Recent Progress in Second Near-Infrared (NIR-II) Fluorescence Imaging in Cancer
Abstract
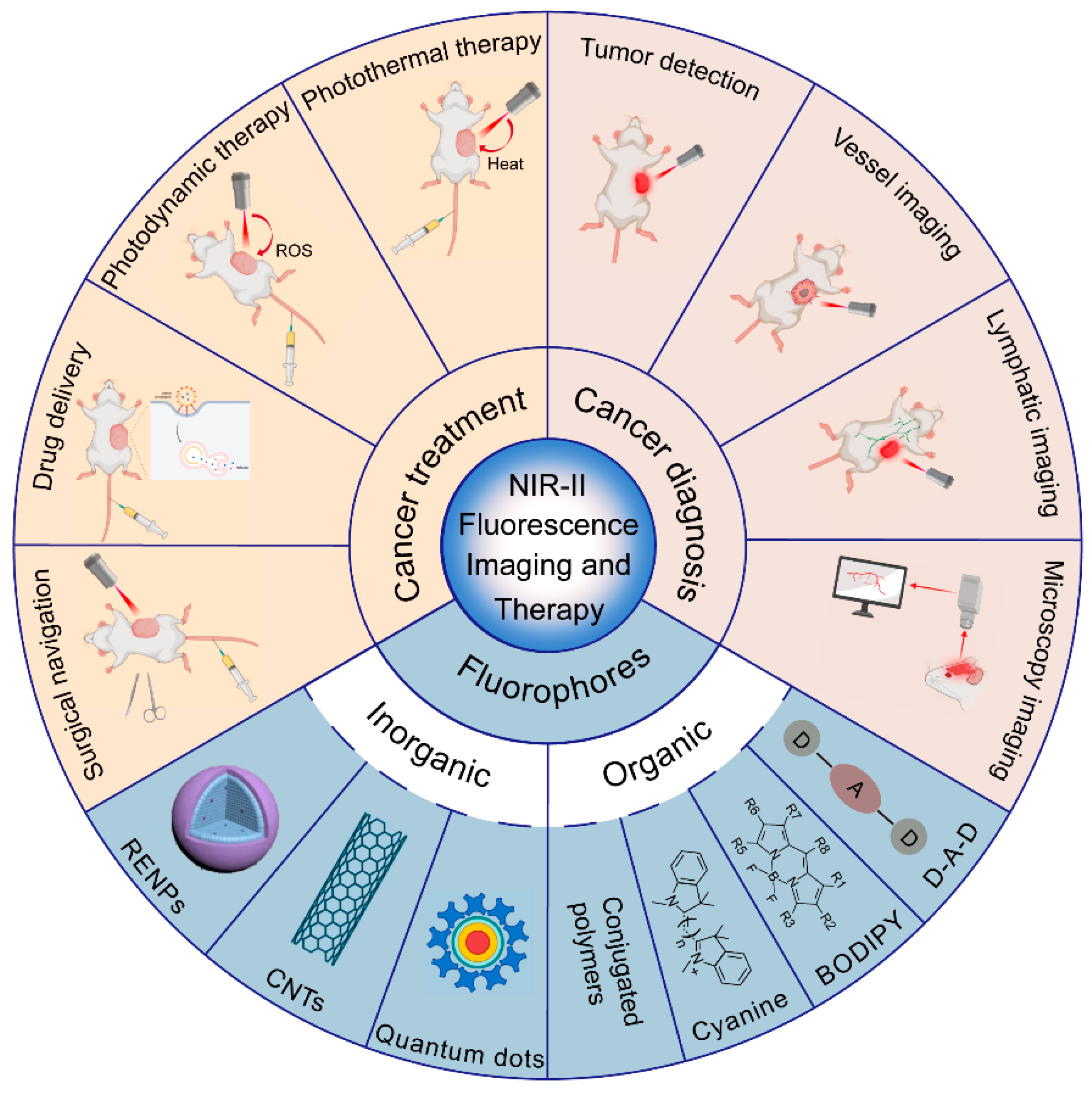
:1. Introduction
2. NIR-II Fluorescent Probes
3. NIR-II Fluorescence and Cancer Diagnosis
3.1. Tumor Detection
3.2. Tumor Vessel Imaging
3.3. Tumor Lymphatic Imaging
3.4. Microscopy Imaging and Optical Detection
4. NIR-II Fluorescence and Cancer Treatment
4.1. NIR-II Fluorescence Imaging-Guided Photothermal Therapy (PTT)
4.2. NIR-II Fluorescence Imaging-Guided Photodynamic Therapy (PDT)
4.3. NIR-II Fluorescence Imaging-Guided Drug Delivery and Chemotherapy
4.4. NIR-II Fluorescence Imaging-Guided Surgical Navigation
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, X.; Ye, H.; Yan, W.; Sun, Y. Factors Associated With Patient’s Refusal of Recommended Cancer Surgery: Based on Surveillance, Epidemiology, and End Results. Front. Public Health 2021, 9, 785602. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekhara, S.H.; Triveni, G.S.; Kumar, R. Imaging of peritoneal deposits in ovarian cancer: A pictorial review. World J. Radiol. 2016, 8, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wu, Z.; Wu, X.; Shan, F.; Zhang, Y.; Ying, X.; Li, Z.; Ji, J. Early diagnosis of anastomotic leakage after colorectal cancer surgery using an inflammatory factors-based score system. BJS Open 2022, 6, zrac069. [Google Scholar] [CrossRef] [PubMed]
- Vandana, J.J.; Lacko, L.A.; Chen, S. Expanding the precision oncology toolkit with micro-organospheres for early cancer diagnosis. Cell. Stem Cell. 2022, 29, 873–875. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Kawai, A.; Wakisaka, M.; Shimoyama, M.; Yasuda, N.; Kin, T.; Arihiro, K. Breast cancer recurrence and survival rates in patients who underwent breast-conserving surgery under non-mechanically ventilated anesthesia. Cancer Rep. 2022, e1643. [Google Scholar] [CrossRef]
- Lee, C.C.; Soon, Y.Y.; Vellayappan, B.; Ho, F.; Tey, J.C.S. Survival rates and safety associated with chemoradiotherapy followed by surgery and chemoradiotherapy alone for patients with T4 esophageal cancer: A systematic review and meta-analysis. Acta Oncol. 2022, 61, 738–748. [Google Scholar] [CrossRef]
- Li, Q.; Feng, Z.; Miao, R.; Liu, X.; Liu, C.; Liu, Z. Prognosis and survival analysis of patients with pancreatic cancer: Retrospective experience of a single institution. World J. Surg. Oncol. 2022, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, Y.; Gao, R.; Liu, X.; Wang, Y.; Yu, J.; Zhan, J.; Huang, Y.; Qin, H.; Zhang, L. Prognostic effects of health-related quality of life at baseline and early change in health-related quality of life on response to treatment and survival in patients with advanced lung cancer: A prospective observational study in China. BMJ Open 2022, 12, e047611. [Google Scholar] [CrossRef]
