Excess Heme Promotes the Migration and Infiltration of Macrophages in Endometrial Hyperplasia Complicated with Abnormal Uterine Bleeding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Sample Preparation and Immunocyte Isolation
2.3. Flow Cytometry Analysis (FCM)
2.4. Cells
2.5. The Treatment of Heme and HO-1 Inhibitor and Nrf2 Inhibitor
2.6. Polymerase Chain Reaction (PCR)
2.7. Integration Analysis of the Protein–Protein Interaction (PPI) Network
2.8. Chemotaxis Assay
2.9. Statistical Analysis
3. Results
3.1. Baseline Characteristics
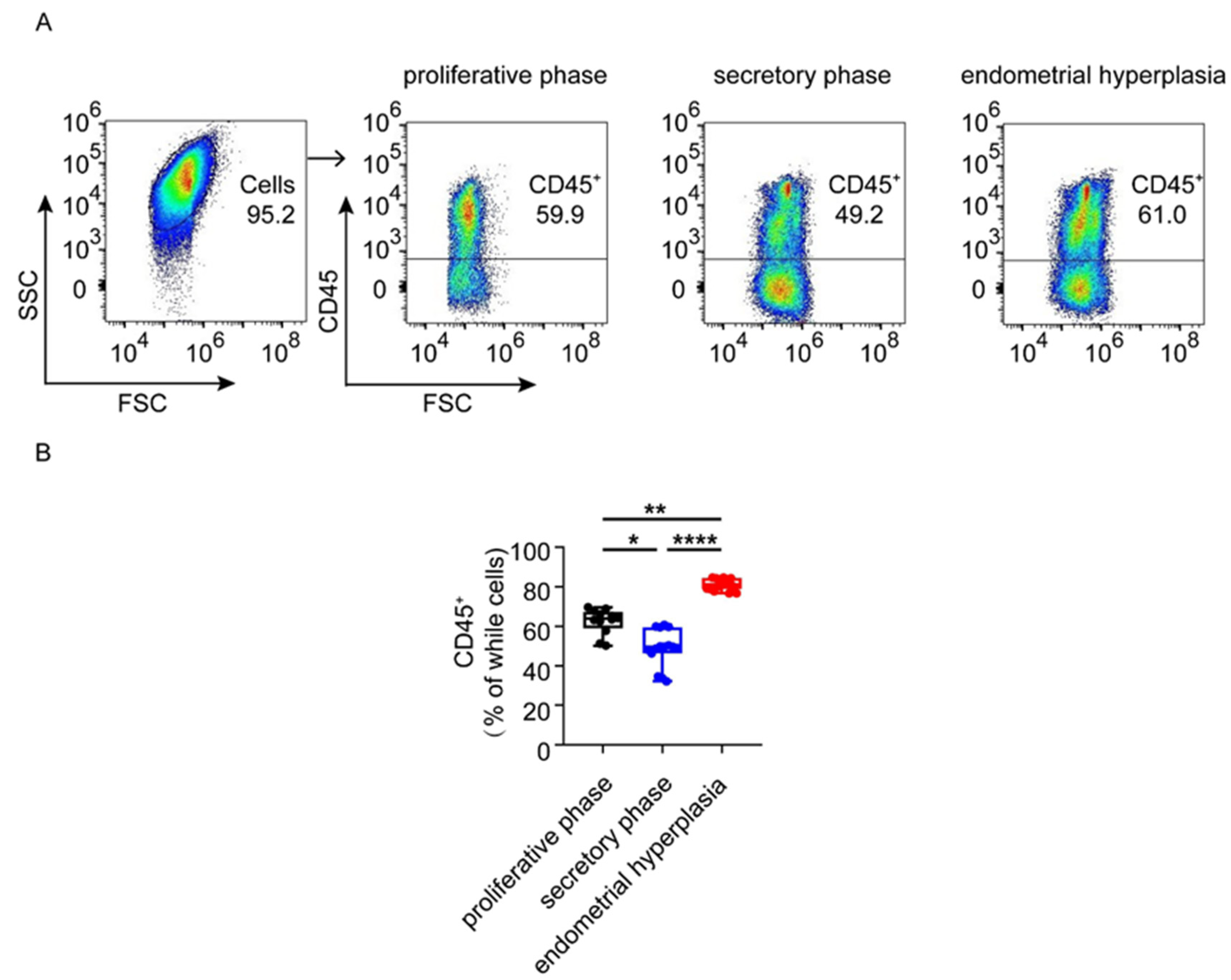
3.2. The Infiltration and Accumulation of CD45+ Immune Cells Are Increased in Endometrium from Patients with EH
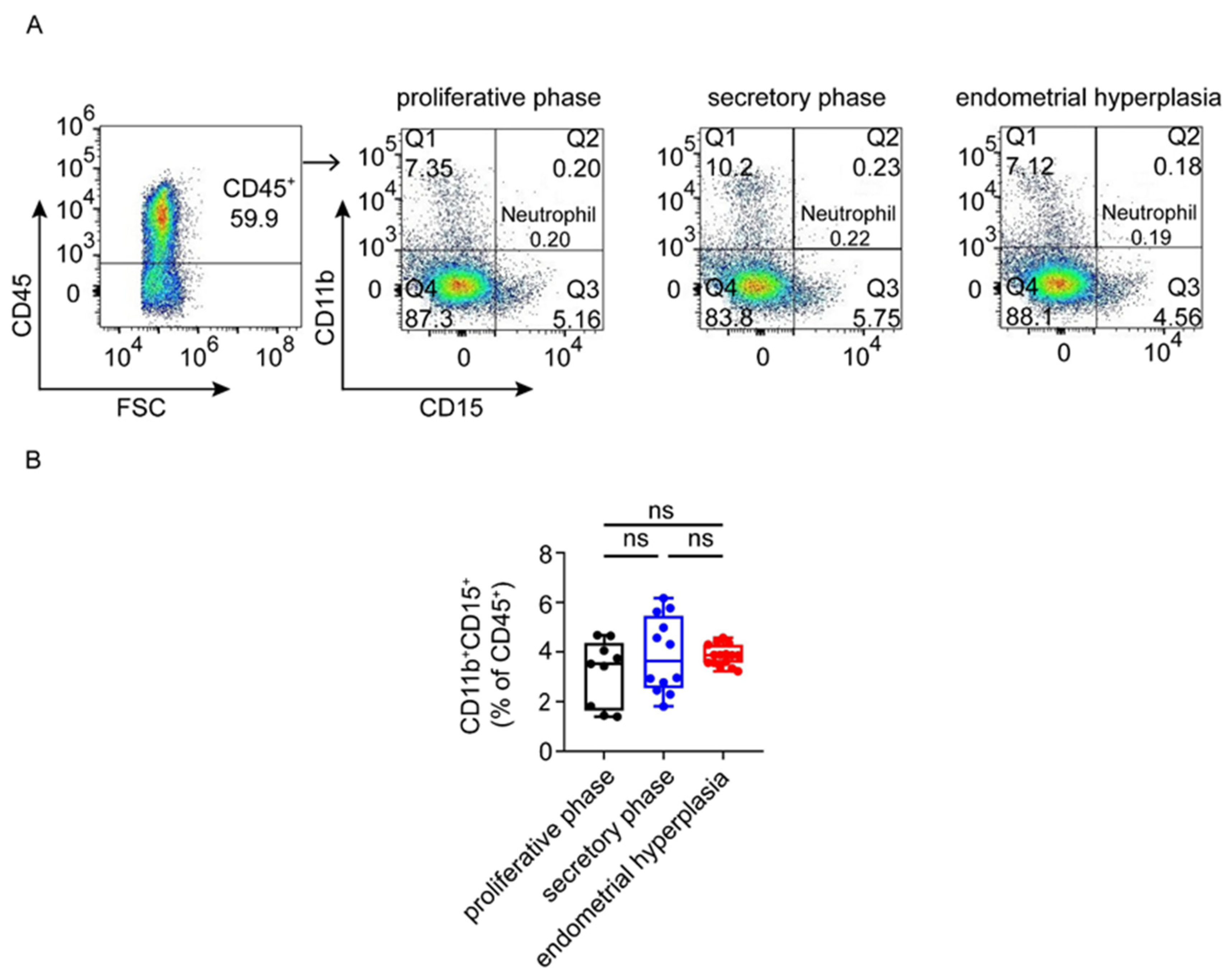
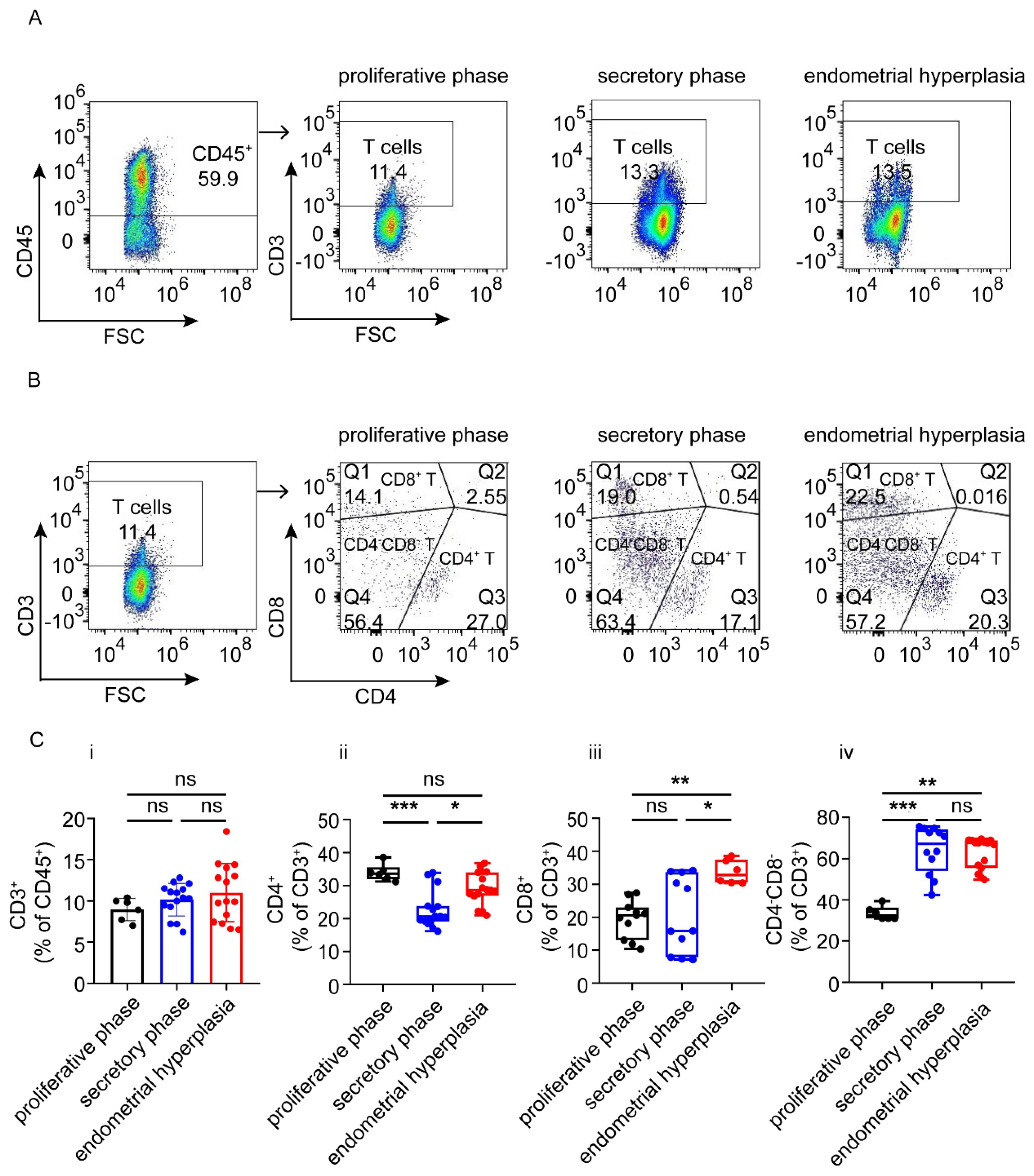
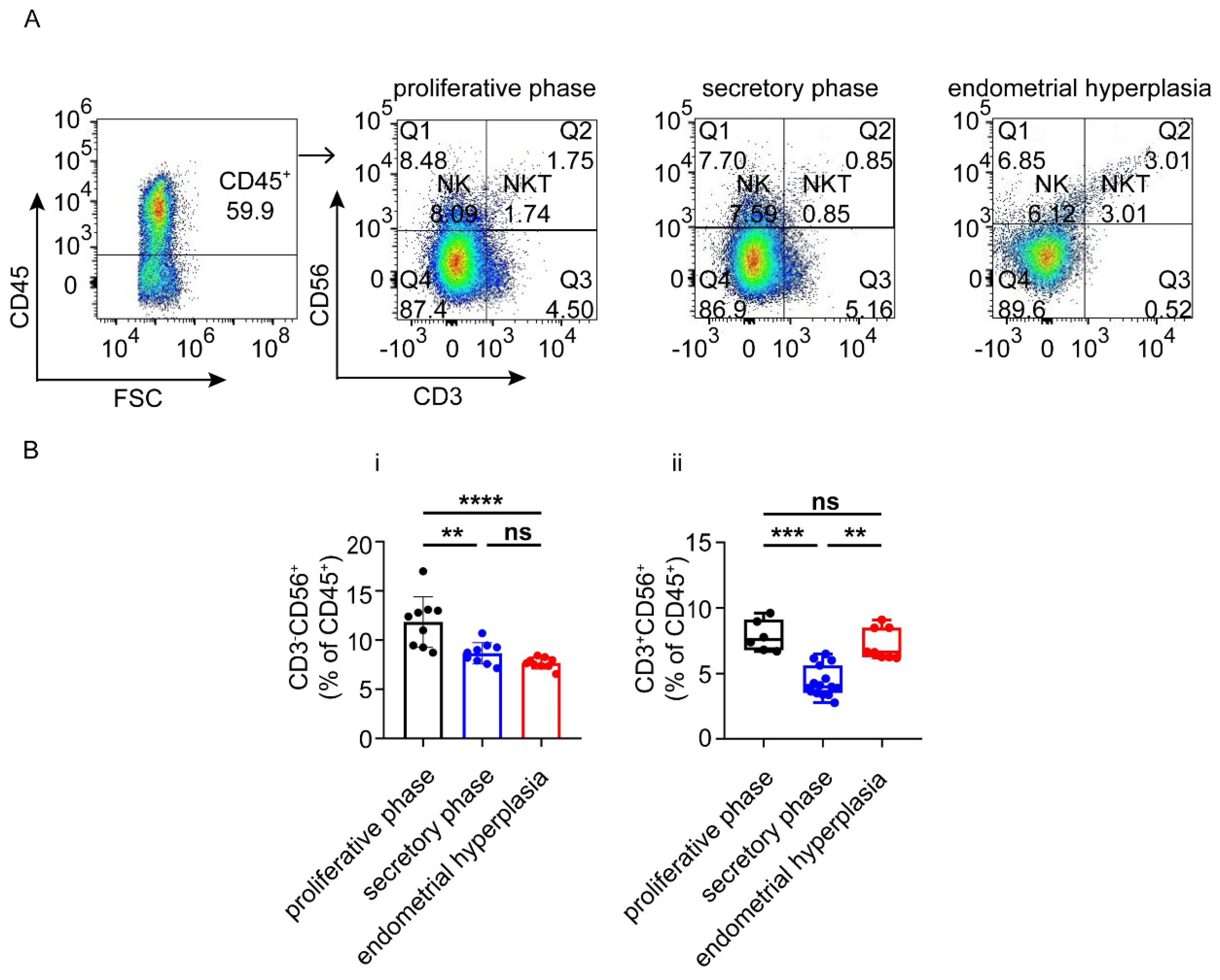
3.3. There Are Increased Ratios of CD8+ Cells and Macrophages, and Decreased NK Cells in Endometrium from EH Patients
3.4. Macrophages Are Largely Infiltrated and Increased in Endometrium from EH Patients
3.5. HO-1 and Nrf2 Inhibitors Increase the Expression of Chemokines in Endometrial Epithelial Cells (EEC)
3.6. Excess Heme Increases the Expression of Chemokines in EECs and Migration of Macrophages in Co-Culture System
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ottnad, E.; Parthasarathy, S.; Sambrano, G.R.; Ramprasad, M.P.; Quehenberger, O.; Kondratenko, N.; Green, S.; Steinberg, D. A macrophage receptor for oxidized low density lipoprotein distinct from the receptor for acetyl low density lipoprotein: Partial purification and role in recognition of oxidatively damaged cells. Proc. Natl. Acad. Sci. USA 1995, 92, 1391–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozdegirmenci, O.; Kayikcioglu, F.; Bozkurt, U.; Akgul, M.A.; Haberal, A. Comparison of the efficacy of three progestins in the treatment of simple endometrial hyperplasia without atypia. Gynecol. Obstet. Invest. 2011, 72, 10–14. [Google Scholar] [CrossRef]
