Intracellular and Extracellular Antifreeze Protein Significantly Improves Mammalian Cell Cryopreservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Reagents
2.2. Transfection of HEK 293T Cells
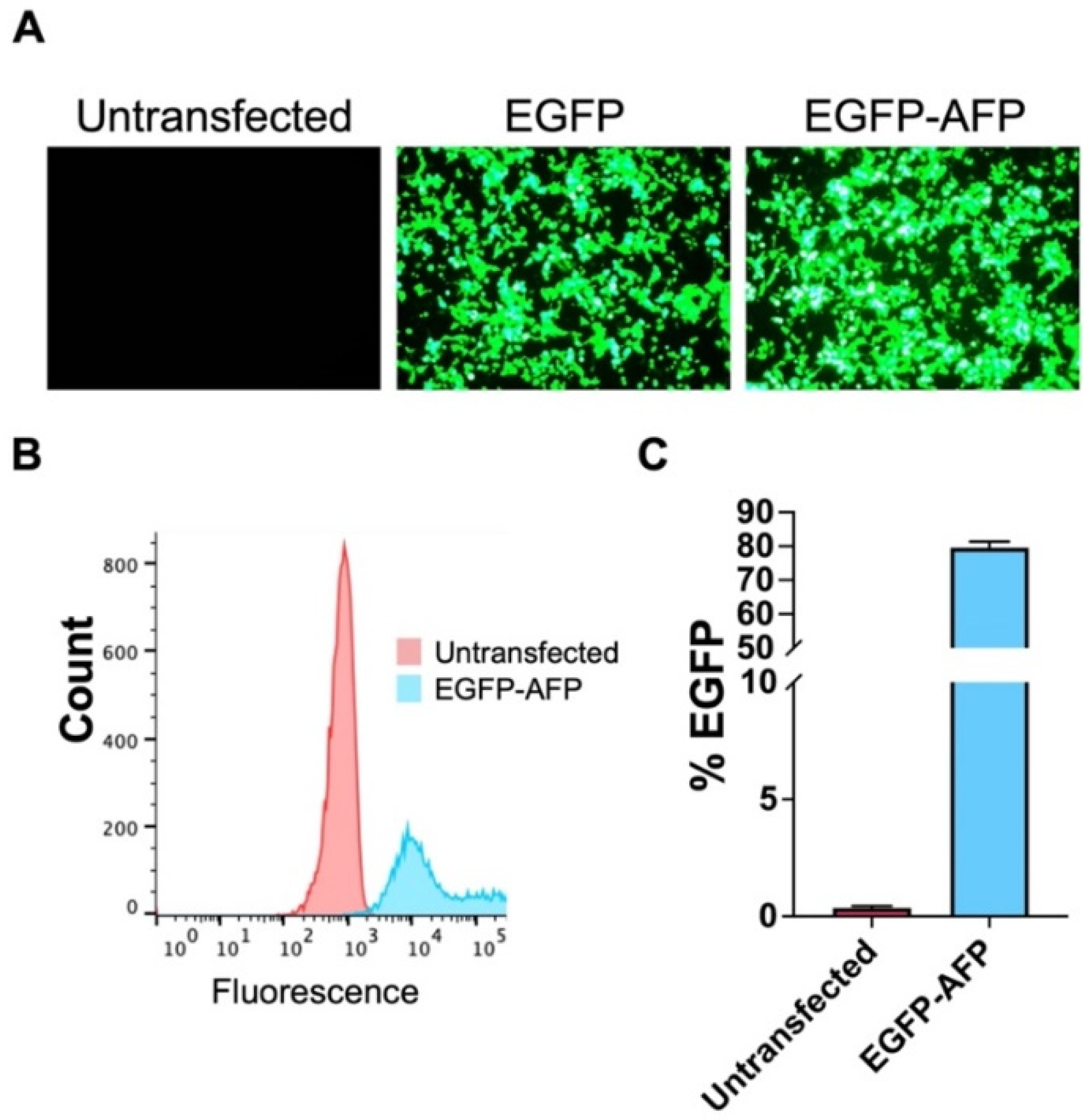
2.3. Flow Cytometry
2.4. Expression and Purification of Recombinant TrxA-ApAFP752
2.5. Cryopreservation and Thawing
- (1)
- 5 µM EC AFP,
- (2)
- 15 µM EC AFP,
- (3)
- IC AFP,
- (4)
- 5 µM EC AFP and IC AFP, and
- (5)
- 15 µM EC AFP and IC AFP.
