Expression of Tumor Suppressor SFRP1 Predicts Biological Behaviors and Prognosis: A Potential Target for Oral Squamous Cell Carcinoma
Abstract
:1. Introduction
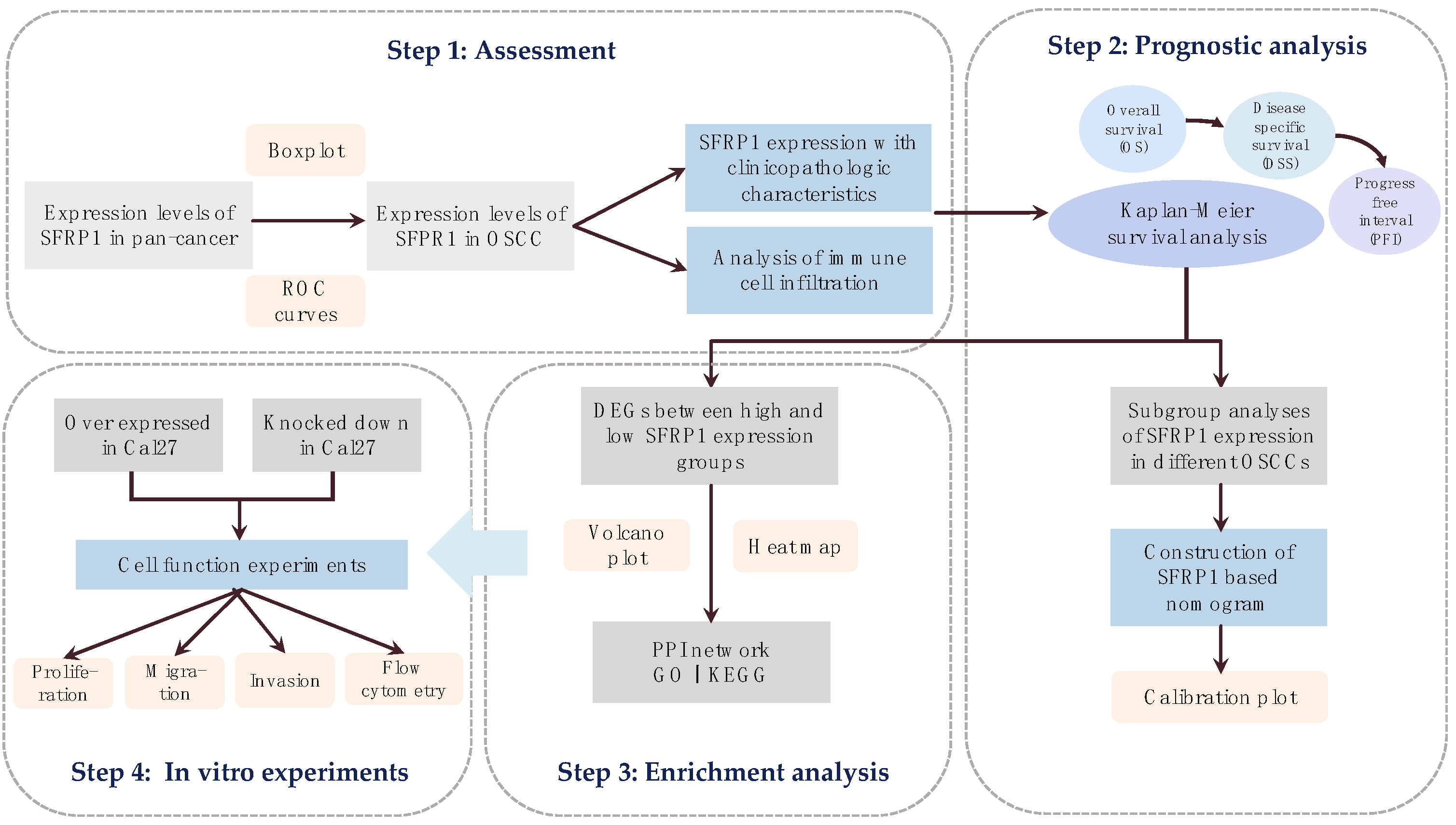
2. Materials and Methods
2.1. Acquisition of Single Gene Matrix
2.2. Tumor Immune Infiltration Analysis
2.3. Prognostic Analysis
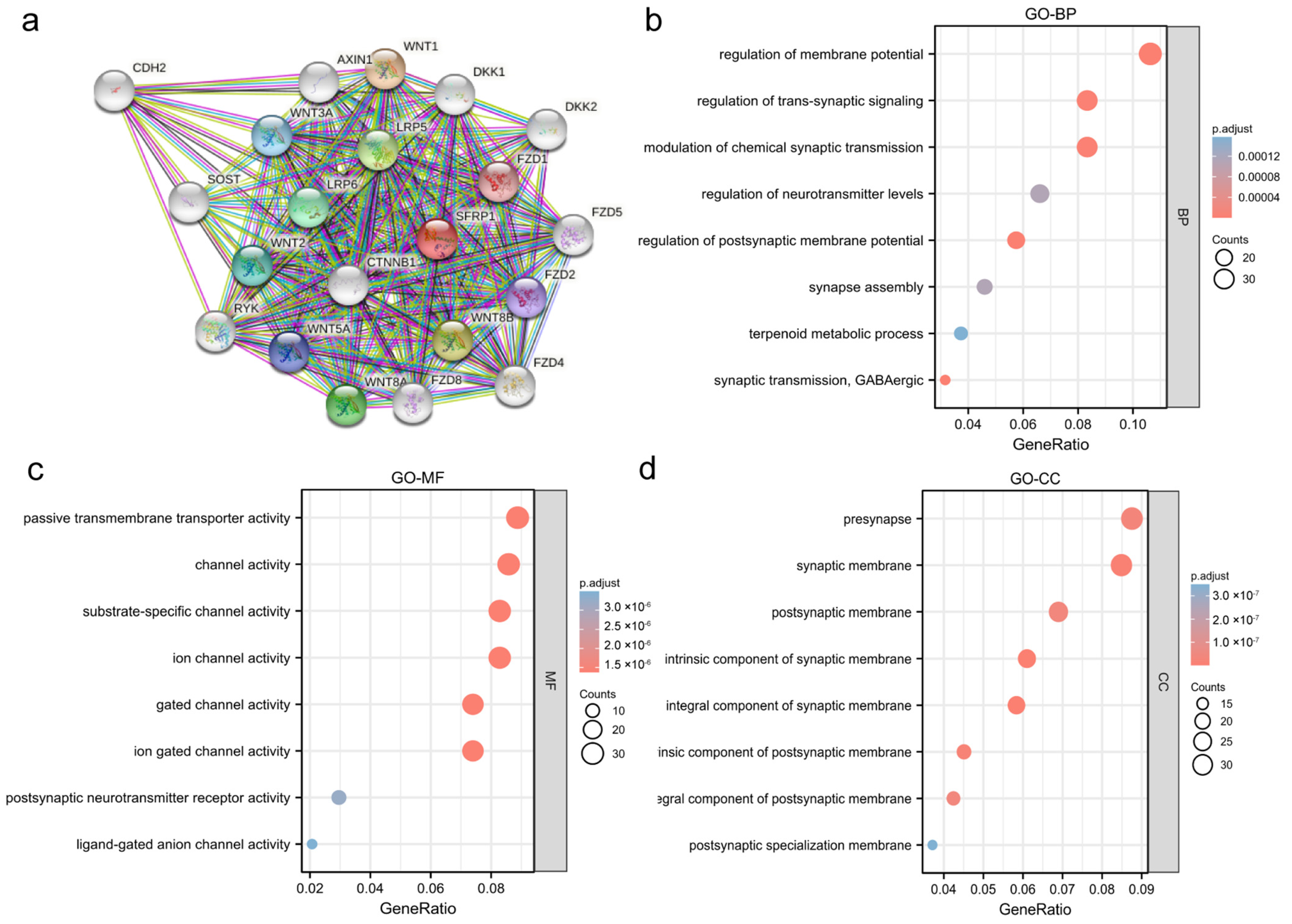
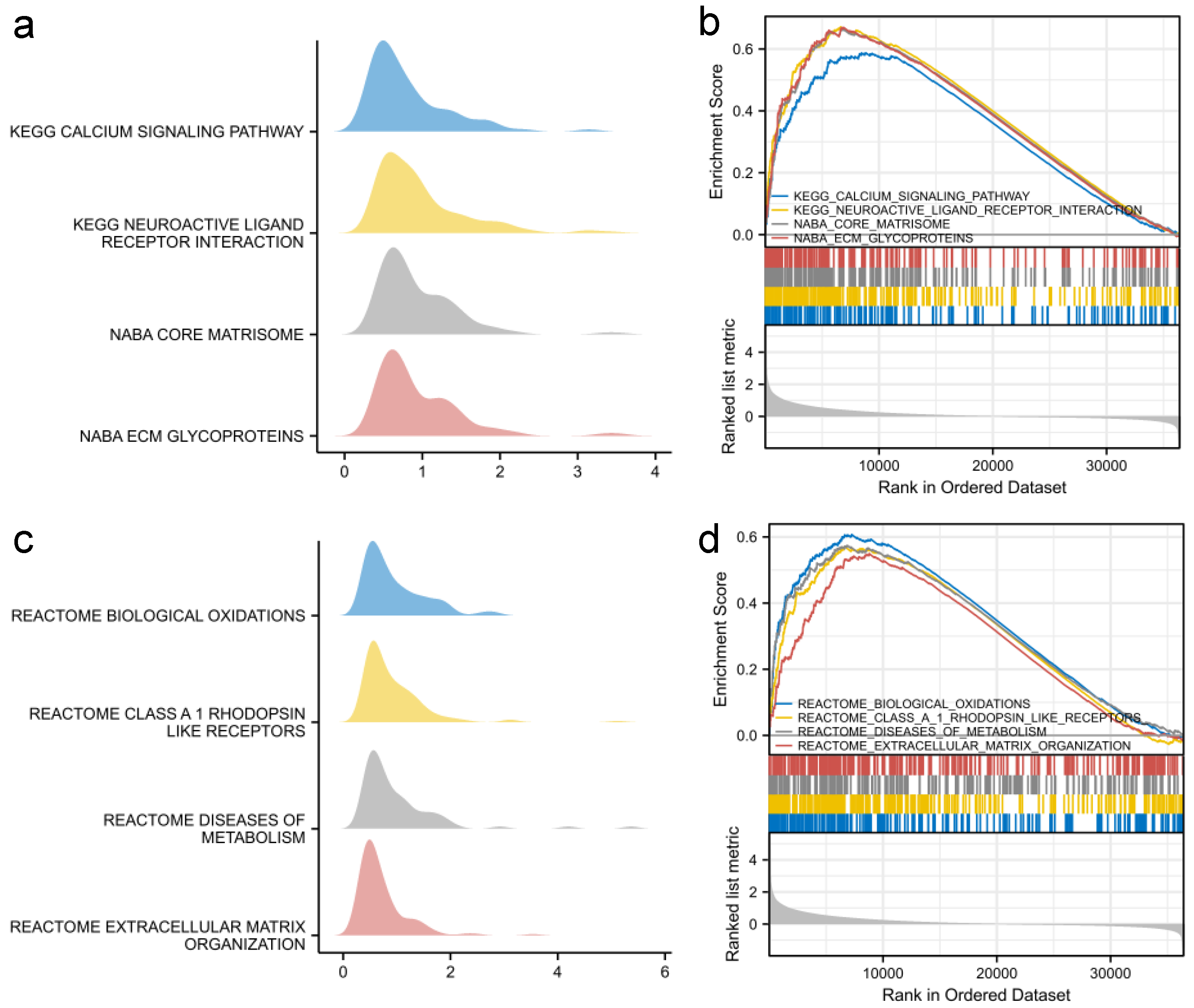
2.4. Enrichment Analysis
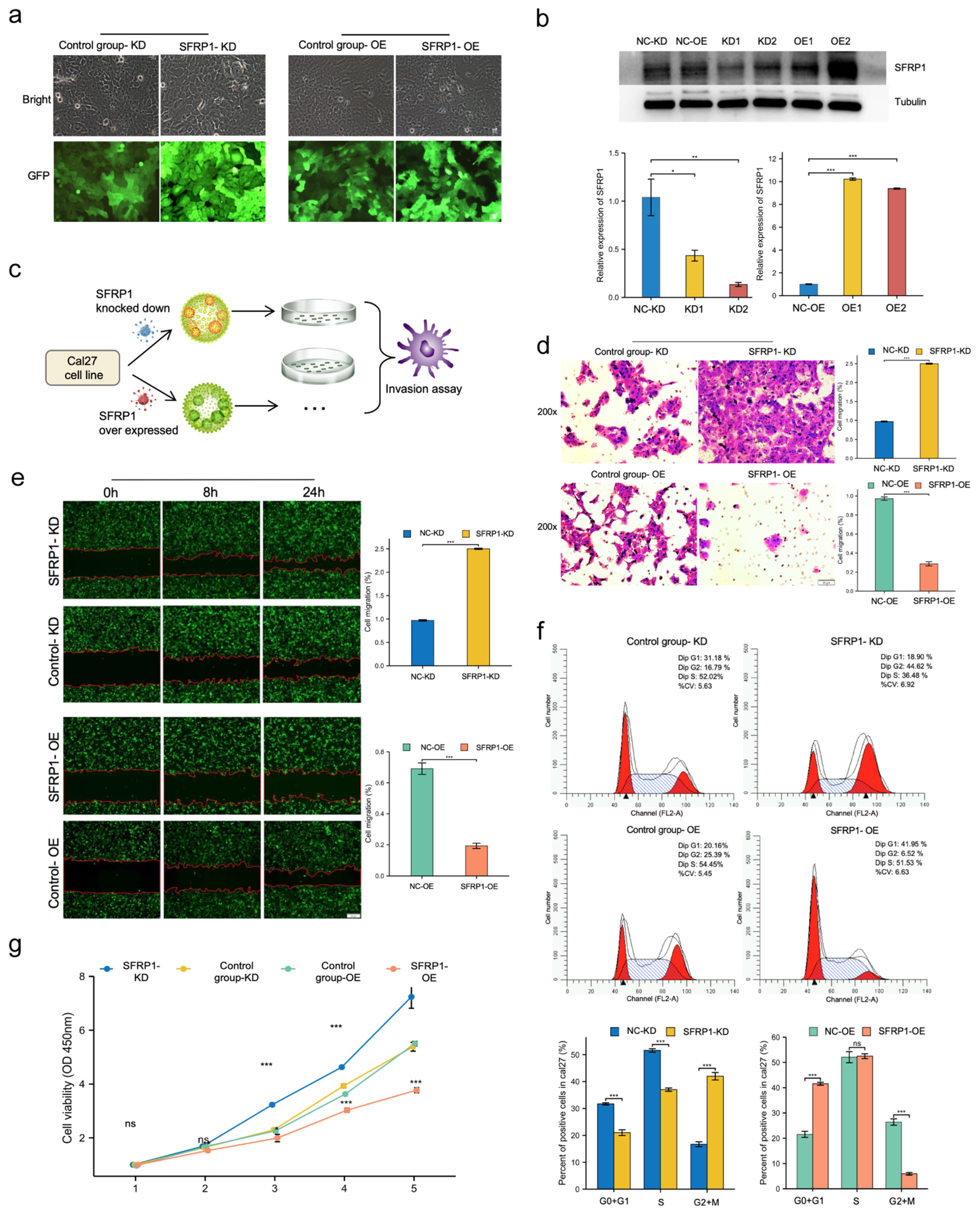
2.5. Cell Culture and Lentiviral Transfection
2.6. Western Blotting Assay
2.7. RT-PCR
2.8. Cholecystokinin-8 (CCK8) Assay
2.9. Transwell Assay
2.10. Wound-Healing Assay
2.11. Flow Cytometry Assay
2.12. Statistical Analysis
3. Results
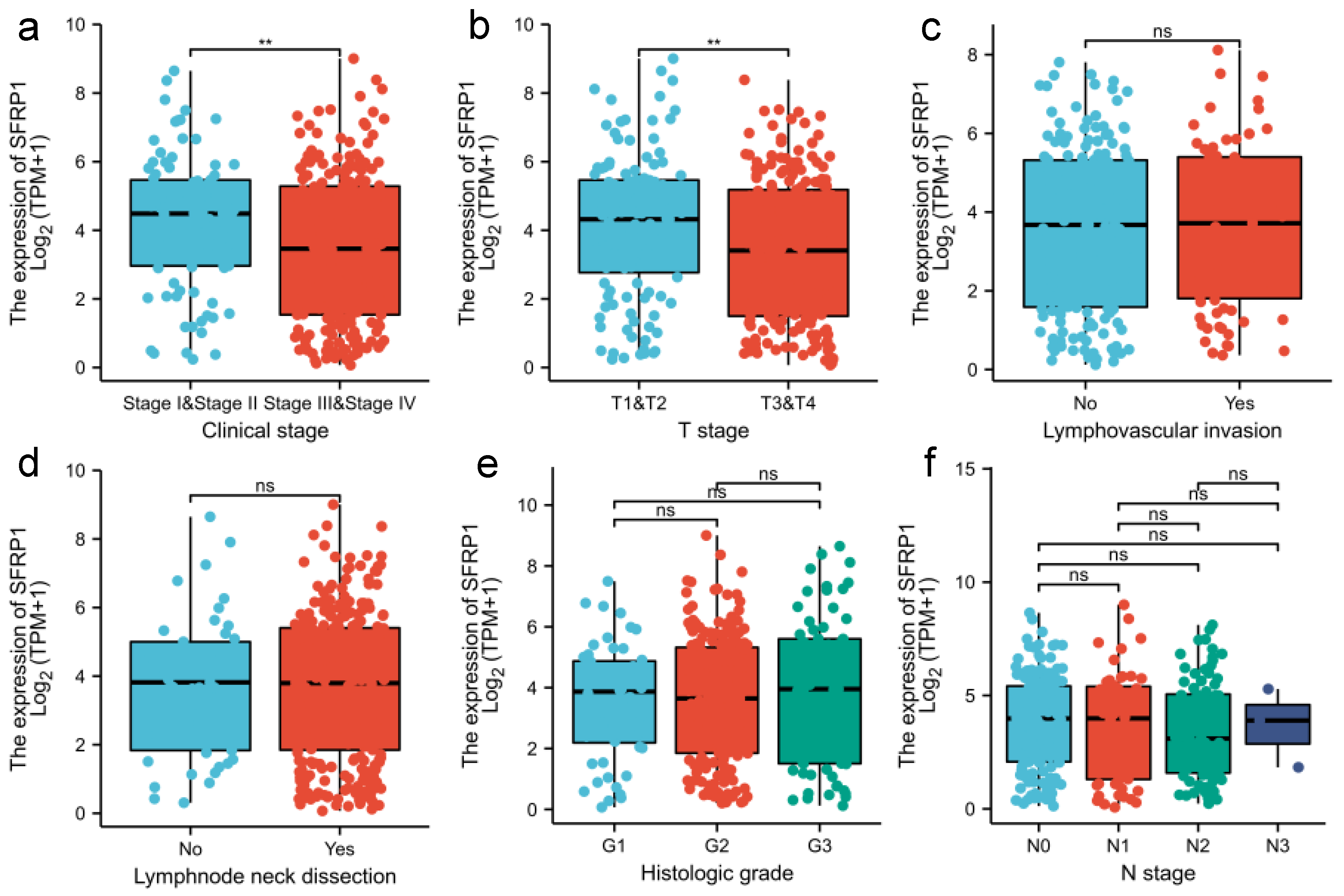
3.1. SFRP1 Is Down-Regulated in OSCC Tissues
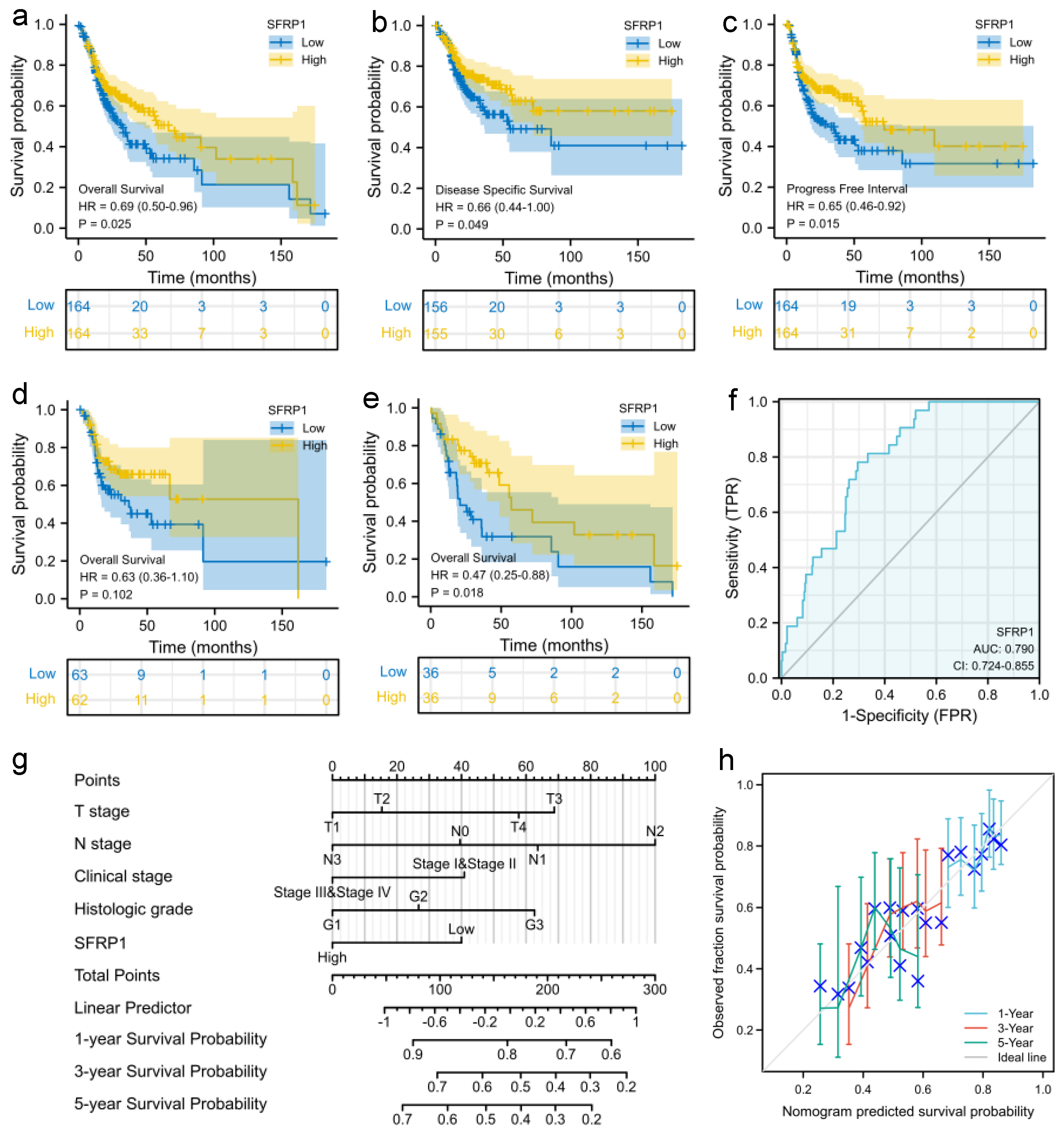
3.2. Prognostic Signature of SFRP1 Expression
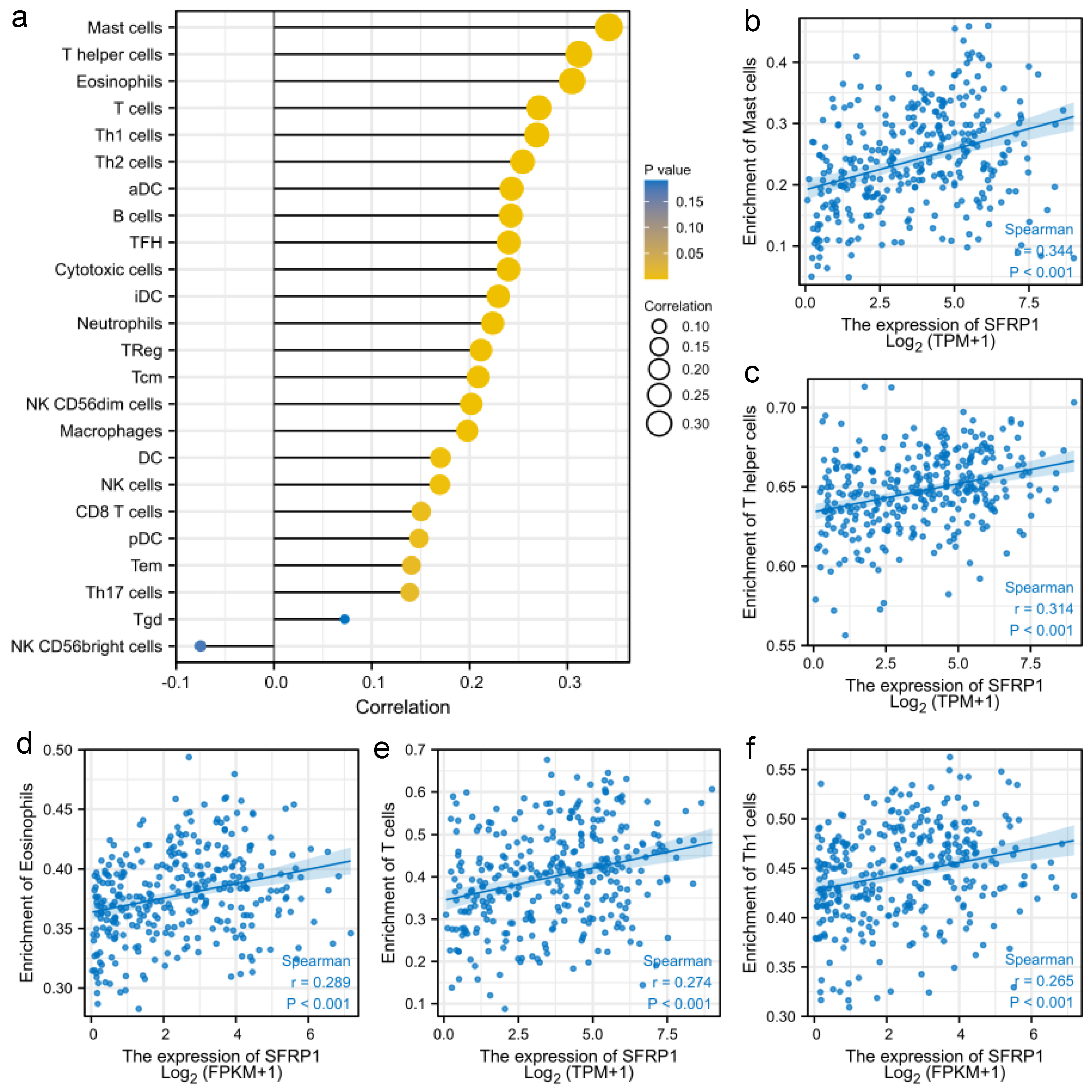
3.3. SFRP1 Is Associated with Tumor Immune Infiltration
3.4. Functional Analysis of SFRP1
3.5. SFRP1 Represses Invasion and Migration of Cal27 Cells In Vitro
