A Novel Method for Identifying the Transition Zone in Long-Segment Hirschsprung Disease: Investigating the Muscle Unit to Ganglion Ratio
Abstract
:1. Introduction
2. Materials and Methods
2.1. Enrolment of Patients and Surgical Procedure
2.2. Tissue Processing and Immunohistochemistry
2.3. Image Analysis and Quantification
2.4. Statistical Analysis
3. Results
3.1. Patients Experienced Post-Operative Enterocolitis despite Intraoperative Histological Assessment of the Proximal “Doughnut” Biopsy
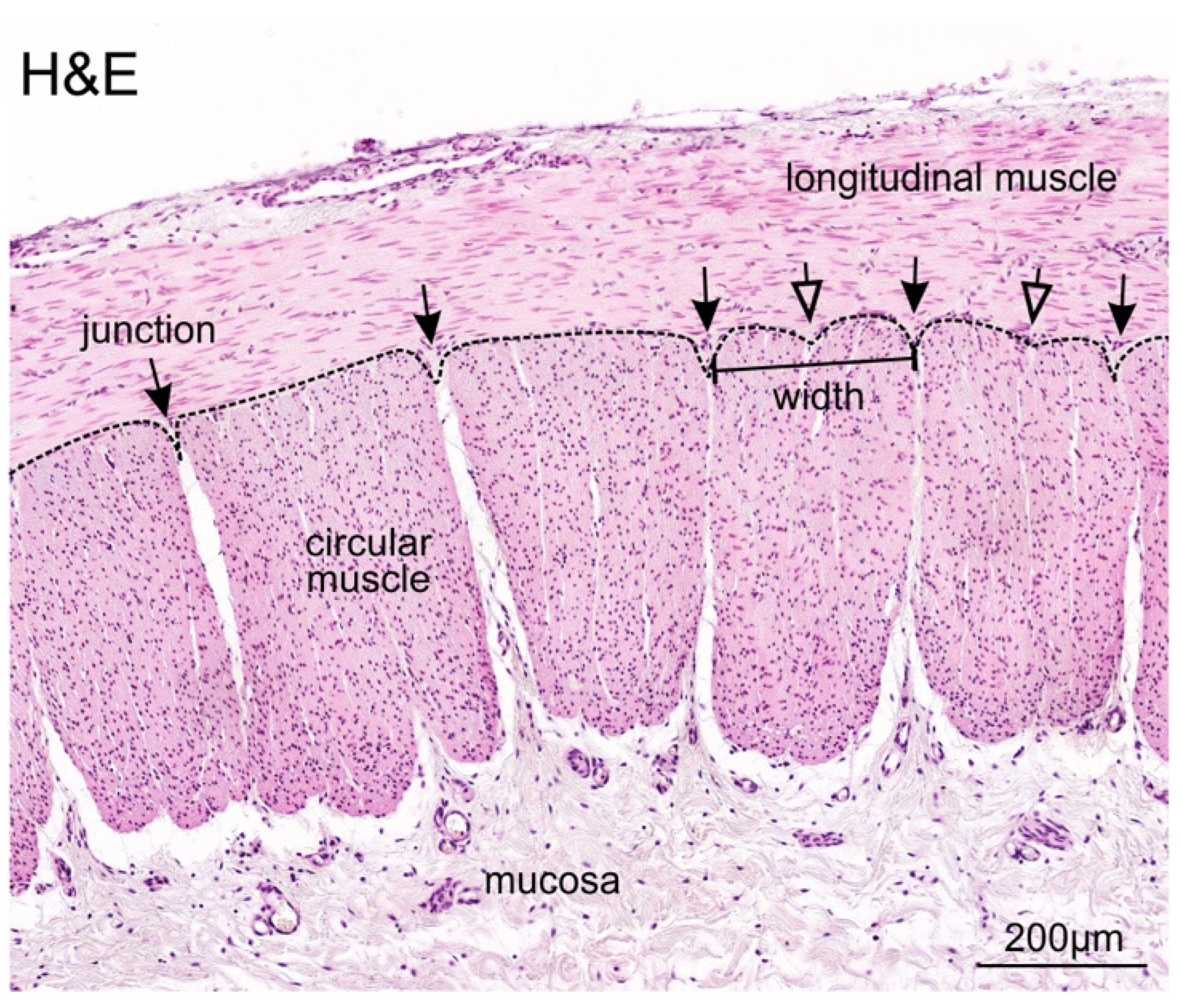
3.2. Identification and Definition of Individual Circular Muscle Units
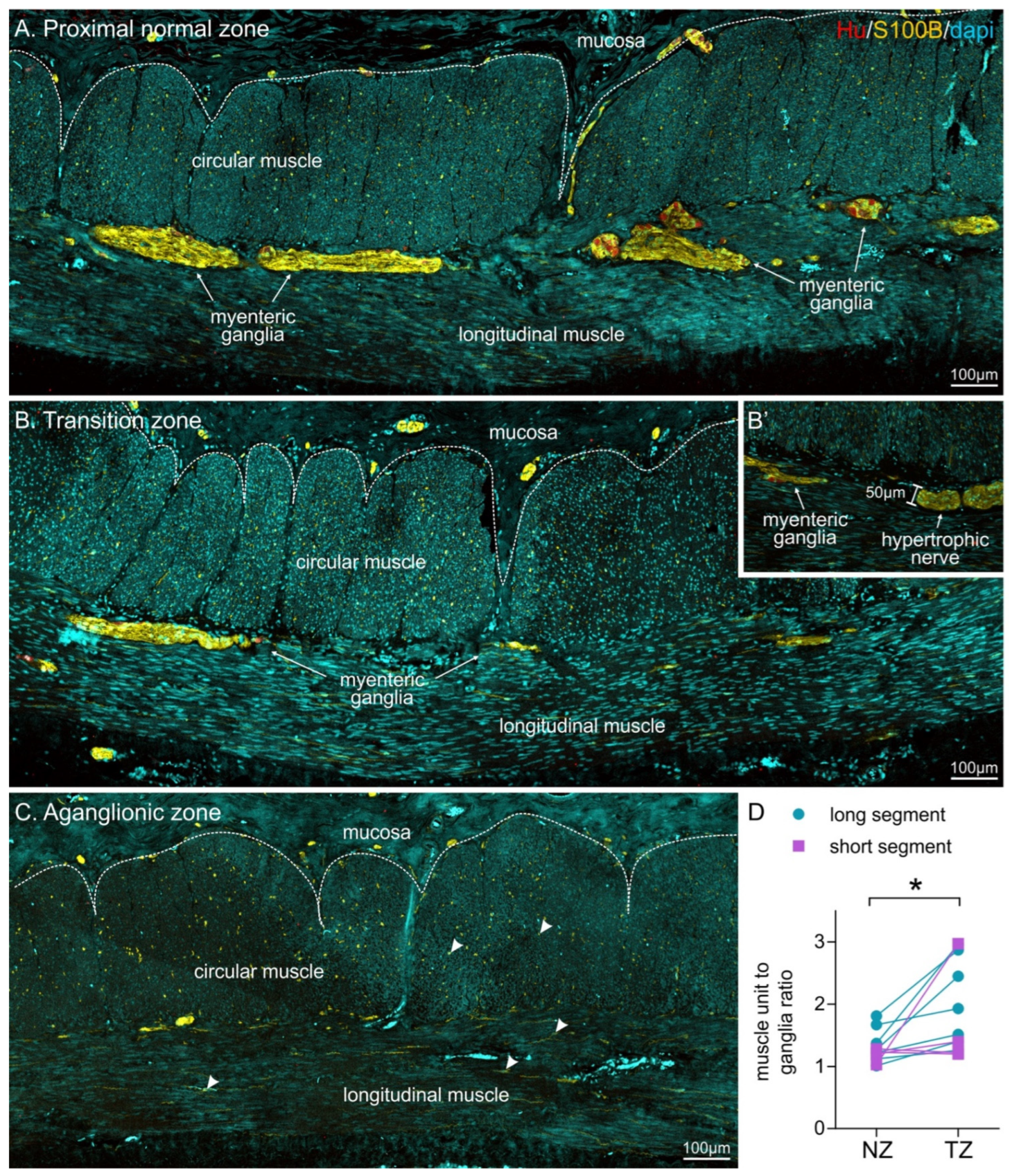
3.3. Muscle Unit to Ganglion Ratio Was Higher in the Transition Zone Compared to Proximal Colon
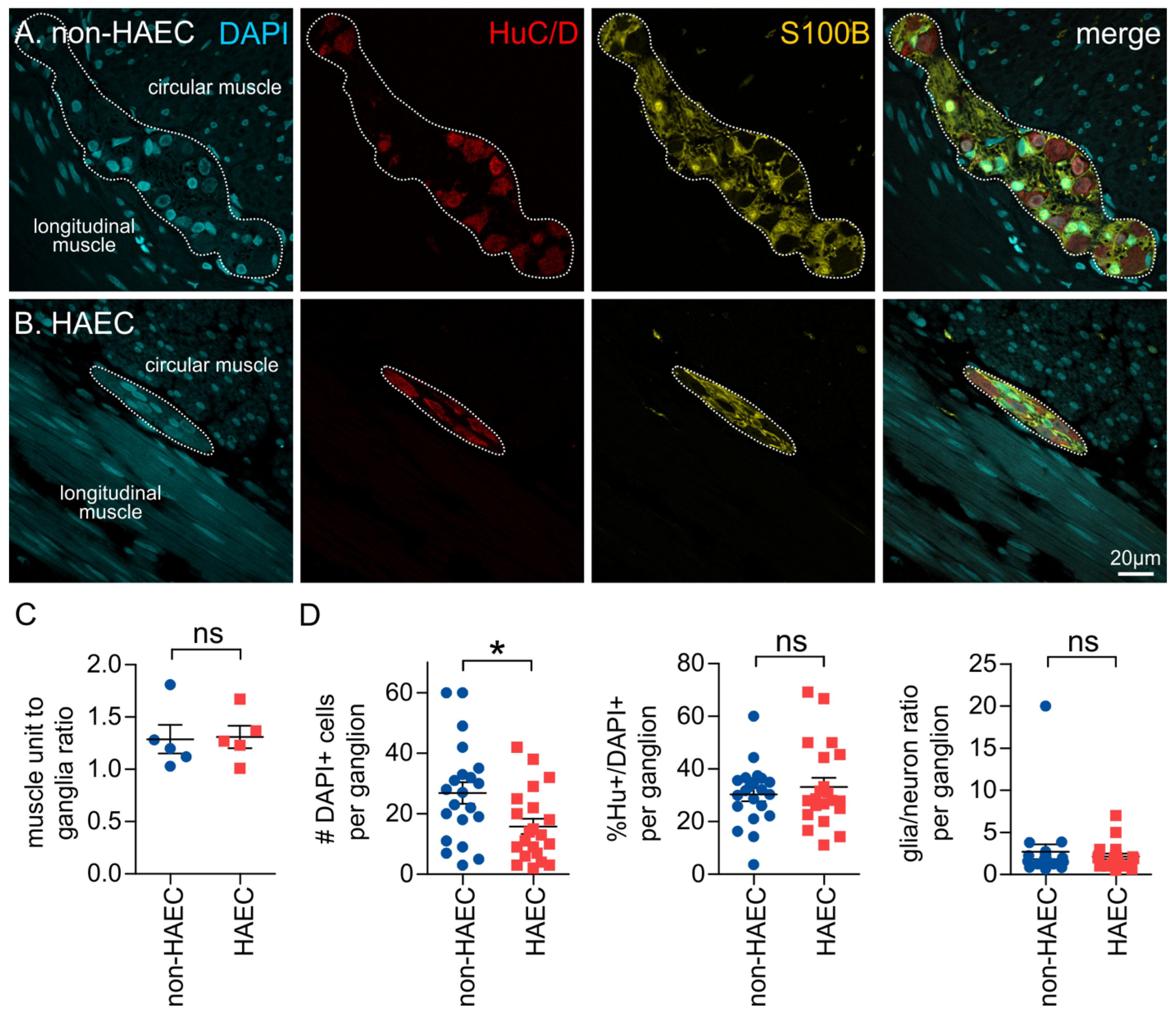
3.4. Ganglia Composition Is Unchanged in the Transition Zone
3.5. Patients That Develop Enterocolitis Have Reduced Enteric Ganglion Size
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Seguella, L.; Gulbransen, B.D. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 571–587. [Google Scholar] [CrossRef]
- Wallace, A.S.; Burns, A.J. Development of the enteric nervous system, smooth muscle and interstitial cells of Cajal in the human gastrointestinal tract. Cell Tissue Res. 2005, 319, 367–382. [Google Scholar] [CrossRef] [PubMed]
- McCann, C.J.; Alves, M.M.; Brosens, E.; Natarajan, D.; Perin, S.; Chapman, C.; Hofstra, R.M.; Burns, A.J.; Thapar, N. Neuronal Development and Onset of Electrical Activity in the Human Enteric Nervous System. Gastroenterology 2019, 156, 1483–1495.e1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, A.J.; Champeval, D.; Le Douarin, N.M. Sacral Neural Crest Cells Colonise Aganglionic Hindgut in Vivo but Fail to Compensate for Lack of Enteric Ganglia. Dev. Biol. 2000, 219, 30–43. [Google Scholar] [CrossRef] [Green Version]
- Uesaka, T.; Nagashimada, M.; Enomoto, H. Neuronal Differentiation in Schwann Cell Lineage Underlies Postnatal Neurogenesis in the Enteric Nervous System. J. Neurosci. 2015, 35, 9879–9888. [Google Scholar] [CrossRef] [Green Version]
