Neuromuscular Active Zone Structure and Function in Healthy and Lambert-Eaton Myasthenic Syndrome States
Abstract
1. Introduction
2. Action Potential Triggered Calcium Entry
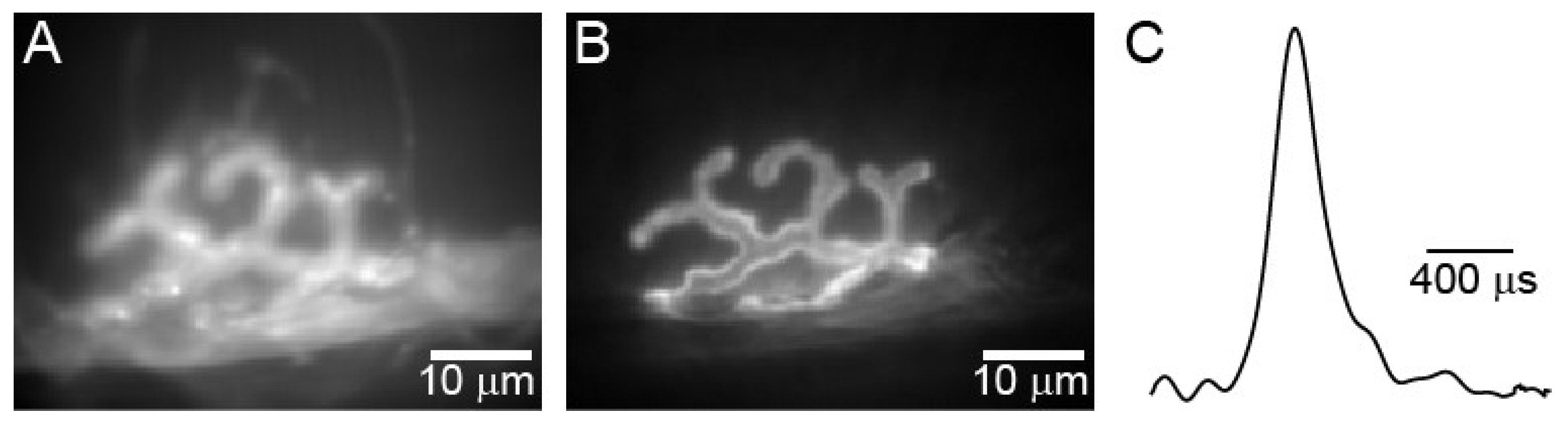
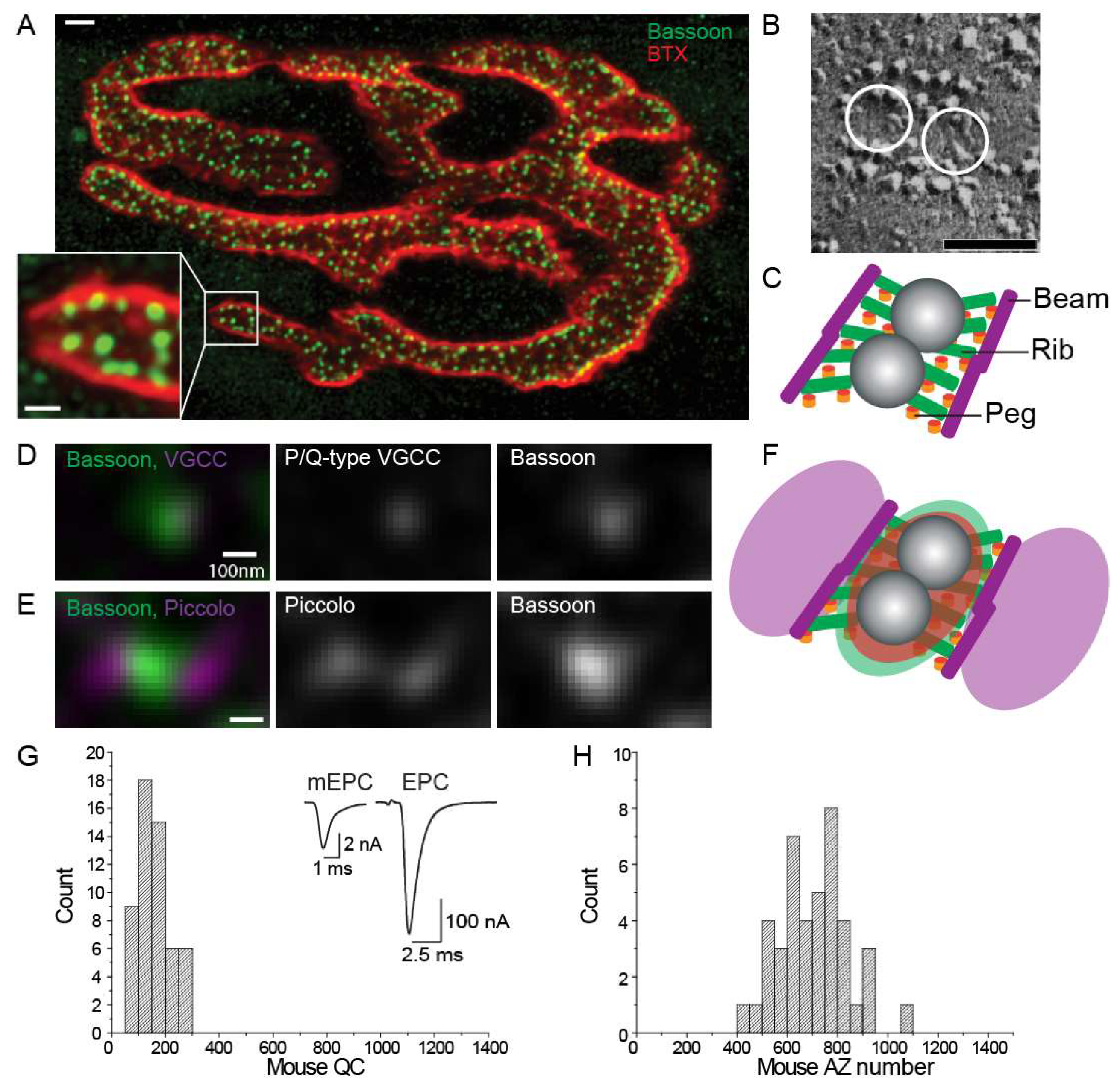
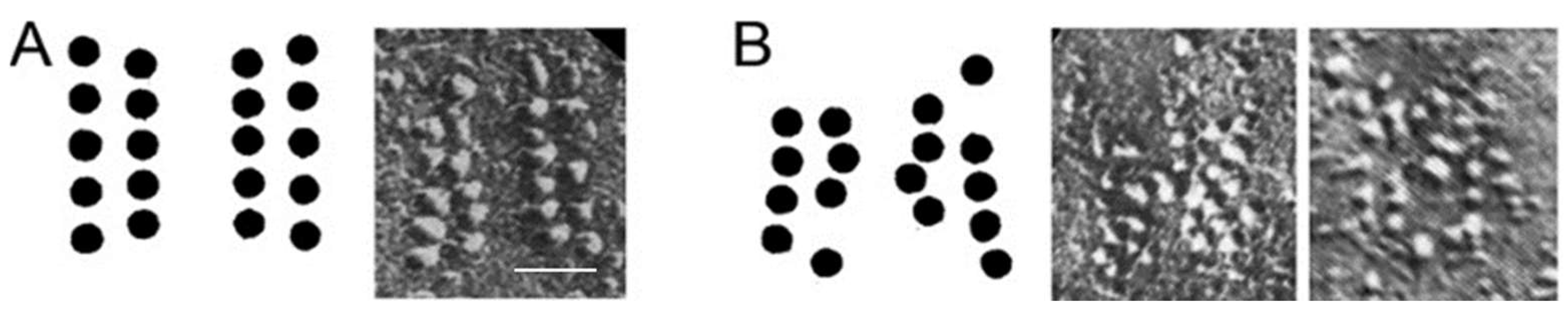
3. Active Zone Structure and Organization at Healthy Synapses
4. Structure-Function Relationships in the NMJ
5. Computer Modeling of Active Zone Structure and Function
6. Lambert-Eaton Myasthenic Syndrome
7. The Passive Transfer Mouse Model for LEMS
8. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Meriney, S.D.; Dittrich, M. Organization and function of transmitter release sites at the neuromuscular junction. J. Physiol. 2013, 591, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.J.; Slater, C.R. Safety factor at the neuromuscular junction. Prog. Neurobiol. 2001, 64, 393–429. [Google Scholar] [CrossRef]
- Sudhof, T.C. The presynaptic active zone. Neuron 2012, 75, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Kochubey, O.; Lou, X.; Schneggenburger, R. Regulation of transmitter release by Ca2+ and synaptotagmin: Insights from a large CNS synapse. Trends Neurosci. 2011, 34, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Laghaei, R.; Ma, J.; Tarr, T.B.; Homan, A.E.; Kelly, L.S.; Tilvawala, M.S.; Vuocolo, B.S.; Rajasekaran, H.P.; Meriney, S.D.; Dittrich, M. Transmitter release site organization can predict synaptic function at the neuromuscular junction. J. Neurophysiol. 2018, 119, 1340–1355. [Google Scholar] [CrossRef] [PubMed]
- Kress, G.; Mennerick, S. Action potential initiation and propagation: Upstream influences on neurotransmission. Neuroscience 2009, 158, 211–222. [Google Scholar] [CrossRef]
- Vincent, A.; Lang, B.; Newsom-Davis, J. Autoimmunity to the voltage-gated calcium channel underlies the Lambert-Eaton myasthenic syndrome, a paraneoplastic disorder. Trends Neurosci. 1989, 12, 496–502. [Google Scholar] [CrossRef]
- Titulaer, M.J.; Lang, B.; Verschuuren, J. Lambert–Eaton myasthenic syndrome: From clinical characteristics to therapeutic strategies. Lancet Neurol. 2011, 10, 1098–1107. [Google Scholar] [CrossRef]
- Fukunaga, H.; Engel, A.G.; Lang, B.; Newsom-Davis, J.; Vincent, A. Passive transfer of Lambert-Eaton myasthenic syndrome with IgG from man to mouse depletes the presynaptic membrane active zones. Proc. Natl. Acad. Sci. USA 1983, 80, 7636–7640. [Google Scholar] [CrossRef]
- Fukuoka, T.; Engel, A.G.; Lang, B.; Newsom-Davis, J.; Prior, C.; W-Wray, D. Lambert-Eaton myasthenic syndrome: I. Early morphological effects of IgG on the presynaptic membrane active zones. Ann. Neurol. 1987, 22, 193–199. [Google Scholar] [CrossRef]
