Triglycerides as Biomarker for Predicting Systemic Lupus Erythematosus Related Kidney Injury of Negative Proteinuria
Abstract
:1. Introduction
2. Materials and Methods
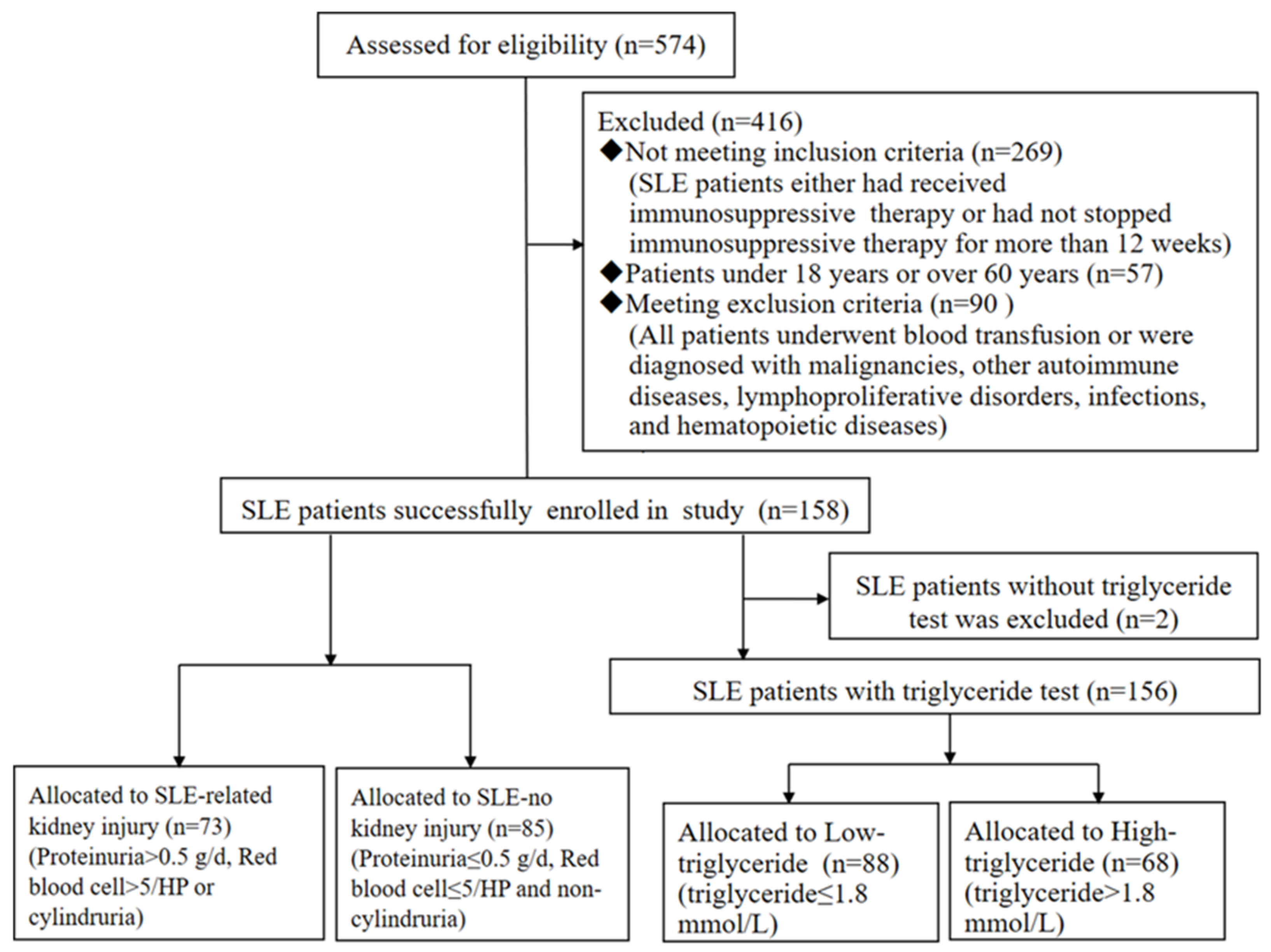
2.1. Study Population
2.2. Laboratory Values and Clinical Assessment
2.3. Association Rule Mining (ARM) and Apriori Algorithm
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Participants
3.2. Data Mining
3.3. Triglycerides May Be Independent Risk Factor for SLE-Related Kidney Injury
3.4. Baseline Characteristics in Low- and High-Triglycerides Patients with SLE
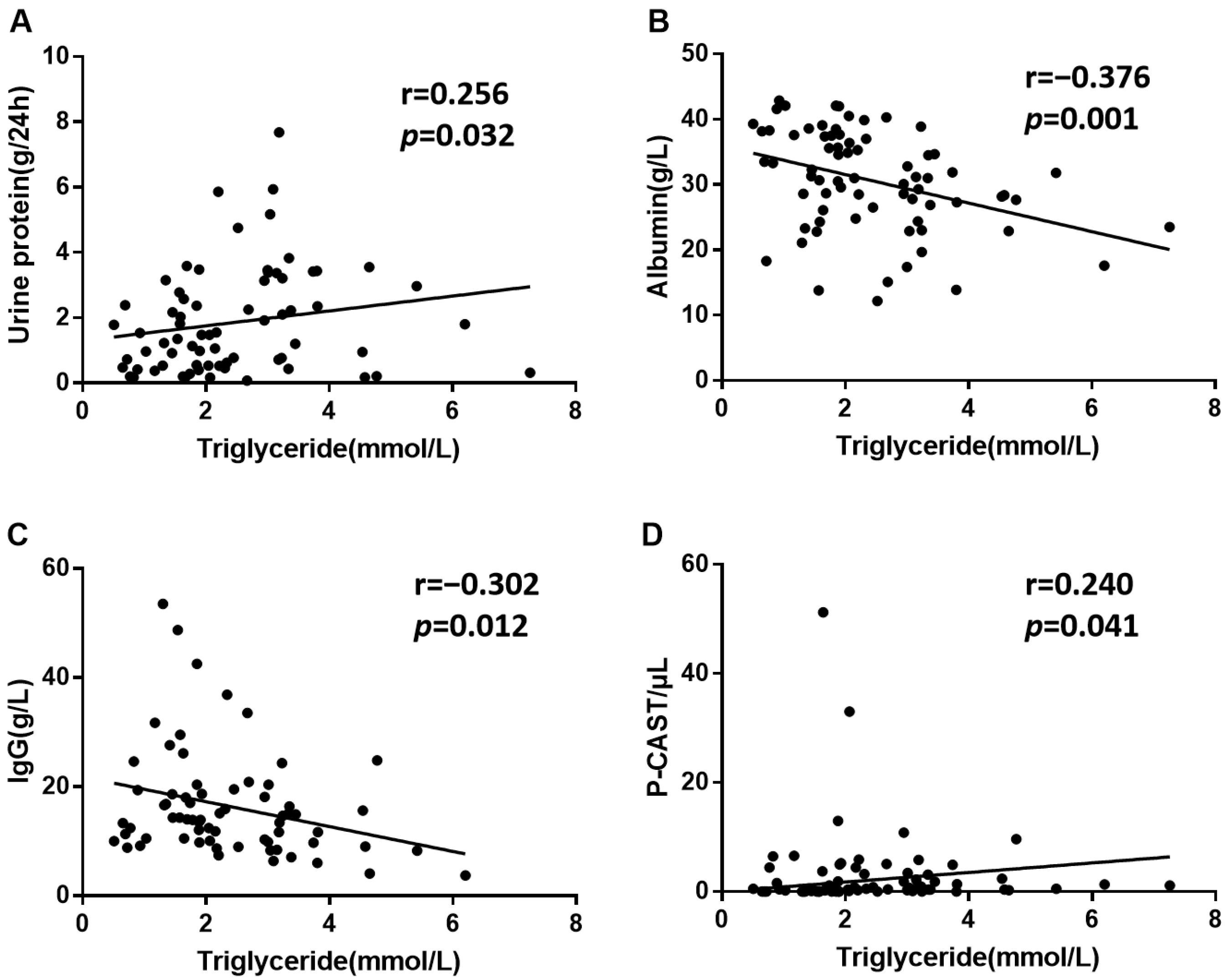
3.5. Triglycerides May Be Correlated with SLE-Related Kidney Injury
3.6. The Predictive Value of Triglycerides for SLE-Related Kidney Injury
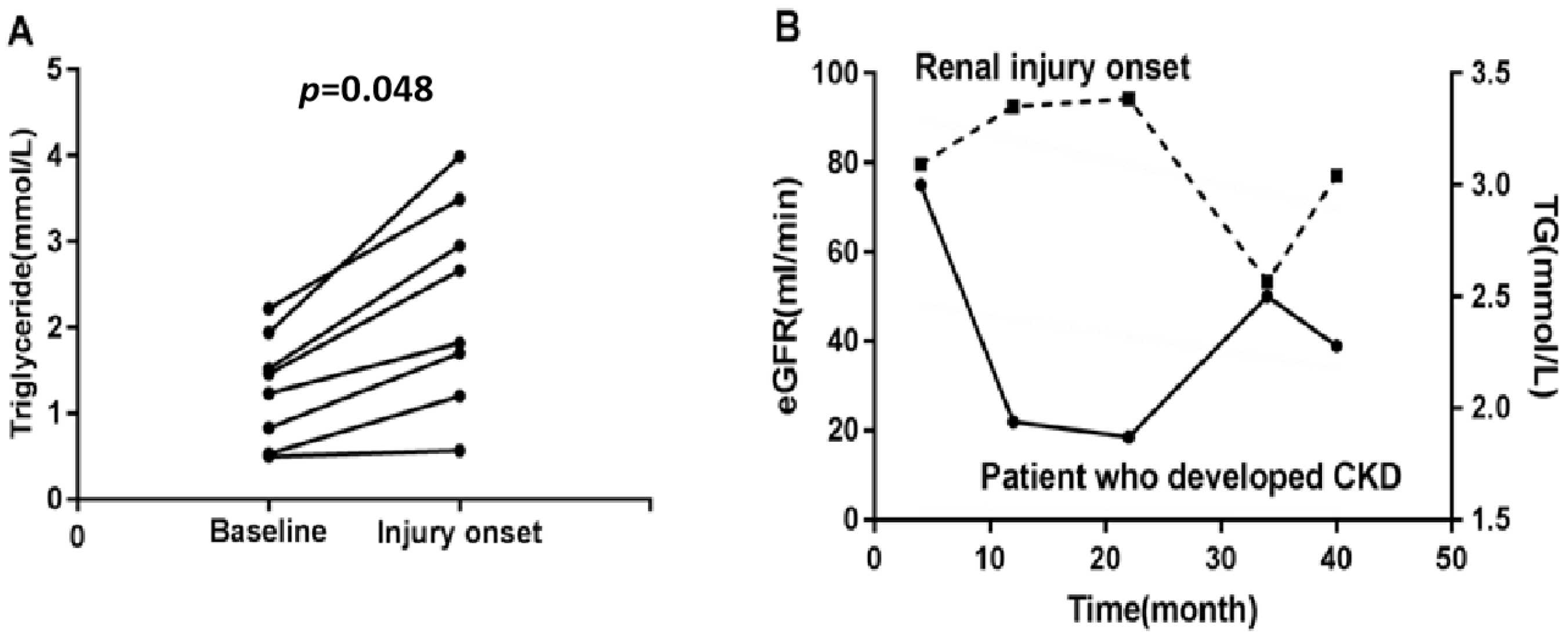
3.7. Triglycerides May Reflect the Process of SLE-Related Kidney Injury
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, W.; Tang, D.; Chen, D.; Zheng, F.; Huang, S.; Xu, Y.; Yu, H.; He, J.; Hong, X.; Yin, L.; et al. Advances in applying of multi-omics approaches in the research of systemic lupus erythematosus. Int. Rev. Immunol. 2020, 39, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Davidson, A. Taming lupus-a new understanding of pathogenesis is leading to clinical advances. Nat. Med. 2012, 18, 871–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, M.J. Neutrophils in the pathogenesis and manifestations of SLE. Nat. Rev. Rheumatol. 2011, 7, 691–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almaani, S.; Meara, A.; Rovin, B.H. Update on Lupus Nephritis. Clin. J. Am. Soc. Nephrol. 2017, 12, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Wahl, T.S.; Graham, L.A.; Morris, M.S.; Richman, J.S.; Hollis, R.H.; Jones, C.E.; Itani, K.M.; Wagner, T.H.; Mull, H.J.; Whittle, J.C.; et al. Association Between Preoperative Proteinuria and Postoperative Acute Kidney Injury and Readmission. JAMA Surg. 2018, 153, e182009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.Y.; Chinchilli, V.M.; Coca, S.; Devarajan, P.; Ghahramani, N.; Go, A.S.; Hsu, R.K.; Ikizler, T.A.; Kaufman, J.; Liu, K.D.; et al. Post-Acute Kidney Injury Proteinuria and Subsequent Kidney Disease Progression: The Assessment, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury (ASSESS-AKI) Study. JAMA Intern. Med. 2020, 180, 402–410. [Google Scholar] [CrossRef]
