Heat Shock Protein 90 (Hsp90) and Hsp70 as Potential Therapeutic Targets in Autoimmune Skin Diseases
Abstract
:1. Introduction
2. Heat Shock Protein 90 (Hsp90)
Inhibition of Hsp90 Activity
3. Heat Shock Protein 70 (Hsp70)
Extracellular Hsp70 Activities
4. Hsp90 and Hsp70 as Potential Therapeutic Targets in Autoimmune Skin Diseases
4.1. Autoimmune Bullous Diseases
4.1.1. Bullous Pemphigoid
4.1.2. Epidermolysis Bullosa Acquisita
4.1.3. Dermatitis Herpetiformis
4.2. Psoriasis
4.3. Vitiligo
4.4. Alopecia Areata
4.5. Systemic Lupus Erythematosus
4.6. Systemic Sclerosis
4.7. Atopic Dermatitis
5. Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Maio, A.; Vazquez, D. Extracellular heat shock proteins: A new location, a new function. Shock 2013, 40, 239–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuehlke, A.D.; Moses, M.A.; Neckers, L. Heat shock protein 90: Its inhibition and function. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutledge, B.S.; Choy, W.Y.; Duennwald, M.L. Folding or holding?—Hsp70 and Hsp90 chaperoning of misfolded proteins in neurodegenerative disease. J. Biol. Chem. 2022, 298, 101905. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.; Wu, K.; Lee, C.; Bardwell, J.C.A. ATP-Independent Chaperones. Annu. Rev. Biophys. 2022, 51, 409–429. [Google Scholar] [CrossRef]
- Calderwood, S.K.; Gong, J.; Murshid, A. Extracellular HSPs: The Complicated Roles of Extracellular HSPs in Immunity. Front. Immunol. 2016, 7, 159. [Google Scholar] [CrossRef] [Green Version]
- Murshid, A.; Borges, T.J.; Bonorino, C.; Lang, B.J.; Calderwood, S.K. Immunological Outcomes Mediated Upon Binding of Heat Shock Proteins to Scavenger Receptors SCARF1 and LOX-1, and Endocytosis by Mononuclear Phagocytes. Front. Immunol. 2019, 10, 3035. [Google Scholar] [CrossRef]
- Murshid, A.; Gong, J.; Calderwood, S.K. The role of heat shock proteins in antigen cross presentation. Front. Immunol. 2012, 3, 63. [Google Scholar] [CrossRef] [Green Version]
- Tukaj, S. Heat Shock Protein 70 as a Double Agent Acting Inside and Outside the Cell: Insights into Autoimmunity. Int. J. Mol. Sci. 2020, 21, 5298. [Google Scholar] [CrossRef]
- Tukaj, S.; Kaminski, M. Heat shock proteins in the therapy of autoimmune diseases: Too simple to be true? Cell Stress Chaperones 2019, 24, 475–479. [Google Scholar] [CrossRef] [Green Version]
- Tukaj, S.; Węgrzyn, G. Anti-Hsp90 therapy in autoimmune and inflammatory diseases: A review of preclinical studies. Cell Stress Chaperones 2016, 21, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Hoter, A.; El-Sabban, M.E.; Naim, H.Y. The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease. Int. J. Mol. Sci. 2018, 19, 2560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Buchner, J. Structure, function and regulation of the hsp90 machinery. Biomed. J. 2013, 36, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Biebl, M.M.; Buchner, J. Structure, Function, and Regulation of the Hsp90 Machinery. Cold Spring Harb. Perspect. Biol. 2019, 11, a034017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell. Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Zuehlke, A.D.; Beebe, K.; Neckers, L.; Prince, T. Regulation and function of the human HSP90AA1 gene. Gene 2015, 570, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Hance, M.W.; Nolan, K.D.; Isaacs, J.S. The double-edged sword: Conserved functions of extracellular hsp90 in wound healing and cancer. Cancers 2014, 6, 1065–1097. [Google Scholar] [CrossRef]
- Li, W.; Sahu, D.; Tsen, F. Secreted heat shock protein-90 (Hsp90) in wound healing and cancer. Biochim. Biophys. Acta 2012, 1823, 730–741. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, A.; Blagg, B.S. Novobiocin and additional inhibitors of the Hsp90 C-terminal nucleotide-binding pocket. Curr. Med. Chem. 2008, 15, 2702–2717. [Google Scholar] [CrossRef] [Green Version]