- Heuckeroth, R.O. Hirschsprung disease-integrating basic science and clinical medicine to improve outcomes. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 152–167. [Google Scholar] [CrossRef]
- Alnajar, H.; Murro, D.; Alsadi, A.; Jakate, S. Spectrum of Clinicopathological Deviations in Long-Segment Hirschsprung Disease Compared with Short-Segment Hirschsprung Disease: A Single-Institution Study. Int. J. Surg. Pathol. 2017, 25, 216–221. [Google Scholar] [CrossRef]
- Wang, X.J.; Camilleri, M. Hirschsprung disease: Insights on genes, penetrance, and prenatal diagnosis. Neurogastroenterol. Motil. 2019, 31, e13732. [Google Scholar] [CrossRef]
- Kawaguchi, A.L.; Guner, Y.S.; Sømme, S.; Quesenberry, A.C.; Arthur, L.G.; Sola, J.E.; Downard, C.D.; Rentea, R.M.; Valusek, P.A.; Smith, C.A.; et al. Management and outcomes for long-segment Hirschsprung disease: A systematic review from the APSA Outcomes and Evidence Based Practice Committee. J. Pediatr. Surg. 2021, 56, 1513–1523. [Google Scholar] [CrossRef]
- Moore, S.W. Total colonic aganglionosis and Hirschsprung’s disease: A review. Pediatr. Surg. Int. 2015, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Henderson, D.; Puri, P. A meta-analysis of clinical outcome of intestinal transplantation in patients with total intestinal aganglionosis. Pediatr. Surg. Int. 2017, 33, 837–841. [Google Scholar] [CrossRef]
- Jarvi, K.; Laitakari, E.M.; Koivusalo, A.; Rintala, R.J.; Pakarinen, M.P. Bowel function and gastrointestinal quality of life among adults operated for Hirschsprung disease during childhood: A population-based study. Ann. Surg. 2010, 252, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, J.; Tomuschat, C.; Puri, P. Long-term results of transanal pull-through for Hirschsprung’s disease: A meta-analysis. Pediatr. Surg. Int. 2016, 32, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Neuvonen, M.I.; Kyrklund, K.; Rintala, R.J.; Pakarinen, M.P. Bowel Function and Quality of Life After Transanal Endorectal Pull-through for Hirschsprung Disease: Controlled Outcomes up to Adulthood. Ann. Surg. 2017, 265, 622–629. [Google Scholar] [CrossRef]