- Aljaberi, N.; Bennett, M.; Brunner, H.I.; Devarajan, P. Proteomic profiling of urine: Implications for lupus nephritis. Expert Rev. Proteom. 2019, 16, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Shidham, G.; Ayoub, I.; Birmingham, D.; Hebert, P.; Rovin, B.; Diamond, B.; Wofsy, D.; Hebert, L. Limited Reliability of the Spot Urine Protein/Creatinine Ratio in the Longitudinal Evaluation of Patients With Lupus Nephritis. Kidney Int. Rep. 2018, 3, 1057–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumestre-Perard, C.; Clavarino, G.; Colliard, S.; Cesbron, J.Y.; Thielens, N.M. Antibodies targeting circulating protective molecules in lupus nephritis: Interest as serological biomarkers. Autoimmun. Rev. 2018, 17, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Lugo, L.; Saenz-Garcia, J.; Navarro Quiroz, E.; Gonzalez Torres, H.; Fang, L.; Diaz-Olmos, Y.; Garavito de Egea, G.; Egea Bermejo, E.; Aroca Martinez, G. Plasma cytokines as potential biomarkers of kidney damage in patients with systemic lupus erythematosus. Lupus 2019, 28, 34–43. [Google Scholar] [CrossRef]
- Jhang, J.; Tzeng, I.; Chou, H.; Jang, S.; Hsieh, C.; Ko, Y.; Huang, H. Association Rule Mining and Prognostic Stratification of 2-Year Longevity in Octogenarians Undergoing Endovascular Therapy for Lower Extremity Arterial Disease: Observational Cohort Study. J. Med. Internet Res. 2020, 22, e17487. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Han, H.; Li, J.; Li, D.; Jiang, L. alpha-hydroxybutyrate dehydrogenase as a biomarker for predicting systemic lupus erythematosus with liver injury. Int. Immunopharmacol. 2019, 77, 105922. [Google Scholar] [CrossRef] [PubMed]
- Zickert, A.; Sundelin, B.; Svenungsson, E.; Gunnarsson, I. Role of early repeated renal biopsies in lupus nephritis. Lupus Sci. Med. 2014, 1, e000018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berland, C.; Montalban, E.; Perrin, E.; Di Miceli, M.; Nakamura, Y.; Martinat, M.; Sullivan, M.; Davis, X.S.; Shenasa, M.A.; Martin, C.; et al. Circulating Triglycerides Gate Dopamine-Associated Behaviors through DRD2-Expressing Neurons. Cell Metab. 2020, 31, 773–790.e11. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Nohara, A.; Kawashiri, M. Serum Triglycerides and Atherosclerotic Cardiovascular Disease: Insights from Clinical and Genetic Studies. Nutrients 2018, 10, 1789. [Google Scholar] [CrossRef] [Green Version]