- Sanderson, P.A.; Critchley, H.O.; Williams, A.R.; Arends, M.J.; Saunders, P.T. New concepts for an old problem: The diagnosis of endometrial hyperplasia. Hum. Reprod. Update 2017, 23, 232–254. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.Z.; Ruan, L.Y.; Wang, Y.; Yang, H.L.; Shi, J.W.; Wu, J.N.; Qiu, X.M.; Ha, S.Y.; Shen, H.H.; Yang, S.L.; et al. Changes in subsets of immunocytes in endometrial hyperplasia. Am. J. Reprod. Immunol. 2020, 84, e13295. [Google Scholar] [CrossRef] [PubMed]
- Acmaz, G.; Aksoy, H.; Unal, D.; Ozyurt, S.; Cingillioglu, B.; Aksoy, U.; Muderris, I. Are neutrophil/lymphocyte and platelet/lymphocyte ratios associated with endometrial precancerous and cancerous lesions in patients with abnormal uterine bleeding? Asian Pac. J. Cancer Prev. 2014, 15, 1689–1692. [Google Scholar] [CrossRef] [Green Version]
- Benetti-Pinto, C.L.; Rosa, E.S.A.; Yela, D.A.; Soares Junior, J.M. Abnormal Uterine Bleeding. Rev. Bras. Ginecol. Obstet. 2017, 39, 358–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahamondes, L.; Ali, M. Recent advances in managing and understanding menstrual disorders. F1000Prime Rep. 2015, 7, 33. [Google Scholar] [CrossRef]
- Cheong, Y.; Cameron, I.T.; Critchley, H.O.D. Abnormal uterine bleeding. Br. Med. Bull. 2017, 123, 103–114. [Google Scholar] [CrossRef]
- Munro, M.G.; Critchley, H.O.D.; Fraser, I.S.; Committee, F.M.D. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J. Gynaecol. Obstet. 2018, 143, 393–408. [Google Scholar] [CrossRef] [Green Version]
- Marnach, M.L.; Laughlin-Tommaso, S.K. Evaluation and Management of Abnormal Uterine Bleeding. Mayo Clin. Proc. 2019, 94, 326–335. [Google Scholar] [CrossRef] [Green Version]
- Munro, M.G.; Critchley, H.; Fraser, I.S. Research and clinical management for women with abnormal uterine bleeding in the reproductive years: More than PALM-COEIN. BJOG 2017, 124, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.; Knapp, S. Heme and hemolysis in innate immunity: Adding insult to injury. Curr. Opin. Immunol. 