The Novel Pimavanserin Derivative ST-2300 with Histamine H3 Receptor Affinity Shows Reduced 5-HT2A Binding, but Maintains Antidepressant- and Anxiolytic-like Properties in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. General Notes
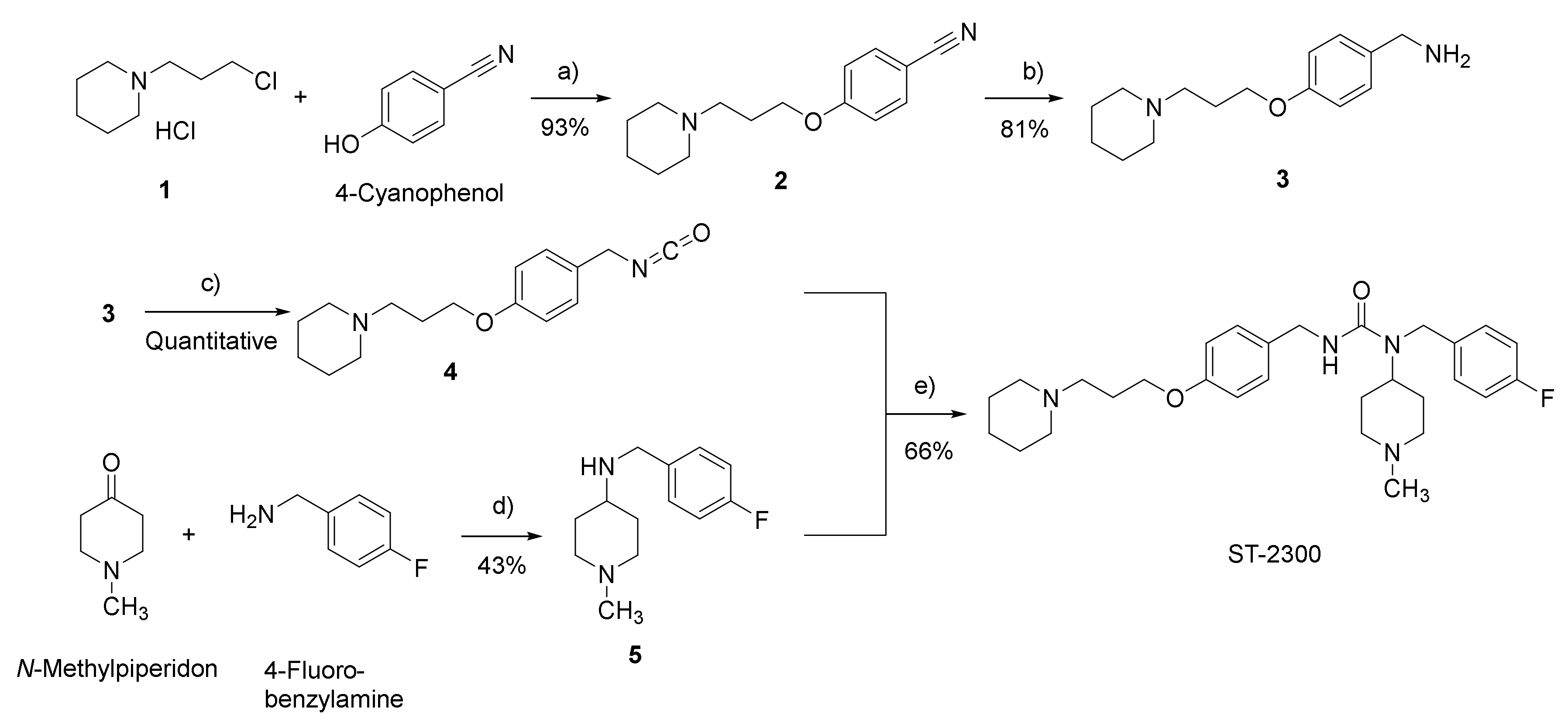
2.1.2. Syntheses and Analyses
2.2. In Vitro Characterization
2.2.1. In Vitro Radioligand Displacement Assays at Different GPCRs
Histamine H3 Receptor
Histamine H1 Receptor
Histamine H4 Receptor
Dopamine D3 and D1 Receptor
Dopamine D2 and Serotonin (5-HT1A, 5-HT2A, 5-HT6, and 5-HT7) Receptor
2.3. In Vivo Assessment
2.3.1. Animals
2.3.2. Drugs
2.3.3. Behavioral Studies
Forced Swim Test
Tail Suspension Test
Open Field Test
2.3.4. Statistics
3. Results
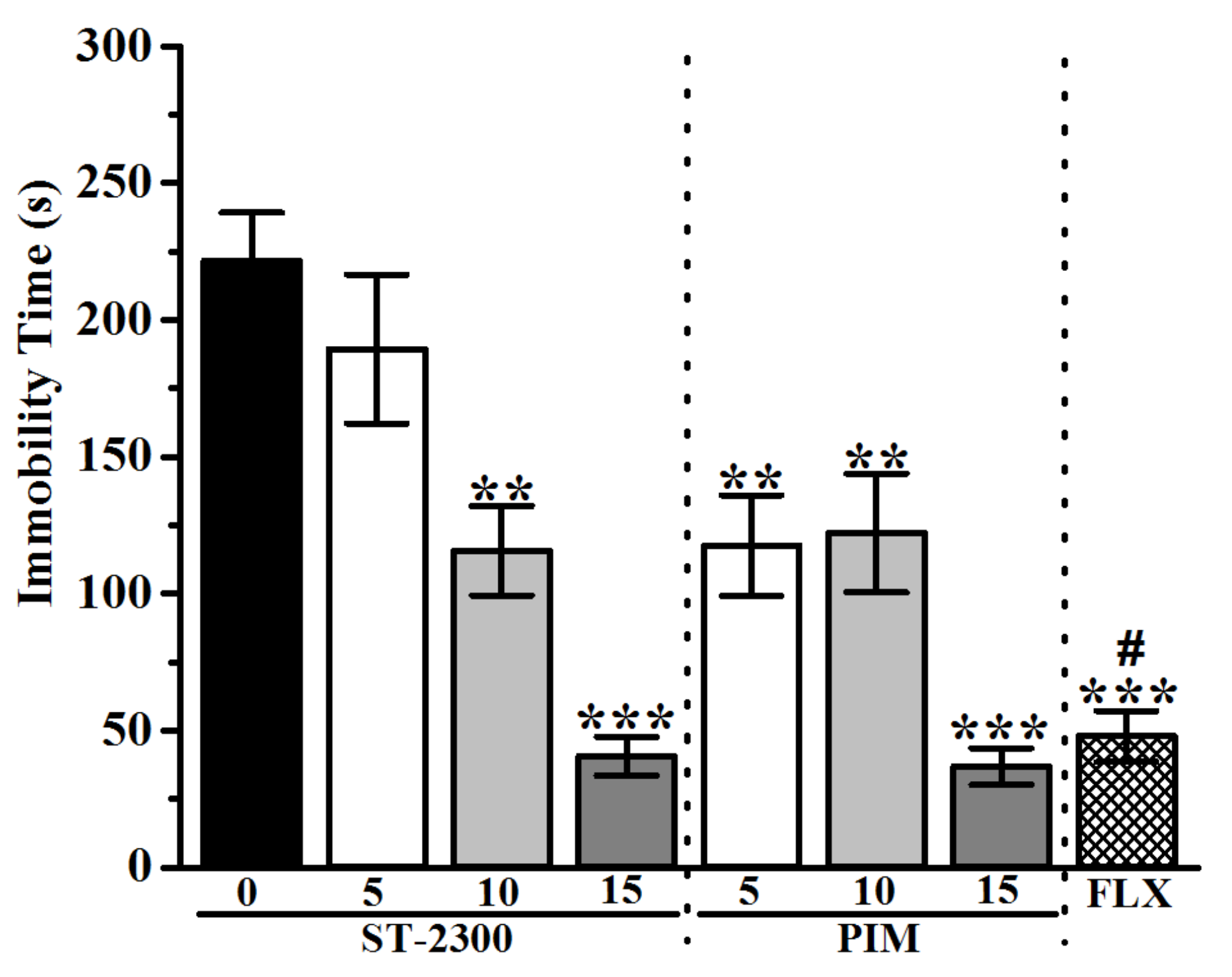
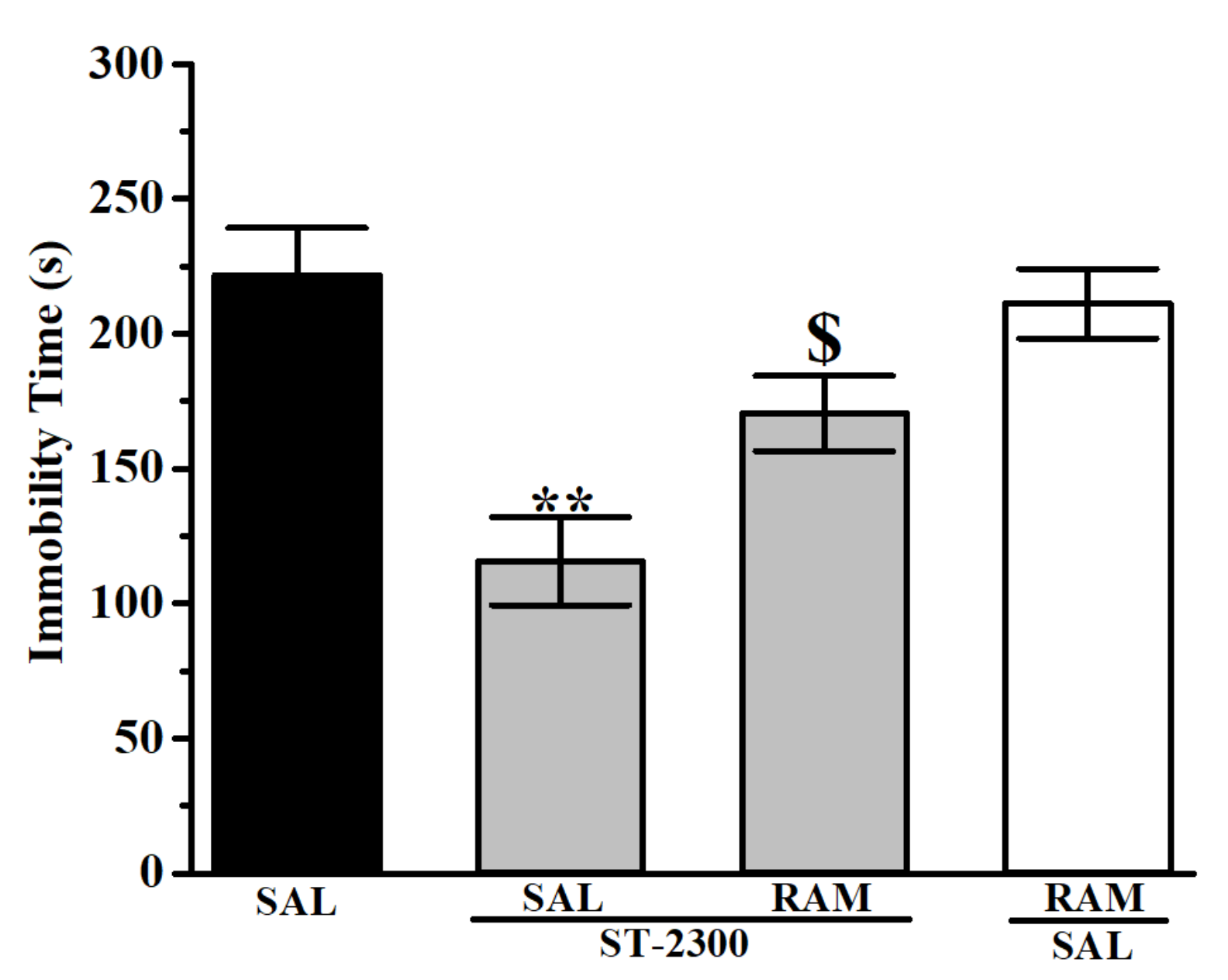
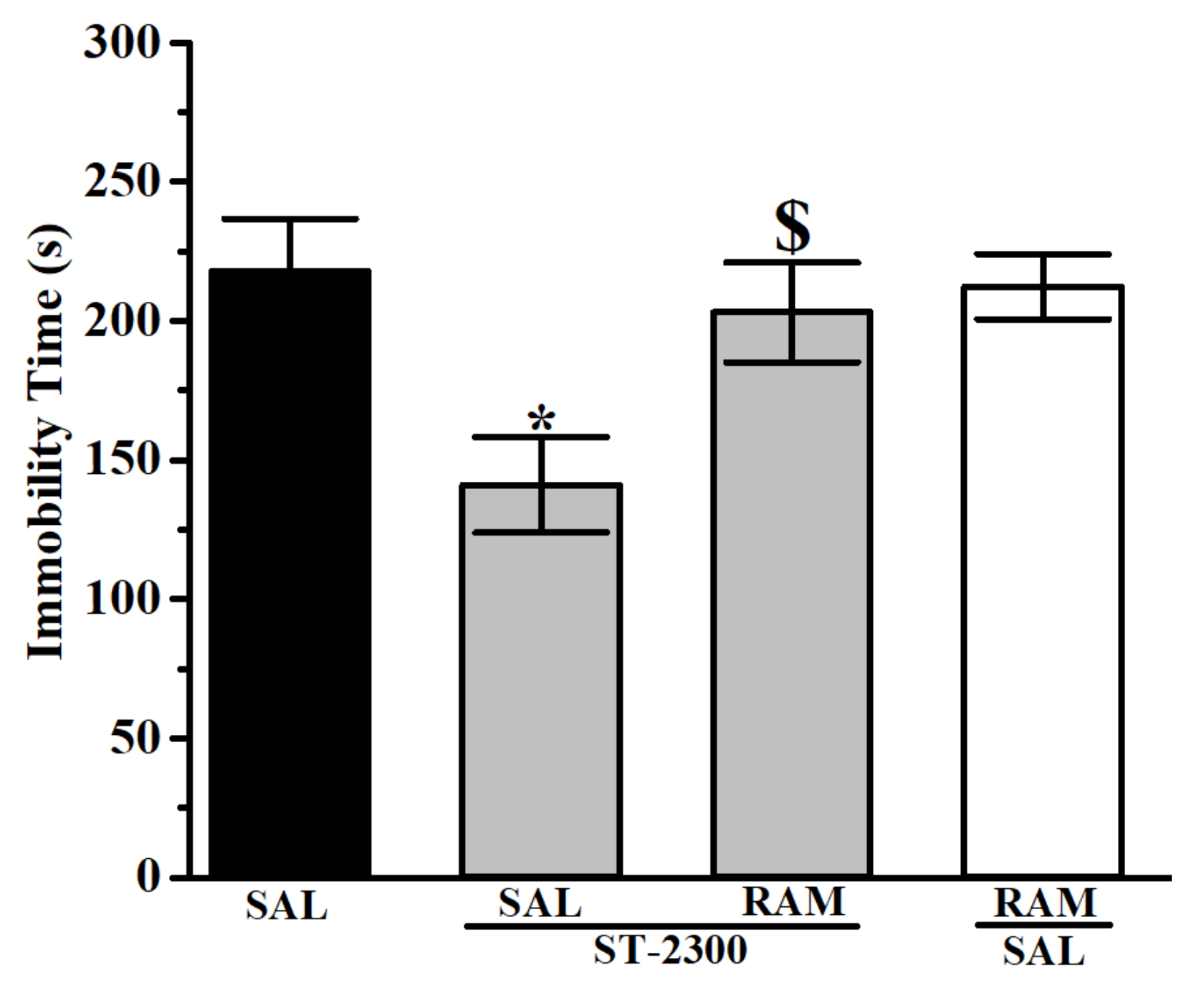
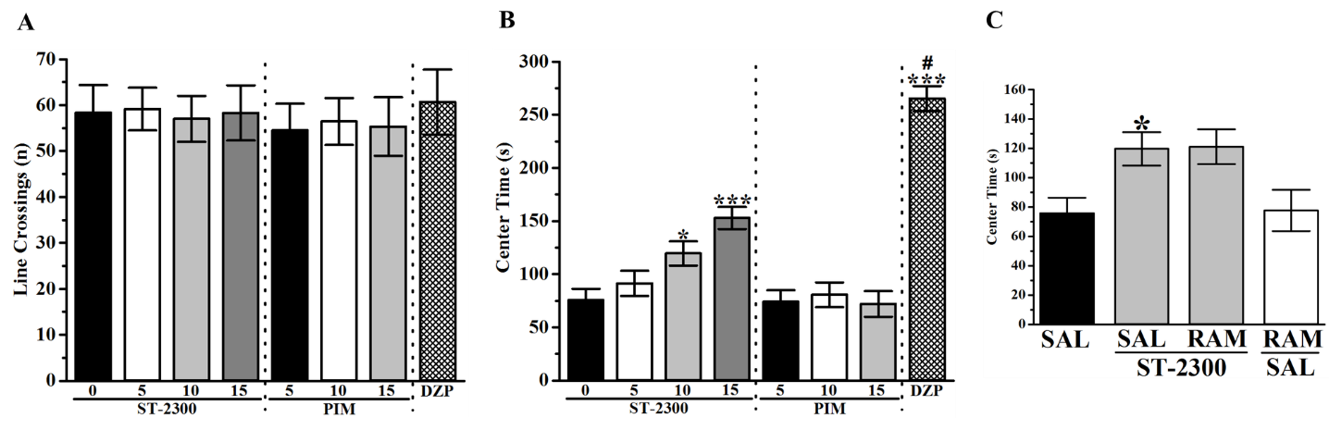
3.1. Forced Swim Test (FST)
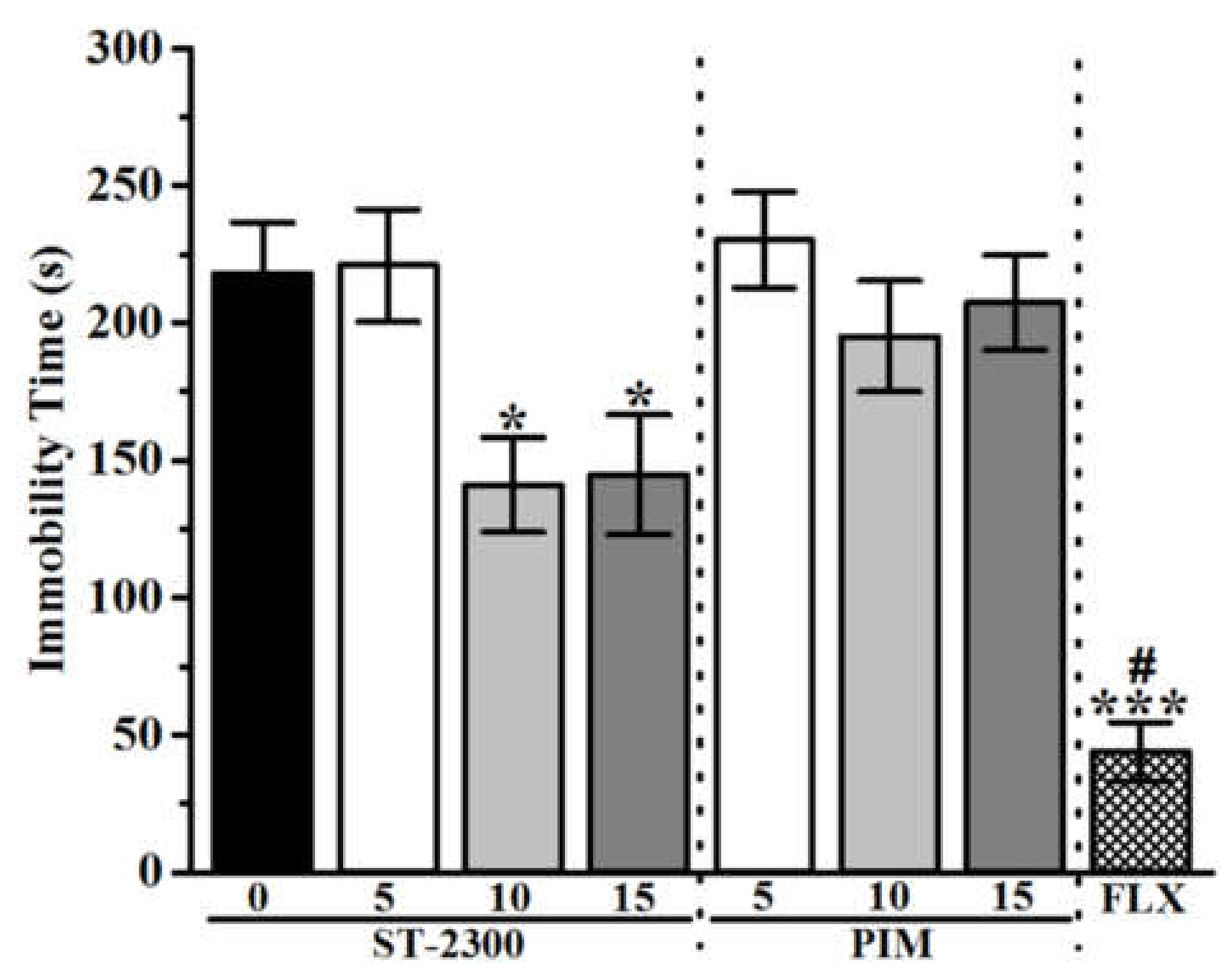
3.2. Tail Suspension Test (TST)
3.3. Open Field Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stevens, D. The mechanism of spontaneous firing in histamine neurons. Behav. Brain Res. 2001, 124, 105–112. [Google Scholar] [CrossRef]
- Schlicker, E.; Malinowska, B.; Kathmann, M.; Göthert, M. Modulation of neurotransmitter releaseviahistamine H3 heteroreceptors. Fundam. Clin. Pharmacol. 1994, 8, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Endou, M.; Poli, E.; Levi, R. Histamine H3-receptor signaling in the heart: Possible involvement of Gi/Go proteins and N-type Ca++ channels. J. Pharmacol. Exp. Ther. 1994, 269, 221–229. [Google Scholar] [PubMed]
- Dauvilliers, Y.; Verbraecken, J.; Partinen, M.; Hedner, J.A.; Saaresranta, T.; Georgiev, O.; Tiholov, R.; LeComte, I.; Tamisier, R.; Lévy, P.; et al. Pitolisant for Daytime Sleepiness in Patients with Obstructive Sleep Apnea Who Refuse Continuous Positive Airway Pressure Treatment. A Randomized Trial. Am. J. Respir. Crit. Care Med. 2020, 201, 1135–1145. [Google Scholar] [CrossRef] [Green Version]