- Ravotti, R.; Worlitschek, J.; Pulham, C.; Stamatiou, A. Triglycerides as Novel Phase-Change Materials: A Review and Assessment of Their Thermal Properties. Molecules 2020, 25, 5572. [Google Scholar] [CrossRef]
- Santos-Baez, L.; Ginsberg, H. Hypertriglyceridemia-Causes, Significance, and Approaches to Therapy. Front. Endocrinol. 2020, 11, 616. [Google Scholar] [CrossRef]
- Ohmura, H. Triglycerides as Residual Risk for Atherosclerotic Cardiovascular Disease. Circ. J. 2019, 83, 969–970. [Google Scholar] [CrossRef] [Green Version]
- Szabo, M.Z.; Szodoray, P.; Kiss, E. Dyslipidemia in systemic lupus erythematosus. Immunol. Res. 2017, 65, 543–550. [Google Scholar] [CrossRef]
- Furie, R.; Rovin, B.H.; Houssiau, F.; Malvar, A.; Teng, Y.K.O.; Contreras, G.; Amoura, Z.; Yu, X.; Mok, C.C.; Santiago, M.B.; et al. Two-Year, Randomized, Controlled Trial of Belimumab in Lupus Nephritis. N. Engl. J. Med. 2020, 383, 1117–1128. [Google Scholar] [CrossRef]
- Qian, G.; Rao, C.R.; Sun, X.; Wu, Y. Boosting association rule mining in large datasets via Gibbs sampling. Proc. Natl. Acad. Sci. USA 2016, 113, 4958–4963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zemedikun, D.T.; Gray, L.J.; Khunti, K.; Davies, M.J.; Dhalwani, N.N. Patterns of Multimorbidity in Middle-Aged and Older Adults: An Analysis of the UK Biobank Data. Mayo Clin. Proc. 2018, 93, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Myoung, S. Comorbidity Study of Attention-deficit Hyperactivity Disorder (ADHD) in Children: Applying Association Rule Mining (ARM) to Korean National Health Insurance Data. Iran. J. Public Health 2018, 47, 481–488. [Google Scholar] [PubMed]
- Ten Doesschate, T.; van Haren, E.; Wijma, R.A.; Koch, B.C.P.; Bonten, M.J.M.; van Werkhoven, C.H. The effectiveness of nitrofurantoin, fosfomycin and trimethoprim for the treatment of cystitis in relation to renal function. Clin. Microbiol. Infect. 2020, 26, 1355–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-Quiroz, E.; Pacheco-Lugo, L.; Lorenzi, H.; Diaz-Olmos, Y.; Almendrales, L.; Rico, E.; Navarro, R.; Espana-Puccini, P.; Iglesias, A.; Egea, E.; et al. High-Throughput Sequencing Reveals Circulating miRNAs as Potential Biomarkers of Kidney Damage in Patients with Systemic Lupus Erythematosus. PLoS ONE 2016, 11, e0166202. [Google Scholar] [CrossRef]
