Culture Media Composition Influences the Antibacterial Effect of Silver, Cupric, and Zinc Ions against Pseudomonas aeruginosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Sources of Metal Ions
2.3. Culture Media
2.4. Ag+, Cu2+ and Zn2+ MIC Determination
2.5. Metal–Medium Interactions
2.6. Bacterial Growth in Selected Media with a Modified pH Range
2.7. The Correlation between Medium Composition and MIC Values of Tested Ions
3. Results
3.1. The Type of Culture Medium Influences the Antibacterial Activity of Metal Ions
3.2. The Chemical Interactions between Metal Ions and Culture Media Components
3.3. Bacterial Growth in the Modified pH Range Depends on Media Composition
3.4. Correlation between Medium Composition and MIC Values of Tested Ions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic Resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azam, M.W.; Khan, A.U. Updates on the Pathogenicity Status of Pseudomonas aeruginosa. Drug Discov. Today 2019, 24, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J.E. Gram-Negative Bacterial Infections; Elsevier Inc.: Amsterdam, The Netherlands, 2013; ISBN 9781437707953. [Google Scholar]
- Wu, W.; Jin, Y.; Bai, F.; Jin, S. Pseudomonas aeruginosa. In Molecular Medical Microbiology: Second Edition; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 2-3, pp. 753–767. ISBN 9780123971692. [Google Scholar]
- Guła, G.; Dorotkiewicz-Jach, A.; Korzekwa, K.; Valvano, M.A.; Drulis-Kawa, Z. Complex Signaling Networks Controlling Dynamic Molecular Changes in Pseudomonas aeruginosa Biofilm. Curr. Med. Chem. 2018, 26, 1979–1993. [Google Scholar] [CrossRef] [PubMed]
- Dorotkiewicz-Jach, A.; Augustyniak, D.; Olszak, T.; Drulis-Kawa, Z. Modern Therapeutic Approaches against Pseudomonas aeruginosa Infections. Curr. Med. Chem. 2015, 22, 1642–1664. [Google Scholar] [CrossRef] [PubMed]
- Hobman, J.L.; Crossman, L.C. Bacterial Antimicrobial Metal Ion Resistance. J. Med. Microbiol. 2015, 64, 471–497. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. At the Nexus of Antibiotics and Metals: The Impact of Cu and Zn on Antibiotic Activity and Resistance. Trends Microbiol. 2017, 25, 820–832. [Google Scholar] [CrossRef]
- Tite, T.; Popa, A.C.; Balescu, L.M.; Bogdan, I.M.; Pasuk, I.; Ferreira, J.M.F.; Stan, G.E. Cationic Substitutions in Hydroxyapatite: Current Status of the Derived Biofunctional Effects and Their in Vitro Interrogation Methods. Materials 2018, 11, 2081. [Google Scholar] [CrossRef] [Green Version]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial Activity of Metals: Mechanisms, Molecular Targets and Applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Atlas, R.M. Handbook of Microbiological Media, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9781439804087. [Google Scholar]
- De Spiegeleer, P.; Sermon, J.; Lietaert, A.; Aertsen, A.; Michiels, C.W. Source of Tryptone in Growth Medium Affects Oxidative Stress Resistance in Escherichia Coli. J. Appl. Microbiol. 2004, 97, 124–133. [Google Scholar] [CrossRef]
- Parija, S.C. Textbook of Microbiology & Immunology; Elsevier India: Chennai, India, 2009; ISBN 978-81-312-2163-1. [Google Scholar]
- Rathnayake, I.V.N.; Megharaj, M.; Krishnamurti, G.S.R.; Bolan, N.S.; Naidu, R. Heavy Metal Toxicity to Bacteria—Are the Existing Growth Media Accurate Enough to Determine Heavy Metal Toxicity? Chemosphere 2013, 90, 1195–1200. [Google Scholar] [CrossRef]
- EUCAST. MIC Determination of Non-Fastidious and Fastidious Organisms. Available online: https://www.eucast.org/ast_of_bacteria/mic_determination/?no_cache=1 (accessed on 17 February 2020).