- Esbenshade, T.A.; Browman, K.E.; Miller, T.R.; Krueger, K.M.; Komater-Roderwald, V.; Zhang, M.; Fox, G.B.; Rueter, L.; Robb, H.M.; Radek, R.J.; et al. Pharmacological Properties and Procognitive Effects of ABT-288, a Potent and Selective Histamine H3 Receptor Antagonist. J. Pharmacol. Exp. Ther. 2012, 343, 233–245. [Google Scholar] [CrossRef] [Green Version]
- Wilson, D.M.; Apps, J.; Bailey, N.; Bamford, M.J.; Beresford, I.J.; Brackenborough, K.; Briggs, M.A.; Brough, S.; Calver, A.R.; Crook, B.; et al. Identification of clinical candidates from the benzazepine class of histamine H3 receptor antagonists. Bioorg. Med. Chem. Lett. 2013, 23, 6890–6896. [Google Scholar] [CrossRef]
- Fox, G.B.; Pan, J.B.; Esbenshade, T.A.; Bennani, Y.L.; Black, L.A.; Faghih, R.; Hancock, A.A.; Decker, M.W. Effects of histamine H3 receptor ligands GT-2331 and ciproxifan in a repeated acquisition avoidance response in the spontaneously hypertensive rat pup. Behav. Brain Res. 2002, 131, 151–161. [Google Scholar] [CrossRef]
- Komater, V.A.; Browman, K.E.; Curzon, P.; Hancock, A.A.; Decker, M.W.; Fox, G.B. H3 receptor blockade by thioperamide enhances cognition in rats without inducing locomotor sensitization. Psychopharmacology 2003, 167, 363–372. [Google Scholar] [CrossRef]
- Bardgett, M.E.; Davis, N.N.; Schultheis, P.J.; Griffith, M.S. Ciproxifan, an H3 receptor antagonist, alleviates hyperactivity and cognitive deficits in the APPTg2576 mouse model of Alzheimer’s disease. Neurobiol. Learn. Mem. 2011, 95, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Eissa, N.; Jayaprakash, P.; Azimullah, S.; Ojha, S.K.; Al-Houqani, M.; Jalal, F.Y.; Łażewska, D.; Kieć-Kononowicz, K.; Sadek, B. The histamine H3R antagonist DL77 attenuates autistic behaviors in a prenatal valproic acid-induced mouse model of autism. Sci. Rep. 2018, 8, 13077. [Google Scholar] [CrossRef]
