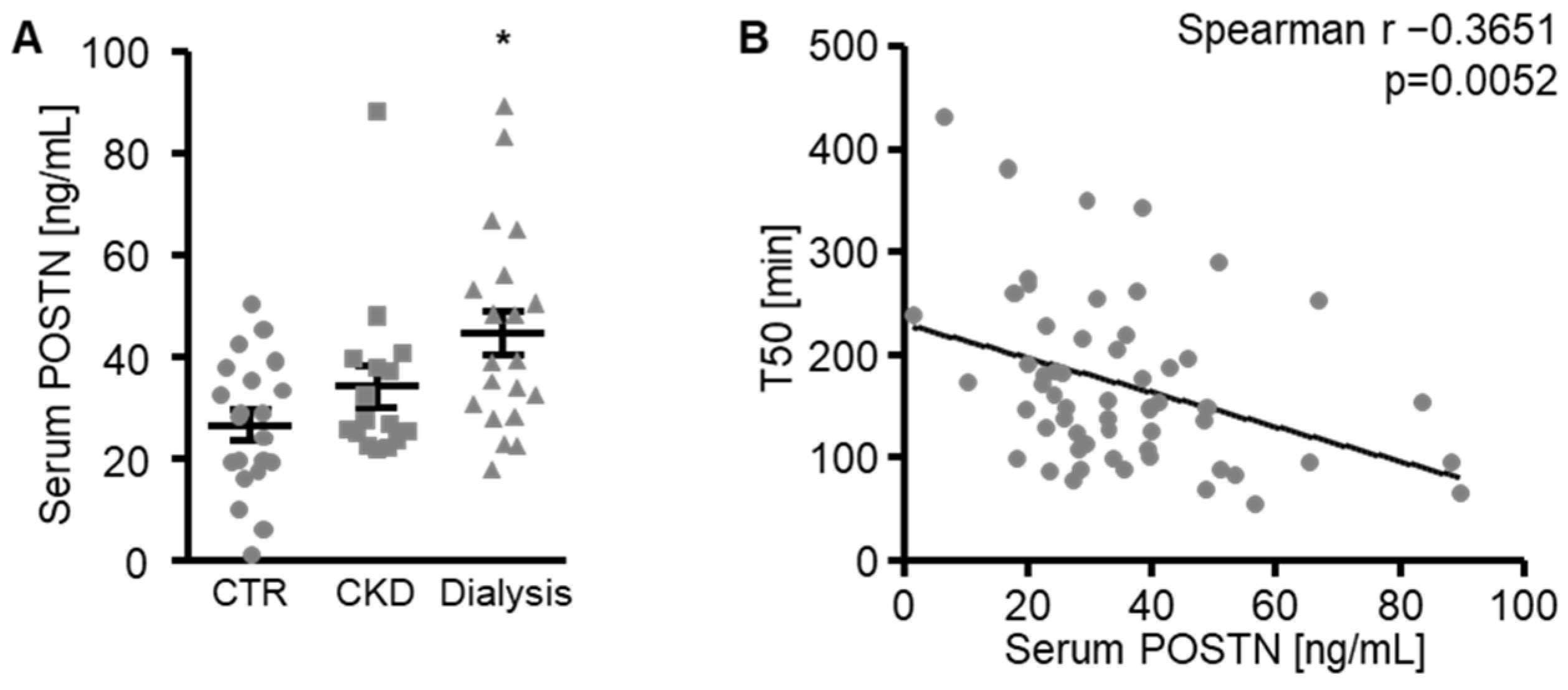
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lanzer, P.; Hannan, F.M.; Lanzer, J.D.; Janzen, J.; Raggi, P.; Furniss, D.; Schuchardt, M.; Thakker, R.; Fok, P.W.; Saez-Rodriguez, J.; et al. Medial Arterial Calcification: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1145–1165. [Google Scholar] [CrossRef] [PubMed]
- Voelkl, J.; Egli-Spichtig, D.; Alesutan, I.; Wagner, C.A. Inflammation: A putative link between phosphate metabolism and cardiovascular disease. Clin. Sci. 2021, 135, 201–227. [Google Scholar] [CrossRef] [PubMed]
- Voelkl, J.; Lang, F.; Eckardt, K.U.; Amann, K.; Kuro, O.M.; Pasch, A.; Pieske, B.; Alesutan, I. Signaling pathways involved in vascular smooth muscle cell calcification during hyperphosphatemia. Cell. Mol. Life Sci. 2019, 76, 2077–2091. [Google Scholar] [CrossRef]
- Tintut, Y.; Honda, H.M.; Demer, L.L. Biomolecules Orchestrating Cardiovascular Calcification. Biomolecules 2021, 11, 1482. [Google Scholar] [CrossRef] [PubMed]
- Alesutan, I.; Luong, T.T.D.; Schelski, N.; Masyout, J.; Hille, S.; Schneider, M.P.; Graham, D.; Zickler, D.; Verheyen, N.; Estepa, M.; et al. Circulating uromodulin inhibits vascular calcification by interfering with pro-inflammatory cytokine signalling. Cardiovasc. Res. 2021, 117, 930–941. [Google Scholar] [CrossRef]
- Conway, S.J.; Izuhara, K.; Kudo, Y.; Litvin, J.; Markwald, R.; Ouyang, G.; Arron, J.R.; Holweg, C.T.; Kudo, A. The role of periostin in tissue remodeling across health and disease. Cell. Mol. Life Sci. 2014, 71, 1279–1288. [Google Scholar] [CrossRef]
- Zhang, Y.; Reif, G.; Wallace, D.P. Extracellular matrix, integrins, and focal adhesion signaling in polycystic kidney disease. Cell. Signal. 2020, 72, 109646. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Mignatti, P.; Abramson, S.B.; Attur, M. Periostin interaction with discoidin domain receptor-1 (DDR1) promotes cartilage degeneration. PLoS ONE 2020, 15, e0231501. [Google Scholar] [CrossRef] [PubMed]
- Idolazzi, L.; Ridolo, E.; Fassio, A.; Gatti, D.; Montagni, M.; Caminati, M.; Martignago, I.; Incorvaia, C.; Senna, G. Periostin: The bone and beyond. Eur. J. Intern. Med. 2017, 38, 12–16. [Google Scholar] [CrossRef]
- Tilman, G.; Mattiussi, M.; Brasseur, F.; van Baren, N.; Decottignies, A. Human periostin gene expression in normal tissues, tumors and melanoma: Evidences for periostin production by both stromal and melanoma cells. Mol. Cancer 2007, 6, 80. [Google Scholar] [CrossRef]
- Kii, I. Practical Application of Periostin as a Biomarker for Pathological Conditions. Adv. Exp. Med. Biol. 2019, 1132, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Hakuno, D.; Kimura, N.; Yoshioka, M.; Mukai, M.; Kimura, T.; Okada, Y.; Yozu, R.; Shukunami, C.; Hiraki, Y.; Kudo, A.; et al. Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and MMP production in humans and rodents. J. Clin. Investig. 2010, 120, 2292–2306. [Google Scholar] [CrossRef] [PubMed]