- Der Sarkissian, S.; Aceros, H.; Williams, P.M.; Scalabrini, C.; Borie, M.; Noiseux, N. Heat shock protein 90 inhibition and multi-target approach to maximize cardioprotection in ischaemic injury. Br. J. Pharmacol. 2020, 177, 3378–3388. [Google Scholar] [CrossRef]
- Sanchez, J.; Carter, T.R.; Cohen, M.S.; Blagg, B.S.J. Old and New Approaches to Target the Hsp90 Chaperone. Curr. Cancer Drug Targets 2020, 20, 253–270. [Google Scholar] [CrossRef]
- Mielczarek-Lewandowska, A.; Hartman, M.L.; Czyz, M. Inhibitors of HSP90 in melanoma. Apoptosis 2020, 25, 12–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bickel, D.; Gohlke, H. C-terminal modulators of heat shock protein of 90 kDa (HSP90): State of development and modes of action. Bioorg. Med. Chem. 2019, 27, 115080. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; McAlpine, S.R. N-terminal and C-terminal modulation of Hsp90 produce dissimilar phenotypes. Chem. Commun. 2015, 51, 1410–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, A.; Shervington, A.; Howl, J.; Jones, S.; Shervington, L. Can RNAi-mediated hsp90α knockdown in combination with 17-AAG be a therapy for glioma? FEBS Open Bio 2013, 3, 271–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada-Kanazawa, S.; Kajihara, I.; Fukushima, S.; Jinnin, M.; Masuzawa, M.; Masuzawa, M.; Amoh, Y.; Hoshina, D.; Abe, R.; Ihn, H. Inhibition of heat shock protein 90 exerts an antitumour effect in angiosarcoma: Involvement of the vascular endothelial growth factor signalling pathway. Br. J. Dermatol. 2017, 177, 456–469. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, C.; Song, C. Pan- and isoform-specific inhibition of Hsp90: Design strategy and recent advances. Eur. J. Med. Chem. 2022, 238, 114516. [Google Scholar] [CrossRef]
- Li, L.; Chen, N.N.; You, Q.D.; Xu, X.L. An updated patent review of anticancer Hsp90 inhibitors (2013-present). Expert Opin. Ther. Pat. 2021, 31, 67–80. [Google Scholar] [CrossRef]
- Shevtsov, M.; Multhoff, G.; Mikhaylova, E.; Shibata, A.; Guzhova, I.; Margulis, B. Combination of Anti-Cancer Drugs with Molecular Chaperone Inhibitors. Int. J. Mol. Sci. 2019, 20, 5284. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wang, L.; You, Q.D.; Xu, X.L. Heat Shock Protein 90 Inhibitors: An Update on Achievements, Challenges, and Future Directions. J. Med. Chem. 2020, 63, 1798–1822. [Google Scholar] [CrossRef]
- Trepel, J.; Mollapour, M.; Giaccone, G.; Neckers, L. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer 2010, 10, 537–549. [Google Scholar] [CrossRef] [Green Version]
- Jackson, S.E. Hsp90: Structure and function. Top Curr. Chem. 2013, 328, 155–240. [Google Scholar] [CrossRef]
- Radons, J. The human HSP70 family of chaperones: Where do we stand? Cell Stress Chaperones 2016, 21, 379–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kityk, R.; Kopp, J.; Sinning, I.; Mayer, M.P. Structure and dynamics of the ATP-bound open conformation of Hsp70 chaperones. Mol. Cell 2012, 48, 863–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenzweig, R.; Nillegoda, N.B.; Mayer, M.P.; Bukau, B. The Hsp70 chaperone network. Nat. Rev. Mol. Cell Biol. 2019, 20, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stokes, J., 3rd; Singh, U.P.; Scissum Gunn, K.; Acharya, A.; Manne, U.; Mishra, M. Targeting Hsp70: A possible therapy for cancer. Cancer Lett. 2016, 374, 156–166. [Google Scholar] [CrossRef] [Green Version]
- Ambrose, A.J.; Chapman, E. Function, Therapeutic Potential, and Inhibition of Hsp70 Chaperones. J. Med. Chem. 2021, 64, 7060–7082. [Google Scholar] [CrossRef]
- Wieten, L.; Broere, F.; van der Zee, R.; Koerkamp, E.K.; Wagenaar, J.; van Eden, W. Cell stress induced HSP are targets of regulatory T cells: A role for HSP inducing compounds as anti-inflammatory immuno-modulators? FEBS Lett. 2007, 581, 3716–3722. [Google Scholar] [CrossRef] [Green Version]