- Eissa, N.; Azimullah, S.; Jayaprakash, P.; Jayaraj, R.L.; Reiner, D.; Ojha, S.K.; Beiram, R.; Stark, H.; Łażewska, D.; Kieć-Kononowicz, K.; et al. The dual-active histamine H3 receptor antagonist and acetylcholine esterase inhibitor E100 ameliorates stereotyped repetitive behavior and neuroinflammmation in sodium valproate induced autism in mice. Chem. Interact. 2019, 312, 108775. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Eissa, N.; Al Awad, M.; Jayaprakash, P.; Zhong, S.; Stölting, F.; Stark, H.; Sadek, B. The histamine H3R and dopamine D2R/D3R antagonist ST-713 ameliorates autism-like behavioral features in BTBR T+tf/J mice by multiple actions. Biomed. Pharmacother. 2021, 138, 111517. [Google Scholar] [CrossRef]
- Sadek, B.; Saad, A.; Subramanian, D.; Shafiullah, M.; Łażewska, D.; Kieć-Kononowiczc, K. Anticonvulsant and procognitive properties of the non-imidazole histamine H3 receptor antagonist DL77 in male adult rats. Neuropharmacology 2016, 106, 46–55. [Google Scholar] [CrossRef]
- Sadek, B.S.; Saad, A.; Latacz, G.; Kuder, K.; Olejarz, A.; Karcz, T.; Stark, H.; Kiec´-Kononowicz, K. Non-imidazole-based histamine H3 receptor antagonists with anticonvulsant activity in different seizure models in male adult rats. Drug Des. Dev. Ther. 2016, 10, 3879–3898. [Google Scholar] [CrossRef] [Green Version]
- Perez-Garcia, C.; Morales, L.; Cano, M.V.; Sancho, I.; Alguacil, L.F. Effects of histamine H3 receptor ligands in experimental models of anxiety and depression. Psychopharmacology 1999, 142, 215–220. [Google Scholar] [CrossRef]
- Sadek, B.S.; Bahi, A.; Schwed, J.S.; Walter, M.; Stark, H. Anxiolytic and antidepressant-like activities of the novel and potent non-imidazole histamine H3 receptor antagonist ST-1283. Drug Des. Dev. Ther. 2014, 8, 627–637. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Hurst, W.J.; Czechtizky, W.; Hall, D.; Moindrot, N.; Nagorny, R.; Pichat, P.; Stefany, D.; Hendrix, J.A.; George, P.G. Identification and profiling of 3,5-dimethyl-isoxazole-4-carboxylic acid [2-methyl-4-((2S,3′S)-2-methyl-[1,3′]bipyrrolidinyl-1′-yl)phenyl] amide as histamine H3 receptor antagonist for the treatment of depression. Bioorg. Med. Chem. Lett. 2013, 23, 6269–6273. [Google Scholar] [CrossRef]
- Jin, C.; Anichtchik, O.; Panula, P. Altered histamine H3 receptor radioligand binding in post-mortem brain samples from subjects with psychiatric diseases. Br. J. Pharmacol. 2009, 157, 118–129. [Google Scholar] [CrossRef] [Green Version]