- Tarr, T.B.; Wipf, P.; Meriney, S.D. Synaptic Pathophysiology and Treatment of Lambert-Eaton Myasthenic Syndrome. Mol. Neurobiol. 2014, 52, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Rowan, M.J.; DelCanto, G.; Yu, J.J.; Kamasawa, N.; Christie, J.M. Synapse-Level Determination of Action Potential Duration by K+ Channel Clustering in Axons. Neuron 2016, 91, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Burkitt, A.N. A Review of the Integrate-and-fire Neuron Model: I. Homogeneous Synaptic Input. Biol. Cybern. 2006, 95, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lapicque, L. Quantitative investigations of electrical nerve excitation treated as polarization. Biol. Cybern. 2007, 97, 341–349. [Google Scholar] [PubMed]
- Hodgkin, A.L.; Huxley, A.F. Resting and action potentials in single nerve fibres. J. Physiol. 1945, 104, 176–195. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. Propagation of electrical signals along giant nerve fibres. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1952, 140, 177–183. [Google Scholar]
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J. Physiol. 1952, 116, 497–506. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. The components of membrane conductance in the giant axon of Loligo. J. Physiol. 1952, 116, 473–496. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J. Physiol. 1952, 116, 449–472. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F.; Katz, B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J. Physiol. 1952, 116, 424–448. [Google Scholar] [CrossRef] [PubMed]
- Bean, B.P. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 2007, 8, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Hoppa, M.B.; Gouzer, G.; Armbruster, M.; Ryan, T.A. Control and Plasticity of the Presynaptic Action Potential Waveform at Small CNS Nerve Terminals. Neuron 2014, 84, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Popovic, M.A.; Foust, A.; McCormick, D.A.; Zecevic, D. The spatio-temporal characteristics of action potential initiation in layer 5 pyramidal neurons: A voltage imaging study. J. Physiol. 2011, 589, 4167–4187. [Google Scholar] [CrossRef]
- Ginebaugh, S.P.; Cyphers, E.D.; Lanka, V.; Ortiz, G.; Miller, E.W.; Laghaei, R.; Meriney, S.D. The Frog Motor Nerve Terminal Has Very Brief Action Potentials and Three Electrical Regions Predicted to Differentially Control Transmitter Release. J. Neurosci. 2020, 40, 3504–3516. [Google Scholar] [CrossRef]
- Ojala, K.S.; Ginebaugh, S.P.; Wu, M.; Miller, E.W.; Ortiz, G.; Covarrubias, M.; Meriney, S.D. A high-affinity, partial antagonist effect of 3,4-diaminopyridine mediates action potential broadening and enhancement of transmitter release at NMJs. J. Biol. Chem. 2021, 296, 100302. [Google Scholar] [CrossRef]