- Wieten, L.; van der Zee, R.; Spiering, R.; Wagenaar-Hilbers, J.; van Kooten, P.; Broere, F.; van Eden, W. A novel heat-shock protein coinducer boosts stress protein Hsp70 to activate T cell regulation of inflammation in autoimmune arthritis. Arthritis Rheum. 2010, 62, 1026–1035. [Google Scholar] [CrossRef]
- Wieten, L.; van der Zee, R.; Goedemans, R.; Sijtsma, J.; Serafini, M.; Lubsen, N.H.; van Eden, W.; Broere, F. Hsp70 expression and induction as a readout for detection of immune modulatory components in food. Cell Stress Chaperones 2010, 15, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Quintana, F.J.; Cohen, I.R. DNA vaccines coding for heat-shock proteins (HSPs): Tools for the activation of HSP-specific regulatory T cells. Expert Opin. Biol. Ther. 2005, 5, 545–554. [Google Scholar] [CrossRef]
- De Maio, A. Extracellular Hsp70: Export and function. Curr. Protein Pept. Sci. 2014, 15, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Hulina, A.; Grdić Rajković, M.; Jakšić Despot, D.; Jelić, D.; Dojder, A.; Čepelak, I.; Rumora, L. Extracellular Hsp70 induces inflammation and modulates LPS/LTA-stimulated inflammatory response in THP-1 cells. Cell Stress Chaperones 2018, 23, 373–384. [Google Scholar] [CrossRef] [PubMed]
- van Eden, W. Immune tolerance therapies for autoimmune diseases based on heat shock protein T-cell epitopes. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Herwijnen, M.J.; Wieten, L.; van der Zee, R.; van Kooten, P.J.; Wagenaar-Hilbers, J.P.; Hoek, A.; den Braber, I.; Anderton, S.M.; Singh, M.; Meiring, H.D.; et al. Regulatory T cells that recognize a ubiquitous stress-inducible self-antigen are long-lived suppressors of autoimmune arthritis. Proc. Natl. Acad. Sci. USA 2012, 109, 14134–14139. [Google Scholar] [CrossRef] [Green Version]
- Tay, S.S.; Roediger, B.; Tong, P.L.; Tikoo, S.; Weninger, W. The Skin-Resident Immune Network. Curr. Dermatol. Rep. 2014, 3, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Vesely, M.D. Getting Under the Skin: Targeting Cutaneous Autoimmune Disease. Yale J. Biol. Med. 2020, 93, 197–206. [Google Scholar]
- Ujiie, H.; Rosmarin, D.; Schön, M.P.; Ständer, S.; Boch, K.; Metz, M.; Maurer, M.; Thaci, D.; Schmidt, E.; Cole, C.; et al. Unmet Medical Needs in Chronic, Non-communicable Inflammatory Skin Diseases. Front. Med. 2022, 9, 875492. [Google Scholar] [CrossRef]
- Kasperkiewicz, M.; Müller, R.; Manz, R.; Magens, M.; Hammers, C.M.; Somlai, C.; Westermann, J.; Schmidt, E.; Zillikens, D.; Ludwig, R.J.; et al. Heat-shock protein 90 inhibition in autoimmunity to type VII collagen: Evidence that nonmalignant plasma cells are not therapeutic targets. Blood 2011, 117, 6135–6142. [Google Scholar] [CrossRef] [Green Version]
- Tukaj, S.; Tiburzy, B.; Manz, R.; de Castro Marques, A.; Orosz, A.; Ludwig, R.J.; Zillikens, D.; Kasperkiewicz, M. Immunomodulatory effects of heat shock protein 90 inhibition on humoral immune responses. Exp. Dermatol. 2014, 23, 585–590. [Google Scholar] [CrossRef]
- Tukaj, S.; Bieber, K.; Kleszczyński, K.; Witte, M.; Cames, R.; Kalies, K.; Zillikens, D.; Ludwig, R.J.; Fischer, T.W.; Kasperkiewicz, M. Topically Applied Hsp90 Blocker 17AAG Inhibits Autoantibody-Mediated Blister-Inducing Cutaneous Inflammation. J. Investig. Dermatol. 2017, 137, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Tukaj, S.; Mantej, J.; Sitko, K.; Bednarek, M.; Zillikens, D.; Ludwig, R.J.; Bieber, K.; Kasperkiewicz, M. Evidence for a role of extracellular heat shock protein 70 in epidermolysis bullosa acquisita. Exp. Dermatol. 2022, 31, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Tukaj, S.; Mantej, J.; Sitko, K.; Zillikens, D.; Ludwig, R.J.; Bieber, K.; Kasperkiewicz, M. Pathological Relevance of Anti-Hsp70 IgG Autoantibodies in Epidermolysis Bullosa Acquisita. Front. Immunol. 2022, 13, 877958. [Google Scholar] [CrossRef] [PubMed]