- Vanover, K.E.; Weiner, D.M.; Makhay, M.; Veinbergs, I.; Gardell, L.R.; Lameh, J.; Del Tredici, A.L.; Piu, F.; Schiffer, H.H.; Ott, T.R.; et al. Pharmacological and Behavioral Profile of N-(4-Fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N′-(4-(2-methylpropyloxy)phenylmethyl) Carbamide (2R,3R)-Dihydroxybutanedioate (2:1) (ACP-103), a Novel 5-Hydroxytryptamine2A Receptor Inverse Agonist. J. Pharmacol. Exp. Ther. 2006, 317, 910–918. [Google Scholar] [CrossRef]
- Ballard, C.; Banister, C.; Khan, Z.; Cummings, J.; Demos, G.; Coate, B.; Youakim, J.M.; Owen, R.; Stankovic, S.; Tomkinson, E.B.; et al. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: A phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018, 17, 213–222. [Google Scholar] [CrossRef]
- Fava, M.; Dirks, B.; Freeman, M.P.; Papakostas, G.I.; Shelton, R.C.; Thase, M.E.; Trivedi, M.H.; Liu, K.; Stankovic, S. A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study of Adjunctive Pimavanserin in Patients with Major Depressive Disorder and an Inadequate Response to Therapy (CLARITY). J. Clin. Psychiatry 2019, 80, 19m12928. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Fava, M.; Freeman, M.P.; Thase, M.E.; Papakostas, G.I.; Shelton, R.C.; Trivedi, M.H.; Dirks, B.; Liu, K.; Stankovic, S. Effect of Adjunctive Pimavanserin on Sleep/Wakefulness in Patients with Major Depressive Disorder. J. Clin. Psychiatry 2020, 82, 20m13425. [Google Scholar] [CrossRef] [PubMed]
- Arias, B.; Gastó, C.; Catalán, R.; Gutiérrez, B.; Pintor, L.; Fañanás, L. The 5-HT2Areceptor gene 102T/C polymorphism is associated with suicidal behavior in depressed patients. Am. J. Med. Genet. 2001, 105, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Niswender, C.M.; Herrick-Davis, K.; Dilley, G.E.; Meltzer, H.Y.; Overholser, J.C.; Stockmeier, C.A.; Emeson, R.B.; Sanders-Bush, E. RNA Editing of the Human Serotonin 5-HT2C Receptor Alterations in Suicide and Implications for Serotonergic Pharmacotherapy. Neuropsychopharmacology 2001, 24, 478–491. [Google Scholar] [CrossRef]