- Brooke, R.E.; Moores, T.S.; Morris, N.P.; Parson, S.; Deuchars, J. Kv3 voltage-gated potassium channels regulate neurotransmitter release from mouse motor nerve terminals. Eur. J. Neurosci. 2004, 20, 3313–3321. [Google Scholar] [CrossRef]
- Flink, M.T.; Atchison, W.D. Iberiotoxin-Induced Block of Ca2+-Activated K+ Channels Induces Dihydropyridine Sensitivity of ACh Release from Mammalian Motor Nerve Terminals. J. Pharmacol. Exp. Ther. 2003, 305, 646–652. [Google Scholar] [CrossRef]
- Luo, F.; Dittrich, M.; Stiles, J.R.; Meriney, S.D. Single-Pixel Optical Fluctuation Analysis of Calcium Channel Function in Active Zones of Motor Nerve Terminals. J. Neurosci. 2011, 31, 11268–11281. [Google Scholar] [CrossRef]
- Luo, F.; Dittrich, M.; Cho, S.; Stiles, J.R.; Meriney, S.D. Transmitter release is evoked with low probability predominately by calcium flux through single channel openings at the frog neuromuscular junction. J. Neurophysiol. 2014, 113, 2480–2489. [Google Scholar] [CrossRef]
- Tarr, T.B.; Dittrich, M.; Meriney, S.D. Are unreliable release mechanisms conserved from NMJ to CNS? Trends Neurosci. 2013, 36, 14–22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Couteaux, R.; Pecot-Dechavassine, M. Synaptic vesicles and pouches at the level of “active zones” of the neuromuscular junction. Comptes Rendus Hebd. Des Seances De L’academie Des Sci. Ser. D Sci. Nat. 1970, 271, 2346–2349. [Google Scholar]
- Heuser, J.E.; Reese, T.S.; Landis, D.M.D. Functional changes in frog neuromuscular junctions studied with freeze-fracture. J. Neurocytol. 1974, 3, 109–131. [Google Scholar] [CrossRef]
- Peper, K.; Dreyer, F.; Sandri, C.; Akert, K.; Moor, H. Structure and ultrastructure of the frog motor endplate. Cell Tissue Res. 1974, 149, 437–455. [Google Scholar] [CrossRef] [PubMed]
- Pfenninger, K.; Akert, K.; Moor, H.; Sandri, C. The fine structure of freeze-fractured presynaptic membranes. J. Neurocytol. 1972, 1, 129–149. [Google Scholar] [CrossRef]
- Nagwaney, S.; Harlow, M.L.; Jung, J.H.; Szule, J.A.; Ress, D.; Xu, J.; Marshall, R.M.; McMahan, U.J. Macromolecular connections of active zone material to docked synaptic vesicles and presynaptic membrane at neuromuscular junctions of mouse. J. Comp. Neurol. 2009, 513, 457–468. [Google Scholar] [CrossRef]
- Ruiz, R.; Cano, R.; Casañas, J.J.; Gaffield, M.A.; Betz, W.J.; Tabares, L. Active Zones and the Readily Releasable Pool of Synaptic Vesicles at the Neuromuscular Junction of the Mouse. J. Neurosci. 2011, 31, 2000–2008. [Google Scholar] [CrossRef]
- Chen, J.; Mizushige, T.; Nishimune, H. Active zone density is conserved during synaptic growth but impaired in aged mice. J. Comp. Neurol. 2011, 520, 434–452. [Google Scholar] [CrossRef]