- Elsherbeny, M.; Abdelhay, S. Obstructive complications after pull-through for Hirschsprung’s disease: Different causes and tailored management. Ann. Pediatr. Surg. 2019, 15, 2. [Google Scholar] [CrossRef] [Green Version]
- Gosain, A. Established and emerging concepts in Hirschsprung’s-associated enterocolitis. Pediatr. Surg. Int. 2016, 32, 313–320. [Google Scholar] [CrossRef]
- Frykman, P.K.; Nordenskjöld, A.; Kawaguchi, A.; Hui, T.T.; Granström, A.L.; Cheng, Z.; Tang, J.; Underhill, D.M.; Iliev, I.; Funari, V.A.; et al. Characterization of Bacterial and Fungal Microbiome in Children with Hirschsprung Disease with and without a History of Enterocolitis: A Multicenter Study. PLoS ONE 2015, 10, e0124172. [Google Scholar] [CrossRef] [Green Version]
- Demehri, F.R.; Frykman, P.K.; Cheng, Z.; Ruan, C.; Wester, T.; Nordenskjöld, A.; Kawaguchi, A.; Hui, T.T.; Granström, A.L.; Funari, V.; et al. Altered fecal short chain fatty acid composition in children with a history of Hirschsprung-associated enterocolitis. J. Pediatr. Surg. 2016, 51, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Tomuschat, C.; Virbel, C.R.; O’Donnell, A.M.; Puri, P. Reduced expression of the NLRP6 inflammasome in the colon of patients with Hirschsprung’s disease. J. Pediatr. Surg. 2019, 54, 1573–1577. [Google Scholar] [CrossRef]
- Keck, S.; Galati-Fournier, V.; Kym, U.; Moesch, M.; Usemann, J.; Müller, I.; Subotic, U.; Tharakan, S.J.; Krebs, T.; Stathopoulos, E.; et al. Lack of Mucosal Cholinergic Innervation Is Associated with Increased Risk of Enterocolitis in Hirschsprung’s Disease. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 507–545. [Google Scholar] [CrossRef] [PubMed]
- Coyle, D.; Murphy, J.M.; Doyle, B.; O’Donnell, A.M.; Gillick, J.; Puri, P. Altered tryptophan hydroxylase 2 expression in enteric serotonergic nerves in Hirschsprung’s-associated enterocolitis. World J. Gastroenterol. 2016, 22, 4662–4672. [Google Scholar] [CrossRef] [PubMed]
- Ambartsumyan, L.; Smith, C.; Kapur, R.P. Diagnosis of Hirschsprung Disease. Pediatr. Dev. Pathol. 2019, 23, 8–22. [Google Scholar] [CrossRef]