- Shelton, R.C.; Fava, M.; Freeman, M.P.; Thase, M.E.; Papakostas, G.I.; Jha, M.K.; Trivedi, M.H.; Dirks, B.; Liu, K.; Stankovic, S. Effect of adjunctive pimavanserin on suicidal ideation in patients with major depression: Analysis of the CLARITY study. J. Affect. Disord. 2020, 277, 478–485. [Google Scholar] [CrossRef]
- Łażewska, D.; Ligneau, X.; Schwartz, J.-C.; Schunack, W.; Stark, H.; Kieć-Kononowicz, K. Ether derivatives of 3-piperidinopropan-1-ol as non-imidazole histamine H3 receptor antagonists. Bioorg. Med. Chem. 2006, 14, 3522–3529. [Google Scholar] [CrossRef]
- Barbier, A.J.; Berridge, C.; Dugovic, C.; Laposky, A.D.; Wilson, S.J.; Boggs, J.; Aluisio, L.; Lord, B.; Mazur, C.; Pudiak, C.M.; et al. Acute wake-promoting actions of JNJ-5207852, a novel, diamine-based H3 antagonist. Br. J. Pharmacol. 2004, 143, 649–661. [Google Scholar] [CrossRef] [Green Version]
- Lutsenko, K.; Hagenow, S.; Affini, A.; Reiner, D.; Stark, H. Rasagiline derivatives combined with histamine H3 receptor properties. Bioorg. Med. Chem. Lett. 2019, 29, 126612. [Google Scholar] [CrossRef]
- Von Coburg, Y.; Kottke, T.; Weizel, L.; Ligneau, X.; Stark, H. Potential utility of histamine H3 receptor antagonist pharmacophore in antipsychotics. Bioorg. Med. Chem. Lett. 2009, 19, 538–542. [Google Scholar] [CrossRef]
- Celanire, S.; Wijtmans, M.; Talaga, P.; Leurs, R.; de Esch, I.J. Keynote review: Histamine H3 receptor antagonists reach out for the clinic. Drug Discov. Today 2005, 10, 1613–1627. [Google Scholar] [CrossRef]
- Affini, A.; Hagenow, S.; Zivkovic, A.; Marco-Contelles, J.; Stark, H. Novel indanone derivatives as MAO B/H3R dual-targeting ligands for treatment of Parkinson’s disease. Eur. J. Med. Chem. 2018, 148, 487–497. [Google Scholar] [CrossRef]
- Thygesen, M.; Schlienger, N.; Tolf, B.-R.; Blatter, F.; Berghausen, J. Salts of N-(4-fluorobenzyl)-N-(1-methylpiperidin-4-yl)-N′-(4-(2-methylpropyloxy)phenylmethyl)carbamide and their preparation. WO2006036874A1, 6 April 2006. [Google Scholar]