- Nishimune, H.; Badawi, Y.; Mori, S.; Shigemoto, K. Dual-color STED microscopy reveals a sandwich structure of Bassoon and Piccolo in active zones of adult and aged mice. Sci. Rep. 2016, 6, 27935. [Google Scholar] [CrossRef]
- Chen, J.; Billings, S.E.; Nishimune, H. Calcium channels link the muscle-derived synapse organizer laminin β2 to Bassoon and CAST/Erc2 to organize presynaptic active zones. J. Neurosci. 2011, 31, 512–525. [Google Scholar] [CrossRef]
- Marques, M.J.; Conchello, J.-A.; Lichtman, J.W. From Plaque to Pretzel: Fold Formation and Acetylcholine Receptor Loss at the Developing Neuromuscular Junction. J. Neurosci. 2000, 20, 3663–3675. [Google Scholar] [CrossRef] [PubMed]
- Patton, B.L.; Cunningham, J.M.; Thyboll, J.; Kortesmaa, J.; Westerblad, H.; Edström, L.; Tryggvason, K.; Sanes, J.R. Properly formed but improperly localized synaptic specializations in the absence of laminin α4. Nat. Neurosci. 2001, 4, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Gundelfinger, E.D.; Reissner, C.; Garner, C.C. Role of Bassoon and Piccolo in Assembly and Molecular Organization of the Active Zone. Front. Synaptic Neurosci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Yang, X.; Gerber, S.H.; Kwon, H.-B.; Ho, A.; Castillo, P.E.; Liu, X.; Südhof, T.C. Piccolo and bassoon maintain synaptic vesicle clustering without directly participating in vesicle exocytosis. Proc. Natl. Acad. Sci. USA 2010, 107, 6504–6509. [Google Scholar] [CrossRef] [PubMed]
- Dieck, S.T.; Sanmartí-Vila, L.; Langnaese, K.; Richter, K.; Kindler, S.; Soyke, A.; Wex, H.; Smalla, K.-H.; Kämpf, U.; Fränzer, J.-T.; et al. Bassoon, a Novel Zinc-finger CAG/Glutamine-repeat Protein Selectively Localized at the Active Zone of Presynaptic Nerve Terminals. J. Cell Biol. 1998, 142, 499–509. [Google Scholar] [CrossRef]
- Cases-Langhoff, C.; Voss, B.; Garner, A.M.; Appeltauer, U.; Takei, K.; Kindler, S.; Veh, R.W.; De Camilli, P.; Gundelfinger, E.D.; Garner, C. Piccolo, a novel 420 kDa protein associated with the presynaptic cytomatrix. Eur. J. Cell Biol. 1996, 69, 214–223. [Google Scholar]
- Badawi, Y.; Nishimune, H. Presynaptic active zones of mammalian neuromuscular junctions: Nanoarchitecture and selective impairments in aging. Neurosci. Res. 2017, 127, 78–88. [Google Scholar] [CrossRef]
- Szule, J.A. Hypothesis Relating the Structure, Biochemistry and Function of Active Zone Material Macromolecules at a Neuromuscular Junction. Front. Synaptic Neurosci. 2022, 13, 798225. [Google Scholar] [CrossRef]
- Zhai, R.; Bellen, H. The Architecture of the Active Zone in the Presynaptic Nerve Terminal. Physiology 2004, 19, 262–270. [Google Scholar] [CrossRef]