2018, 50, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Liu, Y.K.; Hu, W.T.; Tang, L.L.; Sheng, Y.R.; Wei, C.Y.; Li, M.Q.; Zhu, X.Y. Elevated heme impairs macrophage phagocytosis in endometriosis. Reproduction 2019, 158, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Xie, L.; Cheng, Y.; Shi, Y.; Shan, W.; Ning, C.; Xie, B.; Yang, B.; Luo, X.; He, Q.; et al. A20-mediated deubiquitination of ERalpha in the microenvironment of CD163(+) macrophages sensitizes endometrial cancer cells to estrogen. Cancer Lett. 2019, 442, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.J.; Abudukeyoumu, A.; Zhang, X.; Liu, L.B.; Li, M.Q.; Xie, F. Heme oxygenase 1: A novel oncogene in multiple gynecological cancers. Int. J. Biol. Sci. 2021, 17, 2252–2261. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, P.; Vijayan, V.; Gueler, F.; Immenschuh, S. Interplay of Heme with Macrophages in Homeostasis and Inflammation. Int. J. Mol. Sci. 2020, 21, 740. [Google Scholar] [CrossRef] [Green Version]
- Bourdon, M.; Santulli, P.; Jeljeli, M.; Vannuccini, S.; Marcellin, L.; Doridot, L.; Petraglia, F.; Batteux, F.; Chapron, C. Immunological changes associated with adenomyosis: A systematic review. Hum. Reprod. Update 2021, 27, 108–129. [Google Scholar] [CrossRef]
- Raffone, A.; Seracchioli, R.; Raimondo, D.; Maletta, M.; Travaglino, A.; Raimondo, I.; Giaquinto, I.; Orsini, B.; Insabato, L.; Pellicano, M.; et al. Prevalence of adenomyosis in endometrial cancer patients: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2021, 303, 47–53. [Google Scholar] [CrossRef]
- Miura, S.; Khan, K.N.; Kitajima, M.; Hiraki, K.; Moriyama, S.; Masuzaki, H.; Samejima, T.; Fujishita, A.; Ishimaru, T. Differential infiltration of macrophages and prostaglandin production by different uterine leiomyomas. Hum. Reprod. 2006, 21, 2545–2554. [Google Scholar] [CrossRef] [Green Version]
- Wallace, A.E.; Gibson, D.A.; Saunders, P.T.; Jabbour, H.N. Inflammatory events in endometrial adenocarcinoma. J. Endocrinol. 2010, 206, 141–157. [Google Scholar] [CrossRef] [Green Version]
