Growth-Suppressive and Apoptosis-Inducing Effects of Tetrandrine in SW872 Human Malignant Liposarcoma Cells via Activation of Caspase-9, Down-Regulation of XIAP and STAT-3, and ER Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Antibodies
2.2. Cell Culture
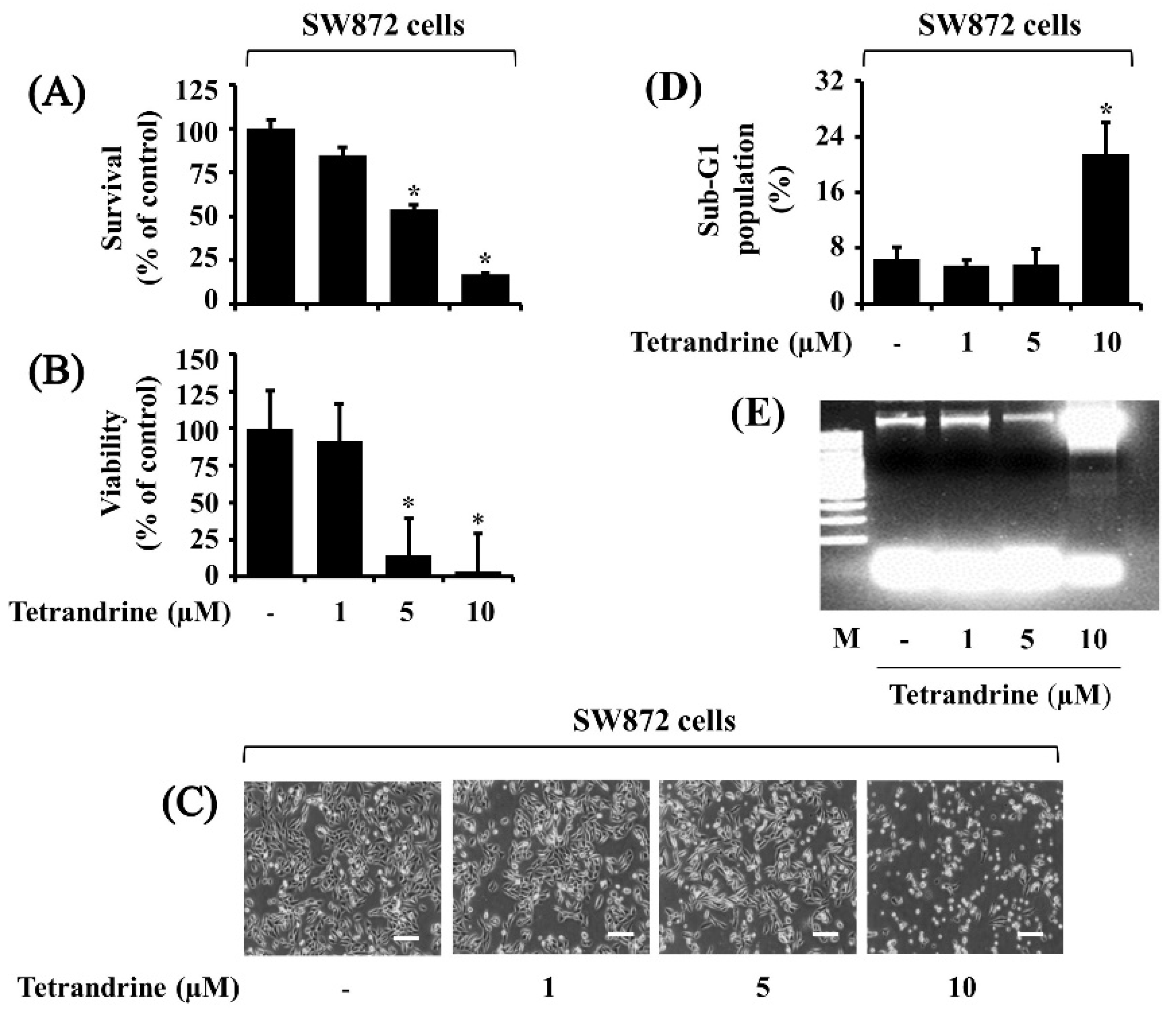
2.3. Cell Counting and Morphological Analysis
2.4. Measurement of DNA Fragmentation
2.5. Measurement of the Sub-G1 Population by Flow Cytometry Analysis
2.6. Preparation of Whole-Cell Lysates
2.7. Western Blot Analysis
2.8. Reverse Transcription-Polymerase Chain Reaction
2.9. Transfection of Small Interfering RNA (siRNA)
2.10. Statistical Analysis
3. Results
3.1. Tetrandrine at 10 μM Vastly Suppresses Growth and Induces Apoptosis of SW872 Cells
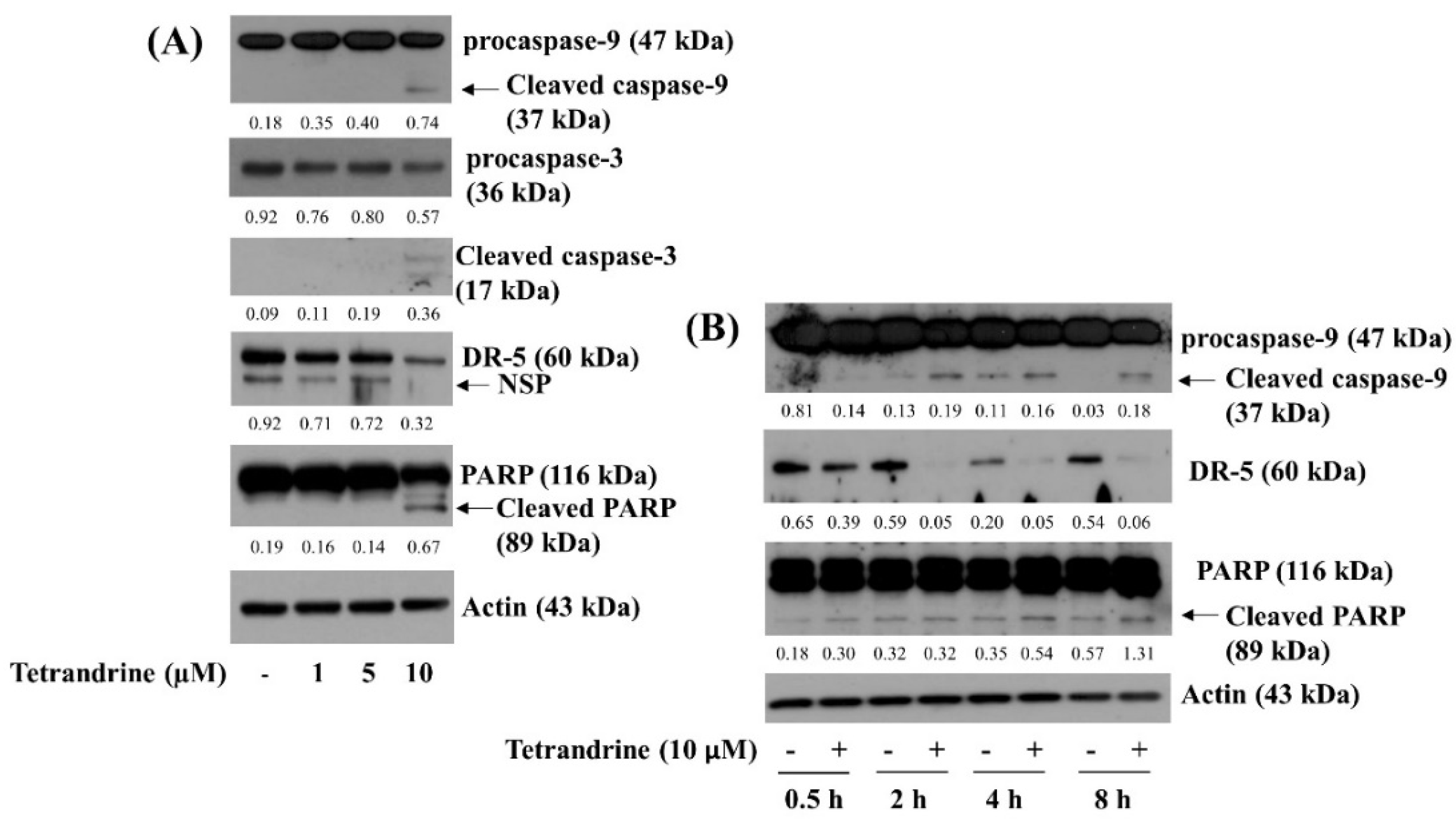
3.2. Tetrandrine at 10 μM Induces Activation of Caspase-9, Caspase-3, and Accumulation of Cleaved PARP in SW872 Cells
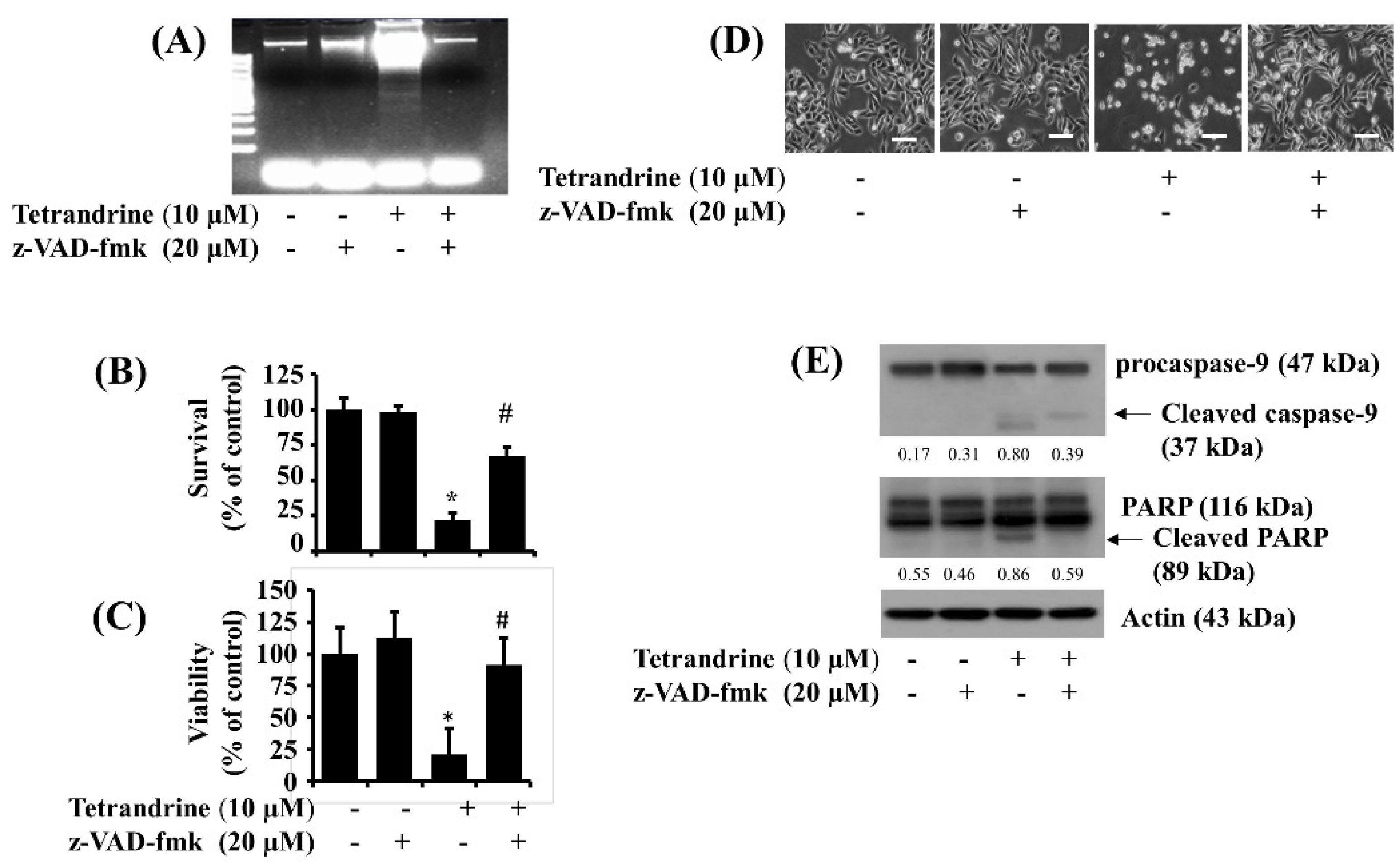
3.3. Activation of Caspases Is Crucial for Tetrandrine (10 μM)-Induced Pro-Apoptotic and Anti-Survival Effects on SW872 Cells
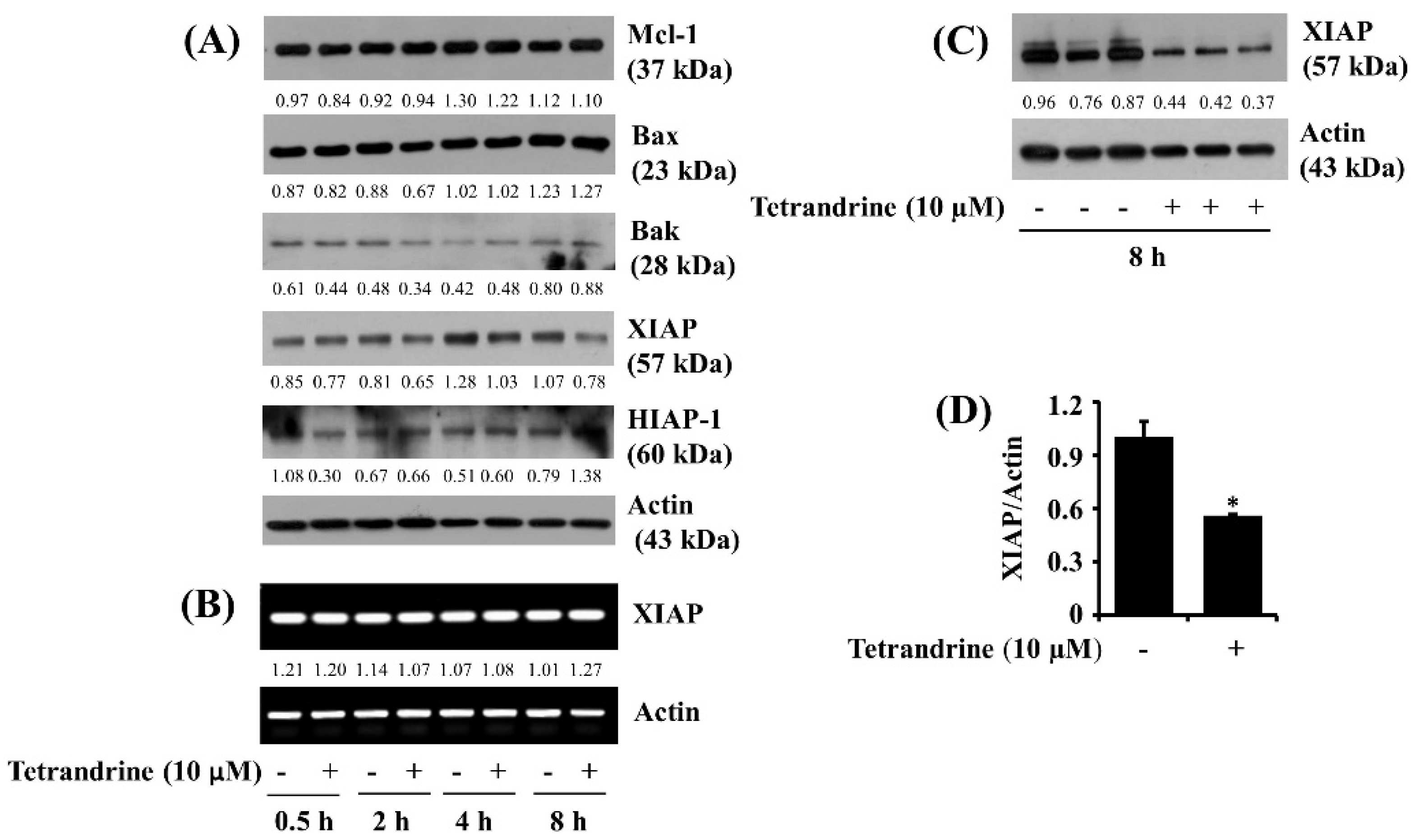
3.4. Tetrandrine at 10 μM Down-Regulates XIAP Expression at the Protein Levels in SW872 Cells
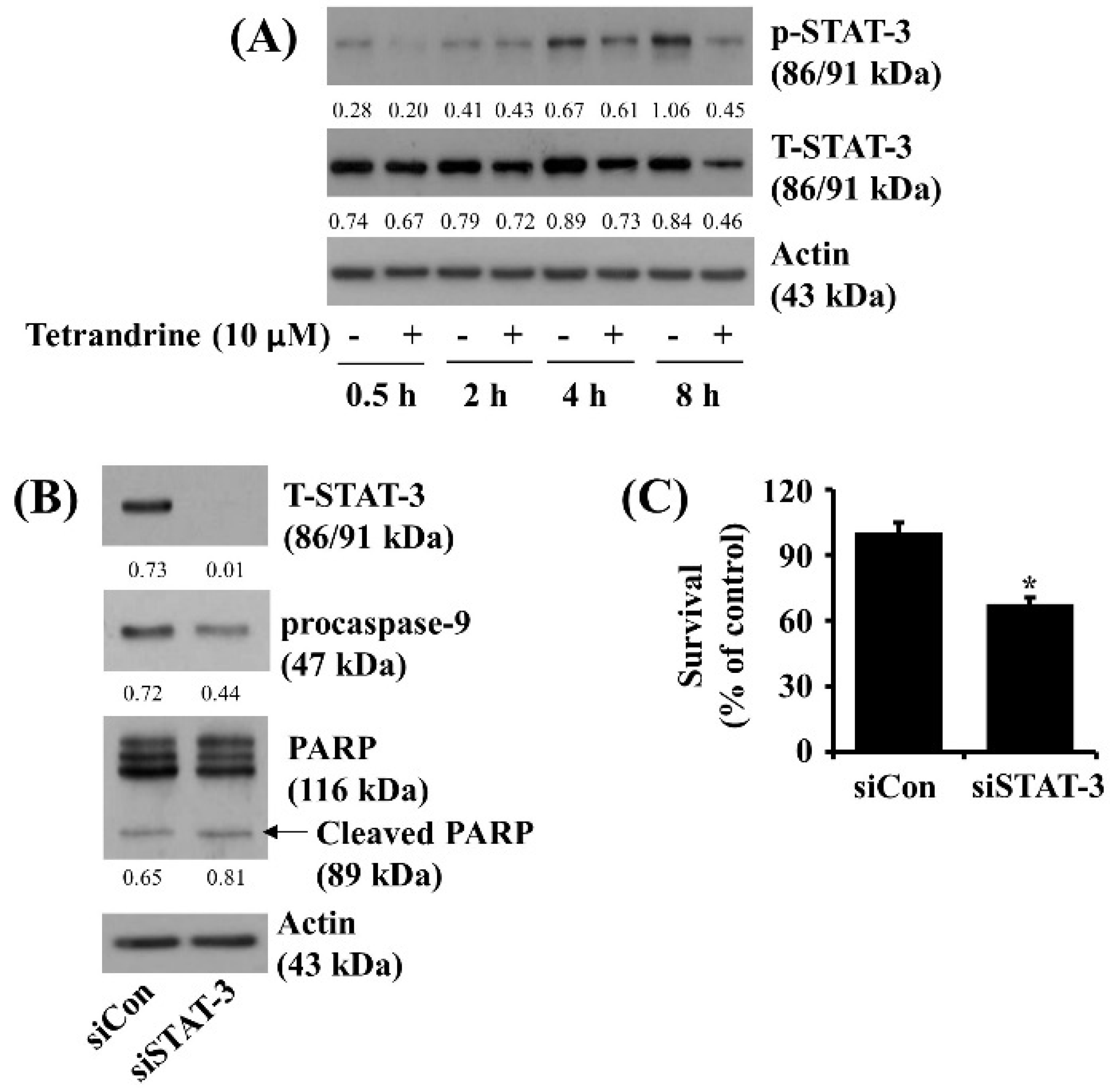
3.5. Tetrandrine at 10 μM Reduces STAT-3 Phosphorylation and Expression in SW872 Cells, and Knockdown of STAT-3 Significantly Decreases the Cell Survival