- Kapur, R.P. Practical pathology and genetics of Hirschsprung’s disease. Semin. Pediatr. Surg. 2009, 18, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, H.S.; Blackburn, S.; Curry, J.; De Coppi, P.; Giuliani, S.; Sebire, N.; Cross, K. Variability of the transition zone length in Hirschsprung disease. J. Pediatr. Surg. 2020, 55, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Friedmacher, F.; Puri, P. Residual aganglionosis after pull-through operation for Hirschsprung’s disease: A systematic review and meta-analysis. Pediatr. Surg. Int. 2011, 27, 1053–1057. [Google Scholar] [CrossRef]
- Ghose, S.I.; Squire, B.R.; Stringer, M.D.; Batcup, G.; Crabbe, D.C. Hirschsprung’s disease: Problems with transition-zone pull-through. J. Pediatr. Surg. 2000, 35, 1805–1809. [Google Scholar] [CrossRef]
- Kapur, R.P.; Kennedy, A.J. Transitional zone pull through: Surgical pathology considerations. Semin. Pediatr. Surg. 2012, 21, 291–301. [Google Scholar] [CrossRef]
- Kapur, R.P. Histology of the Transition Zone in Hirschsprung Disease. Am. J. Surg. Pathol. 2016, 40, 1637–1646. [Google Scholar] [CrossRef]
- Swaminathan, M.; Kapur, R.P. Counting myenteric ganglion cells in histologic sections: An empirical approach. Hum. Pathol. 2010, 41, 1097–1108. [Google Scholar] [CrossRef]
- Knowles, C.H.; Veress, B.; Kapur, R.P.; Wedel, T.; Farrugia, G.; Vanderwinden, J.M.; Geboes, K.; Smith, V.V.; Martin, J.E.; Lindberg, G.; et al. Quantitation of cellular components of the enteric nervous system in the normal human gastrointestinal tract--report on behalf of the Gastro 2009 International Working Group. Neurogastroenterol. Motil. 2011, 23, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Hoff, S.; Zeller, F.; von Weyhern, C.W.; Wegner, M.; Schemann, M.; Michel, K.; Rühl, A. Quantitative assessment of glial cells in the human and guinea pig enteric nervous system with an anti-Sox8/9/10 antibody. J. Comp. Neurol. 2008, 509, 356–371. [Google Scholar] [CrossRef]