- Stenderup, K.; Rosada, C.; Gavillet, B.; Vuagniaux, G.; Dam, T.N. Debio 0932, a new oral Hsp90 inhibitor, alleviates psoriasis in a xenograft transplantation model. Acta Derm. Venereol. 2014, 94, 672–676. [Google Scholar] [CrossRef] [Green Version]
- Raghuwanshi, N.; Yadav, T.C.; Srivastava, A.K.; Raj, U.; Varadwaj, P.; Pruthi, V. Structure-based drug designing and identification of Woodfordia fruticosa inhibitors targeted against heat shock protein (HSP70-1) as suppressor for Imiquimod-induced psoriasis like skin inflammation in mice model. Mater. Sci. Eng. C 2019, 95, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Seifarth, F.G.; Lax, J.E.; Harvey, J.; DiCorleto, P.E.; Husni, M.E.; Chandrasekharan, U.M.; Tytell, M. Topical heat shock protein 70 prevents imiquimod-induced psoriasis-like inflammation in mice. Cell Stress Chaperones 2018, 23, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Tukaj, S.; Mantej, J.; Sobala, M.; Potrykus, K.; Tukaj, Z.; Zillikens, D.; Ludwig, R.J.; Bieber, K.; Kasperkiewicz, M. Therapeutic Implications of Targeting Heat Shock Protein 70 by Immunization or Antibodies in Experimental Skin Inflammation. Front. Immunol. 2021, 12, 614320. [Google Scholar] [CrossRef]
- Bregnhøj, A.; Thuesen, K.K.H.; Emmanuel, T.; Litman, T.; Grek, C.L.; Ghatnekar, G.S.; Johansen, C.; Iversen, L. HSP90 inhibitor RGRN-305 for oral treatment of plaque-type psoriasis: Efficacy, safety and biomarker results in an open-label proof-of-concept study. Br. J. Dermatol. 2022, 186, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Denman, C.J.; McCracken, J.; Hariharan, V.; Klarquist, J.; Oyarbide-Valencia, K.; Guevara-Patiño, J.A.; Le Poole, I.C. HSP70i accelerates depigmentation in a mouse model of autoimmune vitiligo. J. Investig. Dermatol. 2008, 128, 2041–2048. [Google Scholar] [CrossRef] [Green Version]
- Wikramanayake, T.C.; Villasante, A.C.; Mauro, L.M.; Perez, C.I.; Schachner, L.A.; Jimenez, J.J. Prevention and treatment of alopecia areata with quercetin in the C3H/HeJ mouse model. Cell Stress Chaperones 2012, 17, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Hong, L.J.; Chen, A.J.; Li, F.Z.; Chen, K.J.; Fang, S. The HSP90 Inhibitor, 17-AAG, Influences the Activation and Proliferation of T Lymphocytes via AKT/GSK3β Signaling in MRL/lpr Mice. Drug Des. Dev. Ther. 2020, 14, 4605–4612. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, J.; Shin Ogawa, L.; Inoue, T.; Huang, Q.; Chu, J.; Bates, R.C.; Ying, W.; Sonderfan, A.J.; Rao, P.E.; et al. The HSP90 Inhibitor Ganetespib Alleviates Disease Progression and Augments Intermittent Cyclophosphamide Therapy in the MRL/lpr Mouse Model of Systemic Lupus Erythematosus. PLoS ONE 2015, 10, e0127361. [Google Scholar] [CrossRef] [Green Version]
- Shimp, S.K., 3rd; Chafin, C.B.; Regna, N.L.; Hammond, S.E.; Read, M.A.; Caudell, D.L.; Rylander, M.; Reilly, C.M. Heat shock protein 90 inhibition by 17-DMAG lessens disease in the MRL/lpr mouse model of systemic lupus erythematosus. Cell. Mol. Immunol. 2012, 9, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.; Shi, F.D.; Cohen, I.R.; Castaldo, G.; Matarese, G.; Quintana, F.J.; La Cava, A. DNA vaccine encoding heat shock protein 90 protects from murine lupus. Arthritis Res. Ther. 2020, 22, 152. [Google Scholar] [CrossRef]
- Liu, A.; Ferretti, C.; Shi, F.D.; Cohen, I.R.; Quintana, F.J.; La Cava, A. DNA Vaccination With Hsp70 Protects Against Systemic Lupus Erythematosus in (NZB × NZW)F1 Mice. Arthritis Rheumatol. 2020, 72, 997–1002. [Google Scholar] [CrossRef]