3.6. SFRP1 Regulates Cell Cycle Progression and Suppresses Cell Proliferation In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miranda-Filho, A.; Bray, F. Global patterns and trends in cancers of the lip, tongue and mouth. Oral Oncol. 2020, 102, 104551. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botta, L.; Gatta, G.; Trama, A.; Bernasconi, A.; Mariotto, A.B. Incidence and survival of rare cancers in the US and Europe. Cancer Med. 2020, 9, 5632–5642. [Google Scholar] [CrossRef] [PubMed]
- Felice, F.D.; Musio, D.; Terenzi, V.; Valentini, V.; Cassoni, A.; Tombolini, M.; Vincentiis, M.D.; Tombolini, V. Treatment improvement and better patient care: Which is the most important one in oral cavity cancer? Radiat. Oncol. 2014, 9, 263. [Google Scholar] [CrossRef] [Green Version]
- Arteaga, C.L.; Clark, G.M. Inefficacy of methylglyoxal bis (guanylhydrazone) (MGBG) in patients with recurrent head and neck squamous cell carcinoma. Investig. New Drugs 1989, 7, 281. [Google Scholar] [CrossRef] [PubMed]
- Weie, J.; Rosemann, J.; Müller, L.; Kappler, M.; Gutschner, T. Identification of lymphocyte cell-specific protein-tyrosine kinase (LCK) as a driver for invasion and migration of oral cancer by tumor heterogeneity exploitation. Mol. Cancer 2021, 20, 88. [Google Scholar]