2.6. Viability Tests
2.6.1. Trypan Blue Assay
2.6.2. LDH Assay
2.6.3. MTS Assay
2.7. Experimental Design and Statistical Analysis
3. Results
3.1. Transfection
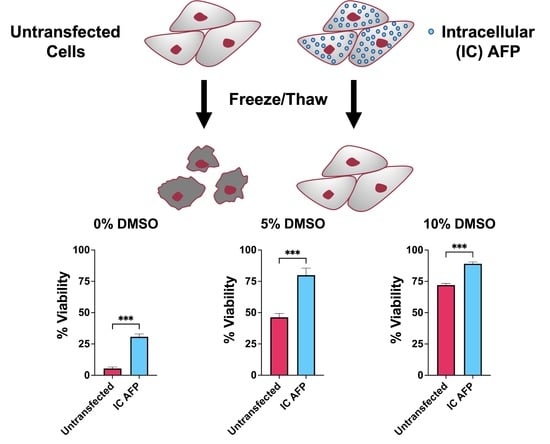
3.2. Untransfected vs. EGFP vs. IC AFP
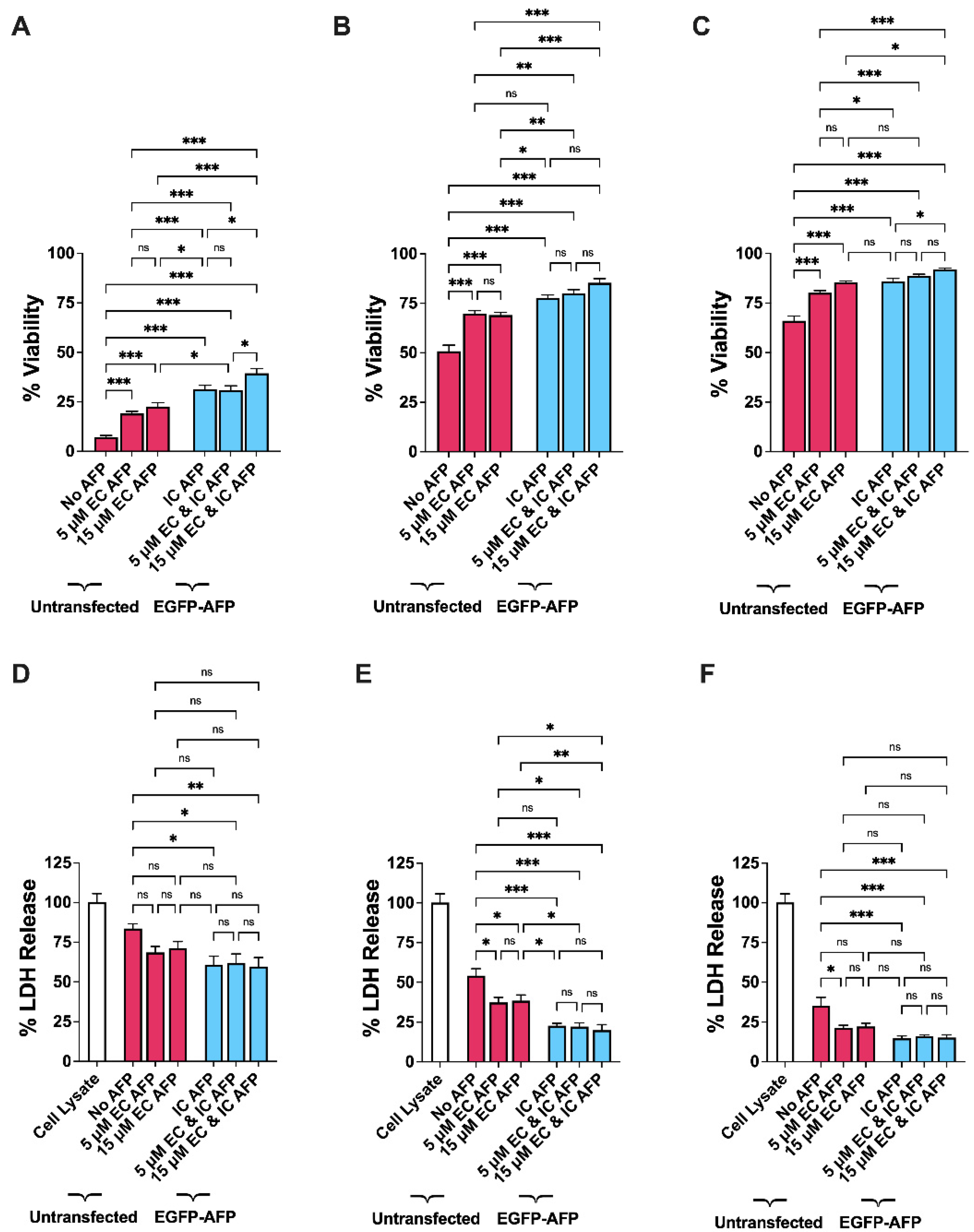
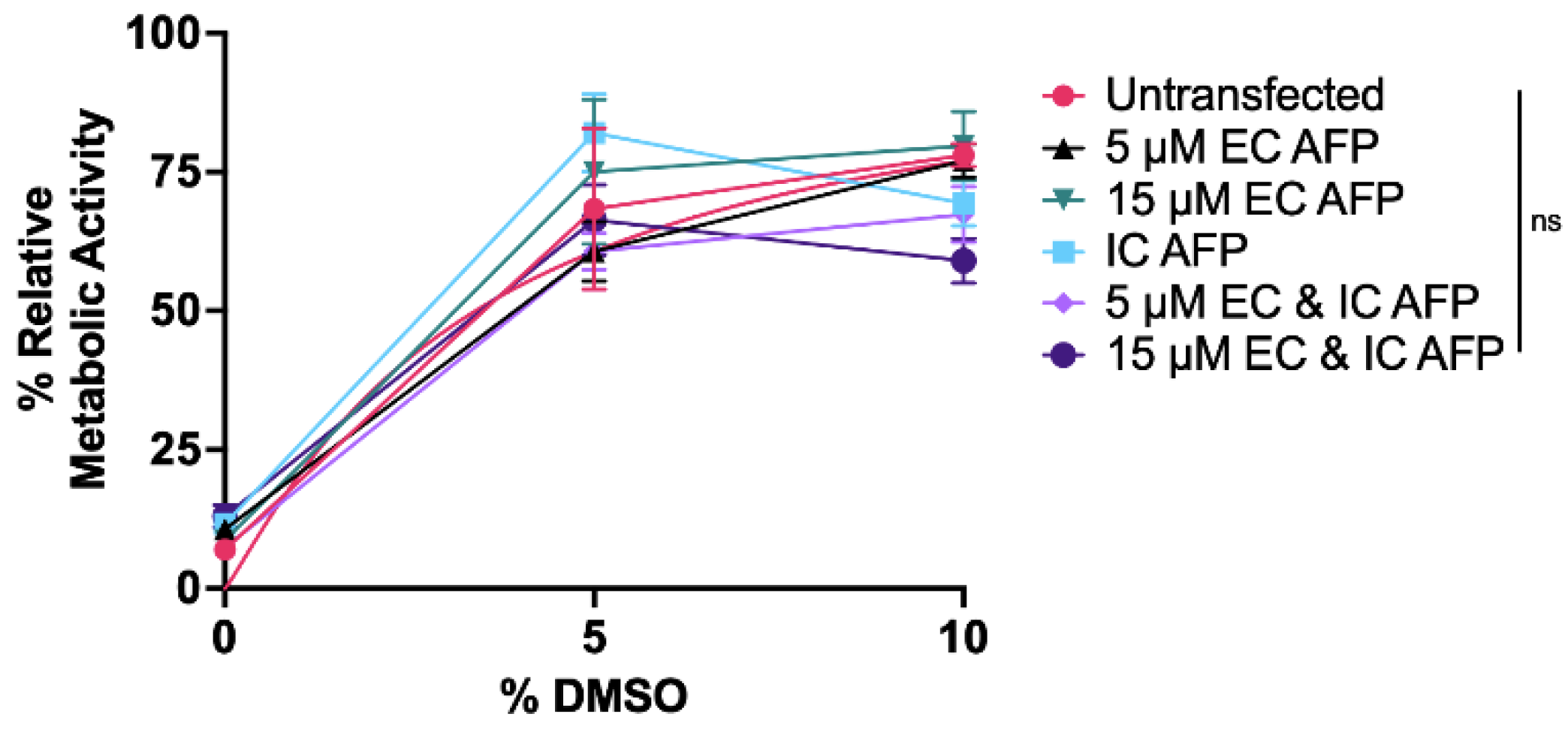
3.3. Intracellular vs. Extracellular AFP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Post, M.J. Cultured meat from stem cells: Challenges and prospects. Meat Sci. 2012, 92, 297–301. [Google Scholar] [CrossRef]
- Stout, A.J.; Mirliani, A.B.; Soule-Albridge, E.L.; Cohen, J.M.; Kaplan, D.L. Engineering carotenoid production in mammalian cells for nutritionally enhanced cell-cultured foods. Metab. Eng. 2020, 62, 126–137. [Google Scholar] [CrossRef]
- Wurm, F.M. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat. Biotechnol. 2004, 22, 1393–1398. [Google Scholar] [CrossRef]
- Tai, W.; Zhang, X.; Drelich, A.; Shi, J.; Hsu, J.C.; Luchsinger, L.; Hillyer, C.D.; Tseng, C.-T.K.; Jiang, S.; Du, L. A novel receptor-binding domain (RBD)-based mRNA vaccine against SARS-CoV-2. Cell Res. 2020, 30, 932–935. [Google Scholar] [CrossRef]
- Raju, R.; Bryant, S.J.; Wilkinson, B.L.; Bryant, G. The need for novel cryoprotectants and cryopreservation protocols: Insights into the importance of biophysical investigation and cell permeability. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2021, 1865, 129749. [Google Scholar] [CrossRef]
- Chen, S.; Du, K.; Zou, C. Current progress in stem cell therapy for type 1 diabetes mellitus. Stem Cell Res. Ther. 2020, 11, 23. [Google Scholar] [CrossRef]
- Stuckey, D.W.; Shah, K. Stem cell-based therapies for cancer treatment: Separating hope from hype. Nat. Cancer 2014, 14, 683–691. [Google Scholar] [CrossRef]
- Scolding, N.J.; Pasquini, M.; Reingold, S.C.; Cohen, J.A.; International Conference on Cell-Based Therapies for Multiple Sclerosis. Cell-based therapeutic strategies for multiple sclerosis. Brain 2017, 140, 2776–2796. [Google Scholar] [CrossRef]
- Kean, L.S. Defining success with cellular therapeutics: The current landscape for clinical end point and toxicity analysis. Blood 2018, 131, 2630–2639. [Google Scholar] [CrossRef]
- Bender, E. Cell-based therapy: Cells on trial. Nature 2016, 540, S106–S108. [Google Scholar] [CrossRef]