- Hoseinzadeh, E.; Makhdoumi, P.; Taha, P.; Hossini, H.; Pirsaheb, M.; Omid Rastegar, S.; Stelling, J. A Review of Available Techniques for Determination of Nano-Antimicrobials Activity. Toxin Rev. 2017, 36, 18–32. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drulis-Kawa, Z.; Gubernator, J.; Dorotkiewicz-Jach, A.; Doroszkiewicz, W.; Kozubek, A. In Vitro Antimicrobial Activity of Liposomal Meropenem against Pseudomonas aeruginosa Strains. Int. J. Pharm. 2006, 315, 59–66. [Google Scholar] [CrossRef]
- Calomiris, J.J.; Armstrong, J.L.; Seidler, R.J. Association of Metal Tolerance with Multiple Antibiotic Resistance of Bacteria Isolated from Drinking Water. Appl. Environ. Microbiol. 1984, 47, 1238–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasman, H.; Bjerrum, M.J.; Christiansen, L.E.; Bruun Hansen, H.C.; Aarestrup, F.M. The Effect of PH and Storage on Copper Speciation and Bacterial Growth in Complex Growth Media. J. Microbiol. Methods 2009, 78, 20–24. [Google Scholar] [CrossRef]
- Pormohammad, A.; Turner, R.J. Silver Antibacterial Synergism Activities with Eight Other Metal(Loid)-Based Antimicrobials against Escherichia Coli, Pseudomonas aeruginosa, and Staphylococcus Aureus. Antibiotics 2020, 9, 853. [Google Scholar] [CrossRef]
- Videira-Quintela, D.; Guillén, F.; Montalvo, G.; Martin, O. Silver, Copper, and Copper Hydroxy Salt Decorated Fumed Silica Hybrid Composites as Antibacterial Agents. Colloids Surf. B Biointerfaces 2020, 195, 111216. [Google Scholar] [CrossRef]