- Berbic, M.; Fraser, I.S. Immunology of normal and abnormal menstruation. Womens Health 2013, 9, 387–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radovic Janosevic, D.; Trandafilovic, M.; Krtinic, D.; Colovic, H.; Milosevic Stevanovic, J.; Pop-Trajkovic Dinic, S. Endometrial immunocompetent cells in proliferative and secretory phase of normal menstrual cycle. Folia Morphol. 2019, 79, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Salamonsen, L.A.; Lathbury, L.J. Endometrial leukocytes and menstruation. Hum. Reprod. Update 2000, 6, 16–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salamonsen, L.A.; Woolley, D.E. Menstruation: Induction by matrix metalloproteinases and inflammatory cells. J. Reprod. Immunol. 1999, 44, 1–27. [Google Scholar] [CrossRef]
- Chernyshov, V.P.; Dons’koi, B.V.; Sudoma, I.O.; Goncharova, Y.O. Comparison of T and NK lymphocyte subsets between human endometrial tissue and peripheral blood. Cent. Eur. J. Immunol. 2019, 44, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.I.; Stankovic, S.; Baxter, A.G. Raising the NKT cell family. Nat. Immunol. 2010, 11, 197–206. [Google Scholar] [CrossRef]
- King, A.; Loke, Y.W. On the nature and function of human uterine granular lymphocytes. Immunol. Today 1991, 12, 432–435. [Google Scholar] [CrossRef]
- Ho, H.N.; Chao, K.H.; Chen, C.K.; Yang, Y.S.; Huang, S.C. Activation status of T and NK cells in the endometrium throughout menstrual cycle and normal and abnormal early pregnancy. Hum. Immunol. 1996, 49, 130–136. [Google Scholar] [CrossRef]
- Hickey, M.; Crewe, J.; Goodridge, J.P.; Witt, C.S.; Fraser, I.S.; Doherty, D.; Christiansen, F.T.; Salamonsen, L.A. Menopausal hormone therapy and irregular endometrial bleeding: A potential role for uterine natural killer cells? J. Clin. Endocrinol. Metab. 2005, 90, 5528–5535. [Google Scholar] [CrossRef] [Green Version]
- Witkiewicz, A.K.; McConnell, T.; Potoczek, M.; Emmons, R.V.; Kurman, R.J. Increased natural killer cells and decreased regulatory T cells are seen in complex atypical endometrial hyperplasia and well-differentiated carcinoma treated with progestins. Hum. Pathol. 2010, 41, 26–32. [Google Scholar] [CrossRef]