- Müller, P.; Lemcke, H.; David, R. Stem Cell Therapy in Heart Diseases—Cell Types, Mechanisms and Improvement Strategies. Cell. Physiol. Biochem. 2018, 48, 2607–2655. [Google Scholar] [CrossRef]
- Meneghel, J.; Kilbride, P.; Morris, G.J. Cryopreservation as a Key Element in the Successful Delivery of Cell-Based Therapies—A Review. Front. Med. 2020, 7, 592242. [Google Scholar] [CrossRef]
- Bachtiger, F.; Congdon, T.R.; Stubbs, C.; Gibson, M.I.; Sosso, G.C. The atomistic details of the ice recrystallisation inhibition activity of PVA. Nat. Commun. 2021, 12, 1323. [Google Scholar] [CrossRef]
- Stacey, G.N.; Connon, C.J.; Coopman, K.; Dickson, A.J.; Fuller, B.; Hunt, C.J.; Kemp, P.; Kerby, J.; Man, J.; Matejtschuk, P.; et al. Preservation and stability of cell therapy products: Recommendations from an expert workshop. Regen. Med. 2017, 12, 553–564. [Google Scholar] [CrossRef]
- Lovelock, J.E.; Bishop, M.W.H. Prevention of Freezing Damage to Living Cells by Dimethyl Sulphoxide. Nature 1959, 183, 1394–1395. [Google Scholar] [CrossRef]
- Stroncek, D.F.; Fautsch, S.K.; Lasky, L.C.; Hurd, D.D.; Ramsay, N.K.; McCullough, J. Adverse reactions in patients transfused with cryopreserved marrow. Transfusion 1991, 31, 521–526. [Google Scholar] [CrossRef]
- DeVries, A.L.; Wohlschlag, D.E. Freezing Resistance in Some Antarctic Fishes. Science 1969, 163, 1073–1075. [Google Scholar] [CrossRef]
- Bar Dolev, M.; Braslavsky, I.; Davies, P.L. Ice-Binding Proteins and Their Function. Annu. Rev. Biochem. 2016, 85, 515–542. [Google Scholar] [CrossRef]
- Voets, I.K. From ice-binding proteins to bio-inspired antifreeze materials. Soft Matter 2017, 13, 4808–4823. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H.; Hur, Y.B.; Lee, C.W.; Park, S.-H.; Koo, B.-W. Marine Antifreeze Proteins: Structure, Function, and Application to Cryopreservation as a Potential Cryoprotectant. Mar. Drugs 2017, 15, 27. [Google Scholar] [CrossRef]
- Griffith, M.; Yaish, M.W.F. Antifreeze proteins in overwintering plants: A tale of two activities. Trends Plant Sci. 2004, 9, 399–405. [Google Scholar] [CrossRef]
- Devries, A.L. Antifreeze glycopeptides and peptides: Interactions with ice and water. Methods Enzymol. 1986, 127, 293–303. [Google Scholar] [CrossRef]
- Bar-Dolev, M.; Celik, Y.; Wettlaufer, J.S.; Davies, P.L.; Braslavsky, I. New insights into ice growth and melting modifications by antifreeze proteins. J. R. Soc. Interface 2012, 9, 3249–3259. [Google Scholar] [CrossRef]