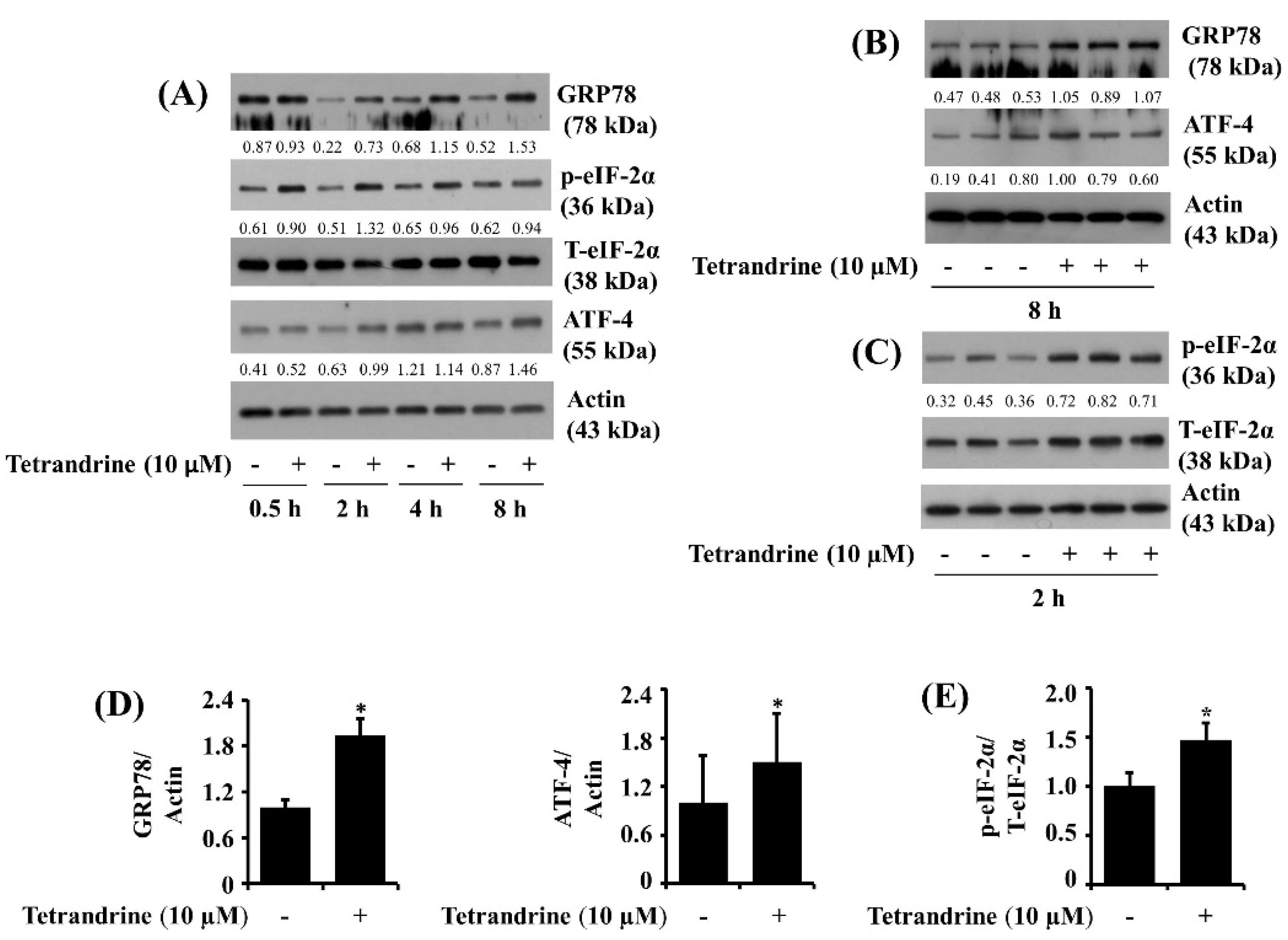
3.6. Tetrandrine at 10 μM Up-Regulates Expression of GRP78 and ATF-4 and Phosphorylation of eIF-2α in SW872 Cells
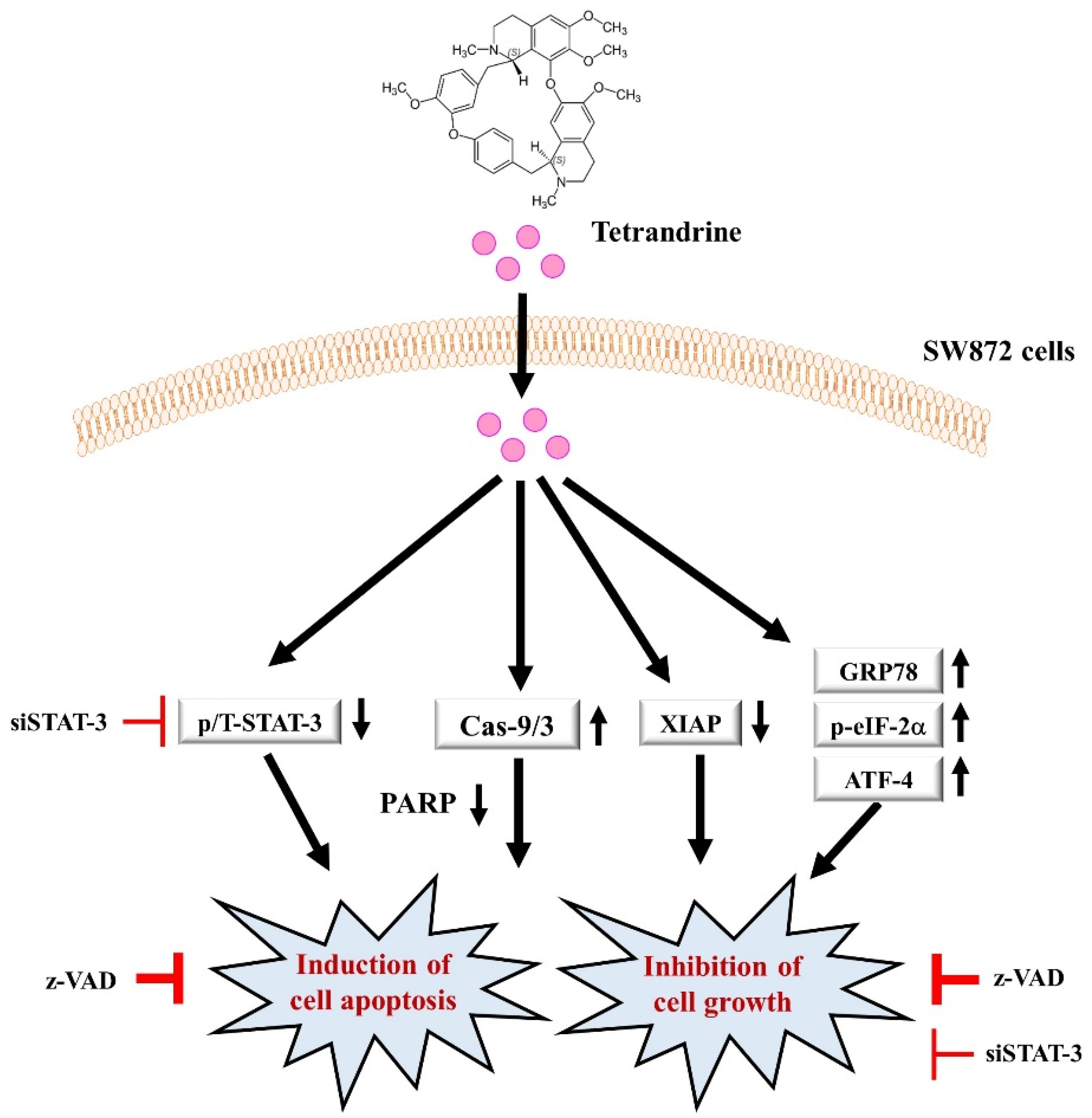
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crago, A.M.; Singer, S. Clinical and molecular approaches to well differentiated and dedifferentiated liposarcoma. Curr. Opin. Oncol. 2011, 23, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Mankin, H.J.; Hornicek, F.J. Diagnosis, Classification, and Management of Soft Tissue Sarcomas. Cancer Control 2005, 12, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.X.; Brown, B.B.; Hanson, H.; Kowaleski-Jones, L.; Smith, K.R.; Zick, C.D. Moderate to vigorous physical activity and weight outcomes: Does every minute count? Am. J. Health Promot. 2013, 28, 41–49. [Google Scholar] [CrossRef]
- Smith, W.A. Phylogenetic analysis of symbionts in feather-feeding lice of the genus Columbicola: Evidence for repeated symbiont replacements. BMC Evol. Biol. 2013, 13, 109. [Google Scholar] [CrossRef]
- De Vreeze, R.S.A. Added Value of Molecular Biological Analysis in Diagnosis and Clinical Management of Liposarcoma: A 30-Year Single-Institution Experience. Ann. Surg. Oncol. 2010, 17, 686–693. [Google Scholar] [CrossRef]