- Zhang, R.R.; Schroeder, A.B.; Grudzinski, J.J.; Rosenthal, E.L.; Warram, J.M.; Pinchuk, A.N.; Eliceiri, K.W.; Kuo, J.S.; Weichert, J.P. Beyond the margins: Real-time detection of cancer using targeted fluorophores. Nat. Rev. Clin. Oncol. 2017, 14, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Vahrmeijer, A.L.; Hutteman, M.; Van Der Vorst, J.R.; Van De Velde, C.J.; Frangioni, J.V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 2013, 10, 507–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thangudu, S.; Huang, E.-Y.; Su, C.-H. Safe magnetic resonance imaging on biocompatible nanoformulations. Biomater. Sci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Pittet, M.J. Imaging in the era of molecular oncology. Nature 2008, 452, 580–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenry; Duan, Y.; Liu, B. Recent Advances of Optical Imaging in the Second Near-Infrared Window. Adv. Mater. 2018, 30, e1802394. [Google Scholar]
- Dumitru, D.; Ghanakumar, S.; Provenzano, E.; Benson, J.R. A Prospective Study Evaluating the Accuracy of Indocyanine Green (ICG) Fluorescence Compared with Radioisotope for Sentinel Lymph Node (SLN) Detection in Early Breast Cancer. Ann. Surg. Oncol. 2022, 29, 3014–3020. [Google Scholar] [CrossRef]
- Martinez-Nunez, S.; del Agua, I.A.; Boza, A.S.; Morales-Conde, S. Individualised splenic hilum lymphadenectomy in gastric cancer: ICG-guided mapping. Cir. Esp. 2022, 100, 173. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wei, Z.; Song, C.; Tang, C.; Han, W.; Dong, X. Optical nano-agents in the second near-infrared window for biomedical applications. Chem. Soc. Rev. 2018, 48, 22–37. [Google Scholar] [CrossRef]
- Zhou, R.; Ohulchanskyy, T.Y.; Xu, Y.; Ziniuk, R.; Xu, H.; Liu, L.; Qu, J. Tumor-Microenvironment-Activated NIR-II Nanotheranostic Platform for Precise Diagnosis and Treatment of Colon Cancer. ACS Appl. Mater. Interfaces 2022, 14, 23206–23218. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, J.; Chen, B.; Ge, X.; Zhang, X.; Gao, S.; Ma, Q.; Song, J. NIR-II Fluorescent Activatable Drug Delivery Nanoplatform for Cancer-Targeted Combined Photodynamic and Chemotherapy. ACS Appl. Bio Mater. 2022, 5, 711–722. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, L.; Zhu, Q.; Feng, Y.; Wu, M. Recent Advances in Second Near-Infrared Region (NIR-II) Fluorophores and Biomedical Applications. Front. Chem. 2021, 9, 750404. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Su, L.; Ge, X.; Ye, J.; Zhao, C.; He, Y.; Yang, H.; Song, J.; Duan, H. Plasmonic-Fluorescent Janus Ag/Ag2S Nanoparticles for In Situ H2O2-Activated NIR-II Fluorescence Imaging. Nano Lett. 2021, 21, 2625–2633. [Google Scholar] [CrossRef]
- Landry, M.P.; Ando, H.; Chen, A.Y.; Cao, J.; Kottadiel, V.I.; Chio, L.; Yang, D.; Dong, J.; Lu, T.K.; Strano, M.S. Single-molecule detection of protein efflux from microorganisms using fluorescent single-walled carbon nanotube sensor arrays. Nat. Nanotechnol. 2017, 12, 368–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, Y.; Dai, H. A mini-review on rare-earth down-conversion nanoparticles for NIR-II imaging of biological systems. Nano Res. 2020, 13, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Min, X.; Zhang, J.; Li, R.-H.; Xia, F.; Cheng, S.-Q.; Li, M.; Zhu, W.; Zhou, W.; Li, F.; Sun, Y. Encapsulation of NIR-II AIEgens in Virus-like Particles for Bioimaging. ACS Appl. Mater. Interfaces 2021, 13, 17372–17379. [Google Scholar] [CrossRef]