- Kratochvílová, I.; Kopečná, O.; Bačíková, A.; Pagáčová, E.; Falková, I.; Follett, S.E.; Elliott, K.W.; Varga, K.; Golan, M.; Falk, M. Changes in Cryopreserved Cell Nuclei Serve as Indicators of Processes during Freezing and Thawing. Langmuir 2019, 35, 7496–7508. [Google Scholar] [CrossRef]
- Tomás, R.M.F.; Bailey, T.L.; Hasan, M.; Gibson, M.I. Extracellular Antifreeze Protein Significantly Enhances the Cryopreservation of Cell Monolayers. Biomacromolecules 2019, 20, 3864–3872. [Google Scholar] [CrossRef]
- Briard, J.G.; Poisson, J.S.; Turner, T.R.; Capicciotti, C.J.; Acker, J.P.; Ben, R.N. Small molecule ice recrystallization inhibitors mitigate red blood cell lysis during freezing, transient warming and thawing. Sci. Rep. 2016, 6, 23619. [Google Scholar] [CrossRef]
- Hirano, Y.; Nishimiya, Y.; Matsumoto, S.; Matsushita, M.; Todo, S.; Miura, A.; Komatsu, Y.; Tsuda, S. Hypothermic preservation effect on mammalian cells of type III antifreeze proteins from notched-fin eelpout. Cryobiology 2008, 57, 46–51. [Google Scholar] [CrossRef]
- Ideta, A.; Aoyagi, Y.; Tsuchiya, K.; Nakamura, Y.; Hayama, K.; Shirasawa, A.; Sakaguchi, K.; Tominaga, N.; Nishimiya, Y.; Tsuda, S. Prolonging hypothermic storage (4 C) of bovine embryos with fish antifreeze protein. J. Reprod. Dev. 2015, 61, 1–6. [Google Scholar] [CrossRef]
- Eskandari, A.; Leow, T.C.; Rahman, M.B.A.; Oslan, S.N. Antifreeze Proteins and Their Practical Utilization in Industry, Medicine, and Agriculture. Biomolecules 2020, 10, 1649. [Google Scholar] [CrossRef]
- Fletcher, G.L.; Hew, C.L.; Davies, P.L. Antifreeze Proteins of Teleost Fishes. Annu. Rev. Physiol. 2001, 63, 359–390. [Google Scholar] [CrossRef]
- Hakim, A.; Nguyen, J.B.; Basu, K.; Zhu, D.F.; Thakral, D.; Davies, P.L.; Isaacs, F.J.; Modis, Y.; Meng, W.Y. Crystal Structure of an Insect Antifreeze Protein and Its Implications for Ice Binding. J. Biol. Chem. 2013, 288, 12295–12304. [Google Scholar] [CrossRef]
- Halwani, D.O.; Brockbank, K.G.M.; Duman, J.G.; Campbell, L.H. Recombinant Dendroides canadensis antifreeze proteins as potential ingredients in cryopreservation solutions. Cryobiology 2014, 68, 411–418. [Google Scholar] [CrossRef]
- Kim, H.J.; Shim, H.E.; Lee, J.H.; Kang, Y.-C.; Hur, Y.B. Ice-Binding Protein Derived from Glaciozyma Can Improve the Viability of Cryopreserved Mammalian Cells. J. Microbiol. Biotechnol. 2015, 25, 1989–1996. [Google Scholar] [CrossRef]
