Cognitive Impairment, Sleep Disturbance, and Depression in Women with Silicone Breast Implants: Association with Autoantibodies against Autonomic Nervous System Receptors
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Patient Recruitment
2.3. Quantification of Circulating Autoantibody Levels
2.4. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen Tervaert, J.W.; Colaris, M.J.; van der Hulst, R.R. Silicone breast implants and autoimmune rheumatic diseases: Myth or reality. Curr. Opin. Rheumatol. 2017, 29, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.H.; III, R.G.; Povoski, S.P. A review of the use of silicone implants in breast surgery. Expert Rev. Med. Devices 2016, 13, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Balk, E.M.; Earley, A.; Avendano, E.A.; Raman, G. Long-term health outcomes in women with silicone gel breast implants. Ann. Intern. Med. 2015, 164, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Colaris, M.J.L.; de Boer, M.; van der Hulst, R.R.; Tervaert, J.W.C. Two hundreds cases of ASIA syndrome following silicone implants: A comparative study of 30 years and a review of current literature. Immunol. Res. 2016, 65, 120–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watad, A.; Bragazzi, N.L.; Amital, H.; Shoenfeld, Y. Hyperstimulation of adaptive immunity as the common pathway for silicone breast implants, autoimmunity, and lymphoma of the breast. Isr. Med. Assoc. J. 2019, 21, 517–519. [Google Scholar] [PubMed]
- Watad, A.; Quaresma, M.; Brown, S.; Cohen Tervaert, J.W.; Rodríguez-Pint, I.; Cervera, R.; Perricone, C.; Shoenfeld, Y. Autoimmune/inflammatory syndrome induced by djuvants (Shoenfeld’s syndrome)—An update. Lupus 2017, 26, 675–681. [Google Scholar] [CrossRef]
- Watad, A.; Bragazzi, N.L.; McGonagle, D.; Adawi, M.; Bridgewood, C.; Damiani, G.; Alijotas-Reig, J.; Esteve-Valverde, E.; Quaresma, M.; Amital, H.; et al. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) demonstrates distinct autoimmune and autoinflammatory disease associations according to the adjuvant subtype: Insights from an analysis of 500 cases. Clin Immunol. 2019, 203, 1–8. [Google Scholar] [CrossRef]
- Zandman-Goddard, G.; Blank, M.; Ehrenfeld, M.; Gilburd, B.; Peter, J.; Shoenfeld, Y. A comparison of autoantibody production in asymptomatic and symptomatic women with silicone breast implants. J. Rheumatol. 1999, 26, 73–77. [Google Scholar]