- Su, Y.; Yu, B.; Wang, S.; Cong, H.; Shen, Y. NIR-II bioimaging of small organic molecule. Biomaterials 2021, 271, 120717. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Liu, C.; Zhang, X.; Fan, Q.; Wu, W.; Jiang, X. NIR-II Dye-Labeled Cylindrical Polymer Brushes for in Vivo Imaging. ACS Macro Lett. 2019, 8, 1623–1628. [Google Scholar] [CrossRef]
- Zhou, H.J.; Ren, T.B. Recent Progress of Cyanine Fluorophores for NIR-II Sensing and Imaging. Chem. Asian J. 2022, 17, e202200147. [Google Scholar] [CrossRef]
- Cui, D.; Li, J.; Zhao, X.; Pu, K.; Zhang, R. Semiconducting Polymer Nanoreporters for Near-Infrared Chemiluminescence Imaging of Immunoactivation. Adv. Mater. 2019, 32, e1906314. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, X. Semiconducting Perylene Diimide Nanostructure: Multifunctional Phototheranostic Nanoplatform. Accounts Chem. Res. 2019, 52, 1245–1254. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, W.; Li, C.; Liu, S.; Hu, X.; Xie, Z. Rational Design of BODIPY-Diketopyrrolopyrrole Conjugated Polymers for Photothermal Tumor Ablation. ACS Appl. Mater. Interfaces 2019, 11, 32720–32728. [Google Scholar] [CrossRef]
- Notaro, S.; Reimer, D.; Fiegl, H.; Schmid, G.; Wiedemair, A.; Rössler, J.; Marth, C.; Zeimet, A.G. Evaluation of folate receptor 1 (FOLR1) mRNA expression, its specific promoter methylation and global DNA hypomethylation in type I and type II ovarian cancers. BMC Cancer 2016, 16, 589. [Google Scholar] [CrossRef] [Green Version]
- Mertens-Walker, I.; Fernandini, B.C.; Maharaj, M.S.; Rockstroh, A.; Nelson, C.C.; Herington, A.C.; Stephenson, S.A. The tumour-promoting receptor tyrosine kinase, EphB4, regulates expression of integrin-beta8 in prostate cancer cells. BMC Cancer 2015, 15, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; You, J.; Ding, H.; Zhang, Z.; Cui, L.; Shen, X.; Bian, X.; Liu, Y.; Chen, J. Vasculogenic mimicry, a negative indicator for progression free survival of lung adenocarcinoma irrespective of first line treatment and epithelial growth factor receptor mutation status. BMC Cancer 2021, 21, 132. [Google Scholar] [CrossRef] [PubMed]
- Oudemans, P.B.; De La Rivière, G.B.; Hart, G.A.M.; Van Heerde, P.; Scholte, G.; Vroom, T.M. Determination of transferrin receptors on frozen sections of malignant B-cell lymphomas by immunofluorescence with a monoclonal antibody. Cancer 1986, 58, 1252–1259. [Google Scholar] [CrossRef]
- Yang, L.; Li, W.; Lu, Z.; Lu, M.; Zhou, J.; Peng, Z.; Zhang, X.; Wang, X.; Shen, L.; Li, J. Clinicopathological features of HER2 positive metastatic colorectal cancer and survival analysis of anti-HER2 treatment. BMC Cancer 2022, 22, 355. [Google Scholar] [CrossRef]
- Vasaikar, S.; Tsipras, G.; Landazuri, N.; Costa, H.; Wilhelmi, V.; Scicluna, P.; Cui, H.L.; Mohammad, A.A.; Davoudi, B.; Shang, M.; et al. Overexpression of endothelin B receptor in glioblastoma: A prognostic marker and therapeutic target? BMC Cancer 2018, 18, 154. [Google Scholar] [CrossRef]
- Nulent, T.J.W.K.; Valstar, M.H.; Smit, L.A.; Smeele, L.E.; Zuithoff, N.P.A.; De Keizer, B.; De Bree, R.; Van Es, R.J.J.; Willems, S.M. Prostate-specific membrane antigen (PSMA) expression in adenoid cystic carcinoma of the head and neck. BMC Cancer 2020, 20, 519. [Google Scholar]
