Effects of Risperidone and Prenatal Poly I:C Exposure on GABAA Receptors and AKT-GSK3β Pathway in the Ventral Tegmental Area of Female Juvenile Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Brain Dissection
2.3. RNA Isolation and Gene Expression Analysis by Real-Time qPCR
2.4. Statistical Analysis
3. Results
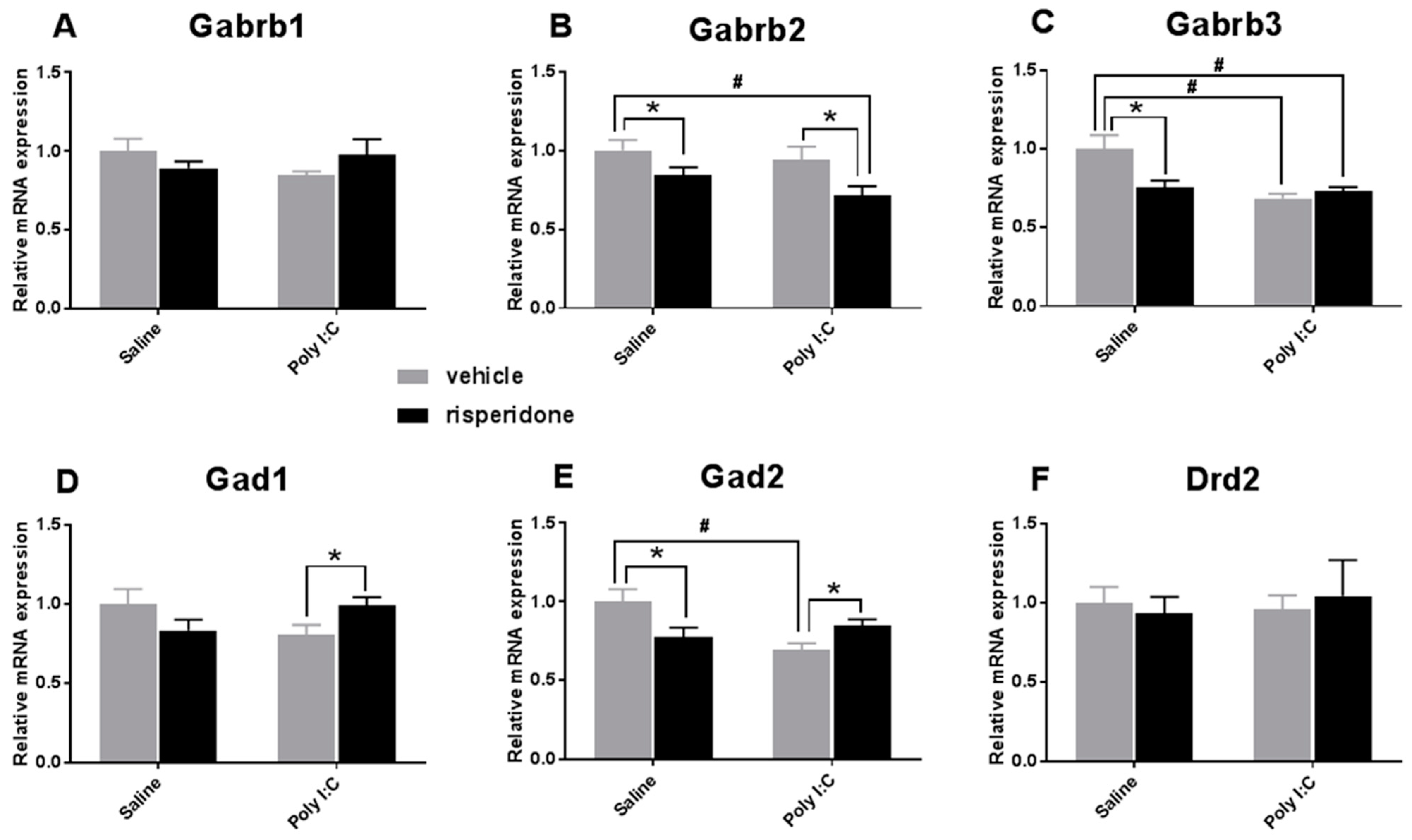
3.1. Effects on the GABAergic Markers
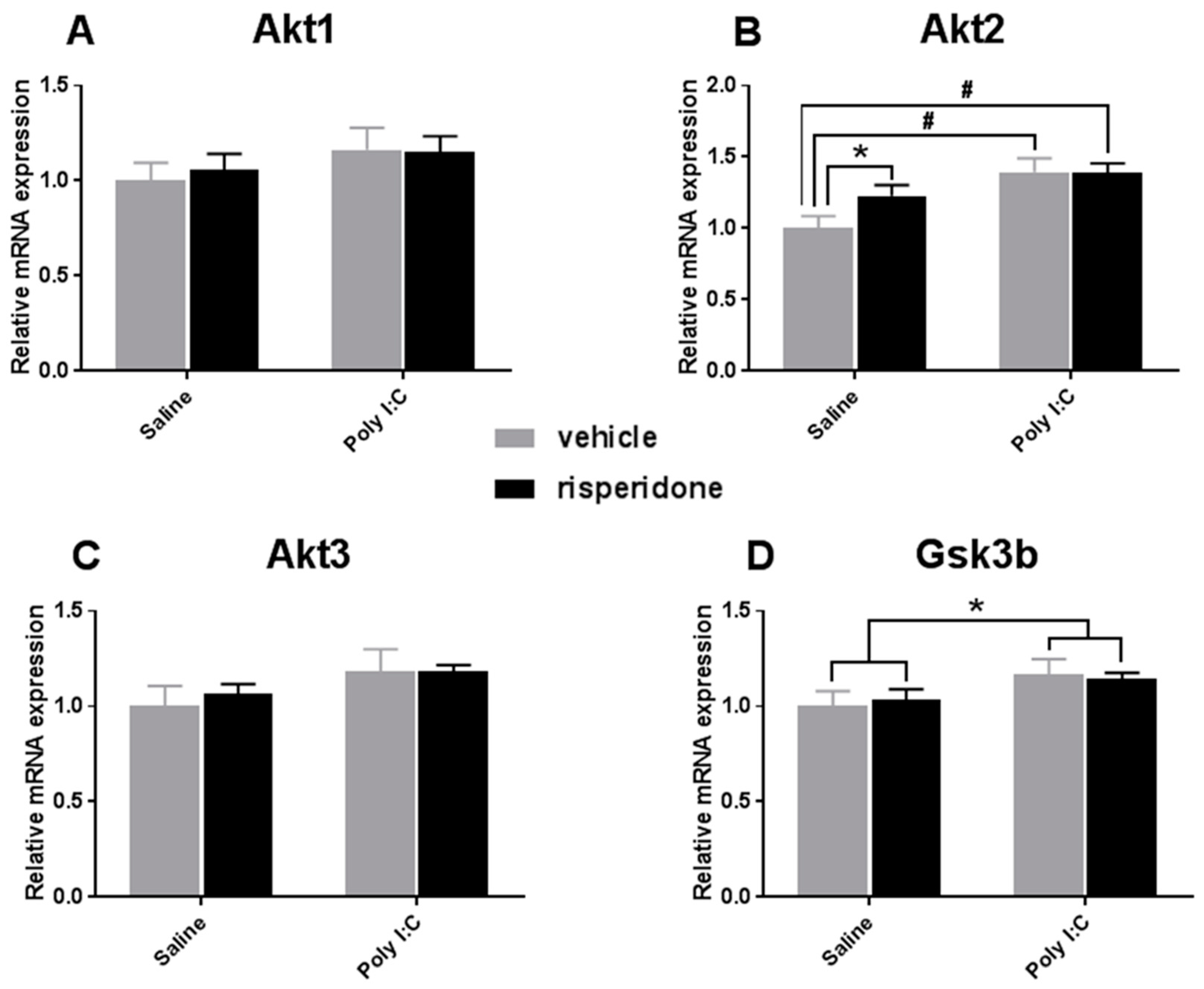
3.2. Effects on Akt-GSK3β Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, A.S.; Susser, E.S. In utero infection and adult schizophrenia. Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Derkits, E.J. Prenatal infection and schizophrenia: A review of epidemiologic and translational studies. Am. J. Psychiatry 2010, 167, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Buka, S.L.; Cannon, T.D.; Torrey, E.F.; Yolken, R.H. Maternal exposure to herpes simplex virus and risk of psychosis among adult offspring. Biol. Psychiatry 2008, 63, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, G.M.; Zimbron, J.; Lewis, G.; Jones, P. Prenatal maternal infection, neurodevelopment and adult schizophrenia: A systematic review of population-based studies. Psychol. Med. 2013, 43, 239–257. [Google Scholar] [CrossRef]
- Haddad, F.L.; Patel, S.V.; Schmid, S. Maternal Immune Activation by Poly I:C as a preclinical Model for Neurodevelopmental Disorders: A focus on Autism and Schizophrenia. Neurosci. Biobehav. Rev. 2020, 113, 546–567. [Google Scholar] [CrossRef]
- Meyer, U. Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol. Psychiatry 2014, 75, 307–315. [Google Scholar] [CrossRef]
- Murray, K.N.; Edye, M.E.; Manca, M.; Vernon, A.C.; Oladipo, J.M.; Fasolino, V.; Harte, M.; Mason, V.; Grayson, B.; McHugh, P.C.; et al. Evolution of a maternal immune activation (mIA) model in rats: Early developmental effects. Brain Behav. Immun. 2018, 75, 48–59. [Google Scholar] [CrossRef]
- Reisinger, S.; Khan, D.; Kong, E.; Berger, A.; Pollak, A.; Pollak, D. The poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol. Ther. 2015, 149, 213–226. [Google Scholar] [CrossRef]
- Gogos, A.; Sbisa, A.; Witkamp, D.; Buuse, M.V.D. Sex differences in the effect of maternal immune activation on cognitive and psychosis-like behaviour in Long Evans rats. Eur. J. Neurosci. 2020, 52, 2614–2626. [Google Scholar] [CrossRef]
- Kokras, N.; Dalla, C. Sex differences in animal models of psychiatric disorders. Br. J. Pharmacol. 2014, 171, 4595–4619. [Google Scholar] [CrossRef]
