Characterization of DAG Binding to TRPC Channels by Target-Dependent cis–trans Isomerization of OptoDArG
Abstract
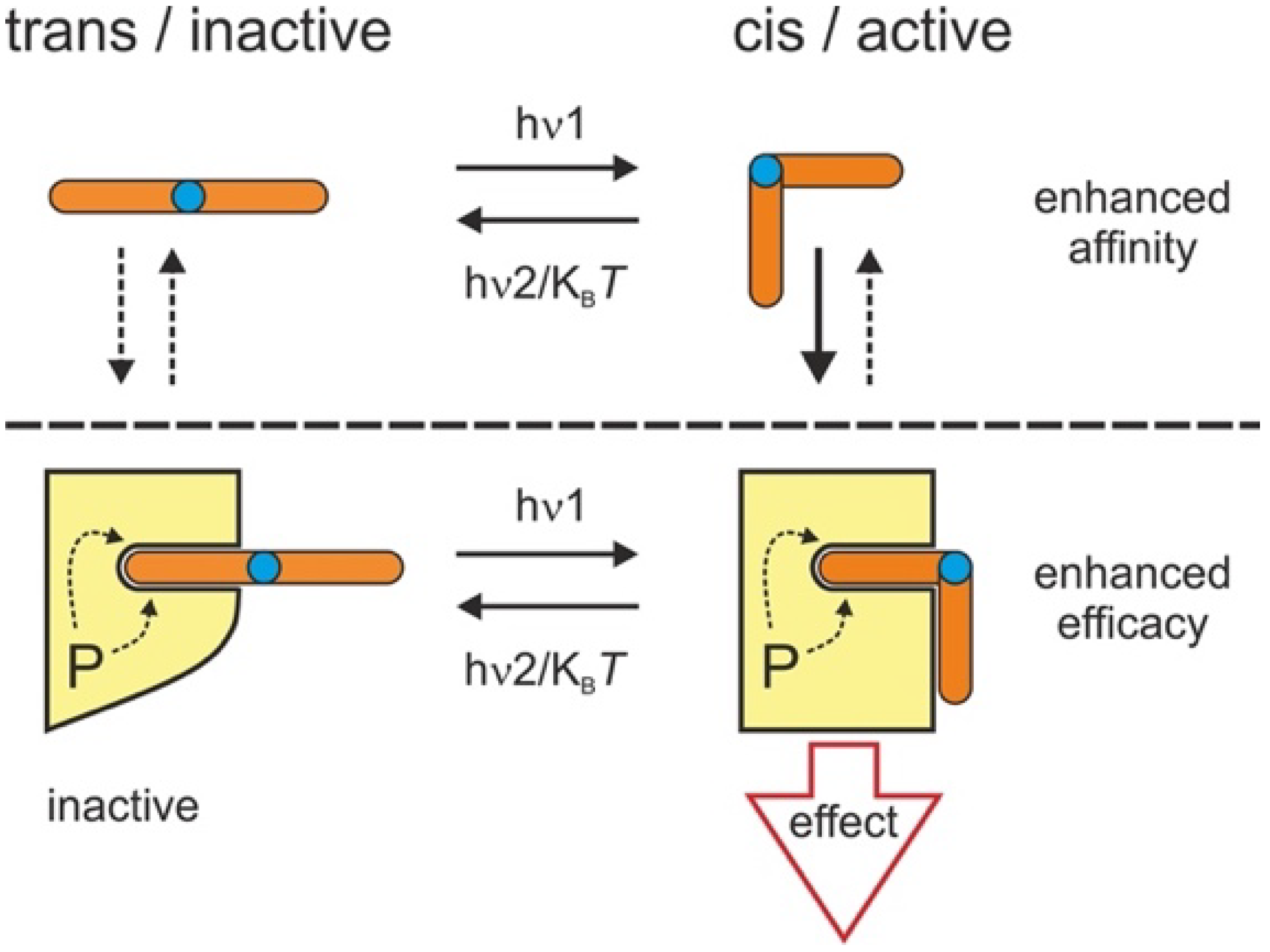
:1. Introduction
2. Materials and Methods
2.1. DNA Constructs and Reagents
2.2. Cell Culture and Transfection
2.3. Electrophysiology
2.4. Giant Unilamellar Vesicle (GUV) Preparation and Methodology
2.5. Statistics
3. Results
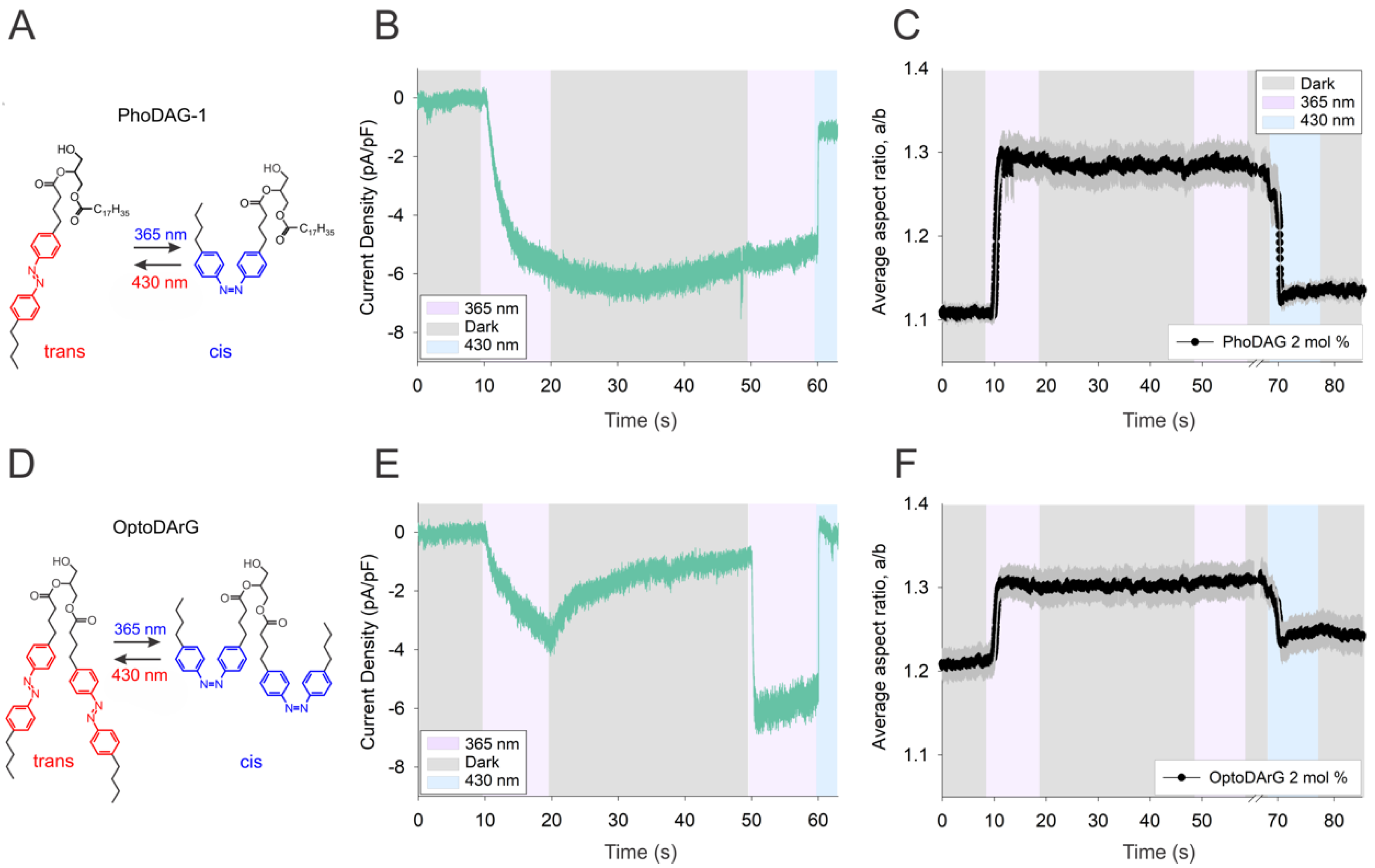
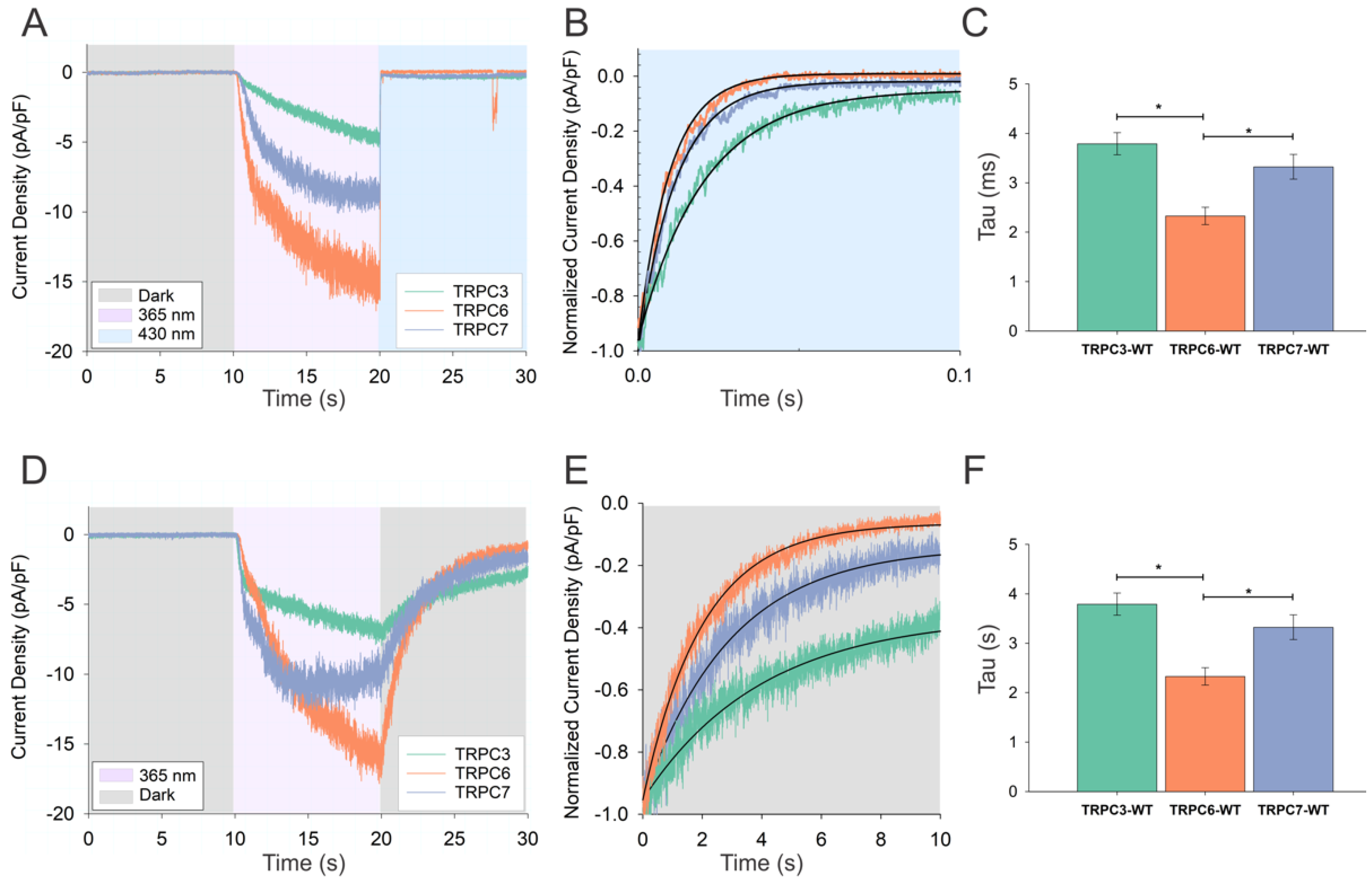
3.1. Activation of TRPC Channels by Cis OptoDArG Promotes Thermal Relaxation of the Photochrome
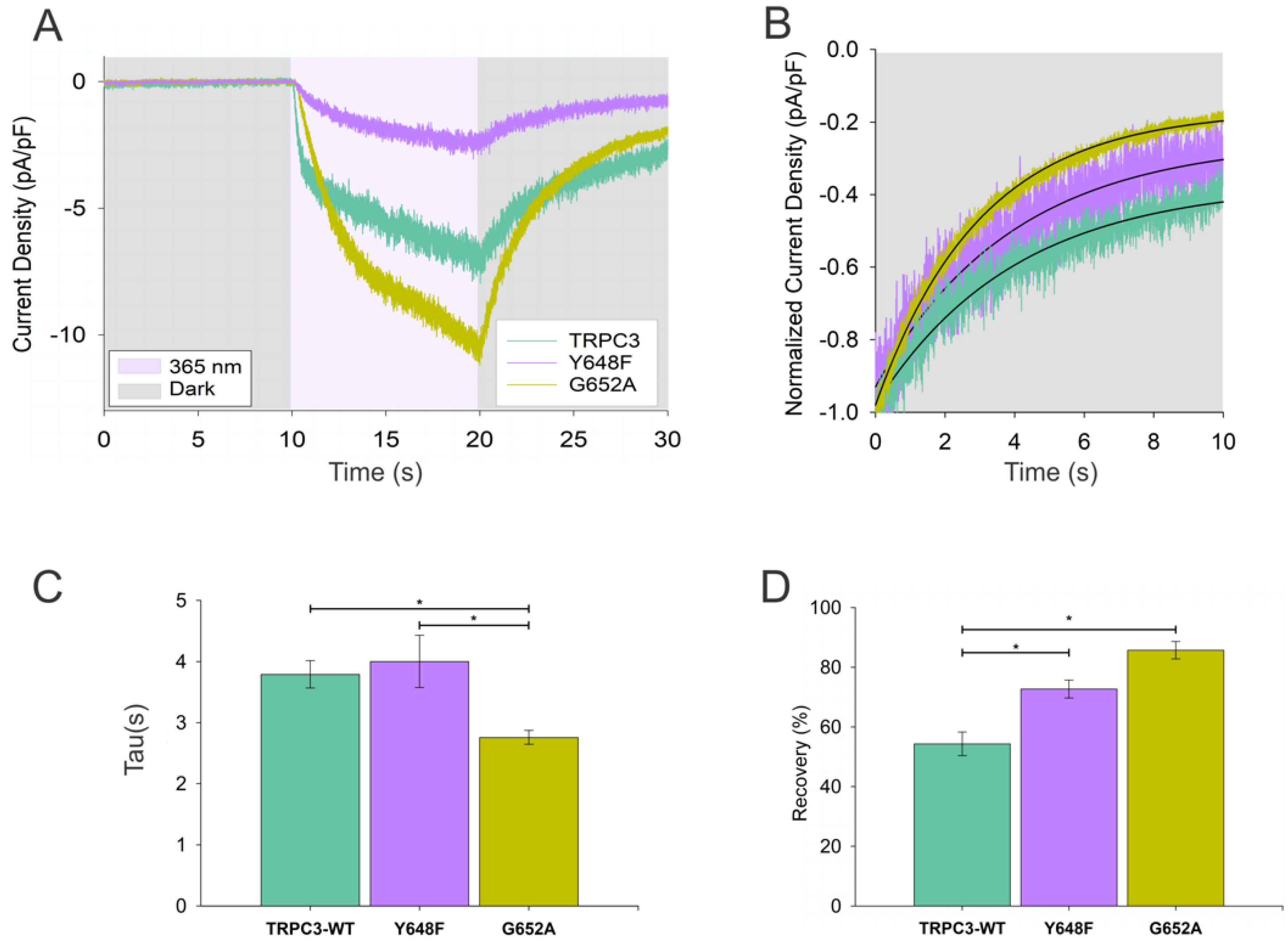
3.2. Kinetics of Thermal Relaxation of OptoDArG within TRPC Complexes Provides Information on Lipid-Binding Structures and Molecular Mechanisms of Channel Regulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frank, J.A.; Yushchenko, D.A.; Hodson, D.J.; Lipstein, N.; Nagpal, J.; Rutter, G.A.; Rhee, J.-S.; Gottschalk, A.; Brose, N.; Schultz, C.; et al. Photoswitchable diacylglycerols enable optical control of protein kinase C. Nat. Chem. Biol. 2016, 12, 755–762. [Google Scholar] [CrossRef] [Green Version]