- Propst, J.; Ko, C. Correlations between active zone ultrastructure and synaptic function studied with freeze-fracture of physiologically identified neuromuscular junctions. J. Neurosci. 1987, 7, 3654–3664. [Google Scholar] [CrossRef]
- Herrera, A.; Grinnell, A.; Wolowske, B. Ultrastructural correlates of experimentally altered transmitter release efficacy in frog motor nerve terminals. Neuroscience 1985, 16, 491–500. [Google Scholar] [CrossRef]
- Herrera, A.A.; Grinnell, A.D. Contralateral denervation causes enhanced transmitter release from frog motor nerve terminals. Nature 1981, 291, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Meriney, S.D.; Wolowske, B.; Ezzati, E.; Grinnell, A.D. Low calcium-induced disruption of active zone structure and function at the frog neuromuscular junction. Synapse 1996, 24, 1–11. [Google Scholar] [CrossRef]
- Bartol, T.; Land, B.; Salpeter, E.; Salpeter, M. Monte Carlo simulation of miniature endplate current generation in the vertebrate neuromuscular junction. Biophys. J. 1991, 59, 1290–1307. [Google Scholar] [CrossRef][Green Version]
- Kerr, R.A.; Bartol, T.M.; Kaminsky, B.; Dittrich, M.; Chang, J.-C.J.; Baden, S.B.; Sejnowski, T.J.; Stiles, J.R. Fast Monte Carlo Simulation Methods for Biological Reaction-Diffusion Systems in Solution and on Surfaces. SIAM J. Sci. Comput. 2008, 30, 3126–3149. [Google Scholar] [CrossRef]
- Stiles, J.R.; Van Helden, D.; Bartol, T.M.; E Salpeter, E.; Salpeter, M.M. Miniature endplate current rise times less than 100 microseconds from improved dual recordings can be modeled with passive acetylcholine diffusion from a synaptic vesicle. Proc. Natl. Acad. Sci. USA 1996, 93, 5747–5752. [Google Scholar] [CrossRef]
- Homan, A.E.; Laghaei, R.; Dittrich, M.; Meriney, S.D. Impact of spatiotemporal calcium dynamics within presynaptic active zones on synaptic delay at the frog neuromuscular junction. J. Neurophysiol. 2018, 119, 688–699. [Google Scholar] [CrossRef]
- Dittrich, M.; Pattillo, J.M.; King, J.D.; Cho, S.; Stiles, J.R.; Meriney, S.D. An Excess-Calcium-Binding-Site Model Predicts Neurotransmitter Release at the Neuromuscular Junction. Biophys. J. 2013, 104, 2751–2763. [Google Scholar] [CrossRef]
- Ma, J.; Kelly, L.; Ingram, J.; Price, T.J.; Meriney, S.D.; Dittrich, M. New insights into short-term synaptic facilitation at the frog neuromuscular junction. J. Neurophysiol. 2015, 113, 71–87. [Google Scholar] [CrossRef]
- Titulaer, M.J.; Maddison, P.; Sont, J.K.; Wirtz, P.W.; Hilton-Jones, D.; Klooster, R.; Willcox, N.; Potman, M.; Smitt, P.A.S.; Kuks, J.B.M.; et al. Clinical Dutch-English Lambert-Eaton Myasthenic Syndrome (LEMS) Tumor Association Prediction Score Accurately Predicts Small-Cell Lung Cancer in the LEMS. J. Clin. Oncol. 2011, 29, 902–908. [Google Scholar] [CrossRef]