- Kaitu’u-Lino, T.J.; Morison, N.B.; Salamonsen, L.A. Neutrophil depletion retards endometrial repair in a mouse model. Cell Tissue Res. 2007, 328, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, C.J.; Kim, D.J.; Kang, J.H. Immune cells in the female reproductive tract. Immune Netw. 2015, 15, 16–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wira, C.R.; Rodriguez-Garcia, M.; Patel, M.V. The role of sex hormones in immune protection of the female reproductive tract. Nat. Rev. Immunol. 2015, 15, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Bonatz, G.; Hansmann, M.L.; Buchholz, F.; Mettler, L.; Radzun, H.J.; Semm, K. Macrophage- and lymphocyte-subtypes in the endometrium during different phases of the ovarian cycle. Int. J. Gynaecol. Obstet. 1992, 37, 29–36. [Google Scholar] [CrossRef]
- Berbic, M.; Schulke, L.; Markham, R.; Tokushige, N.; Russell, P.; Fraser, I.S. Macrophage expression in endometrium of women with and without endometriosis. Hum. Reprod. 2009, 24, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Pires, M.A.; Payan-Carreira, R. Resident Macrophages and Lymphocytes in the Canine Endometrium. Reprod. Domest. Anim. 2015, 50, 740–749. [Google Scholar] [CrossRef]
- Cros, J.; Cagnard, N.; Woollard, K.; Patey, N.; Zhang, S.Y.; Senechal, B.; Puel, A.; Biswas, S.K.; Moshous, D.; Picard, C.; et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 2010, 33, 375–386. [Google Scholar] [CrossRef] [Green Version]
- Jena, M.K.; Nayak, N.; Chen, K.; Nayak, N.R. Role of Macrophages in Pregnancy and Related Complications. Arch. Immunol. Ther. Exp. 2019, 67, 295–309. [Google Scholar] [CrossRef]
- Yang, C.H.; Almomen, A.; Wee, Y.S.; Jarboe, E.A.; Peterson, C.M.; Janat-Amsbury, M.M. An estrogen-induced endometrial hyperplasia mouse model recapitulating human disease progression and genetic aberrations. Cancer Med. 2015, 4, 1039–1050. [Google Scholar] [CrossRef]