- Marx, V. Biology: The big challenges of big data. Nature 2013, 498, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.C.; Yang, Y.; Li, M.T.; Jia, Y.; Chen, S.; Ye, S.; Zeng, X.Z.; Wang, Z.; Zhao, J.X.; Liu, X.Y.; et al. Macrophage activation syndrome in systemic lupus erythematosus: A multicenter, case-control study in China. Clin. Rheumatol. 2018, 37, 93–100. [Google Scholar] [CrossRef]
- Chen, S.Y.; Du, J.; Lu, X.N.; Xu, J.H. Platelet distribution width as a novel indicator of disease activity in systemic lupus erythematosus. J. Res. Med. Sci. 2018, 23. [Google Scholar]
- Zhou, B.; Xia, Y.L.; She, J.Q. Dysregulated serum lipid profile and its correlation to disease activity in young female adults diagnosed with systemic lupus erythematosus: A cross-sectional study. Lipids Health Dis. 2020, 19, 40. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Ma, N.; Fu, H.T.; Wei, T.T.; Tang, Q.Q.; Qin, B.D.; Yang, Z.X.; Zhong, R.Q. Hematocrit Level could Reflect Inflammatory Response and Disease Activity in Patients with Systemic Lupus Erythematosus. Clin. Lab. 2015, 61, 801–807. [Google Scholar] [CrossRef]
- Reiss, A.B.; Jacob, B.; Ahmed, S.; Carsons, S.E.; DeLeon, J. Understanding Accelerated Atherosclerosis in Systemic Lupus Erythematosus: Toward Better Treatment and Prevention. Inflammation 2021, 44, 1663–1682. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D. HDL abnormalities in nephrotic syndrome and chronic kidney disease. Nat. Rev. Nephrol. 2016, 12, 37–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaziri, N.D. Disorders of lipid metabolism in nephrotic syndrome: Mechanisms and consequences. Kidney Int. 2016, 90, 41–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robciuc, M.R.; Naukkarinen, J.; Ortega-Alonso, A.; Tyynismaa, H.; Raivio, T.; Rissanen, A.; Kaprio, J.; Ehnholm, C.; Jauhiainen, M.; Pietilainen, K.H. Serum angiopoietin-like 4 protein levels and expression in adipose tissue are inversely correlated with obesity in monozygotic twins. J. Lipid Res. 2011, 52, 1575–1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saja, M.; Cook, H.; Ruseva, M.; Szajna, M.; Pickering, M.; Woollard, K.; Botto, M. A triglyceride-rich lipoprotein environment exacerbates renal injury in the accelerated nephrotoxic nephritis model. Clin. Exp. Immunol. 