- Nielsen, K.; Binderup, T.; Langer, S.W.; Kjaer, A.; Knigge, P.; Grøndahl, V.; Melchior, L.; Federspiel, B.; Knigge, U. P53, Somatostatin receptor 2a and Chromogranin A immunostaining as prognostic markers in high grade gastroenteropancreatic neuroendocrine neoplasms. BMC Cancer 2020, 20, 27. [Google Scholar]
- E Rogers, B.; Curiel, D.T.; Mayo, M.S.; Laffoon, K.K.; Bright, S.J.; Buchsbaum, D.J. Tumor localization of a radiolabeled bombesin analogue in mice bearing human ovarian tumors induced to express the gastrin-releasing peptide receptor by an adenoviral vector. Cancer 1997, 80, 2419–2424. [Google Scholar] [CrossRef]
- Yu, X.; Liang, C.; Zhang, Y.; Zhang, W.; Chen, H. Inhibitory short peptides targeting EPS8/ABI1/SOS1 tri-complex suppress invasion and metastasis of ovarian cancer cells. BMC Cancer 2019, 19, 878. [Google Scholar]
- Antaris, A.L.; Chen, H.; Cheng, K.; Sun, Y.; Hong, G.; Qu, C.; Diao, S.; Deng, Z.; Hu, X.; Zhang, B.; et al. A small-molecule dye for NIR-II imaging. Nat. Mater. 2016, 15, 235–242. [Google Scholar] [CrossRef]
- Carr, J.A.; Franke, D.; Caram, J.R.; Perkinson, C.F.; Saif, M.; Askoxylakis, V.; Datta, M.; Fukumura, D.; Jain, R.K.; Bawendi, M.G.; et al. Shortwave infrared fluorescence imaging with the clinically approved near-infrared dye indocyanine green. Proc. Natl. Acad. Sci. USA 2018, 115, 4465–4470. [Google Scholar] [PubMed] [Green Version]
- Yang, D.; Wang, H.; Sun, C.; Zhao, H.; Hu, K.; Qin, W.; Ma, R.; Yin, F.; Qin, X.; Zhang, Q.; et al. Development of a high quantum yield dye for tumour imaging. Chem. Sci. 2017, 8, 6322–6326. [Google Scholar] [PubMed] [Green Version]
- Yang, Q.; Hu, Z.; Zhu, S.; Ma, R.; Ma, H.; Ma, Z.; Wan, H.; Zhu, T.; Jiang, Z.; Liu, W.; et al. Donor Engineering for NIR-II Molecular Fluorophores with Enhanced Fluorescent Performance. J. Am. Chem. Soc. 2018, 140, 1715–1724. [Google Scholar]
- Tian, R.; Ma, H.; Yang, Q.; Wan, H.; Zhu, S.; Chandra, S.; Sun, H.; Kiesewetter, D.O.; Niu, G.; Liang, Y.; et al. Rational design of a super-contrast NIR-II fluorophore affords high-performance NIR-II molecular imaging guided microsurgery. Chem. Sci. 2018, 10, 326–332. [Google Scholar]
- Hong, G.; Zou, Y.; Antaris, A.L.; Diao, S.; Wu, D.; Cheng, K.; Zhang, X.; Chen, C.; Liu, B.; He, Y.; et al. Ultrafast fluorescence imaging in vivo with conjugated polymer fluorophores in the second near-infrared window. Nat. Commun. 2014, 5, 4206. [Google Scholar]
- Sun, Y.; Ding, M.; Zeng, X.; Xiao, Y.; Wu, H.; Zhou, H.; Ding, B.; Qu, C.; Hou, W.; Er-Bu, A.; et al. Novel bright-emission small-molecule NIR-II fluorophores for in vivo tumor imaging and image-guided surgery. Chem. Sci. 2017, 8, 3489–3493. [Google Scholar]
- Wang, W.; Ma, Z.; Zhu, S.; Wan, H.; Yue, J.; Ma, H.; Ma, R.; Yang, Q.; Wang, Z.; Li, Q.; et al. Molecular Cancer Imaging in the Second Near-Infrared Window Using a Renal-Excreted NIR-II Fluorophore-Peptide Probe. Adv. Mater. 2018, 30, e1800106. [Google Scholar] [PubMed]