- Watad, A.; Rosenberg, V.; Tiosano, S.; Tervaert, J.W.C.; Yavne, Y.; Shoenfeld, Y.; Shalev, V.; Chodick, G.; Amital, H. Silicone breast implants and the risk of autoimmune/rheumatic disorders: A real-world analysis. Int. J. Epidemiol. 2018, 47, 1846–1854. [Google Scholar] [CrossRef]
- Borba, V.; Malkova, A.; Basantsova, N.; Halpert, G.; Andreoli, L.; Tincani, A.; Amital, H.; Shoenfeld, Y. Classical examples of the concept of the ASIA syndrome. Biomolecules 2020, 10, 1436. [Google Scholar] [CrossRef]
- Herda, L.; Felix, S.; Boege, F. Drug-like actions of autoantibodies against receptors of the autonomous nervous system and their impact on human heart function. Br. J. Pharmacol. 2012, 166, 847–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, M.; Jung, S.T. Engineering therapeutic antibodies targeting G-protein–coupled receptors. Exp. Mol. Med. 2016, 48, e207. [Google Scholar] [CrossRef] [PubMed]
- Marques, O.C.; Marques, A.; Giil, L.M.; De Vito, R.; Rademacher, J.; Günther, J.; Lange, T.; Humrich, J.Y.; Klapa, S.; Schinke, S.; et al. GPCR-specific autoantibody signatures are associated with physiological and pathological immune homeostasis. Nat. Commun. 2018, 9, 5224. [Google Scholar] [CrossRef] [PubMed]
- Mona, M.; Mondello, S.; Hyon, J.Y.; Saleh, W.; Han, K.; Lee, H.-J.; Ha, Y.-J.; Kang, E.H.; Lee, Y.J.; Cha, S. Clinical usefulness of anti-muscarinic type 3 receptor autoantibodies in patients with primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 2020, 39, 795–803. [Google Scholar] [PubMed]
- Namkoong, E.; Lee, S.-W.; Kim, N.; Choi, Y.; Park, K. Effect of anti-muscarinic autoantibodies on leukocyte function in Sjögren’s syndrome. Mol. Immunol. 2017, 90, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Marques, O.; Riemekasten, G. Functional autoantibodies targeting G protein-coupled receptors in rheumatic diseases. Nat. Rev. Rheumatol. 2017, 13, 648–656. [Google Scholar] [CrossRef]
- Altman, J.D.; Trendelenburg, A.U.; MacMillan, L.; Bernstein, D.; Limbird, L.; Starke, K.; Kobilka, B.K.; Hein, L. Abnormal regulation of the sympathetic nervous system in α2a-adrenergic receptor knockout mice. Mol. Pharmacol. 1999, 56, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Turki, J.; Liggett, S.B. Receptor-specific functional properties of β 2-adrenergic receptor autoantibodies in asthma. Am. J. Respir. Cell Mol. Biol. 1995, 12, 531–539. [Google Scholar] [CrossRef]