- Singh, K.; Kumar, Y.; Puri, P.; Sharma, C.; Aneja, K.R. Synthesis, Spectroscopic, Thermal and Antimicrobial Studies of Co(II), Ni(II), Cu(II) and Zn(II) Complexes with Schiff Base Derived from 4-Amino-3-Mercapto-6-Methyl-5-Oxo-1,2,4-Triazine. Med. Chem. Res. 2012, 21, 1708–1716. [Google Scholar] [CrossRef]
- Tarawneh, A.A.; Qaralleh, H.; Al-limoun, M.O.; Khleifat, K.M. Effect of Copper Chemical Form on The Growth of Pseudomonas aeruginosa Isolated from Burned Patients and on Its Cu Uptake. Basic Appl. Res. Biomed. 2019, 5, 107–114. [Google Scholar] [CrossRef]
- McQuillan, J.S.; Shaw, A.M. Differential Gene Regulation in the Ag Nanoparticle and Ag +-Induced Silver Stress Response in Escherichia Coli: A Full Transcriptomic Profile. Nanotoxicology 2014, 8, 177–184. [Google Scholar] [CrossRef]
- Mary, G.; Bajpai, S.; Chand, N. Copper (II) Ions and Copper Nanoparticles-Loaded Chemically Modified Cotton Cellulose Fibers with Fair Antibacterial Properties. J. Appl. Polym. Sci. 2009, 113, 757–766. [Google Scholar] [CrossRef]
- Ramalingam, B.; Parandhaman, T.; Das, S.K. Antibacterial Effects of Biosynthesized Silver Nanoparticles on Surface Ultrastructure and Nanomechanical Properties of Gram-Negative Bacteria Viz. Escherichia Coli and Pseudomonas aeruginosa. ACS Appl. Mater. Interfaces 2016, 8, 4963–4976. [Google Scholar] [CrossRef] [PubMed]