- Tomcik, M.; Zerr, P.; Pitkowski, J.; Palumbo-Zerr, K.; Avouac, J.; Distler, O.; Becvar, R.; Senolt, L.; Schett, G.; Distler, J.H. Heat shock protein 90 (Hsp90) inhibition targets canonical TGF-β signalling to prevent fibrosis. Ann. Rheum. Dis. 2014, 73, 1215–1222. [Google Scholar] [CrossRef] [Green Version]
- Kao, J.K.; Hsu, T.F.; Lee, M.S.; Su, T.C.; Lee, C.H.; Hsu, C.S.; Shieh, J.J.; Wang, J.Y.; Yang, R.C. Subcutaneous injection of recombinant heat shock protein 70 ameliorates atopic dermatitis skin lesions in a mouse model. Kaohsiung J. Med. Sci. 2020, 36, 186–195. [Google Scholar] [CrossRef]
- Schmidt, E.; Kasperkiewicz, M.; Joly, P. Pemphigus. Lancet 2019, 394, 882–894. [Google Scholar] [CrossRef]
- Bieber, K.; Ludwig, R.J. Drug Development in Pemphigoid Diseases. Acta Derm. Venereol. 2020, 100, adv00055. [Google Scholar] [CrossRef] [Green Version]
- Izumi, K.; Bieber, K.; Ludwig, R.J. Current Clinical Trials in Pemphigus and Pemphigoid. Front. Immunol. 2019, 10, 978. [Google Scholar] [CrossRef]
- Schmidt, E.; Zillikens, D. Pemphigoid diseases. Lancet 2013, 381, 320–332. [Google Scholar] [CrossRef]
- Tukaj, S.; Kleszczyński, K.; Vafia, K.; Groth, S.; Meyersburg, D.; Trzonkowski, P.; Ludwig, R.J.; Zillikens, D.; Schmidt, E.; Fischer, T.W.; et al. Aberrant expression and secretion of heat shock protein 90 in patients with bullous pemphigoid. PLoS ONE 2013, 8, e70496. [Google Scholar] [CrossRef]
- Tukaj, S.; Grüner, D.; Zillikens, D.; Kasperkiewicz, M. Hsp90 blockade modulates bullous pemphigoid IgG-induced IL-8 production by keratinocytes. Cell Stress Chaperones 2014, 19, 887–894. [Google Scholar] [CrossRef] [Green Version]
- Kasperkiewicz, M.; Sadik, C.D.; Bieber, K.; Ibrahim, S.M.; Manz, R.A.; Schmidt, E.; Zillikens, D.; Ludwig, R.J. Epidermolysis Bullosa Acquisita: From Pathophysiology to Novel Therapeutic Options. J. Investig. Dermatol. 2016, 136, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Tukaj, S.; Zillikens, D.; Kasperkiewicz, M. Inhibitory effects of heat shock protein 90 blockade on proinflammatory human Th1 and Th17 cell subpopulations. J. Inflamm. 2014, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- Tukaj, S.; Hellberg, L.; Ueck, C.; Hänsel, M.; Samavedam, U.; Zillikens, D.; Ludwig, R.J.; Laskay, T.; Kasperkiewicz, M. Heat shock protein 90 is required for ex vivo neutrophil-driven autoantibody-induced tissue damage in experimental epidermolysis bullosa acquisita. Exp. Dermatol. 2015, 24, 471–473. [Google Scholar] [CrossRef]
- Kárpáti, S. Dermatitis herpetiformis. Clin. Dermatol. 2012, 30, 56–59. [Google Scholar] [CrossRef]
- Kasperkiewicz, M.; Tukaj, S.; Gembicki, A.J.; Silló, P.; Görög, A.; Zillikens, D.; Kárpáti, S. Evidence for a role of autoantibodies to heat shock protein 60, 70, and 90 in patients with dermatitis herpetiformis. Cell Stress Chaperones 2014, 19, 837–843. [Google Scholar] [CrossRef] [Green Version]
- Schön, M.P. Adaptive and Innate Immunity in Psoriasis and Other Inflammatory Disorders. Front. Immunol. 2019, 10, 1764. [Google Scholar] [CrossRef] [Green Version]
- Boehncke, W.H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Kakeda, M.; Arock, M.; Schlapbach, C.; Yawalkar, N. Increased expression of heat shock protein 90 in keratinocytes and mast cells in patients with psoriasis. J. Am. Acad. Dermatol. 2014, 70, 683–690.e681. [Google Scholar] [CrossRef]
- Hansen, R.S.; Thuesen, K.K.H.; Bregnhøj, A.; Moldovan, L.I.; Kristensen, L.S.; Grek, C.L.; Ghatnekar, G.S.; Iversen, L.; Johansen, C. The HSP90 inhibitor RGRN-305 exhibits strong immunomodulatory effects in human keratinocytes. Exp. Dermatol. 2021, 30, 773–781. [Google Scholar] [CrossRef] [PubMed]