- Patel, R.B. Recent translational research into targeted therapy for liposarcoma. Stem Cell Investig. 2017, 4, 21. [Google Scholar] [CrossRef][Green Version]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Wyllie, A.H.; Kerr, J.F.; Currie, A.R. Cell death: The significance of apoptosis. Int. Rev. Cytol. 1980, 68, 251–306. [Google Scholar]
- Cohen, G.M. Caspases: The executioners of apoptosis. Biochem. J. 1997, 326, 1–16. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. The Bcl-2 Protein Family: Arbiters of Cell Survival. Science 1998, 281, 1322–1326. [Google Scholar] [CrossRef]
- Deveraux, Q.L. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998, 17, 2215–2223. [Google Scholar] [CrossRef]
- Groenendyk, J.M.M. Endoplasmic reticulum quality control and apoptosis. Acta Biochim. Pol. 2005, 52, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Cubillos-Ruiz, J.R.; Bettigole, S.E.; Glimcher, L.H. Tumorigenic and Immunosuppressive Effects of Endoplasmic Reticulum Stress in Cancer. Cell 2017, 168, 692–706. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Mohammad, I.S.; Liu, Z. Overview of the STAT-3 signaling pathway in cancer and the development of specific inhibitors. Oncol. Lett. 2020, 19, 2585–2594. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.Y.; Sun, D.; Liu, X.Y.; Pan, Y.; Liang, H. STAT-3 correlates with lymph node metastasis and cell survival in gastric cancer. World J. Gastroenterol. 2010, 16, 5380–5387. [Google Scholar] [CrossRef]
- Gao, P. The roles of signal transducer and activator of transcription factor 3 in tumor angiogenesis. Oncotarget 2017, 8, 69139–69161. [Google Scholar] [CrossRef]