- Lindner, V.; Wang, Q.; Conley, B.A.; Friesel, R.E.; Vary, C.P. Vascular injury induces expression of periostin: Implications for vascular cell differentiation and migration. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 77–83. [Google Scholar] [CrossRef]
- Corjay, M.H.; Diamond, S.M.; Schlingmann, K.L.; Gibbs, S.K.; Stoltenborg, J.K.; Racanelli, A.L. alphavbeta3, alphavbeta5, and osteopontin are coordinately upregulated at early time points in a rabbit model of neointima formation. J. Cell. Biochem. 1999, 75, 492–504. [Google Scholar] [CrossRef]
- Schwanekamp, J.A.; Lorts, A.; Vagnozzi, R.J.; Vanhoutte, D.; Molkentin, J.D. Deletion of Periostin Protects Against Atherosclerosis in Mice by Altering Inflammation and Extracellular Matrix Remodeling. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Jin, R.; Norris, R.A.; Zhang, L.; Yu, S.; Wu, F.; Markwald, R.R.; Nanda, A.; Conway, S.J.; Smyth, S.S.; et al. Periostin mediates vascular smooth muscle cell migration through the integrins alphavbeta3 and alphavbeta5 and focal adhesion kinase (FAK) pathway. Atherosclerosis 2010, 208, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Petho, D.; Gall, T.; Zavaczki, E.; Nyitrai, M.; Posta, J.; Zarjou, A.; Agarwal, A.; Balla, G.; Balla, J. Zinc Inhibits HIF-Prolyl Hydroxylase Inhibitor-Aggravated VSMC Calcification Induced by High Phosphate. Front. Physiol. 2019, 10, 1584. [Google Scholar] [CrossRef]
- Li, P.; Oparil, S.; Feng, W.; Chen, Y.F. Hypoxia-responsive growth factors upregulate periostin and osteopontin expression via distinct signaling pathways in rat pulmonary arterial smooth muscle cells. J. Appl. Physiol. 2004, 97, 1550–1558. [Google Scholar] [CrossRef]
- Gravesen, E.; Nordholm, A.; Mace, M.; Morevati, M.; Hogdall, E.; Nielsen, C.; Kjaer, A.; Olgaard, K.; Lewin, E. Effect of inhibition of CBP-coactivated beta-catenin-mediated Wnt signalling in uremic rats with vascular calcifications. PLoS ONE 2018, 13, e0201936. [Google Scholar] [CrossRef]
- Zhu, Y.; Ji, J.J.; Wang, X.D.; Sun, X.J.; Li, M.; Wei, Q.; Ren, L.Q.; Liu, N.F. Periostin promotes arterial calcification through PPARgamma-related glucose metabolism reprogramming. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H2222–H2239. [Google Scholar] [CrossRef]
- Turczyn, A.; Panczyk-Tomaszewska, M. The role of periostin in kidney diseases. Cent. Eur. J. Immunol. 2021, 46, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Mael-Ainin, M.; Abed, A.; Conway, S.J.; Dussaule, J.C.; Chatziantoniou, C. Inhibition of periostin expression protects against the development of renal inflammation and fibrosis. J. Am. Soc. Nephrol. 2014, 25, 1724–1736. [Google Scholar] [CrossRef] [PubMed]