- Mao, X.; Liu, Z.; Ma, J.; Pang, H.; Zhang, F. Characterization of a novel β-helix antifreeze protein from the desert beetle Anatolica polita. Cryobiology 2011, 62, 91–99. [Google Scholar] [CrossRef]
- Ma, J.; Wang, J.; Mao, X.F.; Wang, Y. Differential expression of two antifreeze proteins in the desert beetle Anatolica polita (Coleoptera: Tenebriondae): Seasonal variation and environmental effects. CryoLetters 2012, 33, 337–348. [Google Scholar]
- Mao, X.; Liu, Z.; Li, H.; Ma, J.; Zhang, F. Calorimetric studies on an insect antifreeze protein ApAFP752 from Anatolica polita. J. Therm. Anal. Calorim. 2011, 104, 343–349. [Google Scholar] [CrossRef]
- Liou, Y.-C.; Tocilj, A.; Davies, P.L.; Jia, Z. Mimicry of ice structure by surface hydroxyls and water of a β-helix antifreeze protein. Nature 2000, 406, 322–324. [Google Scholar] [CrossRef]
- Daley, M.E.; Spyracopoulos, L.; Jia, Z.; Davies, P.L.; Sykes, B.D. Structure and dynamics of a beta-helical antifreeze protein. Biochemistry 2002, 41, 5515–5525. [Google Scholar] [CrossRef]
- Falk, M.; Falková, I.; Kopečná, O.; Bačíková, A.; Pagáčová, E.; Šimek, D.; Golan, M.; Kozubek, S.; Pekarová, M.; Follett, S.E.; et al. Chromatin architecture changes and DNA replication fork collapse are critical features in cryopreserved cells that are differentially controlled by cryoprotectants. Sci. Rep. 2018, 8, 14694. [Google Scholar] [CrossRef]
- Kratochvílová, I.; Golan, M.; Pomeisl, K.; Richter, J.; Sedláková, S.; Šebera, J.; Mičová, J.; Falk, M.; Falková, I.; Řeha, D.; et al. Theoretical and experimental study of the antifreeze protein AFP752, trehalose and dimethyl sulfoxide cryoprotection mechanism: Correlation with cryopreserved cell viability. RSC Adv. 2017, 7, 352–360. [Google Scholar] [CrossRef]
- Jevtić, P.; Elliott, K.W.; Watkins, S.E.; Sreter, J.A.; Jovic, K.; Lehner, I.B.; Baures, P.W.; Tsavalas, J.G.; Levy, D.L.; Varga, K. An insect antifreeze protein from Anatolica polita enhances the cryoprotection of Xenopus laevis eggs and embryos. J. Exp. Biol. 2022, 225, jeb.243662. [Google Scholar] [CrossRef]
- Hunt, M.A.; Currie, M.J.; Robinson, B.A.; Dachs, G.U. Optimizing transfection of primary human umbilical vein endothelial cells using commercially available chemical transfection reagents. J. Biomol. Tech. 2010, 21, 66–72. [Google Scholar]