- Sampath Kumar, T.S.; Madhumathi, K.; Rubaiya, Y.; Doble, M. Dual Mode Antibacterial Activity of Ion Substituted Calcium Phosphate Nanocarriers for Bone Infections. Front. Bioeng. Biotechnol. 2015, 3, 59. [Google Scholar] [CrossRef] [Green Version]
- Swetha, M.; Sahithi, K.; Moorthi, A.; Saranya, N.; Saravanan, S.; Ramasamy, K.; Srinivasan, N.; Selvamurugan, N. Synthesis, Characterization, and Antimicrobial Activity of Nano-Hydroxyapatite-Zinc for Bone Tissue Engineering Applications. J. Nanosci. Nanotechnol. 2012, 12, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Thoendel, M.; Olakanmi, O.; Britigan, B.E.; Singh, P.K. The Transition Metal Gallium Disrupts Pseudomonas aeruginosa Iron Metabolism and Has Antimicrobial and Antibiofilm Activity. J. Clin. Investig. 2007, 117, 877–888. [Google Scholar] [CrossRef]
- Li, M.; Zhu, L.; Lin, D. Toxicity of ZnO Nanoparticles to Escherichia Coli: Mechanism and the Influence of Medium Components. Environ. Sci. Technol. 2011, 45, 1977–1983. [Google Scholar] [CrossRef]
- Ferreira, C.M.H.; Pinto, I.S.S.; Soares, E.V.; Soares, H.M.V.M. (Un)Suitability of the Use of PH Buffers in Biological, Biochemical and Environmental Studies and Their Interaction with Metal Ions-a Review. RSC Adv. 2015, 5, 30989–31003. [Google Scholar] [CrossRef] [Green Version]
- Sobierajska, P.; Dorotkiewicz-Jach, A.; Zawisza, K.; Okal, J.; Olszak, T.; Drulis-Kawa, Z.; Wiglusz, R.J. Preparation and Antimicrobial Activity of the Porous Hydroxyapatite Nanoceramics. J. Alloys Compd. 2018, 748, 179–187. [Google Scholar] [CrossRef]