- Loebel, M.; Grabowski, P.; Heidecke, H.; Bauer, S.; Hanitsch, L.G.; Wittke, K.; Meisel, C.; Reinke, P.; Volk, H.-D.; Fluge, Ø.; et al. Antibodies to β adrenergic and muscarinic cholinergic receptors in patients with chronic fatigue syndrome. Brain Behav. Immun. 2015, 52, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Bynke, A.; Julin, P.; Gottfries, C.-G.; Heidecke, H.; Scheibenbogen, C.; Bergquist, J. Autoantibodies to β-adrenergic and muscarinic cholinergic receptors in Myalgic Encephalomyelitis (ME) patients—A validation study in plasma and cerebrospinal fluid from two Swedish cohorts. Brain Behav. Immun. Health 2020, 7, 100107. [Google Scholar] [CrossRef]
- Cervio, E.; Volta, U.; Verri, M.; Boschi, F.; Pastoris, O.; Granito, A.; Barbara, G.; Parisi, C.; Felicani, C.; Tonini, M.; et al. Sera of Patients with celiac disease and neurologic disorders evoke a mitochondrial-dependent apoptosis in vitro. Gastroenterology 2007, 133, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Cottingham, C.; Wang, Q. α2 adrenergic receptor dysregulation in depressive disorders: Implications for the neurobiology of depression and antidepressant therapy. Neurosci. Biobehav. Rev. 2012, 36, 2214–2225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Ouyang, M.; Ganellin, C.R.; Thomas, S.A. The slow afterhyperpolarization: A target of β1-adrenergic signaling in hippocampus-dependent memory retrieval. J. Neurosci. 2013, 33, 5006–5016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagena, H.; Hansen, N.; Manahan-Vaughan, D. β-adrenergic control of hippocampal function: Subserving the choreography of synaptic information storage and memory. Cereb. Cortex 2016, 26, 1349–1364. [Google Scholar] [CrossRef] [Green Version]
- Weinshenker, D. Functional consequences of locus coeruleus degeneration in Alzheimers disease. Curr. Alzheimer Res. 2008, 5, 342–345. [Google Scholar] [CrossRef]
- Borodovitsyna, O.; Flamini, M.; Chandler, D. Noradrenergic modulation of cognition in health and disease. Neural Plast. 2017, 2017, 6031478. [Google Scholar] [CrossRef] [Green Version]