- Sander, K.; Kottke, T.; Hoffend, C.; Walter, M.; Weizel, L.; Camelin, J.-C.; Ligneau, X.; Schneider, E.H.; Seifert, R.; Schwartz, J.-C.; et al. First Metal-Containing Histamine H3 Receptor Ligands. Org. Lett. 2010, 12, 2578–2581. [Google Scholar] [CrossRef]
- Apelt, J.; Grassmann, S.; Ligneau, X.; Pertz, H.H.; Ganellin, C.R.; Arrang, J.-M.; Schwartz, J.-C.; Schunack, W.; Stark, H. Search for Histamine H3 Receptor Antagonists with Combined Inhibitory Potency at Nτ-Methyltransferase: Ether Derivatives. Pharmazie 2005, 60, 97–106. [Google Scholar] [CrossRef]
- Sander, K.; Kottke, T.; Weizel, L.; Stark, H. Kojic Acid Derivatives as Histamine H3 Receptor Ligands. Chem. Pharm. Bull. 2010, 58, 1353–1361. [Google Scholar] [CrossRef] [Green Version]
- Kottke, T.; Sander, K.; Weizel, L.; Schneider, E.; Seifert, R.; Stark, H. Receptor-specific functional efficacies of alkyl imidazoles as dual histamine H3/H4 receptor ligands. Eur. J. Pharmacol. 2011, 654, 200–208. [Google Scholar] [CrossRef]
- Ligneau, X.; Morisset, S.; Tardivel-Lacombe, J.; Gbahou, F.; Ganellin, C.R.; Stark, H.; Schunack, W.; Schwartz, J.-C.; Arrang, J.-M. Distinct pharmacology of rat and human histamine H3 receptors: Role of two amino acids in the third transmembrane domain. J. Cereb. Blood Flow Metab. 2000, 131, 1247–1250. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.-C.; Prusoff, W.H. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar] [CrossRef]
- Smit, M.; Timmerman, H.; Hijzelendoorn, J.; Fukui, H.; Leurs, R. Regulation of the human histamine H1 receptor stably expressed in Chinese hamster ovary cells. J. Cereb. Blood Flow Metab. 1996, 117, 1071–1080. [Google Scholar] [CrossRef] [Green Version]
- Schneider, E.H.; Schnell, D.; Papa, D.; Seifert, R. High Constitutive Activity and a G-Protein-Independent High-Affinity State of the Human Histamine H4-Receptor. Biochemistry 2009, 48, 1424–1438. [Google Scholar] [CrossRef]
- Sander, K.; Kottke, T.; Tanrikulu, Y.; Proschak, E.; Weizel, L.; Schneider, E.H.; Seifert, R.; Schneider, G.; Stark, H. 2,4-Diaminopyrimidines as histamine H4 receptor ligands—Scaffold optimization and pharmacological characterization. Bioorg. Med. Chem. 2009, 17, 7186–7196. [Google Scholar] [CrossRef]