- Grossi, M.; Morgunova, M.; Cheung, S.; Scholz, D.; Conroy, E.; Terrile, M.; Panarella, A.; Simpson, J.C.; Gallagher, W.M.; O’Shea, D.F. Lysosome triggered near-infrared fluorescence imaging of cellular trafficking processes in real time. Nat. Commun. 2016, 7, 10855. [Google Scholar]
- Wan, H.; Yue, J.; Zhu, S.; Uno, T.; Zhang, X.; Yang, Q.; Yu, K.; Hong, G.; Wang, J.; Li, L.; et al. A bright organic NIR-II nanofluorophore for three-dimensional imaging into biological tissues. Nat. Commun. 2018, 9, 1171. [Google Scholar]
- Li, X.; Jiang, M.; Li, Y.; Xue, Z.; Zeng, S.; Liu, H. 808 nm laser-triggered NIR-II emissive rare-earth nanoprobes for small tumor detection and blood vessel imaging. Mater. Sci. Eng. C 2019, 100, 260–268. [Google Scholar]
- Fan, X.; Li, Y.; Feng, Z.; Chen, G.; Zhou, J.; He, M.; Wu, L.; Li, S.; Qian, J.; Lin, H. Nanoprobes-Assisted Multichannel NIR-II Fluorescence Imaging-Guided Resection and Photothermal Ablation of Lymph Nodes. Adv. Sci. 2021, 8, 2003972. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, B.; Ding, S.; Fan, Y.; Wang, S.; Sun, C.; Zhao, M.; Zhao, C.-X.; Zhang, F. NIR-II bioluminescence for in vivo high contrast imaging and in situ ATP-mediated metastases tracing. Nat. Commun. 2020, 11, 4192. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Sun, P.; Cai, Y.; Xu, W.; Fan, Q.; Hu, Q.; Han, W. A Novel Multimodal NIR-II Nanoprobe for the Detection of Metastatic Lymph Nodes and Targeting Chemo-Photothermal Therapy in Oral Squamous Cell Carcinoma. Theranostics 2019, 9, 391–404. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, X.; Zhang, M.; Jiang, W.; Ou, J. Clinical study of combined application of indocyanine green and methylene blue for sentinel lymph node biopsy in breast cancer. Medicine 2021, 100, e25365. [Google Scholar] [CrossRef] [PubMed]
- Pölcher, M.; Matz, S.; Braun, M.; Brambs, C.; Beer, M.; Hamann, M. Sentinel lymph node mapping with indocyanine green compared to blue dye tracer in gynecologic malignancies—A single center experience of 218 patients. J. Surg. Oncol. 2020, 123, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Chen, Q.; Huang, X.; Lin, G.; Liu, Z.; Chen, J.; Wang, H.; Weng, K.; Li, P.; Xie, J.; et al. Clinical implications of Indocyanine Green Fluorescence Imaging-Guided laparoscopic lymphadenectomy for patients with gastric cancer: A cohort study from two randomized, controlled trials using individual patient data. Int. J. Surg. 2021, 94, 106120. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Mu, X.; Zhang, X.; Ming, D. The Near-Infrared-II Fluorophores and Advanced Microscopy Technologies Development and Application in Bioimaging. Bioconjug. Chem. 2020, 31, 260–275. [Google Scholar] [CrossRef]
- Zhu, S.; Yang, Q.; Antaris, A.L.; Yue, J.; Ma, Z.; Wang, H.; Huang, W.; Wan, H.; Wang, J.; Diao, S.; et al. Molecular imaging of biological systems with a clickable dye in the broad 800- to 1,700-nm near-infrared window. Proc. Natl. Acad. Sci. USA 2017, 114, 962–967. [Google Scholar] [CrossRef] [Green Version]