- Prendergast, B.J.; Onishi, K.G.; Zucker, I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2014, 40, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Lian, J.; Hodgson, J.; Zhang, W.; Deng, C. Prenatal Poly I:C Challenge Affects Behaviors and Neurotransmission via Elevated Neuroinflammation Responses in Female Juvenile Rats. Int. J. Neuropsychopharmacol. 2022, 25, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Bergdolt, L.; Dunaevsky, A. Brain changes in a maternal immune activation model of neurodevelopmental brain disorders. Prog. Neurobiol. 2019, 175, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Osborne, A.L.; Solowij, N.; Babic, I.; Lum, J.S.; Newell, K.; Huang, X.-F.; Weston-Green, K. Effect of cannabidiol on endocannabinoid, glutamatergic and GABAergic signalling markers in male offspring of a maternal immune activation (poly I:C) model relevant to schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 95, 109666. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Pan, B.; Engel, M.; Huang, X.-F. Neuregulin-1 signalling and antipsychotic treatment. Psychopharmacology 2013, 226, 201–215. [Google Scholar] [CrossRef]
- Ginovart, N.; Kapur, S. Role of dopamine D(2) receptors for antipsychotic activity. Curr. Antipsychotics 2012, 212, 27–52. [Google Scholar] [CrossRef]
- Howes, O.; Kapur, S. The dopamine hypothesis of schizophrenia: Version III--the final common pathway. Schizophr. Bull. 2009, 35, 549–562. [Google Scholar] [CrossRef]
- Morikawa, H.; Paladini, C. Dynamic regulation of midbrain dopamine neuron activity: Intrinsic, synaptic, and plasticity mechanisms. Neuroscience 2011, 198, 95–111. [Google Scholar] [CrossRef]
- Pan, B.; Deng, C. Modulation by chronic antipsychotic administration of PKA- and GSK3β-mediated pathways and the NMDA receptor in rat ventral midbrain. Psychopharmacology 2019, 236, 2687–2697. [Google Scholar] [CrossRef]
- Benedetti, F.; Poletti, S.; Radaelli, D.; Bernasconi, A.; Cavallaro, R.; Falini, A.; Lorenzi, C.; Pirovano, A.; Dallaspezia, S.; Locatelli, C.; et al. Temporal lobe grey matter volume in schizophrenia is associated with a genetic polymorphism influencing glycogen synthase kinase 3-β activity. Genes Brain Behav. 2010, 9, 365–371. [Google Scholar] [CrossRef]
- Benedetti, F.; Bollettini, I.; Barberi, I.; Radaelli, D.; Poletti, S.; Locatelli, C.; Pirovano, A.; Lorenzi, C.; Falini, A.; Colombo, C.; et al. Lithium and GSK3-β promoter gene variants influence white matter microstructure in bipolar disorder. Neuropsychopharmacology 2013, 38, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Freyberg, Z.; Ferrando, S.J.; Javitch, J. Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am. J. Psychiatry 2010, 167, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Gould, T.D.; Manji, H.K. Glycogen synthase kinase-3: A putative molecular target for lithium mimetic drugs. Neuropsychopharmacology 2005, 30, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Karege, F.; Méary, A.; Perroud, N.; Jamain, S.; Leboyer, M.; Ballmann, E.; Fernandez, R.; Malafosse, A.; Schürhoff, F. Genetic overlap between schizophrenia and bipolar disorder: A study with AKT1 gene variants and clinical phenotypes. Schizophr. Res. 2012, 135, 8–14. [Google Scholar] [CrossRef]