- Baust, J.M.; Van Buskirk, R.; Baust, J.G. Cell Viability Improves Following Inhibition of Cryopreservation-Induced Apoptosis. In Vitro Cell. Dev. Biol.-Anim. 2000, 36, 262–270. [Google Scholar] [CrossRef]
- Baust, J.M.; Vogel, M.J.; Snyder, K.K.; Van Buskirk, R.G.; Baust, J.G. Activation of Mitochondrial-Associated Apoptosis Contributes to Cryopreservation Failure. Cell Preserv. Technol. 2007, 5, 155–164. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Kim, J.S.; Nam, M.H.; An, S.S.A.; Lim, C.S.; Hur, D.S.; Chung, C.; Chang, J.K. Comparison of the automated fluorescence microscopic viability test with the conventional and flow cytometry methods. J. Clin. Lab. Anal. 2011, 25, 90–94. [Google Scholar] [CrossRef]
- Vembadi, A.; Menachery, A.; Qasaimeh, M.A. Cell Cytometry: Review and Perspective on Biotechnological Advances. Front. Bioeng. Biotechnol. 2019, 7, 147. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb. Protoc. 2018. [Google Scholar] [CrossRef]
- Buttke, T.M.; McCubrey, J.A.; Owen, T.C. Use of an aqueous soluble tetrazolium/formazan assay to measure viability and proliferation of lymphokine-dependent cell lines. J. Immunol. Methods 1993, 157, 233–240. [Google Scholar] [CrossRef]
- Elliott, G.D.; Wang, S.; Fuller, B.J. Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology 2017, 76, 74–91. [Google Scholar] [CrossRef]
- Best, B.P. Cryoprotectant Toxicity: Facts, Issues, and Questions. Rejuvenation Res. 2015, 18, 422–436. [Google Scholar] [CrossRef]
- Eroglu, A.; Russo, M.J.; Bieganski, R.; Fowler, A.; Cheley, S.; Bayley, H.; Toner, M. Intracellular trehalose improves the survival of cryopreserved mammalian cells. Nat. Biotechnol. 2000, 18, 163–167. [Google Scholar] [CrossRef]
- Karow, A. Cryoprotectants—A new class of drugs. J. Pharm. Pharmacol. 1969, 21, 209–223. [Google Scholar] [CrossRef]
- Matsumura, K.; Hayashi, F.; Nagashima, T.; Rajan, R.; Hyon, S.-H. Molecular mechanisms of cell cryopreservation with polyampholytes studied by solid-state NMR. Commun. Mater. 2021, 2, 15. [Google Scholar] [CrossRef]
- Poisson, J.S.; Acker, J.P.; Briard, J.G.; Meyer, J.E.; Ben, R.N. Modulating Intracellular Ice Growth with Cell-Permeating Small-Molecule Ice Recrystallization Inhibitors. Langmuir 2019, 35, 7452–7458. [Google Scholar] [CrossRef]