- Lian, Z.; Niwa, K.; Tagami, K.; Hashimoto, M.; Gao, J.; Yokoyama, Y.; Mori, H.; Tamaya, T. Preventive effects of isoflavones, genistein and daidzein, on estradiol-17beta-related endometrial carcinogenesis in mice. Jpn. J. Cancer Res. 2001, 92, 726–734. [Google Scholar] [CrossRef]
- Mayerhofer, M.; Gleixner, K.V.; Mayerhofer, J.; Hoermann, G.; Jaeger, E.; Aichberger, K.J.; Ott, R.G.; Greish, K.; Nakamura, H.; Derdak, S.; et al. Targeting of heat shock protein 32 (Hsp32)/heme oxygenase-1 (HO-1) in leukemic cells in chronic myeloid leukemia: A novel approach to overcome resistance against imatinib. Blood 2008, 111, 2200–2210. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hannafon, B.N.; Zhou, J.; Ding, W.Q. Clofibrate induces heme oxygenase 1 expression through a PPARalpha-independent mechanism in human cancer cells. Cell Physiol. Biochem. 2013, 32, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Wagener, F.A.; Volk, H.D.; Willis, D.; Abraham, N.G.; Soares, M.P.; Adema, G.J.; Figdor, C.G. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol. Rev. 2003, 55, 551–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, F.F.; Abdelzaher, W.Y.; Ibrahim, R.A.; Elroby Ali, D.M. Amelioration of estrogen-induced endometrial hyperplasia in female rats by hemin via heme-oxygenase-1 expression, suppression of iNOS, p38 MAPK, and Ki67. Can. J. Physiol. Pharmacol. 2019, 97, 1159–1168. [Google Scholar] [CrossRef]
- Vijayan, V.; Wagener, F.; Immenschuh, S. The macrophage heme-heme oxygenase-1 system and its role in inflammation. Biochem. Pharmacol. 2018, 153, 159–167. [Google Scholar] [CrossRef]
- Zhong, H.; Yazdanbakhsh, K. Hemolysis and immune regulation. Curr. Opin. Hematol. 2018, 25, 177–182. [Google Scholar] [CrossRef]
- Raghu, H.; Lepus, C.M.; Wang, Q.; Wong, H.H.; Lingampalli, N.; Oliviero, F.; Punzi, L.; Giori, N.J.; Goodman, S.B.; Chu, C.R.; et al. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann. Rheum. Dis. 2017, 76, 914–922. [Google Scholar] [CrossRef] [Green Version]