- Lichtenegger, M.; Tiapko, O.; Svobodova, B.; Stockner, T.; Glasnov, T.N.; Schreibmayer, W.; Platzer, D.; de la Cruz, G.G.; Krenn, S.; Schober, R.; et al. An optically controlled probe identifies lipid-gating fenestrations within the TRPC3 channel. Nat. Chem. Biol. 2018, 14, 396–404. [Google Scholar] [CrossRef]
- Morstein, J.; Impastato, A.C.; Trauner, D. Photoswitchable Lipids. ChemBioChem 2021, 22, 73–83. [Google Scholar] [CrossRef]
- Curcic, S.; Tiapko, O.; Groschner, K. Photopharmacology and opto-chemogenetics of TRPC channels-some therapeutic visions. Pharmacol. Ther. 2019, 200, 13–26. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, C.K.; Sanchez-Romero, I.; Janovjak, H. Flipping the Photoswitch: Ion Channels Under Light Control. In Novel Chemical Tools to Study Ion Channel Biology; Ahern, C., Pless, S., Eds.; Springer: New York, NY, USA, 2015; pp. 101–117. [Google Scholar]
- Welleman, I.M.; Hoorens, M.W.H.; Feringa, B.L.; Boersma, H.H.; Szymański, W. Photoresponsive molecular tools for emerging applications of light in medicine. Chem. Sci. 2020, 11, 11672–11691. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Zhang, H.; Shen, Z.; Huang, W. Photopharmacology of Proteolysis-Targeting Chimeras: A New Frontier for Drug Discovery. Front. Chem. 2021, 9, 639176. [Google Scholar] [CrossRef] [PubMed]
- Hüll, K.; Morstein, J.; Trauner, D. In Vivo Photopharmacology. Chem. Rev. 2018, 118, 10710–10747. [Google Scholar] [CrossRef]