- Kormann, R.; Kavvadas, P.; Placier, S.; Vandermeersch, S.; Dorison, A.; Dussaule, J.C.; Chadjichristos, C.E.; Prakoura, N.; Chatziantoniou, C. Periostin Promotes Cell Proliferation and Macrophage Polarization to Drive Repair after AKI. J. Am. Soc. Nephrol. 2020, 31, 85–100. [Google Scholar] [CrossRef]
- Satirapoj, B.; Tassanasorn, S.; Charoenpitakchai, M.; Supasyndh, O. Periostin as a tissue and urinary biomarker of renal injury in type 2 diabetes mellitus. PLoS ONE 2015, 10, e0124055. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Kitajima, I.; Yasuda, T.; Seki, S.; Suzuki, K.; Makino, H.; Ujihara, Y.; Ueno, T.; Canh Tung, N.T.; Yahara, Y. Serum Periostin Level Reflects Progression of Ossification of the Posterior Longitudinal Ligament. JBJS Open Access 2022, 7, e21.00111. [Google Scholar] [CrossRef] [PubMed]
- Voelkl, J.; Alesutan, I.; Leibrock, C.B.; Quintanilla-Martinez, L.; Kuhn, V.; Feger, M.; Mia, S.; Ahmed, M.S.; Rosenblatt, K.P.; Kuro, O.M.; et al. Spironolactone ameliorates PIT1-dependent vascular osteoinduction in klotho-hypomorphic mice. J. Clin. Investig. 2013, 123, 812–822. [Google Scholar] [CrossRef]
- Yu, K.W.; Yao, C.C.; Jeng, J.H.; Shieh, H.Y.; Chen, Y.J. Periostin inhibits mechanical stretch-induced apoptosis in osteoblast-like MG-63 cells. J. Formos. Med. Assoc. 2018, 117, 292–300. [Google Scholar] [CrossRef]
- Voelkl, J.; Luong, T.T.; Tuffaha, R.; Musculus, K.; Auer, T.; Lian, X.; Daniel, C.; Zickler, D.; Boehme, B.; Sacherer, M.; et al. SGK1 induces vascular smooth muscle cell calcification through NF-kappaB signaling. J. Clin. Investig. 2018, 128, 3024–3040. [Google Scholar] [CrossRef]
- Cai, T.; Sun, D.; Duan, Y.; Wen, P.; Dai, C.; Yang, J.; He, W. WNT/beta-catenin signaling promotes VSMCs to osteogenic transdifferentiation and calcification through directly modulating Runx2 gene expression. Exp. Cell. Res. 2016, 345, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Xia, P.F.; Chen, L.; Hou, Y.Y. XIST knockdown suppresses vascular smooth muscle cell proliferation and induces apoptosis by regulating miR-1264/WNT5A/beta-catenin signaling in aneurysm. Biosci. Rep. 2021, 41, BSR20201810. [Google Scholar] [CrossRef] [PubMed]
- Alapati, D.; Rong, M.; Chen, S.; Hehre, D.; Hummler, S.C.; Wu, S. Inhibition of beta-catenin signaling improves alveolarization and reduces pulmonary hypertension in experimental bronchopulmonary dysplasia. Am. J. Respir. Cell Mol. Biol. 2014, 51, 104–113. [Google Scholar] [CrossRef]
- Estell, E.G.; Le, P.T.; Vegting, Y.; Kim, H.; Wrann, C.; Bouxsein, M.L.; Nagano, K.; Baron, R.; Spiegelman, B.M.; Rosen, C.J. Irisin directly stimulates osteoclastogenesis and bone resorption in vitro and in vivo. Elife 2020, 9, e58172. [Google Scholar] [CrossRef]