- Van der Zee, E.A.; Luiten, P.G.M. Muscarinic acetylcholine receptors in the hippocampus, neocortex and amygdala: A review of immunocytochemical localization in relation to learning and memory. Prog. Neurobiol. 1999, 58, 409–471. [Google Scholar] [CrossRef]
- Butcher, A.J.; Bradley, S.J.; Prihandoko, R.; Brooke, S.M.; Mogg, A.; Bourgognon, J.-M.; Macedo-Hatch, T.; Edwards, J.M.; Bottrill, A.; Challiss, J.; et al. An antibody biosensor establishes the activation of the M1 muscarinic acetylcholine receptor during learning and memory. J. Biol. Chem. 2016, 291, 8862–8875. [Google Scholar] [CrossRef] [Green Version]
- Halpert, G.; Watad, A.; Tsur, A.M.; Dotan, A.; Quiros-Lim, H.E.; Heidecke, H.; Gilburd, B.; Haik, J.; Levy, Y.; Blank, M.; et al. Autoimmune dysautonomia in women with silicone breast implants. J. Autoimmun. 2021, 120, 102631. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. Lond. Engl. 2014, 12, 1500–1524. [Google Scholar] [CrossRef] [Green Version]
- Zong, S.; Hoffmann, C.; Mané-Damas, M.; Molenaar, P.; Losen, M.; Martinez-Martinez, P. Neuronals Surface autoantibodies in neuropsychiatric disorders: Are there implications for depression? Front. Immunol. 2017, 8, 752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giil, L.M.; Vedeler, C.A.; Kristoffersen, E.K.; Nordrehaug, J.; Heidecke, H.; Dechend, R.; Schulze-Forster, K.; Muller, D.N.; von Goetze, V.S.; Cabral-Marques, O.; et al. Antibodies to signaling molecules and receptors in Alzheimer’s disease are associated with psychomotor slowing, depression, and poor visuospatial function. J. Alzheimers. Dis. 2017, 59, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Graus, F.; Titulaer, M.J.; Balu, R.; Benseler, S.; Bien, C.G.; Cellucci, T.; Cortese, I.; Dale, R.C.; Gelfand, J.M.; Geschwind, M.; et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016, 15, 391–404. [Google Scholar] [CrossRef] [Green Version]
- Leypoldt, F.; Armangue, T.; Dalmau, J. Autoimmune encephalopathies. Ann. N. Y. Acad. Sci. 2015, 1338, 94–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giil, L.M.; Aarsland, D.; Hellton, K.; Lund, A.; Heidecke, H.; Schulze-Forster, K.; Riemekasten, G.; Vik-Mo, A.O.; Kristoffersen, E.K.; Vedeler, C.A.; et al. Antibodies to multiple receptors are associated with neuropsychiatric symptoms and mortality in alzheimer’s disease: A longitudinal study. J. Alzheimer’s Dis. 2018, 64, 761–774. [Google Scholar] [CrossRef]