- Tiapko, O.; Shrestha, N.; Lindinger, S.; Guedes de la Cruz, G.; Graziani, A.; Klec, C.; Butorac, C.; Graier, W.F.; Kubista, H.; Freichel, M.; et al. Lipid-independent control of endothelial and neuronal TRPC3 channels by light. Chem. Sci. 2019, 10, 2837–2842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leinders-Zufall, T.; Storch, U.; Bleymehl, K.; Mederos y Schnitzler, M.; Frank, J.A.; Konrad, D.B.; Trauner, D.; Gudermann, T.; Zufall, F. PhoDAGs Enable Optical Control of Diacylglycerol-Sensitive Transient Receptor Potential Channels. Cell Chem. Biol. 2018, 25, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Liang, R.; Bakhtiiari, A. Effects of Enzyme–Ligand Interactions on the Photoisomerization of a Light-Regulated Chemotherapeutic Drug. J. Phys. Chem. B 2022, 126, 2382–2393. [Google Scholar] [CrossRef]
- Pfeffermann, J.; Eicher, B.; Boytsov, D.; Hannesschlaeger, C.; Galimzyanov, T.R.; Glasnov, T.N.; Pabst, G.; Akimov, S.A.; Pohl, P. Photoswitching of model ion channels in lipid bilayers. J. Photochem. Photobiol. B 2021, 224, 112320. [Google Scholar] [CrossRef]