- van Eden, W. Vaccination against autoimmune diseases moves closer to the clinic. Hum. Vaccines Immunother. 2020, 16, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Karagaiah, P.; Valle, Y.; Sigova, J.; Zerbinati, N.; Vojvodic, P.; Parsad, D.; Schwartz, R.A.; Grabbe, S.; Goldust, M.; Lotti, T.; et al. Emerging drugs for the treatment of vitiligo. Expert Opin. Emerg. Drugs 2020, 25, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Kroll, T.M.; Bommiasamy, H.; Boissy, R.E.; Hernandez, C.; Nickoloff, B.J.; Mestril, R.; Caroline Le Poole, I. 4-Tertiary butyl phenol exposure sensitizes human melanocytes to dendritic cell-mediated killing: Relevance to vitiligo. J. Investig. Dermatol. 2005, 124, 798–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosenson, J.A.; Zloza, A.; Klarquist, J.; Barfuss, A.J.; Guevara-Patino, J.A.; Poole, I.C. HSP70i is a critical component of the immune response leading to vitiligo. Pigment Cell Melanoma Res. 2012, 25, 88–98. [Google Scholar] [CrossRef] [Green Version]
- Abdou, A.G.; Maraee, A.H.; Reyad, W. Immunohistochemical expression of heat shock protein 70 in vitiligo. Ann. Diagn. Pathol. 2013, 17, 245–249. [Google Scholar] [CrossRef]
- Doss, R.W.; El-Rifaie, A.A.; Abdel-Wahab, A.M.; Gohary, Y.M.; Rashed, L.A. Heat Shock Protein-70 Expression in Vitiligo and its Relation to the Disease Activity. Indian J. Dermatol. 2016, 61, 408–412. [Google Scholar] [CrossRef]
- Tian, J.; Wang, Y.; Ding, M.; Zhang, Y.; Chi, J.; Wang, T.; Jiao, B.; Jian, Z.; Yi, X.; Huang, Y.; et al. The Formation of Melanocyte Apoptotic Bodies in Vitiligo and the Relocation of Vitiligo Autoantigens under Oxidative Stress. Oxidative Med. Cell. Longev. 2021, 2021, 7617839. [Google Scholar] [CrossRef]
- Gilhar, A.; Laufer-Britva, R.; Keren, A.; Paus, R. Frontiers in alopecia areata pathobiology research. J. Allergy Clin. Immunol. 2019, 144, 1478–1489. [Google Scholar] [CrossRef] [Green Version]
- Thanomkitti, K.; Kanlaya, R.; Fong-Ngern, K.; Kapincharanon, C.; Sueksakit, K.; Chanchaem, P.; Thuangtong, R.; Thongboonkerd, V. Differential proteomics of lesional vs. non-lesional biopsies revealed non-immune mechanisms of alopecia areata. Sci. Rep. 2018, 8, 521. [Google Scholar] [CrossRef] [Green Version]
- Thanomkitti, K.; Fong-Ngern, K.; Sueksakit, K.; Thuangtong, R.; Thongboonkerd, V. Molecular functional analyses revealed essential roles of HSP90 and lamin A/C in growth, migration, and self-aggregation of dermal papilla cells. Cell Death Discov. 2018, 4, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wikramanayake, T.C.; Alvarez-Connelly, E.; Simon, J.; Mauro, L.M.; Guzman, J.; Elgart, G.; Schachner, L.A.; Chen, J.; Plano, L.R.; Jimenez, J.J. Heat treatment increases the incidence of alopecia areata in the C3H/HeJ mouse model. Cell Stress Chaperones 2010, 15, 985–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanouriakis, A.; Tziolos, N.; Bertsias, G.; Boumpas, D.T. Update οn the diagnosis and management of systemic lupus erythematosus. Ann. Rheum. Dis. 2021, 80, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Latchman, D.S.; Isenberg, D.A. The role of hsp90 in SLE. Autoimmunity 1994, 19, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Minota, S.; Koyasu, S.; Yahara, I.; Winfield, J. Autoantibodies to the heat-shock protein hsp90 in systemic lupus erythematosus. J. Clin. Investig. 1988, 81, 106–109. [Google Scholar] [CrossRef] [Green Version]
- Erkeller-Yüksel, F.M.; Isenberg, D.A.; Dhillon, V.B.; Latchman, D.S.; Lydyard, P.M. Surface expression of heat shock protein 90 by blood mononuclear cells from patients with systemic lupus erythematosus. J. Autoimmun. 1992, 5, 803–814. [Google Scholar] [CrossRef]
- Ripley, B.J.; Isenberg, D.A.; Latchman, D.S. Elevated levels of the 90 kDa heat shock protein (hsp90) in SLE correlate with levels of IL-6 and autoantibodies to hsp90. J. Autoimmun. 2001, 17, 341–346. [Google Scholar] [CrossRef]
- Zhang, M.; Gu, Y.; Huang, S.; Lou, Q.; Xie, Q.; Xu, Z.; Chen, Y.; Pan, F.; Xu, S.; Liu, S.; et al. Copy number variations and polymorphisms in HSP90AB1 and risk of systemic lupus erythematosus and efficacy of glucocorticoids. J. Cell. Mol. Med. 2019, 23, 5340–5348. [Google Scholar] [CrossRef]