- Alimohamad, H.; Sutton, L.; Mouyal, J.; Rajakumar, N.; Rushlow, W.J. The effects of antipsychotics on β-catenin, glycogen synthase kinase-3 and dishevelled in the ventral midbrain of rats. J. Neurochem. 2005, 95, 513–525. [Google Scholar] [CrossRef]
- Alimohamad, H.; Rajakumar, N.; Seah, Y.-H.; Rushlow, W. Antipsychotics alter the protein expression levels of β-catenin and GSK-3 in the rat medial prefrontal cortex and striatum. Biol. Psychiatry 2005, 57, 533–542. [Google Scholar] [CrossRef]
- Allen, J.A.; Yost, J.M.; Setola, V.; Chen, X.; Sassano, M.F.; Chen, M.; Peterson, S.; Yadav, P.N.; Huang, X.-P.; Feng, B.; et al. Discovery of β-Arrestin—Biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc. Natl. Acad. Sci. USA 2011, 108, 18488–18493. [Google Scholar] [CrossRef]
- Beaulieu, J.-M.; Gainetdinov, R.R.; Caron, M.G. Akt/GSK3 signaling in the action of psychotropic drugs. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 327–347. [Google Scholar] [CrossRef]
- Emamian, E.S. AKT/GSK3 signaling pathway and schizophrenia. Front. Mol. Neurosci. 2012, 5, 33. [Google Scholar] [CrossRef]
- Li, X.; Rosborough, K.M.; Friedman, A.B.; Zhu, W.; Roth, K. Regulation of mouse brain glycogen synthase kinase-3 by atypical antipsychotics. Int. J. Neuropsychopharmacol. 2007, 10, 7–19. [Google Scholar] [CrossRef]
- Pan, B.; Huang, X.F.; Deng, C. Chronic administration of aripiprazole activates GSK3β-dependent signalling pathways and up-regulates GABAA receptor expression and CREB1 activity in rats. Sci. Rep. 2016, 6, 30040. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Huang, X.-F.; Deng, C. Aripiprazole and Haloperidol Activate GSK3β-Dependent Signalling Pathway Differentially in Various Brain Regions of Rats. Int. J. Mol. Sci. 2016, 17, 459. [Google Scholar] [CrossRef] [PubMed]
- Sutton, L.P.; Rushlow, W.J. The dopamine D2 receptor regulates Akt and GSK-3 via Dvl-3. Int. J. Neuropsychopharmacol. 2012, 15, 965–979. [Google Scholar] [CrossRef][Green Version]