- Moser, B.; Poetsch, F.; Estepa, M.; Luong, T.T.D.; Pieske, B.; Lang, F.; Alesutan, I.; Voelkl, J. Increased beta-adrenergic stimulation augments vascular smooth muscle cell calcification via PKA/CREB signalling. Pflugers Arch. 2021, 473, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Voelkl, J.; Tuffaha, R.; Luong, T.T.D.; Zickler, D.; Masyout, J.; Feger, M.; Verheyen, N.; Blaschke, F.; Kuro, O.M.; Tomaschitz, A.; et al. Zinc Inhibits Phosphate-Induced Vascular Calcification through TNFAIP3-Mediated Suppression of NF-kappaB. J. Am. Soc. Nephrol. 2018, 29, 1636–1648. [Google Scholar] [CrossRef] [PubMed]
- Hammer, F.; Buehling, S.S.; Masyout, J.; Malzahn, U.; Hauser, T.; Auer, T.; Grebe, S.; Feger, M.; Tuffaha, R.; Degenhart, G.; et al. Protective effects of spironolactone on vascular calcification in chronic kidney disease. Biochem. Biophys. Res. Commun. 2021, 582, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Henze, L.A.; Luong, T.T.D.; Boehme, B.; Masyout, J.; Schneider, M.P.; Brachs, S.; Lang, F.; Pieske, B.; Pasch, A.; Eckardt, K.U.; et al. Impact of C-reactive protein on osteo-/chondrogenic transdifferentiation and calcification of vascular smooth muscle cells. Aging 2019, 11, 5445–5462. [Google Scholar] [CrossRef]
- Wang, X.; Seed, B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 2003, 31, e154. [Google Scholar] [CrossRef]
- Poetsch, F.; Henze, L.A.; Estepa, M.; Moser, B.; Pieske, B.; Lang, F.; Eckardt, K.U.; Alesutan, I.; Voelkl, J. Role of SGK1 in the Osteogenic Transdifferentiation and Calcification of Vascular Smooth Muscle Cells Promoted by Hyperglycemic Conditions. Int. J. Mol. Sci. 2020, 21, 7207. [Google Scholar] [CrossRef]
- Luong, T.T.D.; Tuffaha, R.; Schuchardt, M.; Moser, B.; Schelski, N.; Boehme, B.; Gollmann-Tepekoylu, C.; Schramm, C.; Holfeld, J.; Pieske, B.; et al. Acid sphingomyelinase promotes SGK1-dependent vascular calcification. Clin. Sci. 2021, 135, 515–534. [Google Scholar] [CrossRef]
- Henze, L.A.; Estepa, M.; Pieske, B.; Lang, F.; Eckardt, K.U.; Alesutan, I.; Voelkl, J. Zinc Ameliorates the Osteogenic Effects of High Glucose in Vascular Smooth Muscle Cells. Cells 2021, 10, 3083. [Google Scholar] [CrossRef]
- Alesutan, I.; Moritz, F.; Haider, T.; Shouxuan, S.; Gollmann-Tepekoylu, C.; Holfeld, J.; Pieske, B.; Lang, F.; Eckardt, K.U.; Heinzmann, S.S.; et al. Impact of beta-glycerophosphate on the bioenergetic profile of vascular smooth muscle cells. J. Mol. Med. 2020, 98, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.D.; Eijken, M.; van de Peppel, J.; van Leeuwen, J.P. Calcifying vascular smooth muscle cells and osteoblasts: Independent cell types exhibiting extracellular matrix and biomineralization-related mimicries. BMC Genom. 2014, 15, 965. [Google Scholar] [CrossRef] [PubMed]
- Okubo, Y.; Masuyama, R.; Iwanaga, A.; Koike, Y.; Kuwatsuka, Y.; Ogi, T.; Yamamoto, Y.; Endo, Y.; Tamura, H.; Utani, A. Calcification in dermal fibroblasts from a patient with GGCX syndrome accompanied by upregulation of osteogenic molecules. PLoS ONE 2017, 12, e0177375. [Google Scholar] [CrossRef]