- Pritzl, S.D.; Konrad, D.B.; Ober, M.F.; Richter, A.F.; Frank, J.A.; Nickel, B.; Trauner, D.; Lohmüller, T. Optical Membrane Control with Red Light Enabled by Red-Shifted Photolipids. Langmuir 2022, 38, 385–393. [Google Scholar] [CrossRef]
- Urban, P.; Pritzl, S.D.; Konrad, D.B.; Frank, J.A.; Pernpeintner, C.; Roeske, C.R.; Trauner, D.; Lohmüller, T. Light-Controlled Lipid Interaction and Membrane Organization in Photolipid Bilayer Vesicles. Langmuir 2018, 34, 13368–13374. [Google Scholar] [CrossRef]
- Angelova, M.I.; Dimitrov, D.S. Liposome electroformation. Faraday Discuss. Chem. Soc. 1986, 81, 303–311. [Google Scholar] [CrossRef]
- Aranda, S.; Riske, K.A.; Lipowsky, R.; Dimova, R. Morphological Transitions of Vesicles Induced by Alternating Electric Fields. Biophys. J. 2008, 95, L19–L21. [Google Scholar] [CrossRef] [Green Version]
- Dimova, R.; Bezlyepkina, N.; Jordö, M.D.; Knorr, R.L.; Riske, K.A.; Staykova, M.; Vlahovska, P.M.; Yamamoto, T.; Yang, P.; Lipowsky, R. Vesicles in electric fields: Some novel aspects of membrane behavior. Soft Matter 2009, 5, 3201–3212. [Google Scholar] [CrossRef]
- Georgiev, V.N.; Grafmüller, A.; Bléger, D.; Hecht, S.; Kunstmann, S.; Barbirz, S.; Lipowsky, R.; Dimova, R. Area Increase and Budding in Giant Vesicles Triggered by Light: Behind the Scene. Adv. Sci. 2018, 5, 1800432. [Google Scholar] [CrossRef]