- Karanges, E.A.; Stephenson, C.P.; McGregor, I.S. Longitudinal trends in the dispensing of psychotropic medications in Australia from 2009–2012: Focus on children, adolescents and prescriber specialty. Aust. N. Z. J. Psychiatry 2014, 48, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Memarzia, J.; Tracy, D.; Giaroli, G. The use of antipsychotics in preschoolers: A veto or a sensible last option? J. Psychopharmacol. 2014, 28, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Olfson, M.; Blanco, C.; Wang, S.; Laje, G.; Correll, C.U. National trends in the mental health care of children, adolescents, and adults by office-based physicians. JAMA Psychiatry 2014, 71, 81–90. [Google Scholar] [CrossRef]
- Caccia, S. Safety and pharmacokinetics of atypical antipsychotics in children and adolescents. Paediatr. Drugs 2013, 15, 217–233. [Google Scholar] [CrossRef]
- Fraguas, D.; Correll, C.U.; Merchán-Naranjo, J.; Rapado-Castro, M.; Parellada, M.; Moreno, C.; Arango, C. Efficacy and safety of second-generation antipsychotics in children and adolescents with psychotic and bipolar spectrum disorders: Comprehensive review of prospective head-to-head and placebo-controlled comparisons. Eur. Neuropsychopharmacol. 2011, 21, 621–645. [Google Scholar] [CrossRef]
- Hoekstra, P.J. Risperidone for non-psychotic disorders in paediatric patients: Which child is to benefit? Dev. Med. Child Neurol. 2014, 56, 919–920. [Google Scholar] [CrossRef]
- Olfson, M.; Crystal, S.; Huang, C.; Gerhard, T. Trends in antipsychotic drug use by very young, privately insured children. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 13–23. [Google Scholar] [CrossRef]
- Seida, J.C.; Schouten, J.R.; Boylan, K.; Newton, A.S.; Mousavi, S.S.; Beaith, A.; Vandermeer, B.; Dryden, D.M.; Carrey, N. Antipsychotics for children and young adults: A comparative effectiveness review. Pediatrics 2012, 129, e771–e784. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shaw, S.R. Efficacy of risperidone in managing maladaptive behaviors for children with autistic spectrum disorder: A meta-analysis. J. Pediatr. Health Care 2012, 26, 291–299. [Google Scholar] [CrossRef] [PubMed]
- De Santis, M.; Lian, J.; Huang, X.-F.; Deng, C. Early Antipsychotic Treatment in Juvenile Rats Elicits Long-Term Alterations to the Dopamine Neurotransmitter System. Int. J. Mol. Sci. 2016, 17, 1944. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Lian, J.; Deng, C. Chronic antipsychotic treatment differentially modulates protein kinase A- and glycogen synthase kinase 3 beta-dependent signaling pathways, N-methyl-D-aspartate receptor and γ-aminobutyric acid A receptors in nucleus accumbens of juvenile rats. J. Psychopharmacol. 2018, 32, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Chen, J.; Lian, J.; Huang, X.-F.; Deng, C. Unique Effects of Acute Aripiprazole Treatment on the Dopamine D2 Receptor Downstream cAMP-PKA and Akt-GSK3β Signalling Pathways in Rats. PLoS ONE 2015, 10, e0132722. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Sterotaxic Coordinates, 6th ed.; Academic Press: New York, NY, USA, 2007. [Google Scholar]