- Bhagya, N.; Chandrashekar, K.R. Tetrandrine—A molecule of wide bioactivity. Phytochemistry 2016, 125, 5–13. [Google Scholar] [CrossRef]
- Chen, Y.; Tsai, Y.H.; Tseng, S.H. The potential of tetrandrine as a protective agent for ischemic stroke. Molecules 2011, 16, 8020–8032. [Google Scholar] [CrossRef]
- Lai, Y.L.; Chen, Y.J.; Wu, T.Y.; Wang, S.Y.; Chang, K.H.; Chung, C.H.; Chen, M.L. Induction of apoptosis in human leukemic U937 cells by tetrandrine. Anticancer Drugs 1998, 9, 77–81. [Google Scholar] [CrossRef]
- Li, X.; Su, B.; Liu, R.; Wu, D.; He, D. Tetrandrine Induces Apoptosis and Triggers Caspase Cascade in Human Bladder Cancer Cells. J. Surg. Res. 2011, 166, e45–e51. [Google Scholar] [CrossRef]
- Yoo, S.M.; Oh, S.H.; Lee, S.J.; Lee, B.W.; Ko, W.G.; Moon, C.K.; Lee, B.H. Inhibition of proliferation and induction of apoptosis by tetrandrine in HepG2 cells. J. Ethnopharmacol. 2002, 81, 225–229. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, G.K.; Kim, C.K.; Kim, K.M.; Park, D.I.; Choi, B.T.; Kang, H.S.; Lee, Y.T. Tetrandrine-induced cell cycle arrest and apoptosis in A549 human lung carcinoma cells. Int. J. Oncol. 2002, 21, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Chen, Y.; Chen, J.C.; Lin, T.Y.; Tseng, S.H. Tetrandrine induces apoptosis and growth suppression of colon cancer cells in mice. Cancer Lett. 2010, 287, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, B.; Zhang, Z.; Sheng, X.; Zhang, S. Tetrandrine suppresses cervical cancer growth by inducing apoptosis in vitro and in vivo. Drug Des. Devel. Ther. 2018, 13, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Kou, B.; Liu, W.; He, W.; Zhang, Y.; Zheng, J.; Yan, Y.; Zhang, Y.; Xu, S.; Wang, H. Tetrandrine suppresses metastatic phenotype of prostate cancer cells by regulating Akt/mTOR/MMP-9 signaling pathway. Oncol. Rep. 2016, 35, 2880–2886. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Kumar, V.; Bailey, D.B.; Jang, B.-C. AZD1208, a Pan-Pim Kinase Inhibitor, Has Anti-Growth Effect on 93T449 Human Liposarcoma Cells via Control of the Expression and Phosphorylation of Pim-3, mTOR, 4EBP-1, S6, STAT-3 and AMPK. Int. J. Mol. Sci. 2019, 20, 363. [Google Scholar] [CrossRef]
- Cormier, J.N.; Pollock, R.E. Soft tissue sarcomas. CA Cancer J. Clin. 2004, 54, 94–109. [Google Scholar] [CrossRef]
- Springfield, D. Liposarcoma. Clin. Orthop. Relat. Res. 1993, 289, 50–57. [Google Scholar] [CrossRef]
- Shang, W.; Zhang, J.; Song, H.; Zhu, S.; Zhang, A.; Hua, Y.; Han, S.; Fu, Y. Mechanism of Tetrandrine Against Endometrial Cancer Based on Network Pharmacology. Drug Des. Devel. Ther. 2021, 15, 2907–2919. [Google Scholar] [CrossRef]