- Wirtz, P.W.; Smallegange, T.M.; Wintzen, A.R.; Verschuuren, J. Differences in clinical features between the Lambert-Eaton myasthenic syndrome with and without cancer: An analysis of 227 published cases. Clin. Neurol. Neurosurg. 2002, 104, 359–363. [Google Scholar] [CrossRef]
- Titulaer, M.J.; Verschuuren, J.J. Lambert–Eaton myasthenic syndrome: Tumor versus nontumor forms. Ann. N. Y. Acad. Sci. 2008, 1132, 129–134. [Google Scholar] [CrossRef]
- Kalemkerian, G.P.; Akerley, W.; Bogner, P.; Borghaei, H.; Chow, L.Q.; Downey, R.J.; Hughes, M. Non–small cell lung cancer. J. Natl. Compr. Cancer Netw. 2013, 11, 78–98. [Google Scholar] [CrossRef]
- David, P.; El Far, O.; Martin-Mouto, N.; Poupon, M.F.; Takahashi, M.; Seagar, M.J. Expression of synaptotagmin and syntaxin associated with N-type calcium channels in small cell lung cancer. FEBS Lett. 1993, 326, 135–139. [Google Scholar] [CrossRef]
- Roberts, A.; Perera, S.; Lang, B.; Vincent, A.; Newsom-Davis, J. Paraneoplastic myasthenic syndrome IgG inhibits 45Ca2+ flux in a human small cell carcinoma line. Nature 1985, 317, 737–739. [Google Scholar] [CrossRef]
- Smith, D.O.; Conklin, M.W.; Jensen, P.J.; Atchison, W.D. Decreased calcium currents in motor nerve terminals of mice with Lambert-Eaton myasthenic syndrome. J. Physiol. 1995, 487, 115–123. [Google Scholar] [CrossRef]
- Motomura, M.; Lang, B.; Johnston, I.; Palace, J.; Vincent, A.; Newsom-Davis, J. Incidence of serum anti-P/O-type and anti-N-type calcium channel autoantibodies in the Lambert-Eaton myasthenic syndrome. J. Neurol. Sci. 1997, 147, 35–42. [Google Scholar] [CrossRef]
- Takamori, M.; Takahashi, M.; Yasukawa, Y.; Iwasa, K.; Nemoto, Y.; Suenaga, A.; Nagataki, S.; Nakamura, T. Antibodies to recombinant synaptotagmin and calcium channel subtypes in Lambert-Eaton myasthenic syndrome. J. Neurol. Sci. 1995, 133, 95–101. [Google Scholar] [CrossRef]
- Lennon, V.A.; Kryzer, T.J.; Griesmann, G.E.; O’Suilleabhain, P.; Windebank, A.J.; Woppmann, A.; Miljanich, G.P.; Lambert, E.H. Calcium-Channel Antibodies in the Lambert–Eaton Syndrome and Other Paraneoplastic Syndromes. N. Engl. J. Med. 1995, 332, 1467–1475. [Google Scholar] [CrossRef]
- Kesner, V.G.; Oh, S.J.; Dimachkie, M.M.; Barohn, R.J. Lambert-Eaton myasthenic syndrome. Neurol. Clin. 2018, 36, 379–394. [Google Scholar] [CrossRef]
- Takamori, M. Lambert–Eaton myasthenic syndrome: Search for alternative autoimmune targets and possible compensatory mechanisms based on presynaptic calcium homeostasis. J. Neuroimmunol. 2008, 201–202, 145–152. [Google Scholar] [CrossRef]