- Łażewska, D.; Więcek, M.; Ner, J.; Kamińska, K.; Kottke, T.; Schwed, J.S.; Zygmunt, M.; Karcz, T.; Olejarz, A.; Kuder, K.; et al. Aryl-1,3,5-triazine derivatives as histamine H4 receptor ligands. Eur. J. Med. Chem. 2014, 83, 534–546. [Google Scholar] [CrossRef]
- Bautista-Aguilera, M.; Hagenow, S.; Palomino-Antolin, A.; Farré-Alins, V.; Ismaili, L.; Joffrin, P.-L.; Jimeno, M.L.; Soukup, O.; Janockova, J.; Kalinowsky, L.; et al. Multitarget-Directed Ligands Combining Cholinesterase and Monoamine Oxidase Inhibition with Histamine H3 R Antagonism for Neurodegenerative Diseases. Angew. Chem. Int. Ed. 2017, 56, 12765–12769. [Google Scholar] [CrossRef] [Green Version]
- Bahi, A.; Dreyer, J.-L. Hippocampus-specific deletion of tissue plasminogen activator “tPA” in adult mice impairs depression- and anxiety-like behaviors. Eur. Neuropsychopharmacol. 2012, 22, 672–682. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The Tail Suspension Test. J. Vis. Exp. 2012, 59, e3769. [Google Scholar] [CrossRef] [Green Version]
- Eissa, N.; Azimullah, S.; Jayaprakash, P.; Jayaraj, R.L.; Reiner, D.; Ojha, S.K.; Beiram, R.; Stark, H.; Łażewska, D.; Kieć-Kononowicz, K.; et al. The Dual-Active Histamine H3 Receptor Antagonist and Acetylcholine Esterase Inhibitor E100 Alleviates Autistic-like Behaviors and Oxidative Stress in Valproic Acid Induced Autism in Mice. Int. J. Mol. Sci. 2020, 21, 3996. [Google Scholar] [CrossRef]
- Eissa, N.; Jayaprakash, P.; Stark, H.; Łażewska, D.; Kieć-Kononowicz, K.; Sadek, B. Simultaneous Blockade of Histamine H3 Receptors and Inhibition of Acetylcholine Esterase Alleviate Autistic-like Behaviors in BTBR T+ tf/J Mouse Model of Autism. Biomolecules 2020, 10, 1251. [Google Scholar] [CrossRef]
- Hacksell, U.; Burstein, E.S.; McFarland, K.; Mills, R.G.; Williams, H. On the Discovery and Development of Pimavanserin: A Novel Drug Candidate for Parkinson’s Psychosis. Neurochem. Res. 2014, 39, 2008–2017. [Google Scholar] [CrossRef] [Green Version]