- Cui, X.; Xiao, D.; Wang, X. Inhibition of laryngeal cancer stem cells by tetrandrine. Anticancer Drugs 2019, 30, 886–891. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.L.; Lin, C.C. Tetrandrine-induced cell cycle arrest and apoptosis in Hep G2 cells. Life Sci. 2003, 73, 243–252. [Google Scholar] [CrossRef]
- Oh, S.H.; Lee, B.H. Induction of apoptosis in human hepatoblastoma cells by tetrandrine via caspase-dependent Bid cleavage and cytochrome c release. Biochem. Pharmacol. 2003, 66, 725–731. [Google Scholar] [CrossRef]
- Deveraux, Q.L.; Reed, J.C. IAP family proteins—Suppressors of apoptosis. Genes Dev. 1999, 13, 239–252. [Google Scholar] [CrossRef]
- Shiozaki, E.N.; Chai, J.; Rigotti, D.J.; Riedl, S.J.; Li, P.; Srinivasula, S.M.; Alnemri, E.S.; Fairman, R.; Shi, Y. Mechanism of XIAP-Mediated Inhibition of Caspase-9. Mol. Cell. 2003, 11, 2. [Google Scholar] [CrossRef]
- Bratton, S.B.; Lewis, J.; Butterworth, M.; Duckett, C.S.; Cohen, G.M. XIAP inhibition of caspase-3 preserves its association with the Apaf-1 apoptosome and prevents CD95- and Bax-induced apoptosis. Cell Death Differ. 2002, 9, 881–892. [Google Scholar] [CrossRef]
- Levy, D.E.; Darnell, J.E. STATs: Transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002, 3, 651–662. [Google Scholar] [CrossRef]
- Sahu, R.P.; Srivastava, S.K. The role of STAT-3 in the induction of apoptosis in pancreatic cancer cells by benzyl isothiocyanate. J. Natl. Cancer Inst. 2009, 101, 176–193. [Google Scholar] [CrossRef]
- James, N. Ihle. STATs: Signal Transducers and Activators of Transcription. Cell 1996, 84, 331–334. [Google Scholar]