- Riske, K.A.; Sudbrack, T.P.; Archilha, N.L.; Uchoa, A.F.; Schroder, A.P.; Marques, C.M.; Baptista, M.S.; Itri, R. Giant vesicles under oxidative stress induced by a membrane-anchored photosensitizer. Biophys. J. 2009, 97, 1362–1370. [Google Scholar] [CrossRef] [Green Version]
- Gracià, R.S.; Bezlyepkina, N.; Knorr, R.L.; Lipowsky, R.; Dimova, R. Effect of cholesterol on the rigidity of saturated and unsaturated membranes: Fluctuation and electrodeformation analysis of giant vesicles. Soft Matter 2010, 6, 1472–1482. [Google Scholar] [CrossRef]
- Erkan-Candag, H.; Clarke, A.; Tiapko, O.; Gsell, M.A.F.; Stockner, T.; Groschner, K. Diacylglycerols interact with the L2 lipidation site in TRPC3 to induce a sensitized channel state. EMBO Rep. 2022. [Google Scholar] [CrossRef]
- Rumiana Dimova, C.M.M. The Giant Vesicle Book, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Fan, C.; Choi, W.; Sun, W.; Du, J.; Lü, W. Structure of the human lipid-gated cation channel TRPC3. eLife 2018, 7, e36852. [Google Scholar] [CrossRef]
- Guo, W.; Tang, Q.; Wei, M.; Kang, Y.; Wu, J.-X.; Chen, L. Structural mechanism of human TRPC3 and TRPC6 channel regulation by their intracellular calcium-binding sites. Neuron 2022, 110, 1023–1035.e5. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erkan-Candag, H.; Krivic, D.; Gsell, M.A.F.; Aleksanyan, M.; Stockner, T.; Dimova, R.; Tiapko, O.; Groschner, K. Characterization of DAG Binding to TRPC Channels by Target-Dependent cis–trans Isomerization of OptoDArG. Biomolecules 2022, 12, 799. https://doi.org/10.3390/biom12060799
Erkan-Candag H, Krivic D, Gsell MAF, Aleksanyan M, Stockner T, Dimova R, Tiapko O, Groschner K. Characterization of DAG Binding to TRPC Channels by Target-Dependent cis–trans Isomerization of OptoDArG. Biomolecules. 2022; 12(6):799. https://doi.org/10.3390/biom12060799
Chicago/Turabian StyleErkan-Candag, Hazel, Denis Krivic, Mathias A. F. Gsell, Mina Aleksanyan, Thomas Stockner, Rumiana Dimova, Oleksandra Tiapko, and Klaus Groschner. 2022. "Characterization of DAG Binding to TRPC Channels by Target-Dependent cis–trans Isomerization of OptoDArG" Biomolecules 12, no. 6: 799. https://doi.org/10.3390/biom12060799
APA StyleErkan-Candag, H., Krivic, D., Gsell, M. A. F., Aleksanyan, M., Stockner, T., Dimova, R., Tiapko, O., & Groschner, K. (2022). Characterization of DAG Binding to TRPC Channels by Target-Dependent cis–trans Isomerization of OptoDArG. Biomolecules, 12(6), 799. https://doi.org/10.3390/biom12060799