- Szyszka, K.; Rewak-Soroczynska, J.; Dorotkiewicz-Jach, A.; Ledwa, K.A.; Piecuch, A.; Giersig, M.; Drulis-Kawa, Z.; Wiglusz, R.J. Structural Modification of Nanohydroxyapatite Ca10(PO4)6(OH)2 Related to Eu3+ and Sr2+ Ions Doping and Its Spectroscopic and Antimicrobial Properties. J. Inorg. Biochem. 2020, 203, 110884. [Google Scholar] [CrossRef]
- Sarstedt, M.; Mooi, E. Regression Analysis. In A Concise Guide to Market Research; Springer: Berlin/Heidelberg, Germany, 2014; pp. 193–233. [Google Scholar]
- Henseler, J.; Ringle, C.; Sinkovics, R. The use of partial least squares pathmodeling in international marketing. In Advances in International Marketing; Emerald Group Publishing Limited: Bingley, UK, 2009; pp. 277–319. [Google Scholar]
- LaBauve, A.E.; Wargo, W.J. Growth and Laboratory Maintenance of Pseudomonas aeruginosa. Curr. Protoc. Microbiol. 2012. [Google Scholar] [CrossRef] [Green Version]
- Coffey, B.; Anderson, G. Biofilm Formation in the 96-Well Microtiter Plate. In Pseudomonas Methods and Protocols; Filloux, A., Ramos, J.-M., Eds.; Humana Press: New York, NY, USA, 2014; p. 634. [Google Scholar]
- Atlas, R.M. Handbook of Microbiological Media, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar] [CrossRef]
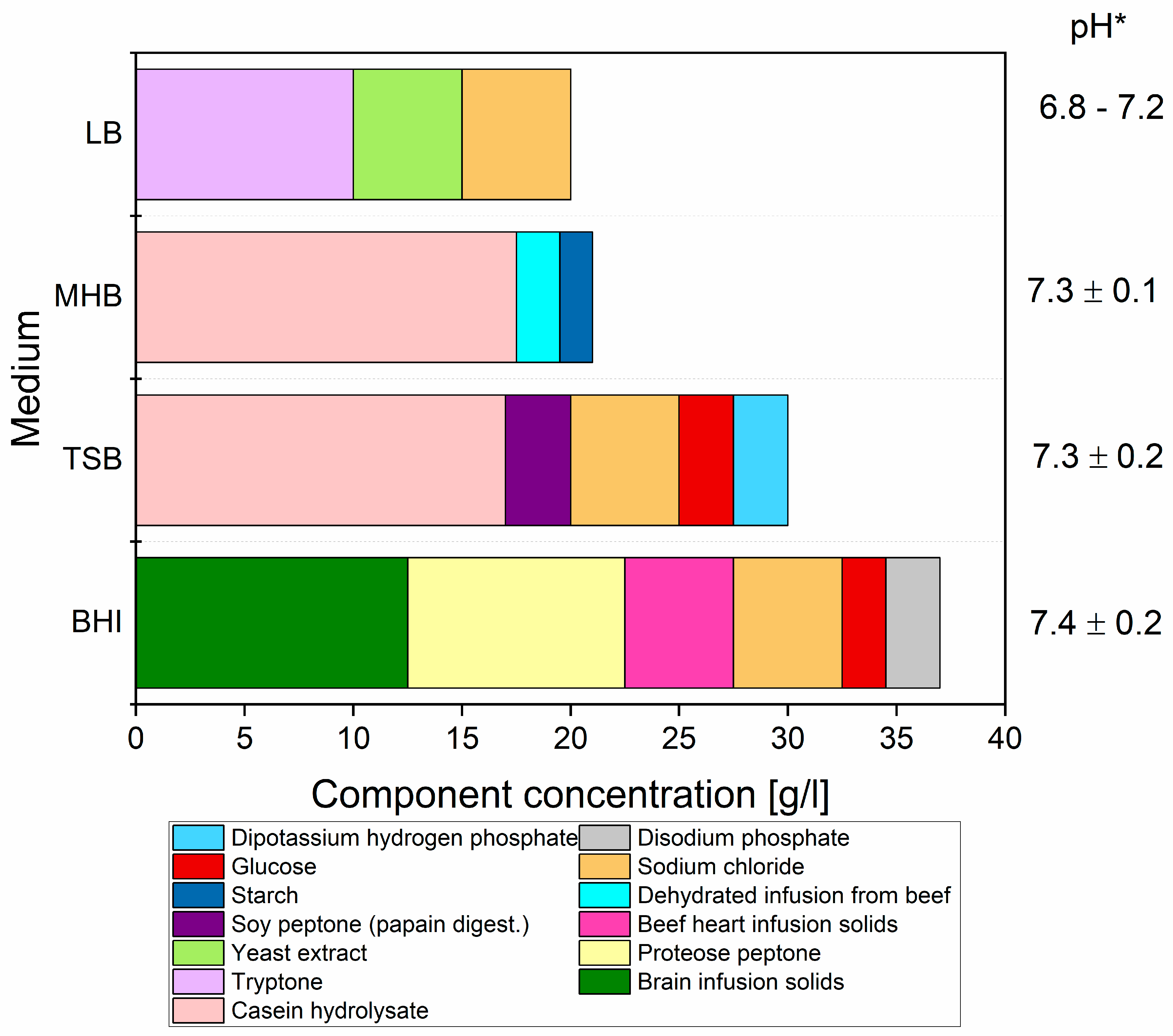
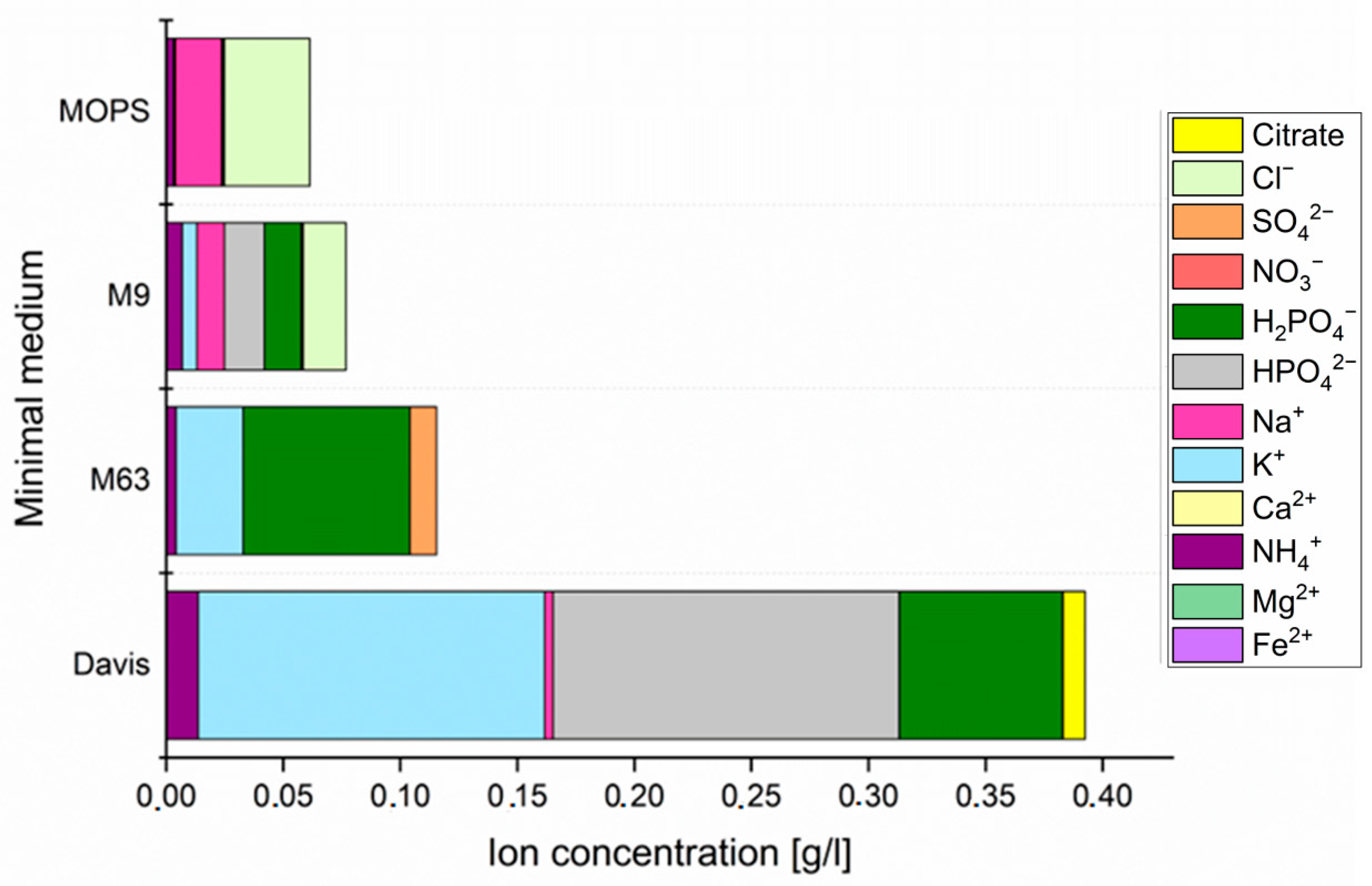
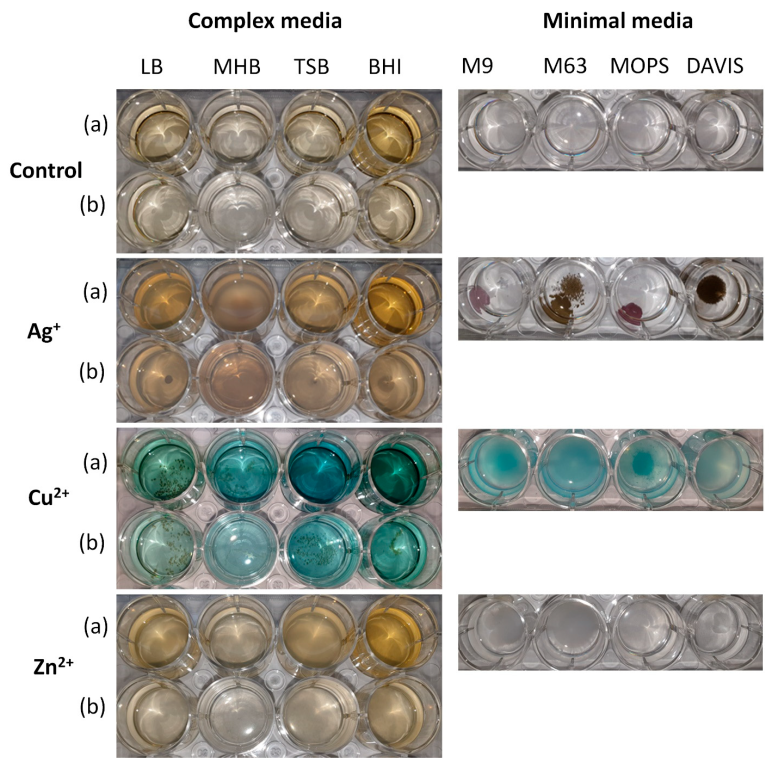
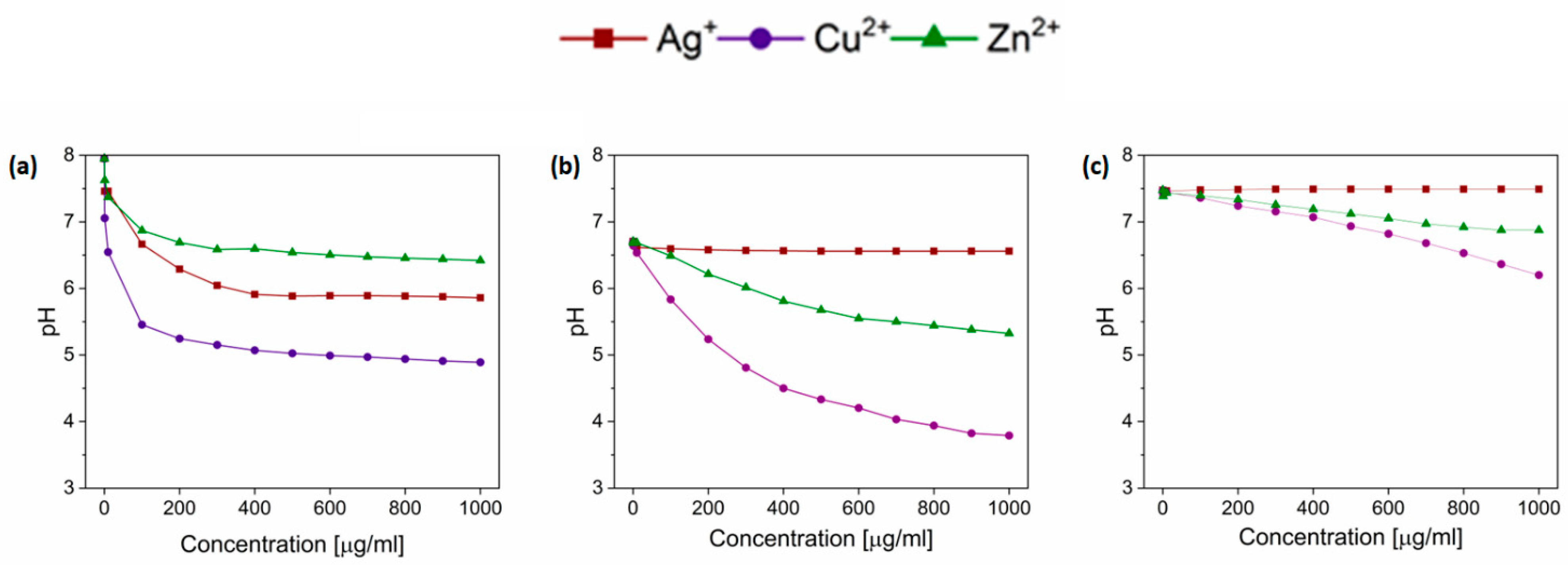

| Minimum Inhibitory Concentrations [µg/mL] | ||||
|---|---|---|---|---|