- Schaer, C.A.; Schoedon, G.; Imhof, A.; Kurrer, M.O.; Schaer, D.J. Constitutive endocytosis of CD163 mediates hemoglobin-heme uptake and determines the noninflammatory and protective transcriptional response of macrophages to hemoglobin. Circ. Res. 2006, 99, 943–950. [Google Scholar] [CrossRef] [Green Version]
- Landis, R.C.; Quimby, K.R.; Greenidge, A.R. M1/M2 Macrophages in Diabetic Nephropathy: Nrf2/HO-1 as Therapeutic Targets. Curr. Pharm. Des. 2018, 24, 2241–2249. [Google Scholar] [CrossRef]
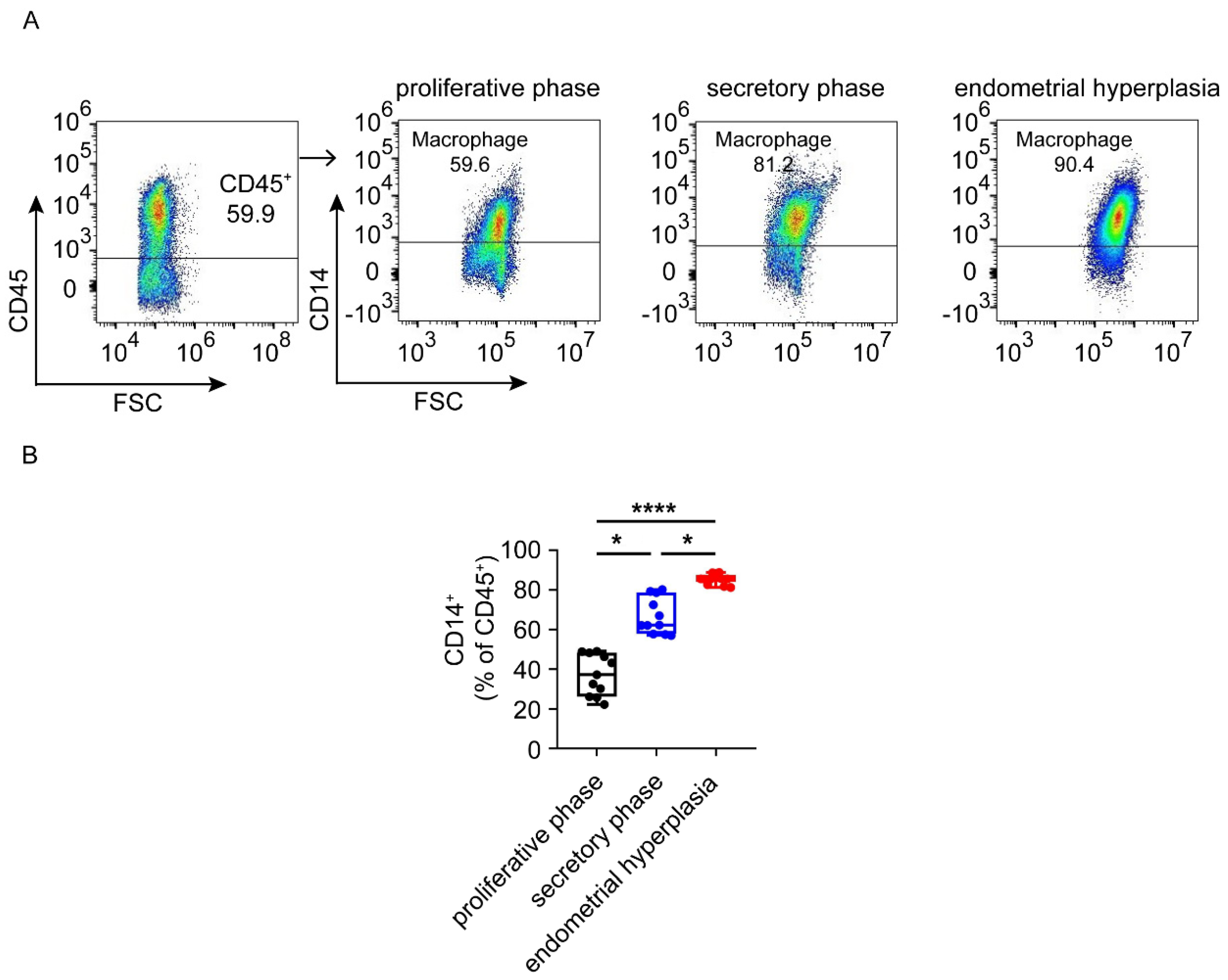
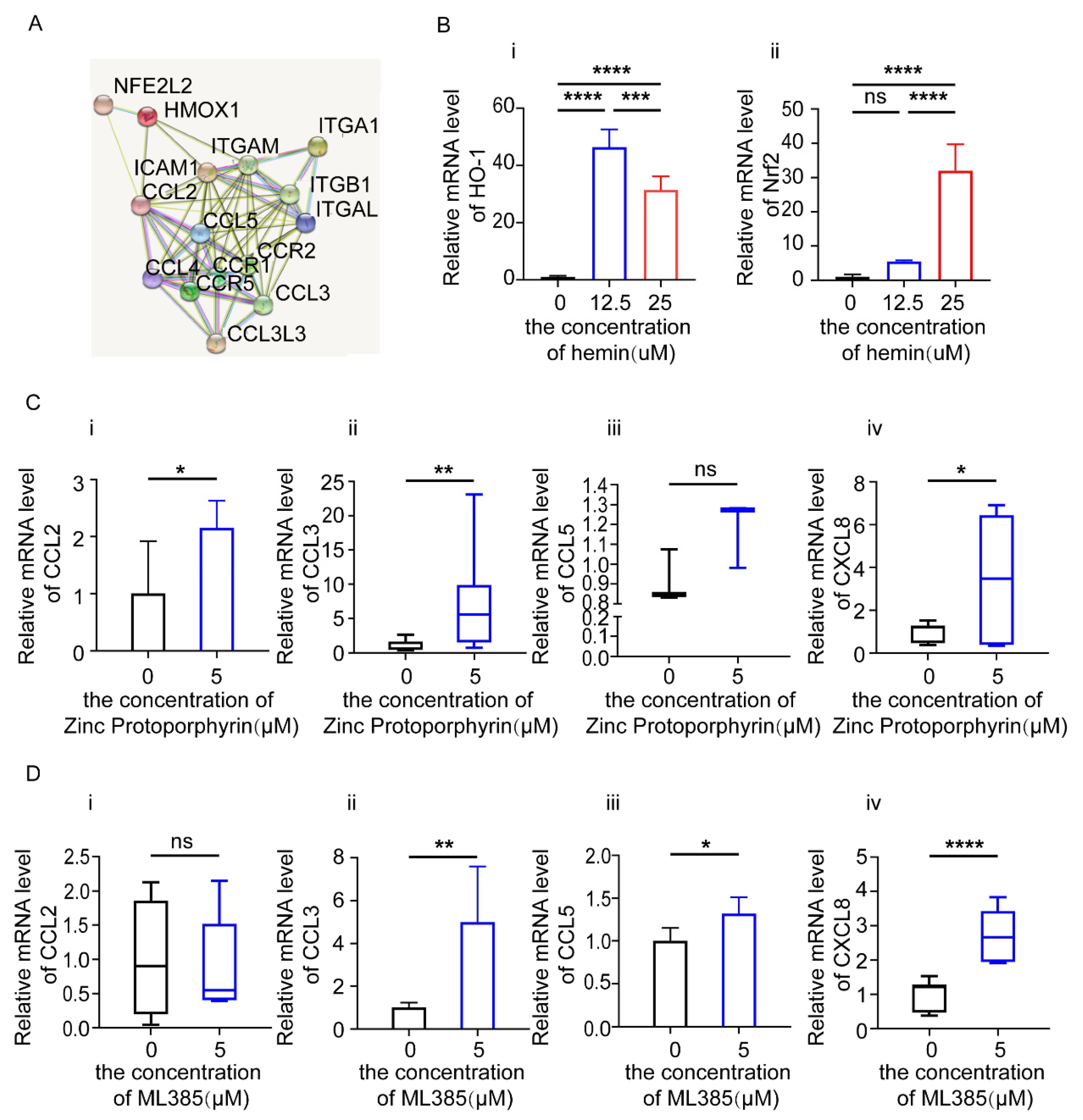
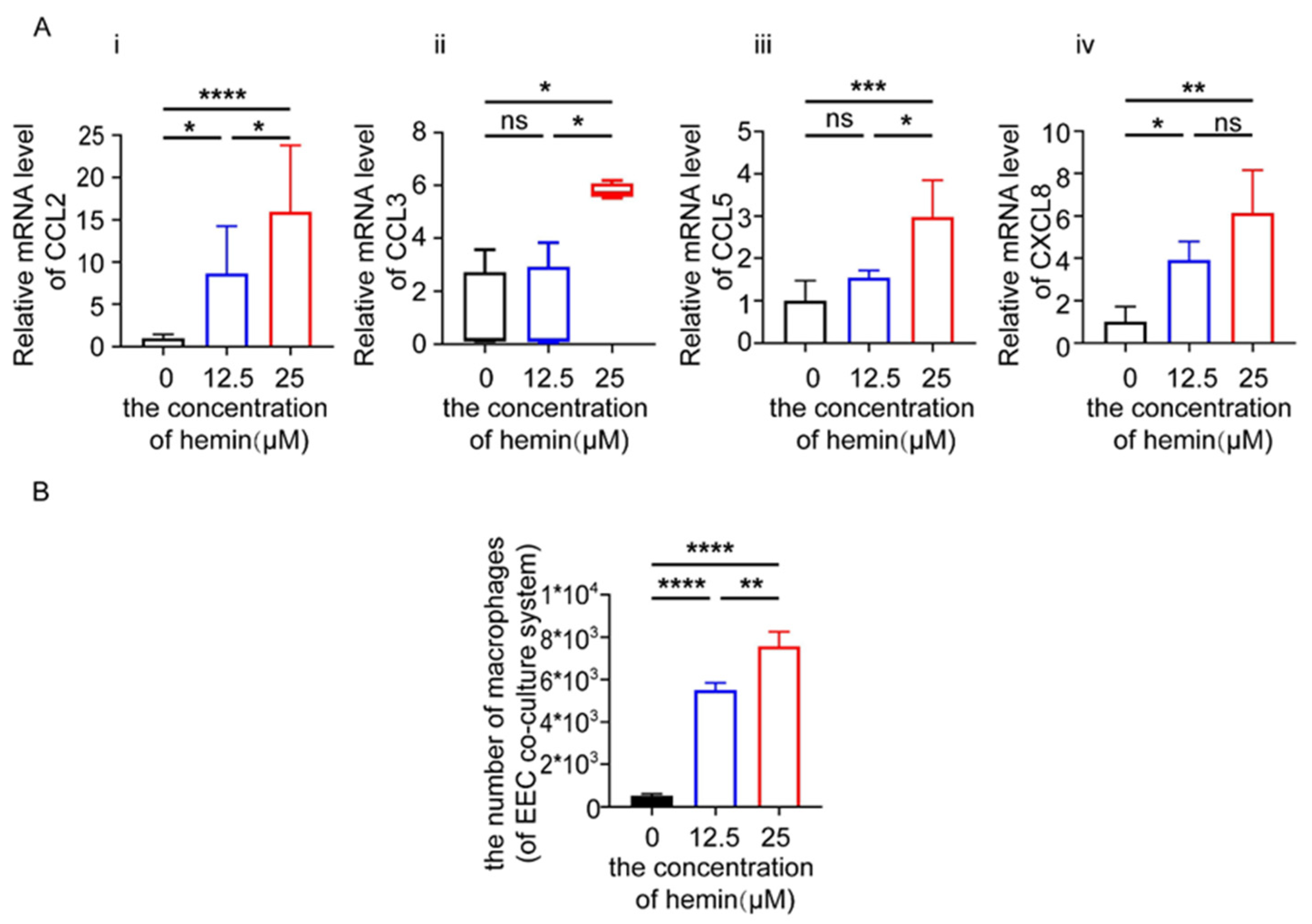
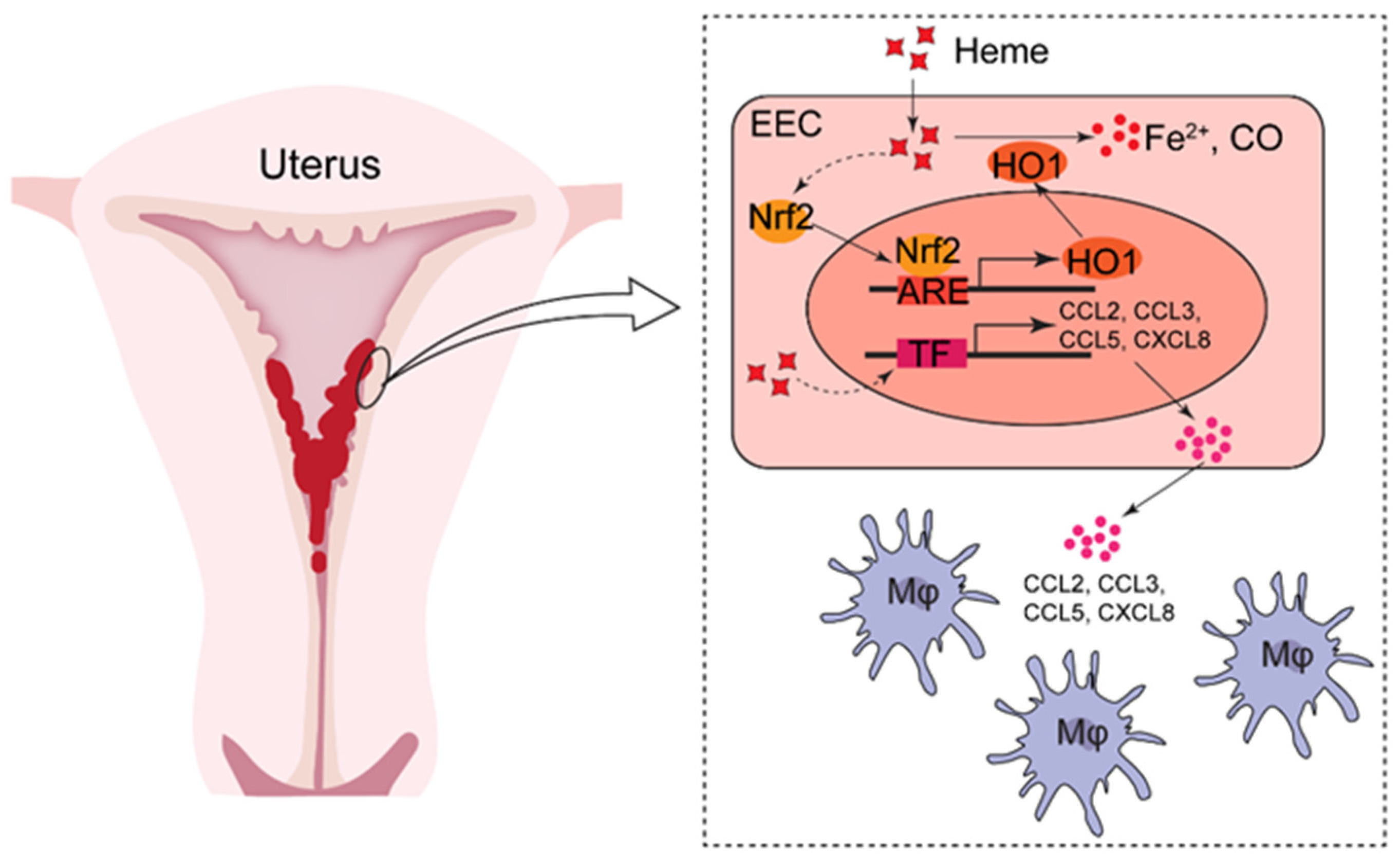
| Proliferative Phase | Secretory Phase | Endometrial Hyperplasia | |
|---|---|---|---|
| Cases | 15 | 15 | 15 |
| Pathology reports | Proliferative period of the menstrual cycle | Secretory phase of the menstrual cycle | Endometrial hyperplasia |
| Age (years) | 35.40 ± 6.98 | 39.0 ± 12.10 | 36.78 ± 2.83 |
| Endometrial thickness (mm) | 9.2 ± 4.96 | 8.2 ± 1.48 | 13.56 ± 1.06 |
| Clinical symptoms | AUB | AUB | AUB |
| Polycystic Ovary Syndrome (PCOS) | Without | Without | Without |
| Glucose level | Normal | Normal | Normal |
| Insulin level | Normal | Normal | Normal |
| Antibody | Manufacturer | Cat No. |
|---|---|---|
| Allophycocyanin/Cyanine 7 (APC/Cy7) Anti-human CD45 | BioLegend | 368515 |
| Phycoerythrin (PE)/ Cy7 Anti-human CD14 | BioLegend | 982510 |
| Brilliant violent (BV) 605 Anti-human CD56 | BioLegend | 362537 |
| Fluorescein isothiocyanate (FITC) Anti-human CD3 | BioLegend | 981002 |
| PE Anti-human CD16 | BioLegend | 980102 |
| PE Anti-human CD4 | BioLegend | 980804 |
| PE Anti-human CD11b | BioLegend | 982606 |
| PE/ Cy7 Anti-human CD8 | BioLegend | 980910 |
| PE/Cy7 Anti-human CD15 | BioLegend | 301923 |
| Gene | Sequence (5’→3’) |
|---|---|
| ACTB | Forwards: GCCGACAGGATGCAGAAGGAGATCA |
| Reverse: AAGCATTTGCGGTGGACGATGGA | |
| CCL2 | Forwards: TCGCTCAGCCAGATGCAATCAATG |
| Reverse: AGATCACAGCTTCTTTGGGACACTTG | |
| CCL3 | Forwards: CATGGCTCTCTGCAACCAGTTCTC |
| Reverse: CTGGCTGCTCGTCTCAAAGTAGTC | |
| CCL5 | Forwards: CTCGCTGTCATCCTCATTGCTACTG |