- Shiozaki, K.; Iseki, E.; Hino, H.; Kosaka, K. Distribution of m1 muscarinic acetylcholine receptors in the hippocampus of patients with Alzheimer’s disease and dementia with Lewy bodies—an immunohistochemical study. J. Neurol. Sci. 2001, 193, 23–28. [Google Scholar] [CrossRef]
- Jurgens, C.W.; Rau, K.E.; Knudson, C.A.; King, J.D.; Carr, P.A.; Porter, J.E.; Doze, V.A. β1 adrenergic receptor-mediated enhancement of hippocampal CA3 network activity. J. Pharmacol. Exp. Ther. 2005, 314, 552–560. [Google Scholar] [CrossRef]
- Hempel, P.; Heinig, B.; Jerosch, C.; Decius, I.; Karczewski, P.; Kassner, U.; Kunze, R.; Steinhagen-Thiessen, E.; Bimmler, M. Immunoadsorption of agonistic autoantibodies against α1-adrenergic receptors in patients with mild to moderate dementia. Ther. Apher. Dial. 2016, 20, 523–529. [Google Scholar] [CrossRef]
- Pohlmann, A.; Karczewski, P.; Ku, M.-C.; Dieringer, B.; Waiczies, H.; Wisbrun, N.; Kox, S.; Palatnik, I.; Reimann, H.M.; Eichhorn, C.; et al. Cerebral blood volume estimation by ferumoxytol-enhanced steady-state MRI at 9.4 T reveals microvascular impact of α1 -adrenergic receptor antibodies. NMR Biomed. 2014, 27, 1085–1093. [Google Scholar] [CrossRef]
- Karczewski, P.; Hempel, P.; Kunze, R.; Bimmler, M. Agonistic autoantibodies to the α(1)-adrenergic receptor and the β(2)-adrenergic receptor in Alzheimer’s and vascular dementia. Scand J. Immunol. 2012, 75, 524–530. [Google Scholar] [CrossRef]
- Thyrian, J.R.; Hertel, J.; Schulze, L.N.; Dörr, M.; Prüss, H.; Hempel, P.; Bimmler, M.; Kunze, R.; Grabe, H.J.; Teipel, S.; et al. Prevalence and determinants of agonistic autoantibodies against α1-adrenergic receptors in patients screened positive for dementia: Results from the population-based DelpHi-study. J. Alzheimers Dis. JAD 2018, 64, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Ernst, D.; Westerbergh, J.; Sogkas, G.; Jablonka, A.; Ahrenstorf, G.; Schmidt, R.E.; Heidecke, H.; Wallentin, L.; Riemekasten, G.; Witte, T. Lowered anti-β1 adrenergic receptor antibody concentrations may have prognostic significance in acute coronary syndrome. Sci. Rep. 2019, 9, 14552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Jiang, T.; Chen, P.; Ouyang, J.; Xu, G.; Zeng, Z.; Sun, Y. Emerging