- Ippolito, C.; Segnani, C.; De Giorgio, R.; Blandizzi, C.; Mattii, L.; Castagna, M.; Moscato, S.; Dolfi, A.; Bernardini, N. Quantitative evaluation of myenteric ganglion cells in normal human left colon: Implications for histopathological analysis. Cell Tissue Res. 2009, 336, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Veras, L.V.; Arnold, M.; Avansino, J.R.; Bove, K.; Cowles, R.A.; Durham, M.M.; Goldstein, A.M.; Krishnan, C.; Langer, J.C.; Levitt, M.; et al. Guidelines for synoptic reporting of surgery and pathology in Hirschsprung disease. J. Pediatr. Surg. 2019, 54, 2017–2023. [Google Scholar] [CrossRef]
- Graham, K.D.; Lopez, S.H.; Sengupta, R.; Shenoy, A.; Schneider, S.; Wright, C.M.; Feldman, M.; Furth, E.; Valdivieso, F.; Lemke, A.; et al. Robust, 3-Dimensional Visualization of Human Colon Enteric Nervous System without Tissue Sectioning. Gastroenterology 2020, 26, 26. [Google Scholar] [CrossRef]
- Altman, J.S. Toluidine Blue as a Rapid Stain for Nerve Cell Bodies in Intact Ganglia. In Neuroanatomical Techniques: Insect Nervous System; Strausfeld, N.J., Miller, T.A., Eds.; Springer: New York, NY, USA, 1980; pp. 21–24. [Google Scholar] [CrossRef]
- Moolenbeek, C.; Ruitenberg, E.J. The “Swiss roll”: A simple technique for histological studies of the rodent intestine. Lab. Anim. 1981, 15, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, H.; Badhe, B.A.; Toi, P.C.; Sambandan, K. Morphometric profile of large intestinal neuronal plexuses in normal perinatal autopsies and Hirschsprung disease. Neurogastroenterol. Motil. 2017, 29, e12939. [Google Scholar] [CrossRef] [PubMed]
- White, F.V.; Langer, J.C. Circumferential distribution of ganglion cells in the transition zone of children with Hirschsprung disease. Pediatr. Dev. Pathol. 2000, 3, 216–222. [Google Scholar] [CrossRef]
- Coyle, D.; O’Donnell, A.M.; Tomuschat, C.; Gillick, J.; Puri, P. The Extent of the Transition Zone in Hirschsprung Disease. J. Pediatr. Surg. 2019, 54, 2318–2324. [Google Scholar] [CrossRef]
- Gunadi; Ryantono, F.; Sethi, R.; Marcellus; Kalim, A.S.; Imelda, P.; Melati, D.; Simanjaya, S.; Widitjiarso, W.; Pitaka, R.T.; et al. Effect of semaphorin 3C gene variants in multifactorial Hirschsprung disease. J. Int. Med. Res. 2021, 49, 300060520987789. [Google Scholar] [CrossRef]