- Andrews, M.; Tousi, B.; Sabbagh, M.N. 5HT6 Antagonists in the Treatment of Alzheimer’s Dementia: Current Progress. Neurol. Ther. 2018, 7, 51–58. [Google Scholar] [CrossRef] [Green Version]

| Receptor Subtype | ST-2300 Ki ± SD (n) or Ki [CI 95%] (n) | Pimavanserin Ki ± SD (n) |
|---|---|---|
| H3R | 14.4 nM [2.98 nM; 70.0 nM] (4) b | inhibition at 10 µM: <50% a |
| 5-HT2A | 1302 ± 331 nM (2) c | 0.7 ± 0.4 nM (2) c |
| 5-HT1A | >10,000 (2) d | >10,000 (2) d |
| 5-HT6 | >10,000 (2) d | 59 ± 11 nM (2) d |
| 5-HT7 | >10,000 (2) d | >10,000 (2) d |
| D1R | >10,000 (3) b | inhibition at 10 µM: <50% a |
| D2R | >10,000 (2) e | 2417 ± 667 nM (2) e |
| D3R | >10,000 (3) f | inhibition at 10 µM: 60% a |
| H1R | >5000 (2) f | inhibition at 10 µM: <50% a |
| H4R | >1000 (2) g | n.d. h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatachalam, K.; Zhong, S.; Dubiel, M.; Satała, G.; Sadek, B.; Stark, H. The Novel Pimavanserin Derivative ST-2300 with Histamine H3 Receptor Affinity Shows Reduced 5-HT2A Binding, but Maintains Antidepressant- and Anxiolytic-like Properties in Mice. Biomolecules 2022, 12, 683. https://doi.org/10.3390/biom12050683
Venkatachalam K, Zhong S, Dubiel M, Satała G, Sadek B, Stark H. The Novel Pimavanserin Derivative ST-2300 with Histamine H3 Receptor Affinity Shows Reduced 5-HT2A Binding, but Maintains Antidepressant- and Anxiolytic-like Properties in Mice. Biomolecules. 2022; 12(5):683. https://doi.org/10.3390/biom12050683
Chicago/Turabian StyleVenkatachalam, Karthikkumar, Sicheng Zhong, Mariam Dubiel, Grzegorz Satała, Bassem Sadek, and Holger Stark. 2022. "The Novel Pimavanserin Derivative ST-2300 with Histamine H3 Receptor Affinity Shows Reduced 5-HT2A Binding, but Maintains Antidepressant- and Anxiolytic-like Properties in Mice" Biomolecules 12, no. 5: 683. https://doi.org/10.3390/biom12050683
APA StyleVenkatachalam, K., Zhong, S., Dubiel, M., Satała, G., Sadek, B., & Stark, H. (2022). The Novel Pimavanserin Derivative ST-2300 with Histamine H3 Receptor Affinity Shows Reduced 5-HT2A Binding, but Maintains Antidepressant- and Anxiolytic-like Properties in Mice. Biomolecules, 12(5), 683. https://doi.org/10.3390/biom12050683