tendency towards autoimmune process in major depressive patients: A novel insight from Th17 cells. Psychiatry Res. 2011, 188, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Iseme, R.A.; McEvoy, M.; Kelly, B.; Agnew, L.; Attia, J.; Walker, F.R. Autoantibodies and depression: Evidence for a causal link? Neurosci. Biobehav. Rev. 2014, 40, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Rada, P.; Colasante, C.; Skirzewski, M.; Hernandez, L.; Hoebel, B. Behavioral depression in the swim test causes a biphasic, long-lasting change in accumbens acetylcholine release, with partial compensation by acetylcholinesterase and muscarinic-1 receptors. Neuroscience 2006, 141, 67–76. [Google Scholar] [CrossRef]
- Postal, M.; Appenzeller, S. The importance of cytokines and autoantibodies in depression. Autoimmun. Rev. 2015, 14, 30–35. [Google Scholar] [CrossRef]
- Lapteva, L.; Nowak, M.; Yarboro, C.H.; Takada, K.; Roebuck-Spencer, T.; Weickert, T.; Bleiberg, J.; Rosenstein, D.; Pao, M.; Patronas, N.; et al. Anti–N-methyl-D-aspartate receptor antibodies, cognitive dysfunction, and depression in systemic lupus erythematosus. Arthritis Rheum. 2006, 54, 2505–2514. [Google Scholar] [CrossRef]
- Ebert, T.; Chapman, J.; Shoenfeld, Y. Anti-ribosomal P-protein and its role in psychiatric manifestations of systemic lupus erythematosus: Myth or reality? Lupus 2005, 14, 571–575. [Google Scholar] [CrossRef]
- Chen, M.; Yan, H.-H.; Shu, S.; Pei, L.; Zang, L.-K.; Fu, Y.; Wang, Z.-F.; Wan, Q.; Bi, L.-L. Amygdalar endothelin-1 regulates pyramidal neuron excitability and affects anxiety. Sci. Rep. 2017, 7, 2316. [Google Scholar] [CrossRef] [Green Version]
- Khoo, T.; Proudman, S.; Limaye, V. Silicone breast implants and depression, fibromyalgia and chronic fatigue syndrome in a rheumatology clinic population. Clin. Rheumatol. 2019, 38, 1271–1276. [Google Scholar] [CrossRef]
- De Boer, M.; Colaris, M.; Van Der Hulst, R.R.; Cohen Tervaert, J.W. Is explantation of silicone breast implants useful in patients with complaints? Immunol. Res. 2017, 65, 25–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nesher, G.; Soriano, A.; Shlomai, G.; Iadgarov, Y.; Shulimzon, T.R.; Borella, E.; Dicker, D.; Shoenfeld, Y. Severe ASIA syndrome associated with lymph node, thoracic, and pulmonary silicone infiltration following breast implant rupture: Experience with four cases. Lupus 2015, 24, 463–468. [Google Scholar] [CrossRef] [PubMed]