| Strain | Complex Media (Non-Diluted/1:1 Diluted) | |||
| LB | MHB | TSB | BHI | |
| Ag+ | ||||
| PAO1 | 1.25/0.625 | 2.5/0.625 | 5/1.25 | 10/10 |
| ATCC 27853 | 1.25/1.25 | 2.5/1.25 | 5/2.5 | 10/10 |
| Cu2+ | ||||
| PAO1 | 240/110 | 290/130 | 690/340 | 730/360 |
| ATCC 27853 | 250/120 | 290/140 | 690/340 | 740/370 |
| Zn2+ | ||||
| PAO1 | 350/160 | 370/160 | >1000/550 | >1000/720 |
| ATCC 27853 | 250/150 | 330/160 | >1000/490 | >1000/700 |
| Minimal media | ||||
| MOPS medium | M9 medium | M63 medium | Davis medium | |
| Ag+ | ||||
| PAO1 | 0.0125 | 0.0125 | 0.00625 | 0.0125 |
| ATCC 27853 | 0.0125 | 0.025 | 0.0125 | 0.0125 |
| Cu2+ | ||||
| PAO1 | 50 | 20 | 5 | 100 |
| ATCC 27853 | 70 | 20 | 20 | 100 |
| Zn2+ | ||||
| PAO1 | 170 | >1000 | >1000 | >1000 |
| ATCC 27853 | 160 | >1000 | >1000 | >1000 |
| MIC [µg/mL] | ||
|---|---|---|
| Strain | LB (Non-Diluted/1:1 Diluted) | MOPS Medium |
| Ag+ | ||
| 9/5 | 1.25/0.625 | 0.0125 |
| 14/3 | 1.25/0.625 | 0.025 |
| 82/3 | 1.25/0.625 | 0.0125 |
| 15/3 | 1.25/0.625 | 0.0125 |
| Cu2+ | ||
| 9/5 | 170/100 | 80 |
| 14/3 | 150/90 | 100 |
| 82/3 | 130/70 | 50 |
| 15/3 | 140/70 | 50 |
| Zn2+ | ||
| 9/5 | 280/150 | 180 |
| 14/3 | 250/100 | 120 |
| 82/3 | 250/110 | 190 |
| 15/3 | 250/110 | 170 |
| No. | MOPS Medium | Davis Medium | M9 Medium | M63 Medium |
| Ag+ | ||||
| 1. | Ag+ + Cl− → AgCl↓ (white)AgCl↓ + hν → Ag0 + Cl2(silver-grey) | 3Ag+ + PO42− → Ag3PO4↓ (yellow-brown) | Ag+ + Cl− → AgCl↓ (white)AgCl↓ + hν → Ag0 + Cl2 (silver-grey) | 3Ag+ + PO42− → Ag3PO4↓ (yellow-brown) |