- Wang, E.; Babbey, C.M.; Dunn, K.W. Performance comparison between the high-speed Yokogawa spinning disc confocal system and single-point scanning confocal systems. J. Microsc. 2005, 218, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wan, H.; Ma, Z.; Zhong, Y.; Sun, Q.; Tian, Y.; Qu, L.; Du, H.; Zhang, M.; Li, L.; et al. Light-sheet microscopy in the near-infrared II window. Nat. Methods 2019, 16, 545–552. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, Y.; Hou, S.; Upputuri, P.K.; Wu, D.; Li, J.; Wang, P.; Zhen, X.; Pramanik, M.; Pu, K.; et al. Compact Plasmonic Blackbody for Cancer Theranosis in the Near-Infrared II Window. ACS Nano 2018, 12, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Qiu, F.; Shen, L.; Chen, D.; Su, Y.; Yang, C.; Li, B.; Yan, D.; Zhu, X. Combining Two-Photon-Activated Fluorescence Resonance Energy Transfer and Near-Infrared Photothermal Effect of Unimolecular Micelles for Enhanced Photodynamic Therapy. ACS Nano 2016, 10, 10489–10499. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Zou, Q.; Li, S.; Yan, X. Self-Assembled Peptide- and Protein-Based Nanomaterials for Antitumor Photodynamic and Photothermal Therapy. Adv. Mater. 2017, 29, 1605021. [Google Scholar] [CrossRef]
- Hu, Z.; Fang, C.; Li, B.; Zhang, Z.; Cao, C.; Cai, M.; Su, S.; Sun, X.; Shi, X.; Li, C.; et al. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat. Biomed. Eng. 2020, 4, 259–271. [Google Scholar] [CrossRef]
- Cao, Z.; Feng, L.; Zhang, G.; Wang, J.; Shen, S.; Li, D.; Yang, X. Semiconducting polymer-based nanoparticles with strong absorbance in NIR-II window for in vivo photothermal therapy and photoacoustic imaging. Biomaterials 2018, 155, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Wang, Y.; Zou, R.; Bian, K.; Liu, P.; Shen, S.; Yang, W.; Zhang, B.; Wang, D. Molecular Engineered Squaraine Nanoprobe for NIR-II/Photoacoustic Imaging and Photothermal Therapy of Metastatic Breast Cancer. ACS Appl. Mater. Interfaces 2020, 12, 4276–4284. [Google Scholar] [CrossRef]
- Lu, X.; Chen, J.; Li, J.; Xia, B.; Xu, J.; Wang, Q.; Xie, C.; Fan, Q.; Huang, W. Single nanoparticles as versatile phototheranostics for tri-modal imaging-guided photothermal therapy. Biomater. Sci. 2019, 7, 3609–3613. [Google Scholar] [PubMed]
- Sun, T.; Han, J.; Liu, S.; Wang, X.; Wang, Z.Y.; Xie, Z. Tailor-Made Semiconducting Polymers for Second Near-Infrared Photothermal Therapy of Orthotopic Liver Cancer. ACS Nano 2019, 13, 7345–7354. [Google Scholar]
- Wei, Z.; Wu, M.; Lan, S.; Li, J.; Zhang, X.; Zhang, D.; Liu, X.; Liu, J. Semiconducting polymer-based nanoparticles for photothermal therapy at the second near-infrared window. Chem. Commun. 2018, 54, 13599–13602. [Google Scholar] [CrossRef]
- Xu, C.; Jiang, Y.; Han, Y.; Pu, K.; Zhang, R. A Polymer Multicellular Nanoengager for Synergistic NIR-II Photothermal Immunotherapy. Adv. Mater. 2021, 33, 2008061. [Google Scholar]
- Hackbarth, S.; Islam, W.; Fang, J.; Šubr, V.; Röder, B.; Etrych, T.; Maeda, H. Singlet oxygen phosphorescence detection in vivo identifies PDT-induced anoxia in solid tumors. Photochem. Photobiol. Sci. 2019, 18, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J. Photodynamic therapy (PDT) of malignant tumors. Crit. Rev. Oncol. 1984, 2, 83–116. [Google Scholar] [CrossRef]