- Valastyan, S.; Weinberg, R. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.B.; Qiang, F.L.; Dong, J.; Cai, J.; Zhou, S.H.; Shi, M.X.; Chen, K.P.; Hu, Z.B. Plasma DNA methylation of Wnt antagonists predicts recurrence of esophageal squamous cell carcinoma. World J. Gastroenterol. 2011, 17, 4917–4921. [Google Scholar] [CrossRef]
- Caspi, M.; Wittenstein, A.; Kazelnik, M.; Shor-Nareznoy, Y.; Rosin-Arbesfeld, R. Therapeutic targeting of the oncogenic Wnt signaling pathway for treating colorectal cancer and other colonic disorders. Adv. Drug Deliv. Rev. 2020, 169, 118–136. [Google Scholar] [CrossRef]
- Peifer, M.; Polakis, P. Wnt signaling in oncogenesis and embryogenesis—A look outside the nucleus. Science 2000, 287, 1606–1609. [Google Scholar] [CrossRef]
- Hnzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-Seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.; Li, X.; Da, M.; Liu, X.; Zhi, W. Prognostic significance of tumor infiltrating immune cells in oral squamous cell carcinoma. BMC Cancer 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Gosipatala, S.B.; Pandey, A.; Singh, P. Prognostic Relevance of SFRP1 Gene Promoter Methylation in Colorectal Carcinoma. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 1571–1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Chen, L.; Mashrah, M.; Zhu, Y.; Liu, J.; Yang, X.; He, Z.; Wang, L.; Xiang, T.; Yao, Z.; et al. Deregulation of secreted frizzled-related proteins is associated with aberrant beta-catenin activation in the carcinogenesis of oral submucous fibrosis. OncoTargets Ther. 2015, 8, 2923–2931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemi, A.; Rostami, S.; Chahardouli, B.; Alizad Ghandforosh, N.; Ghotaslou, A.; Nadali, F. Study of SFRP1 and SFRP2 methylation status in patients with de novo Acute Myeloblastic Leukemia. Int. J. Hematol.-Oncol. Stem Cell Res. 2015, 9, 15–21. [Google Scholar] [PubMed]
- Chakraborty, B.; Mukhopadhyay, D.; Roychowdhury, A.; Basu, M.; Alam, N.; Chatterjee, K.; Chakrabarti, J.; Panda, C. Differential Wnt-β-catenin pathway activation in HPV positive and negative oral epithelium is transmitted during head and neck tumorigenesis: Clinical implications. Med. Microbiol. Immunol. 2021, 210, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Alamoud, K.A.; Kukuruzinska, M.A. Emerging Insights into Wnt/beta-catenin Signaling in Head and Neck Cancer. J. Dent. Res. 2018, 97, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Koo, B.S.; Kim, J.M.; Huang, S.; Rho, Y.S.; Bae, W.J.; Kang, H.J.; Kim, Y.S.; Moon, J.H.; Lim, Y.C. Wnt/beta-catenin signalling maintains self-renewal and tumourigenicity of head and neck squamous cell carcinoma stem-like cells by activating Oct4. J. Pathol. 2014, 234, 99–107. [Google Scholar] [CrossRef]
- Kakinouchi, K.; Yoshie, S.; Tsuji, S.; Murono, S.; Hazama, A. Dysfunction of Cl channels promotes epithelial to mesenchymal transition in oral squamous cell carcinoma via activation of Wnt/β-catenin signaling pathway. Biochem. Biophys. Res. Commun. 2021, 555, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Baharudin, R.; Tieng, F.; Lee, L.H.; Mutalib, N. Epigenetics of SFRP1: The Dual Roles in Human Cancers. Cancers 2020, 12, 445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakhry, C.; Blackford, A.L.; Neuner, G.; Xiao, W.; Gillison, M.L. Association