- Da Violante, G.; Zerrouk, N.; Richard, I.; Provot, G.; Chaumeil, J.C.; Arnaud, P. Evaluation of the Cytotoxicity Effect of Dimethyl Sulfoxide (DMSO) on Caco2/TC7 Colon Tumor Cell Cultures. Biol. Pharm. Bull. 2002, 25, 1600–1603. [Google Scholar] [CrossRef]
- Castor, L. Control of division by cell contact and serum concentration in cultures of 3T3 cells. Exp. Cell Res. 1971, 68, 17–24. [Google Scholar] [CrossRef]
- Dulbecco, R. Topoinhibition and Serum Requirement of Transformed and Untransformed Cells. Nature 1970, 227, 802–806. [Google Scholar] [CrossRef]
- Bereiter-Hahn, J.; Münnich, A.; Woiteneck, P. Dependence of Energy Metabolism on the Density of Cells in Culture. Cell Struct. Funct. 1998, 23, 85–93. [Google Scholar] [CrossRef][Green Version]
- Nitsch, S.; Chatterjee, A.; Hofmann, N.; Glasmacher, B. Impact of cryopreservation on histone modifications of mesenchymal stem cells. Biomed. Tech. 2014, 59, S294–S297. [Google Scholar]
- Baboo, J.; Kilbride, P.; Delahaye, M.; Milne, S.; Fonseca, F.; Blanco, M.; Meneghel, J.; Nancekievill, A.; Gaddum, N.; Morris, G.J. The Impact of Varying Cooling and Thawing Rates on the Quality of Cryopreserved Human Peripheral Blood T Cells. Sci. Rep. 2019, 9, 3417. [Google Scholar] [CrossRef]
- Lauterboeck, L.; Saha, D.; Chatterjee, A.; Hofmann, N.; Glasmacher, B. Xeno-Free Cryopreservation of Bone Marrow-Derived Multipotent Stromal Cells from Callithrix jacchus. Biopreserv. Biobank. 2016, 14, 530–538. [Google Scholar] [CrossRef]
- Lee, H.H.; Lee, H.J.; Kim, H.J.; Lee, J.H.; Ko, Y.; Kim, S.M.; Lee, J.R.; Suh, C.S.; Kim, S.H. Effects of antifreeze proteins on the vitrification of mouse oocytes: Comparison of three different antifreeze proteins. Hum. Reprod. 2015, 30, 2110–2119. [Google Scholar] [CrossRef]
- Carpenter, J.F.; Hansen, T.N. Antifreeze protein modulates cell survival during cryopreservation: Mediation through influence on ice crystal growth. Proc. Natl. Acad. Sci. USA 1992, 89, 8953–8957. [Google Scholar] [CrossRef]
- Knight, C.A.; Duman, J.G. Inhibition of recrystallization of ice by insect thermal hysteresis proteins: A possible cryoprotective role. Cryobiology 1986, 23, 256–262. [Google Scholar] [CrossRef]
- Jia, Z.C.; Davies, P.L. Antifreeze proteins: An unusual receptor-ligand interaction. Trends Biochem. Sci. 2002, 27, 101–106. [Google Scholar] [CrossRef]