- Song, C.; Ran, J.; Wei, Z.; Wang, Y.; Chen, S.; Lin, L.; Zhang, G.; Cai, Y.; Han, W. Organic Near-Infrared-II Nanophotosensitizer for Safe Cancer Phototheranostics and Improving Immune Microenvironment against Metastatic Tumor. ACS Appl. Mater. Interfaces 2021, 13, 3547–3558. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, J.; Wang, Y.; Li, J. Recent Advances in Engineering Nanomedicines for Second Near-Infrared Photothermal-Combinational Immunotherapy. Nanomaterials 2022, 12, 1656. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, H.; Fan, M.; Gao, S.; Fan, D.; Wang, Z.; Chang, J.; Zhang, J.; Ge, K. Near-infrared-II photothermal ultra-small carbon dots promoting anticancer efficiency by enhancing tumor penetration. J. Colloid Interface Sci. 2022, 616, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, Q.; Yang, J.; Hou, M.; Zheng, B.; Xu, J.; Chai, Y.; Xiong, L.; Zhang, C. Tumor microenvironment-responsive nanohybrid for hypoxia amelioration with photodynamic and near-infrared II photothermal combination therapy. Acta Biomater. 2022, 146, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, Q.; Sun, J.; Gong, H.; Cao, Y.; Duan, L.; Yi, S.; Ying, B.; Xiao, B. Near-Infrared-Enpowered Nanomotor-Mediated Targeted Chemotherapy and Mitochondrial Phototherapy to Boost Systematic Antitumor Immunity. Adv. Health Mater. 2022, 11, e2200255. [Google Scholar] [CrossRef]
- Liu, H.; Xu, C.; Meng, M.; Li, S.; Sheng, S.; Zhang, S.; Ni, W.; Tian, H.; Wang, Q. Metal-organic framework-mediated multifunctional nanoparticles for combined chemo-photothermal therapy and enhanced immunotherapy against colorectal cancer. Acta Biomater. 2022, 144, 132–141. [Google Scholar] [CrossRef]
- Li, C.; Li, T.; Niu, K.; Xiao, Z.; Huang, J.; Pan, X.; Sun, Y.; Wang, Y.; Ma, D.; Xie, P.; et al. Mild phototherapy mediated by manganese dioxide-loaded mesoporous polydopamine enhances immunotherapy against colorectal cancer. Biomater. Sci. 2022, 10, 3647–3656. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, X.; Zhang, Z.; Zhang, H.; Liu, H.; Zhu, X.; Li, H.; Chi, X.; Yin, Z.; Gao, J. Real-Time Monitoring of Arsenic Trioxide Release and Delivery by Activatable T1 Imaging. ACS Nano 2015, 9, 2749–2759. [Google Scholar] [CrossRef]
- Li, J.; Jiang, R.; Wang, Q.; Li, X.; Hu, X.; Yuan, Y.; Lu, X.; Wang, W.; Huang, W.; Fan, Q. Semiconducting polymer nanotheranostics for NIR-II/Photoacoustic imaging-guided photothermal initiated nitric oxide/photothermal therapy. Biomaterials 2019, 217, 119304. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhou, L.; Wang, W.; Li, X.; Zhang, F. In vivo gastrointestinal drug-release monitoring through second near-infrared window fluorescent bioimaging with orally delivered microcarriers. Nat. Commun. 2017, 8, 14702. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.; Liu, C.; Hu, H.; Guo, X.-L.; Jiang, B.-P.; Liang, H.; Shen, X.-C. NIR-II light-modulated thermosensitive hydrogel for light-triggered cisplatin release and repeatable chemo-photothermal therapy. Chem. Sci. 2019, 10, 4699–4706. [Google Scholar] [CrossRef] [Green Version]
- Hettie, K.S.; Teraphongphom, N.T.; Ertsey, R.; Chin, F.T. Off-Peak Near-Infrared-II (NIR-II) Bioimaging of an Immunoconjugate Having Peak Fluorescence Emission in the NIR-I Spectral Region for Improving Tumor Margin Delineation. ACS Appl. Bio Mater. 2020, 3, 8658–8666. [Google Scholar] [CrossRef]