| 2. | AgCl↓ + 2NH3·H2O → [Ag(NH3)2]+ + Cl− + H2O (colourless) | AgCl↓ + 2NH3·H2O → [Ag(NH3)2]+ + Cl− + H2O (colourless) | ||
| 3. | [Ag(NH3)2]+ + Cl− + 2H+ → AgCl↓ + 2NH4− (white)AgCl↓ + hν → Ag0 + Cl2 (silver-grey) | [Ag(NH3)2]+ + Cl− + 2H+ → AgCl↓ + 2NH4− (white)AgCl↓ + hν → Ag0 + Cl2 (silver-grey) | ||
| 4. | 3Ag+ + PO42− → Ag3PO4↓ (yellow-brown) | 3Ag+ + PO42− → Ag3PO4↓ (yellow-brown) | ||
| Cu2+ | ||||
| 1. | Cu2+ + H2O → [Cu(H2O)4]+ (blue) | Cu2+ + n (H2Cit−) → Cu(HCit)nn(d−3)+2 + n (2-d)H+ | Cu2+ + OH− + C6H12O6 → Cu2O↓ + C6H12O7 + H2O (brown-red) | Cu2+ + OH− + C6H12O6 → Cu2O↓ + C6H12O7 + H2O (brown-red) |
| 2 | 4Fe2+ + 3HPO42− → FeHPO4↓ + Fe3(PO4)2↓ + H+ (white) | Cu2+ + OH− + C6H12O6 → Cu2O↓ + C6H12O7 + H2O (brown-red) | ||
| 3 | Fe2+ + NO3− + H+ → Fe3+ + NO↑ + H2O (yellow) | |||
| 4 | Fe3+ + HPO42− → FePO4↓ + H+ (yellow) | |||
| 5 | Cu2+ + OH− + C6H12O6 → Cu2O↓ + C6H12O7 + H2O (brown-red) | |||
| Zn2+ | ||||
| 1. | 3Zn2+ + 2HPO42− → Zn3(PO4)2↓ + 2H+ (white) | 3Zn2+ + 2HPO42− → Zn3(PO4)2↓ + 2H+ (white) | 3Zn2+ + 2HPO42− → Zn3(PO4)2↓ + 2H+ (white) | 3Zn2+ + 2HPO42− → Zn3(PO4)2↓ + 2H+ (white) |
| 2. | 3Zn2+ + 2H2PO42− → Zn3(PO4)2↓ + 4H+ (white) | 3Zn2+ + 2H2PO42− → Zn3(PO4)2↓ + 4H+ (white) | ||
| Control | Ag+ | Cu2+ | Zn2+ | |
|---|---|---|---|---|
| Complex media (non-diluted/1:1 diluted) | ||||
| LB | 6.71/6.81 | 7.17/6.92 | 3.77/3.52 | 5.14/5.12 |
| MHB | 6.99/7.05 | 7.00/7.09 | 3.80/3.58 | 5.18/5.14 |