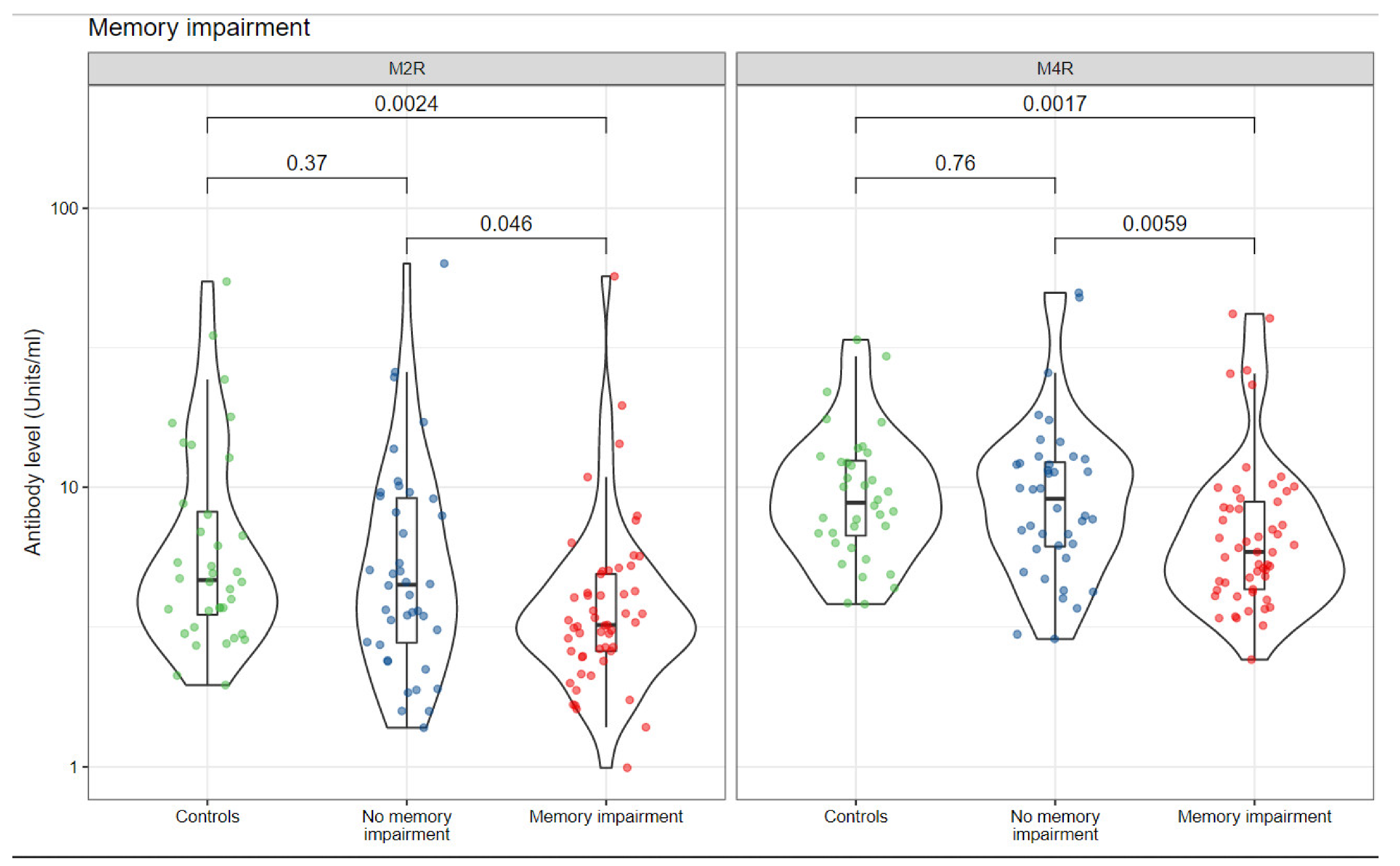
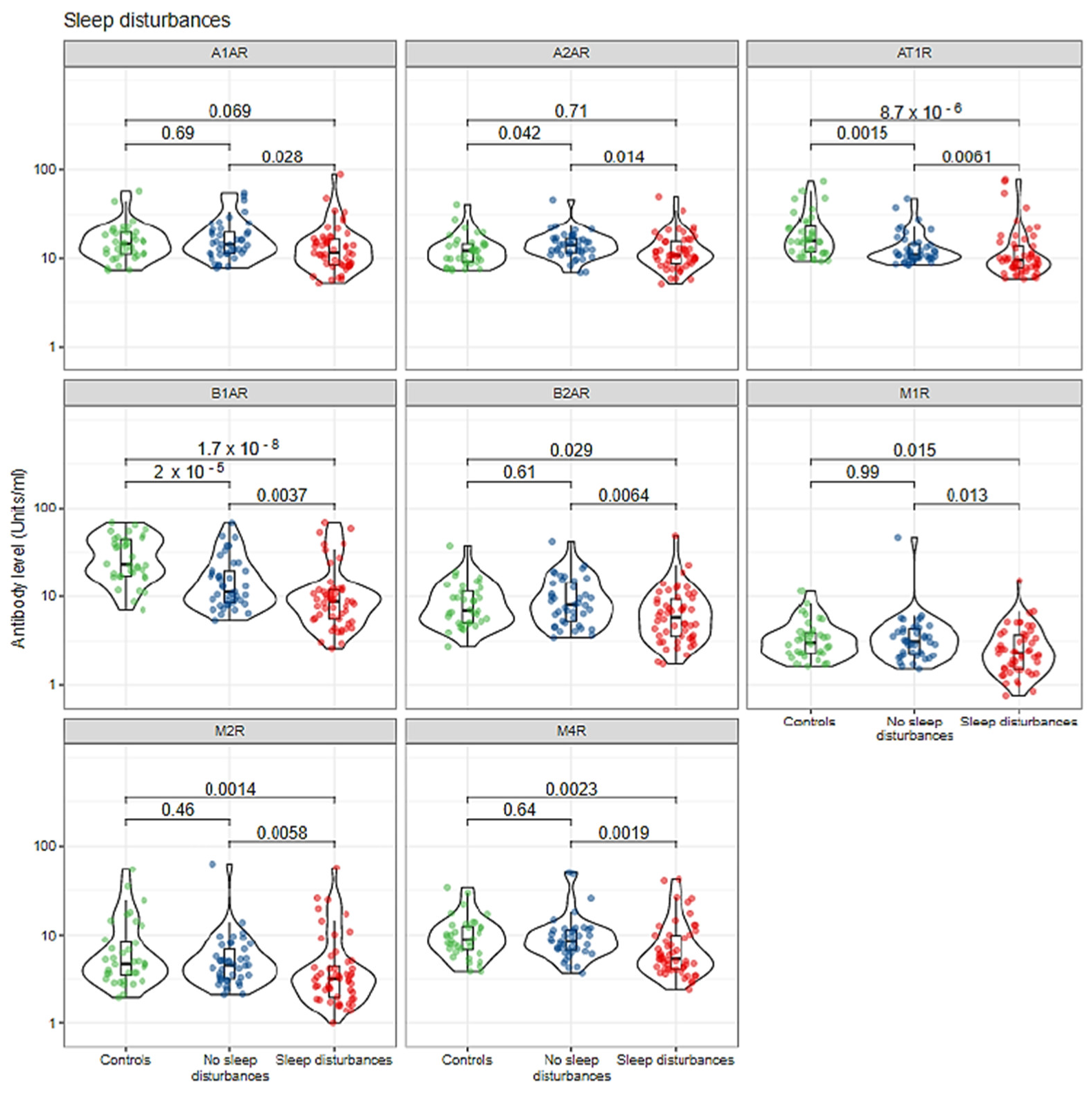


| Characteristic | Controls, N = 36 1 | Without Symptom, N = 40 1 | With Symptom, N = 53 1 |
|---|---|---|---|
| A1AR | 15 (11, 20) | 14 (11, 20) | 13 (9, 17) |
| A2AR | 12.2 (9.1, 14.5) | 13.8 (9.7, 16.9) | 11.5 (9.9, 14.8) |
| B1AR | 23 (17, 44) | 11 (8, 19) | 9 (7, 13) |
| B2AR | 6.9 (5.1, 11.5) | 7.0 (4.3, 10.8) | 6.8 (4.5, 10.0) |
| M1R | 3.04 (2.28, 3.92) | 3.09 (2.16, 4.38) | 2.38 (1.83, 3.83) |
| M2R | 5 (3, 8) | 4 (3, 9) | 3 (3, 5) |
| M3R | 7.9 (6.4, 10.1) | 6.8 (5.2, 9.7) | 7.0 (5.3, 8.6) |
| M4R | 9 (7, 12) | 9 (6, 12) | 6 (4, 9) |
| M5R | 6.8 (5.3, 9.3) | 7.7 (5.4, 10.8) | 6.7 (5.4, 8.6) |
| AT1R | 16 (12, 23) | 11 (9, 17) | 10 (8, 12) |
| ETAR | 11.3 (9.4, 14.2) | 9.3 (7.5, 12.3) | 8.4 (6.9, 10.2) |
| Characteristic | Controls, N = 36 1 | Without Symptom, N = 41 1 | With Symptom, N = 52 1 |
|---|---|---|---|
| A1AR | 15 (11, 20) | 14 (11, 20) | 12 (8, 16) |
| A2AR | 12.2 (9.1, 14.5) | 14.1 (11.5, 16.7) | 10.9 (8.7, 15.5) |
| B1AR | 23 (17, 44) | 11 (9, 20) | 9 (6, 12) |
| B2AR | 6.9 (5.1, 11.5) | 8.1 (5.3, 14.1) | 5.8 (3.6, 9.3) |
| M1R | 3.04 (2.28, 3.92) | 3.12 (2.19, 4.34) | 2.31 (1.49, 3.70) |
| M2R | 5 (3, 8) | 5 (3, 7) | 3 (2, 4) |
| M3R | 7.9 (6.4, 10.1) | 7.7 (5.8, 8.9) | 6.7 (4.9, 9.3) |
| M4R | 9 (7, 12) | 8 (7, 11) | 5 (4, 10) |
| M5R | 6.8 (5.3, 9.3) | 7.5 (5.8, 11.2) | 6.7 (5.1, 8.9) |
| AT1R | 16 (12, 23) | 11 (10, 14) | 10 (8, 14) |
| ETAR | 11.3 (9.4, 14.2) | 9.3 (7.9, 11.8) | 8.2 (6.3, 11.2) |
| Characteristic | Controls, N = 36 1 | Without Symptom, N = 51 1 | With Symptom, N = 42 1 |
|---|---|---|---|
| A1AR | 15 (11, 20) | 15 (11, 21) | 12 (8, 15) |
| A2AR | 12.2 (9.1, 14.5) | 12.7 (10.5, 16.6) | 11.5 (9.6, 15.9) |
| B1AR | 23 (17, 44) | 11 (8, 17) | 9 (6, 11) |
| B2AR | 6.9 (5.1, 11.5) | 6.7 (4.4, 10.5) | 7.0 (4.2, 10.3) |
| M1R | 3.04 (2.28, 3.92) | 2.58 (1.97, 4.22) | 2.54 (1.81, 3.87) |
| M2R | 5 (3, 8) | 4 (3, 7) | 3 (2, 5) |
| M3R | 7.9 (6.4, 10.1) | 7.1 (5.3, 9.6) | 6.8 (5.0, 8.7) |
| M4R | 9 (7, 12) | 8 (6, 12) | 5 (4, 8) |
| M5R | 6.8 (5.3, 9.3) | 7.6 (5.6, 11.6) | 6.7 (4.9, 8.3) |
| AT1R | 16 (12, 23) | 11 (9, 16) | 10 (8, 13) |