| Reverse: TTGCCACTGGTGTAGAAATACTCCTTG | |
| CXCL8 | Forwards: CTCTCTTGGCAGCCTTCCTGATTTC |
| Reverse: TTTGGGGTGGAAAGGTTTGGAGTATG | |
| HO-1 | Forwards: TGCCAGTGCCACCAAGTTCAAG |
| Reverse: TGTTGAGCAGGAACGCAGTCTTG | |
| Nrf2 | Forwards: AGTCCAGAAGCCAAACTGACAGAAG |
| Reverse: GGAGAGGATGCTGCTGAAGGAATC |
| Immunocytes Subsets | Proliferative Phase | Secretory Phase | Endometrial Hyperplasia |
|---|---|---|---|
| Immunocytes | +++ | +++ ↓ * | ++++ ↑ ** |
| Macrophages | +++ | ++++ ↑ * | ++++ ↑ **** |
| T cells total | + | + ns | + ns |
| CD4+ | ++ | + ↓ *** | ++ ↑ # |
| CD8+ | + | + ↓ $ | ++ ↑ ** |
| CD4− CD8− | +++ | ++++ ↑ *** | +++ ↑ ** |
| NK | −/+ | −/+ ↓ ** | −/+ ↓ **** |
| NKT | − | − ↓ *** | − ↓ ## |
| Neutrophils | − | − ns | − ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, L.-Y.; Lai, Z.-Z.; Shi, J.-W.; Yang, H.-L.; Ye, J.-F.; Xie, F.; Qiu, X.-M.; Zhu, X.-Y.; Li, M.-Q. Excess Heme Promotes the Migration and Infiltration of Macrophages in Endometrial Hyperplasia Complicated with Abnormal Uterine Bleeding. Biomolecules 2022, 12, 849. https://doi.org/10.3390/biom12060849
Ruan L-Y, Lai Z-Z, Shi J-W, Yang H-L, Ye J-F, Xie F, Qiu X-M, Zhu X-Y, Li M-Q. Excess Heme Promotes the Migration and Infiltration of Macrophages in Endometrial Hyperplasia Complicated with Abnormal Uterine Bleeding. Biomolecules. 2022; 12(6):849. https://doi.org/10.3390/biom12060849
Chicago/Turabian StyleRuan, Lu-Yu, Zhen-Zhen Lai, Jia-Wei Shi, Hui-Li Yang, Jiang-Feng Ye, Feng Xie, Xue-Min Qiu, Xiao-Yong Zhu, and Ming-Qing Li. 2022. "Excess Heme Promotes the Migration and Infiltration of Macrophages in Endometrial Hyperplasia Complicated with Abnormal Uterine Bleeding" Biomolecules 12, no. 6: 849. https://doi.org/10.3390/biom12060849
APA StyleRuan, L.-Y., Lai, Z.-Z., Shi, J.-W., Yang, H.-L., Ye, J.-F., Xie, F., Qiu, X.-M., Zhu, X.-Y., & Li, M.-Q. (2022). Excess Heme Promotes the Migration and Infiltration of Macrophages in Endometrial Hyperplasia Complicated with Abnormal Uterine Bleeding. Biomolecules, 12(6), 849. https://doi.org/10.3390/biom12060849