2018, 192, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.Q.; Zhang, W.; Wang, S.; Zhao, L.; Ma, R.; Hu, J.W.; Wang, L.J.; Meng, J.; Zhou, C.L.; Lin, G.; et al. The pentapeptide PLNPK inhibits systemic lupus erythematosus-associated renal damage. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2010, 59, 1081–1089. [Google Scholar] [CrossRef]
- Touzani, S.; Al-Waili, N.; Imtara, H.; Aboulghazi, A.; Hammas, N.; Falcao, S.; Vilas-Boas, M.; Arabi, I.E.; Al-Waili, W.; Lyoussi, B. Arbutus Unedo Honey and Propolis Ameliorate Acute Kidney Injury, Acute Liver Injury, and Proteinuria via Hypoglycemic and Antioxidant Activity in Streptozotocin-Treated Rats. Cell Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2022, 56, 66–81. [Google Scholar]
- Porrini, E.; Ruggenenti, P.; Luis-Lima, S.; Carrara, F.; Jimenez, A.; de Vries, A.P.J.; Torres, A.; Gaspari, F.; Remuzzi, G. Estimated GFR: Time for a critical appraisal. Nat. Rev. Nephrol. 2019, 15, 177–190. [Google Scholar] [CrossRef]

| SLE Patients | Healthy Subjects | p-Value | |
|---|---|---|---|
| (n = 158) | (n = 158) | ||
| Patient demographics | |||
| Age, years | 37.05 (24.74–49.36) | 36.72 (25.59–47.85) | 0.953 |
| Gender, n (%) | 1.000 | ||
| Male | 11 (6.96) | 11 (6.96) | |
| Female | 147 (93.04) | 147 (93.04) | |
| SLE damage index, score | 0.00 (0.00–1.00) | - | - |
| SLEDAI-2K, score | 7.00 (4.00–11.00) | - | - |
| BMI, kg/m2 | 21.09 (19.14–23.05) | - | - |
| Kidney injury, n (%) | 73 (46.20) | - | - |
| Renal biopsy class | 26 (35.62) | - | - |
| III/III + V | 4 (15.4) | - | - |
| IV/IV +V | 18 (69.2) | - | - |
| V only | 4 (15.4) | - | - |
| Creatinine > 108 μmol/L | 14 (19.18) | - | - |
| Proteinuria > 0.5 g/d | 55 (75.34) | - | - |
| Red blood cell > 5/HP | 50 (68.49) | - | - |
| P-CAST | 30 (41.09) | - | - |
| Laboratory indicators | |||
| Triglycerides, mmol/L | 1.68 (1.20–2.44) | 1.08 (0.81–1.45) | <0.000 *** |
| HDL, mmol/L | 0.86 (0.65–1.11) | 1.31 (1.16–1.51) | <0.000 *** |
| LDL, mmol/L | 2.01 (1.58–2.75) | 2.40 (1.69–3.11) | 0.005 ** |