| TSB | 7.21/7.33 | 7.23/7.32 | 4.73/4.16 | 5.48/5.10 |
| BHI | 7.17/7.26 | 7.15/7.23 | 4.92/4.22 | 5.60/5.17 |
| Minimal media | ||||
| MOPS | 7.48 | 7.46 | 6.15 | 6.60 |
| M9 | 7.27 | 7.23 | 6.53 | 6.61 |
| M63 | 7.14 | 7.02 | 6.66 | 6.62 |
| Davis | 7.24 | 7.05 | 6.19 | 6.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rewak-Soroczynska, J.; Dorotkiewicz-Jach, A.; Drulis-Kawa, Z.; Wiglusz, R.J. Culture Media Composition Influences the Antibacterial Effect of Silver, Cupric, and Zinc Ions against Pseudomonas aeruginosa. Biomolecules 2022, 12, 963. https://doi.org/10.3390/biom12070963
Rewak-Soroczynska J, Dorotkiewicz-Jach A, Drulis-Kawa Z, Wiglusz RJ. Culture Media Composition Influences the Antibacterial Effect of Silver, Cupric, and Zinc Ions against Pseudomonas aeruginosa. Biomolecules. 2022; 12(7):963. https://doi.org/10.3390/biom12070963
Chicago/Turabian StyleRewak-Soroczynska, Justyna, Agata Dorotkiewicz-Jach, Zuzanna Drulis-Kawa, and Rafal J. Wiglusz. 2022. "Culture Media Composition Influences the Antibacterial Effect of Silver, Cupric, and Zinc Ions against Pseudomonas aeruginosa" Biomolecules 12, no. 7: 963. https://doi.org/10.3390/biom12070963
APA StyleRewak-Soroczynska, J., Dorotkiewicz-Jach, A., Drulis-Kawa, Z., & Wiglusz, R. J. (2022). Culture Media Composition Influences the Antibacterial Effect of Silver, Cupric, and Zinc Ions against Pseudomonas aeruginosa. Biomolecules, 12(7), 963. https://doi.org/10.3390/biom12070963