- Darnell, J.E. STATs and Gene Regulation. Science 1997, 277, 1630–1635. [Google Scholar] [CrossRef]
- Shodeinde, A.; Ginjupalli, K.; Lewis, H.D.; Riaz, S.; Barton, B.E. STAT3 Inhibition Induces Apoptosis in Cancer Cells Independent of STAT1 or STAT2. J. Mol. Biochem. 2013, 2, 18–26. [Google Scholar] [PubMed]
- Yadav, A.K.; Jang, B.C. Anti-Survival and Pro-Apoptotic Effects of 6-Shogaol on SW872 Human Liposarcoma Cells via Control of the Intrinsic Caspase Pathway, STAT-3, AMPK, and ER Stress. Biomolecules 2020, 10, 1380. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Miguel, T.; Fonteriz, R.I.; Vay, L.; Gajate, C.; López-Hernández, S.; Mollinedo, F. Endoplasmic Reticulum Stress in the Proapoptotic Action of Edelfosine in Solid Tumor Cells. Cancer Res. 2007, 67, 10368–10378. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S. The glucose-regulated proteins: Stress induction and clinical applications. Trends Biochem. Sci. 2001, 26, 504–510. [Google Scholar] [CrossRef]
- De Haro, C.; Méndez, R.; Santoyo, J. The eIF-2alpha kinases and the control of protein synthesis. FASEB J. 1996, 10, 1378–1387. [Google Scholar] [CrossRef]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The integrated stress response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef]