- Richetto, J.; Calabrese, F.; Riva, M.A.; Meyer, U. Prenatal immune activation induces maturation-dependent alterations in the prefrontal GABAergic transcriptome. Schizophr. Bull. 2014, 40, 351–361. [Google Scholar] [CrossRef]
- De Jonge, J.C.; Vinkers, C.H.; Pol, H.E.H.; Marsman, A. GABAergic Mechanisms in Schizophrenia: Linking Postmortem and In Vivo Studies. Front. Psychiatry 2017, 8, 118. [Google Scholar] [CrossRef]
- Hoftman, G.D.; Volk, D.W.; Bazmi, H.H.; Li, S.; Sampson, A.R.; Lewis, D.A. Altered cortical expression of GABA-related genes in schizophrenia: Illness progression vs developmental disturbance. Schizophr. Bull. 2015, 41, 180–191. [Google Scholar] [CrossRef]
- Richetto, J.; Labouesse, M.; Poe, M.M.; Cook, J.M.; Grace, A.A.; Riva, M.A.; Meyer, U. Behavioral Effects of the Benzodiazepine-Positive Allosteric Modulator SH-053-2′F-S-CH3 in an Immune-Mediated Neurodevelopmental Disruption Model. Int. J. Neuropsychopharmacol. 2015, 18, pyu055. [Google Scholar] [CrossRef]
- Lazarus, M.S.; Krishnan, K.; Huang, Z.J. GAD67 deficiency in parvalbumin interneurons produces deficits in inhibitory transmission and network disinhibition in mouse prefrontal cortex. Cereb. Cortex 2015, 25, 1290–1296. [Google Scholar] [CrossRef]
- Luoni, A.; Richetto, J.; Longo, L.; Riva, M. Chronic lurasidone treatment normalizes GABAergic marker alterations in the dorsal hippocampus of mice exposed to prenatal immune activation. Eur. Neuropsychopharmacol. 2017, 27, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Grimm, J.W.; See, R.E. Unique activation of extracellular striato-pallidal neurotransmitters in rats following acute risperidone. Brain Res. 1998, 801, 182–189. [Google Scholar] [CrossRef]
- Xu, S.; Gullapalli, R.P.; Frost, D.O. Olanzapine antipsychotic treatment of adolescent rats causes long term changes in glutamate and GABA levels in the nucleus accumbens. Schizophr. Res. 2015, 161, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Lian, J.; Huang, X.-F.; Deng, C. Aripiprazole Increases the PKA Signalling and Expression of the GABAA Receptor and CREB1 in the Nucleus Accumbens of Rats. J. Mol. Neurosci. 2016, 59, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Chadha, R.; Meador-Woodruff, J.H. Downregulated AKT-mTOR signaling pathway proteins in dorsolateral prefrontal cortex in Schizophrenia. Neuropsychopharmacology 2020, 45, 1059–1067. [Google Scholar] [CrossRef]
- Dummler, B.; Hemmings, B.A. Physiological roles of PKB/Akt isoforms in development and disease. Biochem. Soc. Trans. 2007, 35, 231–235. [Google Scholar] [CrossRef]