- Saito, K.; Kukita, K.; Kutomi, G.; Okuya, K.; Asanuma, H.; Tabeya, T.; Naishiro, Y.; Yamamoto, M.; Takahashi, H.; Torigoe, T.; et al. Heat shock protein 90 associates with Toll-like receptors 7/9 and mediates self-nucleic acid recognition in SLE. Eur. J. Immunol. 2015, 45, 2028–2041. [Google Scholar] [CrossRef]
- Shukla, H.D.; Pitha, P.M. Role of hsp90 in systemic lupus erythematosus and its clinical relevance. Autoimmune Dis. 2012, 2012, 728605. [Google Scholar] [CrossRef]
- Xie, Q.M.; Lou, Q.Y.; Huang, S.W.; Hu, H.Q.; Li, S.S.; Zhang, M.; Sun, X.X.; Xu, J.H.; Jiang, S.Q.; Liu, S.X.; et al. Hsp70 Gene Polymorphisms Are Associated With Disease Susceptibility and HRQOL Improvement in Chinese Han Population With Systemic Lupus Erythematosus. J. Clin. Rheumatol. 2020, 26, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Fürnrohr, B.G.; Wach, S.; Kelly, J.A.; Haslbeck, M.; Weber, C.K.; Stach, C.M.; Hueber, A.J.; Graef, D.; Spriewald, B.M.; Manger, K.; et al. Polymorphisms in the Hsp70 gene locus are genetically associated with systemic lupus erythematosus. Ann. Rheum. Dis. 2010, 69, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Mišunová, M.; Svitálková, T.; Pleštilová, L.; Kryštufková, O.; Tegzová, D.; Svobodová, R.; Hušáková, M.; Tomčík, M.; Bečvář, R.; Závada, J.; et al. Molecular markers of systemic autoimmune disorders: The expression of MHC-located HSP70 genes is significantly associated with autoimmunity development. Clin. Exp. Rheumatol. 2017, 35, 33–42. [Google Scholar]
- Page, N.; Gros, F.; Schall, N.; Décossas, M.; Bagnard, D.; Briand, J.P.; Muller, S. HSC70 blockade by the therapeutic peptide P140 affects autophagic processes and endogenous MHCII presentation in murine lupus. Ann. Rheum. Dis. 2011, 70, 837–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef]
- RuizdelRio, J.; Muñoz, P.; Carreira, P.; Maestro, D.; Pablos, J.L.; Palanca, A.; Merino, J.; Serrano-Mollar, A.; Merino, R.; Tamayo, E.; et al. Profibrotic Role of Inducible Heat Shock Protein 90α Isoform in Systemic Sclerosis. J. Immunol. 2022, 209, 38–48. [Google Scholar] [CrossRef]
- Štorkánová, H.; Oreská, S.; Špiritović, M.; Heřmánková, B.; Bubová, K.; Komarc, M.; Pavelka, K.; Vencovský, J.; Distler, J.H.W.; Šenolt, L.; et al. Plasma Hsp90 levels in patients with systemic sclerosis and relation to lung and skin involvement: A cross-sectional and longitudinal study. Sci. Rep. 2021, 11, 1. [Google Scholar] [CrossRef]
- Ogawa, F.; Shimizu, K.; Hara, T.; Muroi, E.; Hasegawa, M.; Takehara, K.; Sato, S. Serum levels of heat shock protein 70, a biomarker of cellular stress, are elevated in patients with systemic sclerosis: Association with fibrosis and vascular damage. Clin. Exp. Rheumatol. 2008, 26, 659–662. [Google Scholar]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Roesner, L.M.; Werfel, T. Autoimmunity (or Not) in Atopic Dermatitis. Front. Immunol. 2019, 10, 2128. [Google Scholar] [CrossRef]
- Lu, Z.; Zeng, N.; Cheng, Y.; Chen, Y.; Li, Y.; Lu, Q.; Xia, Q.; Luo, D. Atopic dermatitis and risk of autoimmune diseases: A systematic review and meta-analysis. Allergy Asthma Clin. Immunol. 2021, 17, 96. [Google Scholar] [CrossRef] [PubMed]
- Sitko, K.; Bednarek, M.; Mantej, J.; Trzeciak, M.; Tukaj, S. Circulating heat shock protein 90 (Hsp90) and autoantibodies to Hsp90 are increased in patients with atopic dermatitis. Cell Stress Chaperones 2021, 26, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Ghoreishi, M.; Yokozeki, H.; Hua, W.M.; Nishioka, K. Expression of 27 KD, 65 KD and 72/73 KD heat shock protein in atopic dermatitis: Comparison with those in normal skin and contact dermatitis. J. Dermatol. 2000, 27, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.B.; Nakayama, H.; Shmyhlo, M.; Inoue, S.; Kondo, M.; Ikezawa, Z.; Ouchi, Y.; Cyong, J.C. High positive frequency of antibodies to metallothionein and heat shock protein 70 in sera of patients with metal allergy. Clin. Exp. Immunol. 2003, 131, 275–279. [Google Scholar] [CrossRef]