- Yang, W.; Chen, S.-C.; Lai, J.-Y.; Ming, Y.-C.; Chen, J.-C.; Chen, P.-L. Distinctive genetic variation of long-segment Hirschsprung’s disease in Taiwan. Neurogastroenterol. Motil. 2019, 31, e13665. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.; Tang, C.S.-M.; Tam, P.K.-H. The Emerging Genetic Landscape of Hirschsprung Disease and Its Potential Clinical Applications. Front. Pediatr. 2021, 9, 638093. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, D.M.; Friedmacher, F.; Puri, P. Total colonic aganglionosis: A systematic review and meta-analysis of long-term clinical outcome. Pediatr. Surg. Int. 2012, 28, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Thomson, D.; Allin, B.; Long, A.M.; Bradnock, T.; Walker, G.; Knight, M. Laparoscopic assistance for primary transanal pull-through in Hirschsprung’s disease: A systematic review and meta-analysis. BMJ Open 2015, 5, e006063. [Google Scholar] [CrossRef]
- Tomuschat, C.; Zimmer, J.; Puri, P. Laparoscopic-assisted pull-through operation for Hirschsprung’s disease: A systematic review and meta-analysis. Pediatr. Surg. Int. 2016, 32, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Müller, I.; Kym, U.; Galati, V.; Tharakan, S.; Subotic, U.; Krebs, T.; Stathopoulos, E.; Schmittenbecher, P.; Cholewa, D.; Romero, P.; et al. Cholinergic Signaling Attenuates Pro-Inflammatory Interleukin-8 Response in Colonic Epithelial Cells. Front. Immunol. 2021, 12, 781147. [Google Scholar] [CrossRef]
- Wang, H.; Foong, J.P.P.; Harris, N.L.; Bornstein, J.C. Enteric neuroimmune interactions coordinate intestinal responses in health and disease. Mucosal. Immunol. 2022, 15, 27–39. [Google Scholar] [CrossRef]
- Populin, L.; Stebbing, M.J.; Furness, J.B. Neuronal regulation of the gut immune system and neuromodulation for treating inflammatory bowel disease. FASEB Bioadv. 2021, 3, 953–966. [Google Scholar] [CrossRef]
- Gershon, M.D.; Margolis, K.G. The gut, its microbiome, and the brain: Connections and communications. J. Clin. Investig. 2021, 131, e143768. [Google Scholar] [CrossRef]
- Udit, S.; Blake, K.; Chiu, I.M. Somatosensory and autonomic neuronal regulation of the immune response. Nat. Rev. Neurosci. 2022, 23, 157–171. [Google Scholar] [CrossRef]