- Matsuda, S.; Ikeda, Y.; Murakami, M.; Nakagawa, Y.; Tsuji, A.; Kitagishi, Y. Roles of PI3K/AKT/GSK3 Pathway Involved in Psychiatric Illnesses. Diseases 2019, 7, 22. [Google Scholar] [CrossRef]
- Bitanihirwe, B.K.Y.; Weber, L.; Feldon, J.; Meyer, U. Cognitive impairment following prenatal immune challenge in mice correlates with prefrontal cortical AKT1 deficiency. Int. J. Neuropsychopharmacol. 2010, 13, 981–996. [Google Scholar] [CrossRef]
- Willi, R.; Harmeier, A.; Giovanoli, S.; Meyer, U. Altered GSK3β signaling in an infection-based mouse model of developmental neuropsychiatric disease. Neuropharmacology 2013, 73, 56–65. [Google Scholar] [CrossRef]
- Bitanihirwe, B.K.Y.; Peleg-Raibstein, D.; Mouttet, F.; Feldon, J.; Meyer, U. Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuropsychopharmacology 2010, 35, 2462–2478. [Google Scholar] [CrossRef]
- Baghel, M.S.; Singh, B.; Patro, N.; Khanna, V.K.; Patro, I.K.; Thakur, M.K. Poly (I:C) Exposure in Early Life Alters Methylation of DNA and Acetylation of Histone at Synaptic Plasticity Gene Promoter in Developing Rat Brain Leading to Memory Impairment. Ann. Neurosci. 2019, 26, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liu, X.; Lian, J.; Deng, C. Epigenetic histone modulations of PPARγ and related pathways contribute to olanzapine-induced metabolic disorders. Pharmacol. Res. 2020, 155, 104703. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Jia, H.; Kast, R.J.; Thomas, E.A. Epigenetic changes at gene promoters in response to immune activation in utero. Brain Behav. Immun. 2013, 30, 168–175. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Lian, J.; Su, Y.; Deng, C. Effects of Risperidone and Prenatal Poly I:C Exposure on GABAA Receptors and AKT-GSK3β Pathway in the Ventral Tegmental Area of Female Juvenile Rats. Biomolecules 2022, 12, 732. https://doi.org/10.3390/biom12050732
Chen S, Lian J, Su Y, Deng C. Effects of Risperidone and Prenatal Poly I:C Exposure on GABAA Receptors and AKT-GSK3β Pathway in the Ventral Tegmental Area of Female Juvenile Rats. Biomolecules. 2022; 12(5):732. https://doi.org/10.3390/biom12050732
Chicago/Turabian StyleChen, Shiyan, Jiamei Lian, Yueqing Su, and Chao Deng. 2022. "Effects of Risperidone and Prenatal Poly I:C Exposure on GABAA Receptors and AKT-GSK3β Pathway in the Ventral Tegmental Area of Female Juvenile Rats" Biomolecules 12, no. 5: 732. https://doi.org/10.3390/biom12050732
APA StyleChen, S., Lian, J., Su, Y., & Deng, C. (2022). Effects of Risperidone and Prenatal Poly I:C Exposure on GABAA Receptors and AKT-GSK3β Pathway in the Ventral Tegmental Area of Female Juvenile Rats. Biomolecules, 12(5), 732. https://doi.org/10.3390/biom12050732