- Ashwin, K.; Somashekhar, S.; Arvind, R.; Kumar, C.; Ahuja, V. Sentinel node mapping using indocyanine green and near-infrared fluorescence imaging technology for endometrial cancer: A prospective study using a surgical algorithm in Indian patients. J. Minimal Access Surg. 2021, 17, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Achterberg, F.B.; Mulder, B.G.S.; Meijer, R.P.J.; Bonsing, B.A.; Hartgrink, H.H.; Mieog, J.S.D.; Zlitni, A.; Park, S.-M.; Sarasqueta, A.F.; Vahrmeijer, A.L.; et al. Real-time surgical margin assessment using ICG-fluorescence during laparoscopic and robot-assisted resections of colorectal liver metastases. Ann. Transl. Med. 2020, 8, 1448. [Google Scholar] [CrossRef]
- Piccolo, G.; Barabino, M.; Pesce, A.; Diana, M.; Lecchi, F.; Santambrogio, R.; Opocher, E.; Bianchi, P.P.; Piozzi, G.N. Role of Indocyanine Green Fluorescence Imaging in Minimally Invasive Resection of Colorectal Liver Metastases. Surg. Laparosc. Endosc. Percutaneous Tech. 2022, 32, 259–265. [Google Scholar] [CrossRef]
- Wen, Q.; Zhang, Y.; Li, C.; Ling, S.; Yang, X.; Chen, G.; Yang, Y.; Wang, Q. NIR-II Fluorescent Self-Assembled Peptide Nanochain for Ultrasensitive Detection of Peritoneal Metastasis. Angew. Chem. Int. Ed. 2019, 58, 11001–11006. [Google Scholar] [CrossRef] [PubMed]
| NIR-II materials | Advantages | Disadvantages |
|---|---|---|
| Inorganic | ||
| QDs | narrow emission wavelength, broad excitation wavelength, superior quantum yield, steady optical properties, long fluorescence lifetime | toxicity(containing heavy metal elements Cd and Pb) |
| CNTs | good photostability | high excitation intensity, low quantum yield |
| RENPs | narrow emission spectra, high emission efficiency, low photo-bleaching | toxicity, low water solubility |
| Organic | ||
| organic small molecules | high biocompatibility, rapid excretion capacity, excellent resolution | low photostability low water solubility |
| conjugated polymers | rapid emission rate, higher fluorescence brightness, good photostability, good biocompatibility, good water dispersibility | limitation of excitation and emission wavelength |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Chen, Y.; Wang, B.; Gao, X.; Wu, M. Recent Progress in Second Near-Infrared (NIR-II) Fluorescence Imaging in Cancer. Biomolecules 2022, 12, 1044. https://doi.org/10.3390/biom12081044
Wang T, Chen Y, Wang B, Gao X, Wu M. Recent Progress in Second Near-Infrared (NIR-II) Fluorescence Imaging in Cancer. Biomolecules. 2022; 12(8):1044. https://doi.org/10.3390/biom12081044
Chicago/Turabian StyleWang, Tian, Yingying Chen, Bo Wang, Xiaofan Gao, and Mingfu Wu. 2022. "Recent Progress in Second Near-Infrared (NIR-II) Fluorescence Imaging in Cancer" Biomolecules 12, no. 8: 1044. https://doi.org/10.3390/biom12081044
APA StyleWang, T., Chen, Y., Wang, B., Gao, X., & Wu, M. (2022). Recent Progress in Second Near-Infrared (NIR-II) Fluorescence Imaging in Cancer. Biomolecules, 12(8), 1044. https://doi.org/10.3390/biom12081044