| Total cholesterol, mmol/L | 3.52 (2.88–4.66) | 4.58 (0.82–8.34) | <0.000 *** |
| AST/ALT | 1.53 (1.12–2.09) | 1.33 (1.14–1.65) | 0.003 ** |
| Hemoglobin, g/L | 108.50 (88.00–121.25) | 139.19 (125.68–142.7) | <0.000 *** |
| PDW, fl | 13.70 (12.20–16.20) | 16.75 (15.93–17.57) | <0.000 *** |
| Hematocrit, % | 32.03 (24.48–39.58) | 42.85 (39.09–46.61) | <0.000 *** |
| RDW-SD, fl | 47.50 (43.90–52.90) | 42.55 (39.96–45.14) | <0.000 *** |
| LYM, 109/L | 1.01 (0.68–1.46) | 1.93 (1.44–2.42) | <0.000 *** |
| BUN, mmol/L | 4.71 (3.51–6.83) | 4.54 (3.56–5.52) | 0.087 |
| Creatinine, μmol/L | 61.25 (51.00–77.28) | 57.20 (52.50–65.75) | 0.068 |
| Uric acid, μmol/L | 294.00 (236.00–381.25) | 304.75 (227.71–381.79) | 0.827 |
| LDH, U/L | 213.00 (173.00–314.75) | - | - |
| α-HBDH, U/L | 192.00 (150.00–273.00) | - | - |
| Proteinuria, g/d | 0.43 (0.14–1.81) | - | - |
| Albumin, g/L | 34.21 (26.71–41.71) | 46.40 (44.2–48.6) | <0.000 *** |
| P-CAST/μL | 0.39 (0.00–1.13) | - | - |
| Total protein, g/L | 67.14 (55.38–78.90) | 77.31 (72.58–82.04) | <0.000 *** |
| GFR, ml/min/1.73 m2 | 128.80 (76.23–181.37) | 138.45 (113.3–163.6) | 0.040 * |
| Antecedent | Consequent | Lift | Support (%) | Confidence (%) |
|---|---|---|---|---|
| SLE-kidney dysfunction | Triglyceride | 1.462 | 46.203 | 71.233 |
| SLE-kidney dysfunction | LDH | 1.341 | 46.203 | 60.274 |
| SLE-kidney dysfunction | AST/ALT | 1.176 | 46.203 | 68.493 |
| SLE-kidney dysfunction | α-HBDH | 1.173 | 46.203 | 61.644 |
| SLE-kidney dysfunction | Total cholesterol | 1.169 | 46.203 | 73.973 |
| SLE-kidney dysfunction | Hemoglobin | 1.092 | 46.203 | 78.082 |
| SLE-kidney dysfunction | PDW | 1.036 | 46.203 | 61.644 |
| SLE-kidney dysfunction | Hematocrit | 1.024 | 46.203 | 84.932 |
| SLE-kidney dysfunction | RDW | 1.006 | 46.203 | 63.014 |
| SLE-kidney dysfunction | LYM | 1.003 | 46.203 | 78.082 |
| SLE-Related Kidney Injury | SLE-No Kidney Injury | p-Value | |
|---|---|---|---|
| (n = 73) | (n = 85) | ||
| Patient demographics | |||
| Age, years | 34.00 (25.00–41.50) | 39.00 (28.50–46.00) | 0.019 * |
| Gender (M/F) | 5/68 | 6/79 | 0.959 |
| Disease duration, months | 12.00 (1.40–36.00) | 7.00 (2.00–30.00) | 0.789 |
| BMI, kg/m2 | 21.09 (18.83–23.44) | 21.00 (19.36–22.88) | 0.936 |
| Indicators mined by association rules | |||