- Matilla, L.; Garaikoetxea, M.; Arrieta, V.; Garcia-Pena, A.; Fernandez-Celis, A.; Navarro, A.; Gainza, A.; Alvarez, V.; Sadaba, R.; Jover, E.; et al. Sex-Differences in Aortic Stenosis: Mechanistic Insights and Clinical Implications. Front. Cardiovasc. Med. 2022, 9, 818371. [Google Scholar] [CrossRef]
- Bagnato, C.; Thumar, J.; Mayya, V.; Hwang, S.I.; Zebroski, H.; Claffey, K.P.; Haudenschild, C.; Eng, J.K.; Lundgren, D.H.; Han, D.K. Proteomics analysis of human coronary atherosclerotic plaque: A feasibility study of direct tissue proteomics by liquid chromatography and tandem mass spectrometry. Mol. Cell. Proteom. 2007, 6, 1088–1102. [Google Scholar] [CrossRef]
- Pohjolainen, V.; Rysa, J.; Napankangas, J.; Koobi, P.; Eraranta, A.; Ilves, M.; Serpi, R.; Porsti, I.; Ruskoaho, H. Left ventricular periostin gene expression is associated with fibrogenesis in experimental renal insufficiency. Nephrol. Dial. Transplant. 2012, 27, 115–122. [Google Scholar] [CrossRef][Green Version]
- Zhu, X.; Ma, K.; Zhou, K.; Pan, X.; Liu, J.; Nurnberg, B.; Alesutan, I.; Volkl, J.; Lang, F. Requirement of Na+/H+ Exchanger NHE1 for Vasopressin-Induced Osteogenic Signaling and Calcification in Human Aortic Smooth Muscle Cells. Kidney Blood Press. Res. 2022, 47, 399–409. [Google Scholar] [CrossRef]
- Tkatchenko, T.V.; Moreno-Rodriguez, R.A.; Conway, S.J.; Molkentin, J.D.; Markwald, R.R.; Tkatchenko, A.V. Lack of periostin leads to suppression of Notch1 signaling and calcific aortic valve disease. Physiol. Genom. 2009, 39, 160–168. [Google Scholar] [CrossRef]
- Bonnet, N.; Garnero, P.; Ferrari, S. Periostin action in bone. Mol. Cell. Endocrinol. 2016, 432, 75–82. [Google Scholar] [CrossRef]
- Zhang, F.; Luo, K.; Rong, Z.; Wang, Z.; Luo, F.; Zhang, Z.; Sun, D.; Dong, S.; Xu, J.; Dai, F. Periostin Upregulates Wnt/beta-Catenin Signaling to Promote the Osteogenesis of CTLA4-Modified Human Bone Marrow-Mesenchymal Stem Cells. Sci. Rep. 2017, 7, 41634. [Google Scholar] [CrossRef]
- Cheng, S.L.; Shao, J.S.; Halstead, L.R.; Distelhorst, K.; Sierra, O.; Towler, D.A. Activation of vascular smooth muscle parathyroid hormone receptor inhibits Wnt/beta-catenin signaling and aortic fibrosis in diabetic arteriosclerosis. Circ. Res. 2010, 107, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Shao, J.S.; Lai, C.F.; Huang, E.; Cai, J.; Behrmann, A.; Cheng, S.L.; Towler, D.A. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr-/- mice. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2589–2596. [Google Scholar] [CrossRef] [PubMed]
- Tuffaha, R.; Voelkl, J.; Pieske, B.; Lang, F.; Alesutan, I. Role of PKB/SGK-dependent phosphorylation of GSK-3alpha/beta in vascular calcification during cholecalciferol overload in mice. Biochem. Biophys. Res. Commun. 2018, 503, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Riascos-Bernal, D.F.; Chinnasamy, P.; Gross, J.N.; Almonte, V.; Egana-Gorrono, L.; Parikh, D.; Jayakumar, S.; Guo, L.; Sibinga, N.E.S. Inhibition of Smooth Muscle beta-Catenin Hinders Neointima Formation After Vascular Injury. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 879–888. [Google Scholar] [CrossRef]