| ETAR | 11.3 (9.4, 14.2) | 9.2 (7.8, 12.0) | 8.1 (6.6, 10.6) |
| Characteristic | Controls, N = 36 1 | Without Symptom, N = 56 1 | With Symptom, N = 37 1 |
|---|---|---|---|
| A1AR | 15 (11, 20) | 14 (11, 19) | 11 (8, 16) |
| A2AR | 12.2 (9.1, 14.5) | 14.2 (10.9, 16.9) | 10.3 (8.4, 12.4) |
| B1AR | 23 (17, 44) | 11 (8, 21) | 8 (6, 11) |
| B2AR | 6.9 (5.1, 11.5) | 7.5 (4.9, 10.8) | 5.1 (3.5, 8.4) |
| M1R | 3.04 (2.28, 3.92) | 3.05 (2.21, 4.37) | 2.13 (1.51, 3.67) |
| M2R | 5 (3, 8) | 4 (3, 8) | 3 (2, 4) |
| M3R | 7.9 (6.4, 10.1) | 7.7 (5.7, 9.7) | 5.9 (4.9, 8.1) |
| M4R | 9 (7, 12) | 9 (6, 11) | 5 (4, 7) |
| M5R | 6.8 (5.3, 9.3) | 7.6 (5.8, 11.7) | 6.3 (4.9, 8.2) |
| AT1R | 16 (12, 23) | 11 (9, 16) | 10 (8, 11) |
| ETAR | 11.3 (9.4, 14.2) | 9.3 (8.0, 12.1) | 7.7 (6.2, 9.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tocut, M.; Halpert, G.; Tsur, A.M.; Sharif, K.; Heidecke, H.; Levy, Y.; Watad, A.; Amital, H.; Shoenfeld, Y. Cognitive Impairment, Sleep Disturbance, and Depression in Women with Silicone Breast Implants: Association with Autoantibodies against Autonomic Nervous System Receptors. Biomolecules 2022, 12, 776. https://doi.org/10.3390/biom12060776
Tocut M, Halpert G, Tsur AM, Sharif K, Heidecke H, Levy Y, Watad A, Amital H, Shoenfeld Y. Cognitive Impairment, Sleep Disturbance, and Depression in Women with Silicone Breast Implants: Association with Autoantibodies against Autonomic Nervous System Receptors. Biomolecules. 2022; 12(6):776. https://doi.org/10.3390/biom12060776
Chicago/Turabian StyleTocut, Milena, Gilad Halpert, Avishai M. Tsur, Kassem Sharif, Harald Heidecke, Yair Levy, Abdulla Watad, Howard Amital, and Yehuda Shoenfeld. 2022. "Cognitive Impairment, Sleep Disturbance, and Depression in Women with Silicone Breast Implants: Association with Autoantibodies against Autonomic Nervous System Receptors" Biomolecules 12, no. 6: 776. https://doi.org/10.3390/biom12060776
APA StyleTocut, M., Halpert, G., Tsur, A. M., Sharif, K., Heidecke, H., Levy, Y., Watad, A., Amital, H., & Shoenfeld, Y. (2022). Cognitive Impairment, Sleep Disturbance, and Depression in Women with Silicone Breast Implants: Association with Autoantibodies against Autonomic Nervous System Receptors. Biomolecules, 12(6), 776. https://doi.org/10.3390/biom12060776