- Lang, B.; Molenaar, P.C.; Newsom-Davis, J.; Vincent, A. Passive Transfer of Lambert-Eaton Myasthenic Syndrome in Mice: Decreased Rates of Resting and Evoked Release of Acetylcholine from Skeletal Muscle. J. Neurochem. 1984, 42, 658–662. [Google Scholar] [CrossRef]
- Fukunaga, H.; Engel, A.G.; Osame, M.; Lambert, E.H. Paucity and disorganization of presynaptic membrane active zones in the Lambert-Eaton myasthenic syndrome. Muscle Nerve 1982, 5, 686–697. [Google Scholar] [CrossRef]
- Xu, Y.F.; Hewett, S.J.; Atchison, W.D. Passive transfer of Lambert-Eaton myasthenic syndrome induces dihydropyridine sensitivity of ICa in mouse motor nerve terminals. J. Neurophysiol. 1998, 80, 1056–1069. [Google Scholar] [CrossRef]
- Nagel, A.; Engel, A.G.; Lang, B.; Newsom-Davis, J.; Fukuoka, T. Lambert-eaton myasthenic syndrome IgG depletes presynaptic membrane active zone particles by antigenic modulation. Ann. Neurol. 1988, 24, 552–558. [Google Scholar] [CrossRef]
- Uchitel, O.D.; A Protti, D.; Sanchez, V.; Cherksey, B.D.; Sugimori, M.; Llinás, R. P-type voltage-dependent calcium channel mediates presynaptic calcium influx and transmitter release in mammalian synapses. Proc. Natl. Acad. Sci. USA 1992, 89, 3330–3333. [Google Scholar] [CrossRef]
- Katz, E.; Ferro, P.A.; Weisz, G.; Uchitel, O. Calcium channels involved in synaptic transmission at the mature and regenerating mouse neuromuscular junction. J. Physiol. 1996, 497, 687–697. [Google Scholar] [CrossRef]
- Flink, M.T.; Atchison, W.D. Passive transfer of Lambert-Eaton syndrome to mice induces dihydropyridine sensitivity of neuromuscular transmission. J. Physiol. 2002, 543, 567–576. [Google Scholar] [CrossRef]
- Lang, B.; Newsom-Davis, J.; Prior, C.; Wray, D. Antibodies to motor nerve terminals: An electrophysiological study of a human myasthenic syndrome transferred to mouse. J. Physiol. 1983, 344, 335–345. [Google Scholar] [CrossRef]
- Tarr, T.B.; Lacomis, D.; Reddel, S.W.; Liang, M.; Valdomir, G.; Frasso, M.; Wipf, P.; Meriney, S.D. Complete reversal of Lambert-Eaton myasthenic syndrome synaptic impairment by the combined use of a K+ channel blocker and a Ca2+ channel agonist. J. Physiol. 2014, 592, 3687–3696. [Google Scholar] [CrossRef]
- Tarr, T.B.; Malick, W.; Liang, M.; Valdomir, G.; Frasso, M.; Lacomis, D.; Reddel, S.W.; Garcia-Ocano, A.; Wipf, P.; Meriney, S.D. Evaluation of a novel calcium channel agonist for therapeutic potential in Lambert-Eaton myasthenic syndrome. J. Neurosci. 2013, 33, 10559–10567. [Google Scholar] [CrossRef]
- Catterall, W.A. Interactions of Presynaptic Ca2+ Channels and Snare Proteins in Neurotransmitter Release. Ann. N. Y. Acad. Sci. 1999, 868, 144–159. [Google Scholar] [CrossRef]