of Oral Human Papillomavirus DNA Persistence With Cancer Progression After Primary Treatment for Oral Cavity and Oropharyngeal Squamous Cell Carcinoma. JAMA Oncol. 2019, 5, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Peltanova, B.; Raudenska, M.; Masarik, M. Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: A systematic review. Mol. Cancer 2019, 18, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Sun, C.; Kim, N.; Bhan, C.; Zhang, K. Comprehensive Analysis of SFRP Family Members Prognostic Value and Immune Infiltration in Gastric Cancer. Life 2021, 11, 522. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.H.; Zhang, Y.J.; Yue, J.X.; Zhou, T. Comprehensive Analysis of the Expression and Prognosis for SFRPs in Breast Carcinoma. Cell Transplant. 2020, 29, 96. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Wang, Q.; Zhang, H.; Zhu, G.; Lang, J. Two immune-enhanced molecular subtypes differ in inflammation, checkpoint signaling and outcome of advanced head and neck squamous cell carcinoma. Oncoimmunology 2017, 7, e1392427. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, R.R.; Sarate, R.M.; Setia, P.; Shah, S.; Waghmare, S.K. SFRP1 in Skin Tumor Initiation and Cancer Stem Cell Regulation with Potential Implications in Epithelial Cancers. Stem Cell Rep. 2020, 14, 271–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Xie, Y.; Liu, Y.; Wang, F.; Li, M.; Qi, J. MBD2 and EZH2 regulate the expression of SFRP1 without affecting its methylation status in a colorectal cancer cell line. Exp. Ther. Med. 2020, 20, 242. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.; Kim, J.H.; Lee, Y.S.; Lee, Y.C. Change in gene expression profiles of secreted frizzled-related proteins (SFRPs) by sodium butyrate in gastric cancers: Induction of promoter demethylation and histone modification causing inhibition of Wnt signaling. Int. J. Oncol. 2012, 40, 1533–1542. [Google Scholar] [PubMed] [Green Version]
- Warrier, S.; Bhuvanalakshmi, G.; Arfuso, F.; Rajan, G.; Millward, M.; Dharmarajan, A. Cancer stem-like cells from head and neck cancers are chemosensitized by the Wnt antagonist, sFRP4, by inducing apoptosis, decreasing stemness, drug resistance and epithelial to mesenchymal transition. Cancer Gene Ther. 2014, 21, 381–388. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Zhang, Y.; Liu, Y.; Hang, L.; Yang, J. Expression of Tumor Suppressor SFRP1 Predicts Biological Behaviors and Prognosis: A Potential Target for Oral Squamous Cell Carcinoma. Biomolecules 2022, 12, 1034. https://doi.org/10.3390/biom12081034
Chen C, Zhang Y, Liu Y, Hang L, Yang J. Expression of Tumor Suppressor SFRP1 Predicts Biological Behaviors and Prognosis: A Potential Target for Oral Squamous Cell Carcinoma. Biomolecules. 2022; 12(8):1034. https://doi.org/10.3390/biom12081034
Chicago/Turabian StyleChen, Chun, Yifei Zhang, Yupeng Liu, Lei Hang, and Jun Yang. 2022. "Expression of Tumor Suppressor SFRP1 Predicts Biological Behaviors and Prognosis: A Potential Target for Oral Squamous Cell Carcinoma" Biomolecules 12, no. 8: 1034. https://doi.org/10.3390/biom12081034
APA StyleChen, C., Zhang, Y., Liu, Y., Hang, L., & Yang, J. (2022). Expression of Tumor Suppressor SFRP1 Predicts Biological Behaviors and Prognosis: A Potential Target for Oral Squamous Cell Carcinoma. Biomolecules, 12(8), 1034. https://doi.org/10.3390/biom12081034