- Muller, P.A.; Koscso, B.; Rajani, G.M.; Stevanovic, K.; Berres, M.-L.; Hashimoto, D.; Mortha, A.; Leboeuf, M.; Li, X.-M.; Mucida, D.; et al. Crosstalk between Muscularis Macrophages and Enteric Neurons Regulates Gastrointestinal Motility. Cell 2014, 158, 300–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grubišić, V.; McClain, J.L.; Fried, D.E.; Grants, I.; Rajasekhar, P.; Csizmadia, E.; Ajijola, O.A.; Watson, R.E.; Poole, D.P.; Robson, S.C.; et al. Enteric Glia Modulate Macrophage Phenotype and Visceral Sensitivity following Inflammation. Cell Rep. 2020, 32, 108100. [Google Scholar] [CrossRef] [PubMed]
- Progatzky, F.; Shapiro, M.; Chng, S.H.; Garcia-Cassani, B.; Classon, C.H.; Sevgi, S.; Laddach, A.; Bon-Frauches, A.C.; Lasrado, R.; Rahim, M.; et al. Regulation of intestinal immunity and tissue repair by enteric glia. Nature 2021, 599, 125–130. [Google Scholar] [CrossRef] [PubMed]

| Patient | Sex | Age at Operation (months) | Phenotype | Procedure(s) | Follow-Up Duration (Months from Date of Last Procedure) | Complication |
|---|---|---|---|---|---|---|
| HSCR | ||||||
| Patient 1 | Female | 6 | Long—proximal transverse colon | 3: Ileostomy + Swenson + stoma closure | 17 | none |
| Patient 2 * | Male | 6 | Long—proximal descending colon | 3: Ileostomy + Swenson + stoma closure | 11 | none |
| Patient 3 | Male | 7 | Long—distal transverse colon | 3: Ileostomy + Swenson + stoma closure | 11 | Three episodes of enterocolitis |
| Patient 4 | Male | 4 | Short | 3: Ileostomy + Swenson + stoma closure | 12 | none |
| Patient 5 | Male | 5 | Long—distal descending colon | 1: Swenson (no stoma) | 15 | Three episodes of enterocolitis |
| Patient 6 | Male | 3 | Short | 1: Swenson (no stoma) | 10 | none |
| Patient 7 | Male | 21 | Short | 3: Ileostomy + Swenson + stoma closure | 2 | none |
| Patient 8 | Male | 4 | Short—rectal sigmoid (skip) | 2: Colostomy + Swenson | 7 | Several episodes of enterocolitis |
| Patient 9 | Male | 6 | Long | 1: Swenson (no stoma) | 6 | Three episodes of enterocolitis |
| Patient 10 | Male | 3 | Short | 1: Swenson (no stoma) | 6 | Constipation |