- van Eden, W.; Jansen, M.A.A.; Ludwig, I.S.; Leufkens, P.; van der Goes, M.C.; van Laar, J.M.; Broere, F. Heat Shock Proteins Can Be Surrogate Autoantigens for Induction of Antigen Specific Therapeutic Tolerance in Rheumatoid Arthritis. Front. Immunol. 2019, 10, 279. [Google Scholar] [CrossRef]
- van Eden, W.; Jansen, M.A.A.; Ludwig, I.; van Kooten, P.; van der Zee, R.; Broere, F. The Enigma of Heat Shock Proteins in Immune Tolerance. Front. Immunol. 2017, 8, 1599. [Google Scholar] [CrossRef] [PubMed]
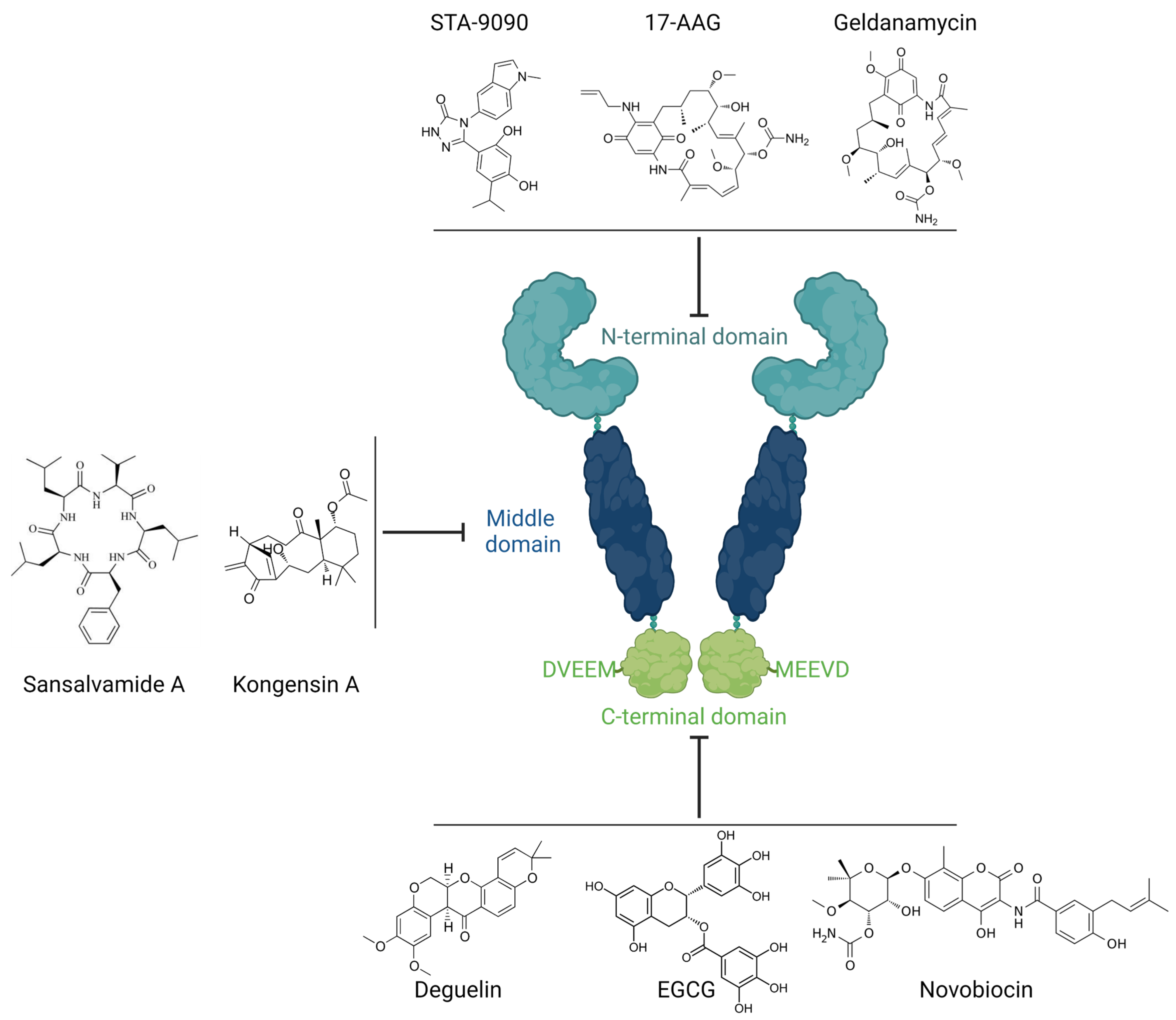
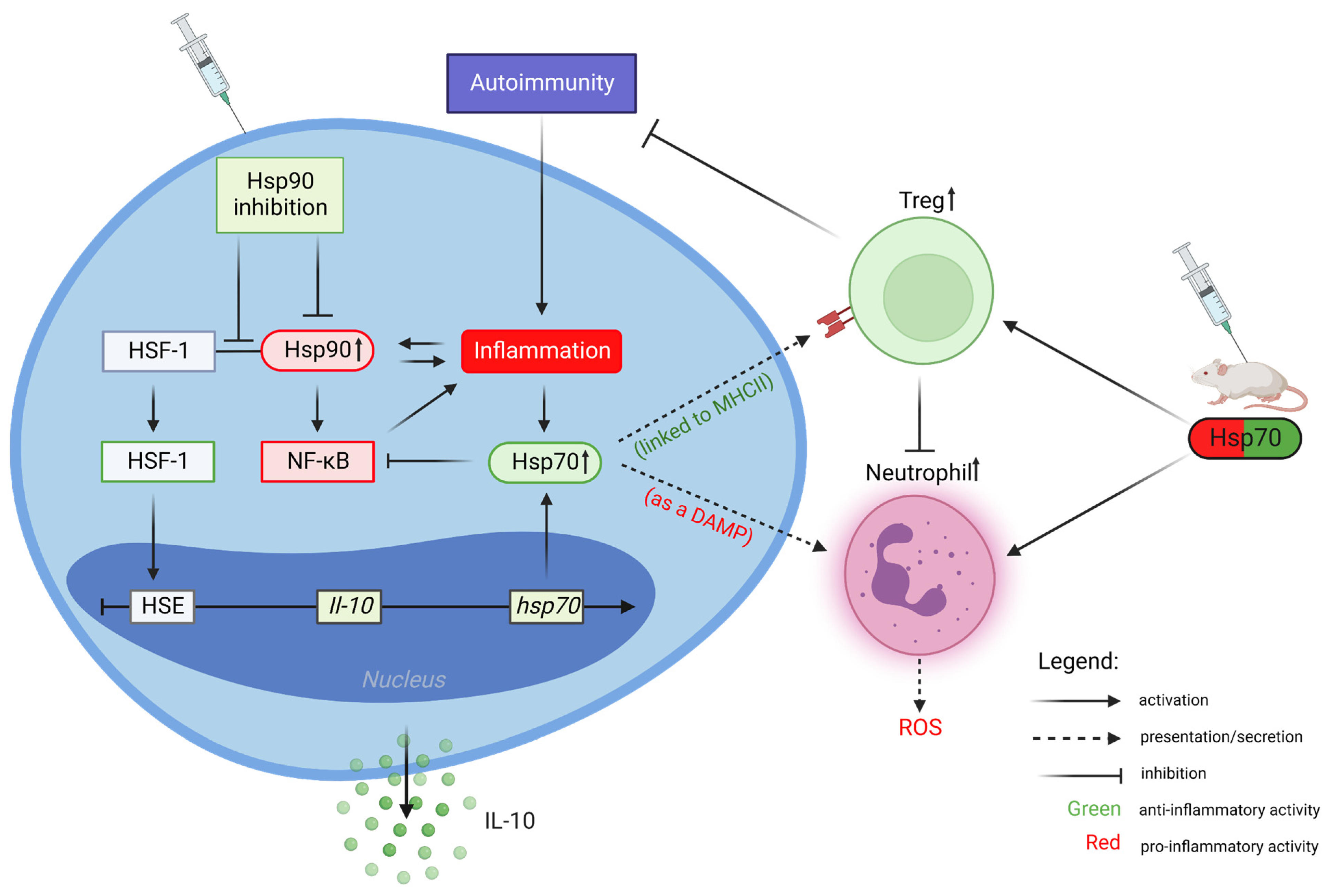
| Disease | Animal Model/ Clinical Observation | Target | Inhibitor | Outcome | Literature |
|---|---|---|---|---|---|
| Epidermolysis bullosa acquisita | COL7 or anti-COL7 IgG immunized mouse models | Hsp90 | TCBL-145 | Clinical and histological improvement | [48] |
| 17-DMAG | [48,49] | ||||
| 17-AAG | [50] | ||||
| Hsp70 | None | Hsp70- or anti-Hsp70 IgG-treated EBA mice had more intense disease activity | [51,52] | ||
| Psoriasis | Mouse xenograft transplantation model | Hsp90 | Debio 0932 | Clinical and histological improvement | [53] |
| Imiquimod-induced mouse model | Hsp70 | Myricetin Quercetin Ellagic acid | Clinical and histological improvement | [54] | |
| Hsp70 | None | Topically applied Hsp70 ameliorated disease activity | [55] | ||
| Hsp70 | None | Hsp70-based immunization ameliorated disease activity | [56] | ||
| Phase I–II evaluation of safety and efficacy | Hsp90 | Debio 0932 | Clinical improvement during unrelated (oncological diseases) clinical trial | [53] | |
| Phase Ib proof-of-concept study | Hsp90 | RGRN-305 | Clinical and serological improvement | [57] | |
| Vitiligo | Mouse model of autoimmune vitiligo | Hsp70 | None | Human- and mouse-derived inducible Hsp70-vaccinated mice displayedaccelerated depigmentation | [58] |
| Alopecia areata | C3H/HeJ spontaneous mouse model of AA | Hsp70 | Quercetin | Clinical and histological improvement | [59] |
| Systemic lupus erythematosus | MRL/lpr mouse model of SLE | Hsp90 | 17-AAG | Clinical and functional improvement | [60] |
| STA-9090 | [61] | ||||
| 17-DMAG | [62] | ||||
| (NZB × NZW)F1 mouse model of SLE | Hsp90 | None | Vaccination with DNA encoding Hsp90 protected from murine lupus | [63] | |
| Hsp70 | None | Vaccination with DNA encoding Hsp70 led to disease suppression | [64] | ||
| Systemic sclerosis | Mouse model of Ssc | Hsp90 | 17-DMAG | Histological and functional improvement | [65] |
| Atopic dermatitis | OVA-induced mouse model | Hsp70 | None | Subcutaneous administration of recombinant Hsp70 led to clinical, histological, and serological improvement | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tukaj, S.; Sitko, K. Heat Shock Protein 90 (Hsp90) and Hsp70 as Potential Therapeutic Targets in Autoimmune Skin Diseases. Biomolecules 2022, 12, 1153. https://doi.org/10.3390/biom12081153
Tukaj S, Sitko K. Heat Shock Protein 90 (Hsp90) and Hsp70 as Potential Therapeutic Targets in Autoimmune Skin Diseases. Biomolecules. 2022; 12(8):1153. https://doi.org/10.3390/biom12081153
Chicago/Turabian StyleTukaj, Stefan, and Krzysztof Sitko. 2022. "Heat Shock Protein 90 (Hsp90) and Hsp70 as Potential Therapeutic Targets in Autoimmune Skin Diseases" Biomolecules 12, no. 8: 1153. https://doi.org/10.3390/biom12081153
APA StyleTukaj, S., & Sitko, K. (2022). Heat Shock Protein 90 (Hsp90) and Hsp70 as Potential Therapeutic Targets in Autoimmune Skin Diseases. Biomolecules, 12(8), 1153. https://doi.org/10.3390/biom12081153