- Yao, L.; Sun, Y.T.; Sun, W.; Xu, T.H.; Ren, C.; Fan, X.; Sun, L.; Liu, L.L.; Feng, J.M.; Ma, J.F.; et al. High phosphorus level leads to aortic calcification via beta-catenin in chronic kidney disease. Am. J. Nephrol. 2015, 41, 28–36. [Google Scholar] [CrossRef]
- Han, L.; Gong, S.; Wang, R.; Liu, S.; Wang, B.; Chen, G.; Gong, T.; Xu, W. Knockdown of POSTN Inhibits Osteogenic Differentiation of Mesenchymal Stem Cells From Patients With Steroid-Induced Osteonecrosis. Front. Cell Dev. Biol. 2020, 8, 606289. [Google Scholar] [CrossRef]
- Mi, Z.; Bhattacharya, S.D.; Kim, V.M.; Guo, H.; Talbot, L.J.; Kuo, P.C. Osteopontin promotes CCL5-mesenchymal stromal cell-mediated breast cancer metastasis. Carcinogenesis 2011, 32, 477–487. [Google Scholar] [CrossRef]
- Huang, J.; Hu, M.; Niu, H.; Wang, J.; Si, Y.; Cheng, S.; Ding, W. Osteopontin isoform c promotes the survival of cisplatin-treated NSCLC cells involving NFATc2-mediated suppression on calcium-induced ROS levels. BMC Cancer 2021, 21, 750. [Google Scholar] [CrossRef]
- Kumar, P.; Smith, T.; Raeman, R.; Chopyk, D.M.; Brink, H.; Liu, Y.; Sulchek, T.; Anania, F.A. Periostin promotes liver fibrogenesis by activating lysyl oxidase in hepatic stellate cells. J. Biol. Chem. 2018, 293, 12781–12792. [Google Scholar] [CrossRef]
- Jenkins, W.S.; Vesey, A.T.; Vickers, A.; Neale, A.; Moles, C.; Connell, M.; Joshi, N.V.; Lucatelli, C.; Fletcher, A.M.; Spratt, J.C.; et al. In vivo alpha-V beta-3 integrin expression in human aortic atherosclerosis. Heart 2019, 105, 1868–1875. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, J.; Wei, F.; Wang, Y.; Chen, S.; Li, G.; Dong, N. Micromechanical force promotes aortic valvular calcification. J. Thorac. Cardiovasc. Surg. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.T.; Wang, C.G.; Zhang, T.L.; Wang, K. Fibronectin enhances in vitro vascular calcification by promoting osteoblastic differentiation of vascular smooth muscle cells via ERK pathway. J. Cell. Biochem. 2006, 99, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Charo, I.F.; Nannizzi, L.; Smith, J.W.; Cheresh, D.A. The vitronectin receptor alpha v beta 3 binds fibronectin and acts in concert with alpha 5 beta 1 in promoting cellular attachment and spreading on fibronectin. J. Cell Biol. 1990, 111, 2795–2800. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, A.; Alvarez, J.; Greenfield, E.M.; Teti, A.; Grano, M.; Colucci, S.; Zambonin-Zallone, A.; Ross, F.P.; Teitelbaum, S.L.; Cheresh, D.; et al. Recognition of osteopontin and related peptides by an alpha v beta 3 integrin stimulates immediate cell signals in osteoclasts. J. Biol. Chem. 1991, 266, 20369–20374. [Google Scholar] [CrossRef]