| Patient 11 | Female | 27 | Long—transverse colon | 2: Colostomy + Swenson (ileostomy formed following pull-through) | - | Stoma not yet closed |
| Patient 12 | Female | 5 | Long—descending colon | 1: Swenson (no stoma) | 13 | One episode of enterocolitis |
| Patient 13 # | Male | 27 | Total colonic aganglionosis | 2: Ileostomy + Duhamel | - | Stoma not yet closed |
| Control | ||||||
| Patient 1 | Male | 36 | Neonatal necrotizing enterocolitis | 2: Ileostomy + stoma closure with resection of strictured bowel | 7 | N/A (no follow-up) |
| HSCR Type | Proximal/Normal | Transition Zone | Statistical Analysis |
|---|---|---|---|
| All patients (pooled) | 1.29 ± 0.07 | 1.93 ± 0.22 | * p = 0.012; n = 11 |
| Long-segment | 1.35 ± 0.1 | 2.06 ± 0.3 | * p = 0.017; n = 7 |
| Short-segment | 1.19 ± 0.05 | 1.70 ± 0.43 | ns: p = 0.36; n = 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Pham, J.; King, S.K.; Newgreen, D.F.; Young, H.M.; Stamp, L.A.; Hao, M.M. A Novel Method for Identifying the Transition Zone in Long-Segment Hirschsprung Disease: Investigating the Muscle Unit to Ganglion Ratio. Biomolecules 2022, 12, 1101. https://doi.org/10.3390/biom12081101
Yang W, Pham J, King SK, Newgreen DF, Young HM, Stamp LA, Hao MM. A Novel Method for Identifying the Transition Zone in Long-Segment Hirschsprung Disease: Investigating the Muscle Unit to Ganglion Ratio. Biomolecules. 2022; 12(8):1101. https://doi.org/10.3390/biom12081101
Chicago/Turabian StyleYang, Wendy, Jenny Pham, Sebastian K. King, Donald F. Newgreen, Heather M. Young, Lincon A. Stamp, and Marlene M. Hao. 2022. "A Novel Method for Identifying the Transition Zone in Long-Segment Hirschsprung Disease: Investigating the Muscle Unit to Ganglion Ratio" Biomolecules 12, no. 8: 1101. https://doi.org/10.3390/biom12081101
APA StyleYang, W., Pham, J., King, S. K., Newgreen, D. F., Young, H. M., Stamp, L. A., & Hao, M. M. (2022). A Novel Method for Identifying the Transition Zone in Long-Segment Hirschsprung Disease: Investigating the Muscle Unit to Ganglion Ratio. Biomolecules, 12(8), 1101. https://doi.org/10.3390/biom12081101