- Liu, K.C.; Lin, Y.J.; Hsiao, Y.T.; Lin, M.L.; Yang, J.L.; Huang, Y.P.; Chu, Y.L.; Chung, Y.L. Tetrandrine Induces Apoptosis in Human Nasopharyngeal Carcinoma NPC-TW 039 Cells by Endoplasmic Reticulum Stress and Ca2+/Calpain Pathways. Anticancer Res. 2017, 37, 6107–6118. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samsuzzaman, M.; Jang, B.-C. Growth-Suppressive and Apoptosis-Inducing Effects of Tetrandrine in SW872 Human Malignant Liposarcoma Cells via Activation of Caspase-9, Down-Regulation of XIAP and STAT-3, and ER Stress. Biomolecules 2022, 12, 843. https://doi.org/10.3390/biom12060843
Samsuzzaman M, Jang B-C. Growth-Suppressive and Apoptosis-Inducing Effects of Tetrandrine in SW872 Human Malignant Liposarcoma Cells via Activation of Caspase-9, Down-Regulation of XIAP and STAT-3, and ER Stress. Biomolecules. 2022; 12(6):843. https://doi.org/10.3390/biom12060843
Chicago/Turabian StyleSamsuzzaman, Mohammed, and Byeong-Churl Jang. 2022. "Growth-Suppressive and Apoptosis-Inducing Effects of Tetrandrine in SW872 Human Malignant Liposarcoma Cells via Activation of Caspase-9, Down-Regulation of XIAP and STAT-3, and ER Stress" Biomolecules 12, no. 6: 843. https://doi.org/10.3390/biom12060843
APA StyleSamsuzzaman, M., & Jang, B.-C. (2022). Growth-Suppressive and Apoptosis-Inducing Effects of Tetrandrine in SW872 Human Malignant Liposarcoma Cells via Activation of Caspase-9, Down-Regulation of XIAP and STAT-3, and ER Stress. Biomolecules, 12(6), 843. https://doi.org/10.3390/biom12060843