- Steitz, S.A.; Speer, M.Y.; McKee, M.D.; Liaw, L.; Almeida, M.; Yang, H.; Giachelli, C.M. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am. J. Pathol. 2002, 161, 2035–2046. [Google Scholar] [CrossRef]
- Kantauskaite, M.; Bolten, K.; Boschheidgen, M.; Schmidt, C.; Kolb, T.; Eckardt, K.U.; Pasch, A.; Schimmoller, L.; Rump, L.C.; Voelkl, J.; et al. Serum Calcification Propensity and Calcification of the Abdominal Aorta in Patients With Primary Aldosteronism. Front. Cardiovasc. Med. 2022, 9, 771096. [Google Scholar] [CrossRef]
- Bundy, J.D.; Cai, X.; Scialla, J.J.; Dobre, M.A.; Chen, J.; Hsu, C.Y.; Leonard, M.B.; Go, A.S.; Rao, P.S.; Lash, J.P.; et al. Serum Calcification Propensity and Coronary Artery Calcification Among Patients With CKD: The CRIC (Chronic Renal Insufficiency Cohort) Study. Am. J. Kidney Dis. 2019, 73, 806–814. [Google Scholar] [CrossRef]
- Bundy, J.D.; Cai, X.; Mehta, R.C.; Scialla, J.J.; de Boer, I.H.; Hsu, C.Y.; Go, A.S.; Dobre, M.A.; Chen, J.; Rao, P.S.; et al. Serum Calcification Propensity and Clinical Events in CKD. Clin J. Am. Soc. Nephrol. 2019, 14, 1562–1571. [Google Scholar] [CrossRef]
- Bojic, M.; Koller, L.; Cejka, D.; Niessner, A.; Bielesz, B. Propensity for Calcification in Serum Associates With 2-Year Cardiovascular Mortality in Ischemic Heart Failure With Reduced Ejection Fraction. Front. Med. 2021, 8, 672348. [Google Scholar] [CrossRef]
- Eelderink, C.; Te Velde-Keyzer, C.A.; Frenay, A.S.; Vermeulen, E.A.; Bachtler, M.; Aghagolzadeh, P.; van Dijk, P.R.; Gansevoort, R.T.; Vervloet, M.G.; Hillebrands, J.L.; et al. Serum Calcification Propensity and the Risk of Cardiovascular and All-Cause Mortality in the General Population: The PREVEND Study. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1942–1951. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alesutan, I.; Henze, L.A.; Boehme, B.; Luong, T.T.D.; Zickler, D.; Pieske, B.; Eckardt, K.-U.; Pasch, A.; Voelkl, J. Periostin Augments Vascular Smooth Muscle Cell Calcification via β-Catenin Signaling. Biomolecules 2022, 12, 1157. https://doi.org/10.3390/biom12081157
Alesutan I, Henze LA, Boehme B, Luong TTD, Zickler D, Pieske B, Eckardt K-U, Pasch A, Voelkl J. Periostin Augments Vascular Smooth Muscle Cell Calcification via β-Catenin Signaling. Biomolecules. 2022; 12(8):1157. https://doi.org/10.3390/biom12081157
Chicago/Turabian StyleAlesutan, Ioana, Laura A. Henze, Beate Boehme, Trang T. D. Luong, Daniel Zickler, Burkert Pieske, Kai-Uwe Eckardt, Andreas Pasch, and Jakob Voelkl. 2022. "Periostin Augments Vascular Smooth Muscle Cell Calcification via β-Catenin Signaling" Biomolecules 12, no. 8: 1157. https://doi.org/10.3390/biom12081157
APA StyleAlesutan, I., Henze, L. A., Boehme, B., Luong, T. T. D., Zickler, D., Pieske, B., Eckardt, K.-U., Pasch, A., & Voelkl, J. (2022). Periostin Augments Vascular Smooth Muscle Cell Calcification via β-Catenin Signaling. Biomolecules, 12(8), 1157. https://doi.org/10.3390/biom12081157