| Triglycerides, mmol/L | 2.07 (1.58–3.19) | 1.42 (1.07–1.82) | <0.001 *** |
| HDL, mmol/L | 0.85 (0.60–1.11) | 0.88 (0.72–1.11) | 0.526 |
| LDL, mmol/L | 2.07 (1.45–2.89) | 2.12 (1.33–2.91) | 0.687 |
| LDH, U/L | 256.25 (187.95–350.48) | 196.45 (167.40–240.00) | 0.001 *** |
| AST/ALT | 1.71 (1.10–2.58) | 1.45 (1.13–1.85) | 0.093 |
| α-HBDH, U/L | 231.50 (156.00–316.75) | 183.00 (149.00–213.00) | 0.003 ** |
| Total cholesterol, mmol/L | 3.47 (2.82–5.26) | 3.53 (2.88–4.32) | 0.272 |
| Hemoglobin, g/L | 100.30 (74.09–126.51) | 110.06 (76.75–124.45) | 0.015 * |
| PDW, fl | 13.70 (11.60–16.05) | 13.65 (12.48–16.25) | 0.424 |
| Hematocrit, % | 29.95 (21.78–38.12) | 34.30 (29.45–37.25) | 0.001 *** |
| RDW-SD, fl | 47.50 (44.00–52.50) | 47.65 (43.83–53.45) | 0.938 |
| LYM, 109/L | 0.99 (0.61–1.44) | 1.05 (0.70–1.48) | 0.438 |
| Index | ORs (95% CIs) | p-Value |
|---|---|---|
| Triglycerides, mmol/L | 2.44 (1.48–4.03) | 0.001 ** |
| LDH, U/L | 1.01 (0.10–1.03) | 0.179 |
| α-HBDH, U/L | 0.99 (0.98–1.01) | 0.371 |
| Hemoglobin, g/L | 1.08 (0.99–1.18) | 0.074 |
| Hematocrit, % | 0.73 (0.54–1.00) | 0.050 |
| Age, years | 0.99 (0.95–1.02) | 0.410 |
| Gender, male/female | 0.48 (0.09–2.54) | 0.388 |
| BMI, kg/m2 | 1.02 (0.89–1.16) | 0.828 |
| Low-Triglycerides (n = 88) | High-Triglycerides (n = 68) | p-Value | |
|---|---|---|---|
| Patient demographics | |||
| Age, years | 39.10 (27.4–50.8) | 32.50 (24–45) | 0.028 * |
| Gender, male/female | 6/82 | 5/63 | 1.000 |
| SLEDAI-2K, score | 5.00 (3.25–9.00) | 9.00 (4.00–14.00) | <0.001 *** |
| Disease duration, months | 10.50 (2.00–36.00) | 7.75 (2.25–24.88) | 0.857 |
| BMI | 20.75 (19.23–23.18) | 21.31 (18.38–24.24) | 0.781 |
| Kidney injury, n (%) | 26 (29.55) | 47 (69.12) | <0.001 *** |
| Gallbladder disorders, n (%) | 19 (21.59) | 13 (19.12) | 0.704 |
| Cardiovascular diseases, n (%) | 16 (18.18) | 20 (29.41) | 0.099 |
| Hematologic disorders, n (%) | 15 (17.05) | 11 (16.18) | 0.885 |
| Liver injury, n (%) | 12 (13.64) | 8 (11.76) | 0.729 |
| Thyroid disorders, n (%) | 11 (12.50) | 9 (13.24) | 0.892 |
| Splenic disorders, n (%) | 7 (7.95) | 4 (5.88) | 0.852 |
| Neurological symptoms, n (%) | 3 (3.41) | 4 (5.88) | 0.726 |
| Laboratory indicators | |||
| Blood urea nitrogen, mmol/L | 4.35 (3.36–5.49) | 5.66 (3.79–9.29) | 0.004 ** |