- Mochida, S.; Westenbroek, R.E.; Yokoyama, C.T.; Zhong, H.; Myers, S.J.; Scheuer, T.; Itoh, K.; Catterall, W.A. Requirement for the synaptic protein interaction site for reconstitution of synaptic transmission by P/Q-type calcium channels. Proc. Natl. Acad. Sci. USA 2003, 100, 2819–2824. [Google Scholar] [CrossRef]
- Sheng, Z.-H.; Westenbroek, R.E.; Catterall, W.A. Physical Link and Functional Coupling of Presynaptic Calcium Channels and the Synaptic Vesicle Docking/Fusion Machinery. J. Bioenerg. Biomembr. 1998, 30, 335–345. [Google Scholar] [CrossRef]
- Flink, M.T. The role of L-type calcium ion channels in release of acetylcholine from motor nerve terminals following passive transfer of lambert-eaton myasthenic syndrome to mature mice. In Department of Pharmacology and Toxicology; Michigan State University: East Lansing, MI, USA, 2003; p. 235. [Google Scholar]
- Nakao, Y.K.; Motomura, M.; Fukudome, T.; Fukuda, T.; Shiraishi, H.; Yoshimura, T.; Tsujihata, M.; Eguchi, K. Seronegative Lambert-Eaton myasthenic syndrome: Study of 110 Japanese patients. Neurology 2002, 59, 1773–1775. [Google Scholar] [CrossRef]
- Maddison, P.; Lang, B.; Mills, K.; Newsom-Davis, J. Long term outcome in Lambert-Eaton myasthenic syndrome without lung cancer. J. Neurol. Neurosurg. Psychiatry 2001, 70, 212–217. [Google Scholar] [CrossRef]
- Slater, C.R. The Structure of Human Neuromuscular Junctions: Some Unanswered Molecular Questions. Int. J. Mol. Sci. 2017, 18, 2183. [Google Scholar] [CrossRef]
- Jones, R.A.; Harrison, C.; Eaton, S.L.; Hurtado, M.L.; Graham, L.C.; Alkhammash, L.; Oladiran, O.A.; Gale, A.; Lamont, D.J.; Simpson, H.; et al. Cellular and Molecular Anatomy of the Human Neuromuscular Junction. Cell Rep. 2017, 21, 2348–2356. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ginebaugh, S.P.; Badawi, Y.; Tarr, T.B.; Meriney, S.D. Neuromuscular Active Zone Structure and Function in Healthy and Lambert-Eaton Myasthenic Syndrome States. Biomolecules 2022, 12, 740. https://doi.org/10.3390/biom12060740
Ginebaugh SP, Badawi Y, Tarr TB, Meriney SD. Neuromuscular Active Zone Structure and Function in Healthy and Lambert-Eaton Myasthenic Syndrome States. Biomolecules. 2022; 12(6):740. https://doi.org/10.3390/biom12060740
Chicago/Turabian StyleGinebaugh, Scott P., Yomna Badawi, Tyler B. Tarr, and Stephen D. Meriney. 2022. "Neuromuscular Active Zone Structure and Function in Healthy and Lambert-Eaton Myasthenic Syndrome States" Biomolecules 12, no. 6: 740. https://doi.org/10.3390/biom12060740
APA StyleGinebaugh, S. P., Badawi, Y., Tarr, T. B., & Meriney, S. D. (2022). Neuromuscular Active Zone Structure and Function in Healthy and Lambert-Eaton Myasthenic Syndrome States. Biomolecules, 12(6), 740. https://doi.org/10.3390/biom12060740