| Creatinine, μmol/L | 58.90 (51.00–66.15) | 65.35 (51.48–98.95) | 0.024 * |
| Anti-ds-DNA antibody (+), n (%) | 17 (19.32%) | 14 (20.59%) | 0.888 |
| Anti-sm antibody (+), n (%) | 26 (29.55%) | 23 (33.82%) | 0.396 |
| ESR, mm/h | 52.00 (18.75–86.00) | 43.00 (27.00–79.00) | 0.644 |
| Immunoglobulin G, g/L | 17.85 (13.90–25.13) | 14.90 (9.89–19.50) | 0.002 ** |
| Immunoglobulin A, g/L | 2.66 (2.01–3.70) | 3.01 (2.21–3.52) | 0.436 |
| Immunoglobulin M, g/L | 1.38 (0.96–2.03) | 1.24 (0.90–1.81) | 0.650 |
| Complement 3, g/L | 0.71 (0.40–0.96) | 0.50 (0.32–0.77) | 0.010 * |
| Complement 4, g/L | 0.14 (0.06–0.23) | 0.095 (0.06–0.17) | 0.168 |
| CRP, mg/L | 3.89 (3.27–13.05) | 3.34 (3.08–9.68) | 0.267 |
| Proteinuria, g/24 h | 0.25 (0.13–0.83) | 0.95 (0.30–3.14) | <0.001 *** |
| Albumin, g/L | 37.55 (31.63–42.05) | 31.37 (23.93–38.81) | <0.001 *** |
| P-CAST/μL | 0.25 (0.00–0.59) | 0.52 (0.12–2.29) | 0.001 ** |
| Total protein, g/L | 70.67 (60.5–80.84) | 62.21 (50.22–74.2) | <0.001 *** |
| Proteinuria (+), n | Proteinuria (−), n | Total | |
|---|---|---|---|
| High triglycerides, n | 37 | 8 | 45 |
| Low triglycerides, n | 17 | 8 | 25 |
| Total | 54 | 16 | 70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, M.; Li, D.; Liu, T.; Cai, Y.; Yang, J.; Jiang, L.; Yu, H. Triglycerides as Biomarker for Predicting Systemic Lupus Erythematosus Related Kidney Injury of Negative Proteinuria. Biomolecules 2022, 12, 945. https://doi.org/10.3390/biom12070945
Si M, Li D, Liu T, Cai Y, Yang J, Jiang L, Yu H. Triglycerides as Biomarker for Predicting Systemic Lupus Erythematosus Related Kidney Injury of Negative Proteinuria. Biomolecules. 2022; 12(7):945. https://doi.org/10.3390/biom12070945
Chicago/Turabian StyleSi, Mingjun, Danyang Li, Ting Liu, Yuanyan Cai, Jingyu Yang, Lili Jiang, and Haitao Yu. 2022. "Triglycerides as Biomarker for Predicting Systemic Lupus Erythematosus Related Kidney Injury of Negative Proteinuria" Biomolecules 12, no. 7: 945. https://doi.org/10.3390/biom12070945
APA StyleSi, M., Li, D., Liu, T., Cai, Y., Yang, J., Jiang, L., & Yu, H. (2022). Triglycerides as Biomarker for Predicting Systemic Lupus Erythematosus Related Kidney Injury of Negative Proteinuria. Biomolecules, 12(7), 945. https://doi.org/10.3390/biom12070945