- Drori, R.; Celik, Y.; Davies, P.L.; Braslavsky, I. Ice-binding proteins that accumulate on different ice crystal planes produce distinct thermal hysteresis dynamics. J. R. Soc. Interface 2014, 11, 10. [Google Scholar] [CrossRef]
- Knight, C.A.; Cheng, C.C.; Devries, A. Adsorption of alpha-helical antifreeze peptides on specific ice crystal surface planes. Biophys. J. 1991, 59, 409–418. [Google Scholar] [CrossRef]
- Braslavsky, I.; Drori, R. LabVIEW-operated Novel Nanoliter Osmometer for Ice Binding Protein Investigations. J. Vis. Exp. 2013, 6, e4189. [Google Scholar] [CrossRef]
- Pertaya, N.; Marshall, C.B.; DiPrinzio, C.L.; Wilen, L.; Thomson, E.S.; Wettlaufer, J.S.; Davies, P.L.; Braslavsky, I. Fluorescence Microscopy Evidence for Quasi-Permanent Attachment of Antifreeze Proteins to Ice Surfaces. Biophys. J. 2007, 92, 3663–3673. [Google Scholar] [CrossRef]
- DeLuca, C.I.; Comley, R.; Davies, P.L. Antifreeze Proteins Bind Independently to Ice. Biophys. J. 1998, 74, 1502–1508. [Google Scholar] [CrossRef][Green Version]
- Drori, R.; Davies, P.L.; Braslavsky, I. Experimental correlation between thermal hysteresis activity and the distance between antifreeze proteins on an ice surface. RSC Adv. 2015, 5, 7848–7853. [Google Scholar] [CrossRef]
- Baust, J.M.; Campbell, L.H.; Harbell, J.W. Best practices for cryopreserving, thawing, recovering, and assessing cells. In Vitro Cell. Dev. Biol.-Anim. 2017, 53, 855–871. [Google Scholar] [CrossRef]
- Matissek, S.J.; Han, W.; Karbalivand, M.; Sayed, M.; Reilly, B.M.; Mallat, S.; Ghazal, S.M.; Munshi, M.; Yang, G.; Treon, S.P.; et al. Epigenetic targeting of Waldenström macroglobulinemia cells with BET inhibitors synergizes with BCL2 or histone deacetylase inhibition. Epigenomics 2021, 13, 129–144. [Google Scholar] [CrossRef]
- Karbalivand, M.; Almada, L.L.; Ansell, S.M.; Fernandez-Zapico, M.E.; Elsawa, S.F. MLL1 inhibition reduces IgM levels in Waldenström macroglobulinemia. Leuk. Res. 2022, 116, 106841. [Google Scholar] [CrossRef]
- Scandella, V.; Paolicelli, R.C.; Knobloch, M. A novel protocol to detect green fluorescent protein in unfixed, snap-frozen tissue. Sci. Rep. 2020, 10, 14642. [Google Scholar] [CrossRef]
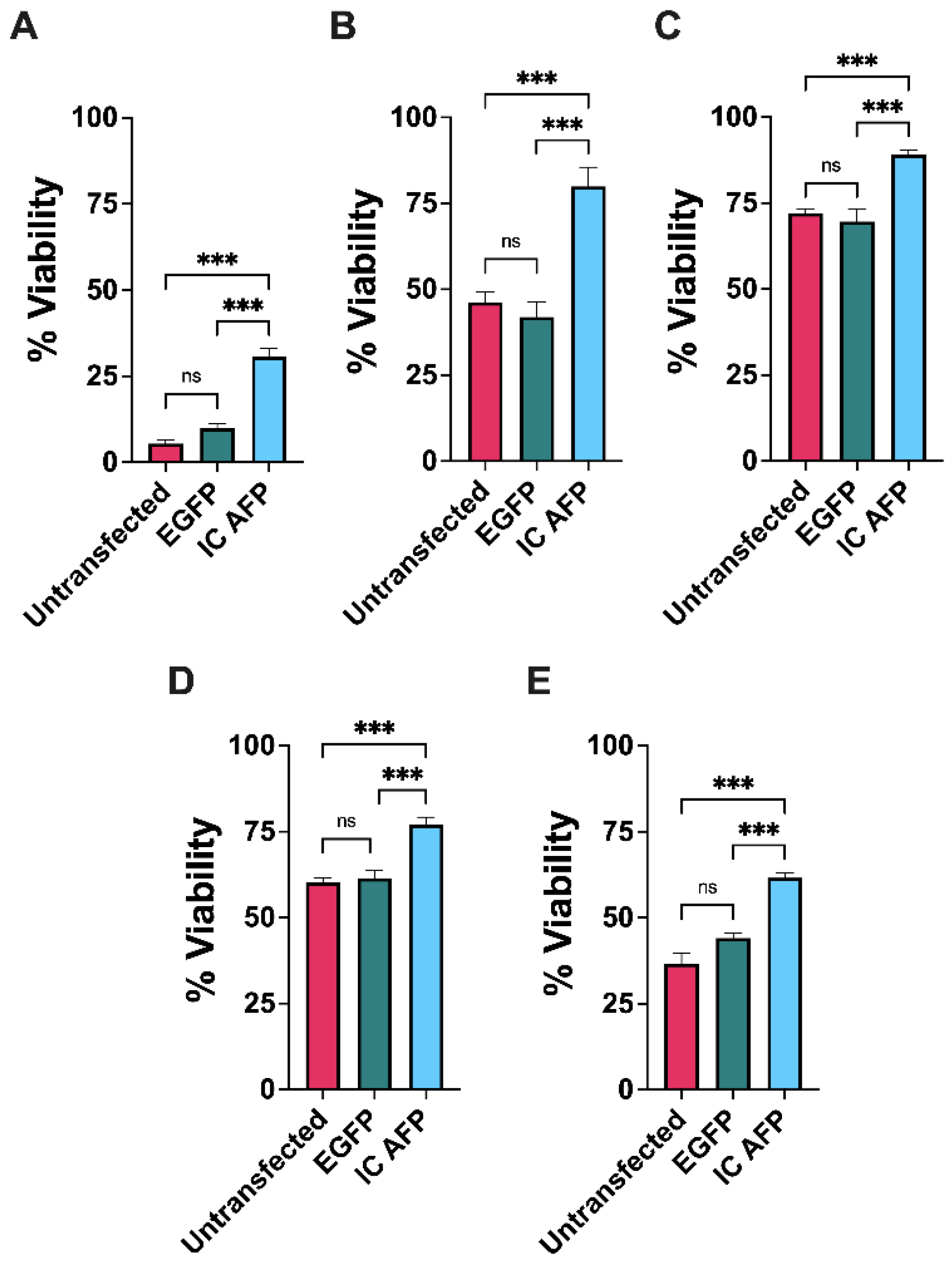


| 0% DMSO | 5% DMSO | 10% DMSO | 15% DMSO | 20% DMSO | |
|---|---|---|---|---|---|
| Untransfected | 5 | 46 | 72 | 60 | 37 |
| EGFP | 10 | 42 | 70 | 62 | 44 |
| IC AFP | 31 | 80 | 89 | 77 | 62 |
| IC AFP vs. Untransfected | +26 (***) | +34 (***) | +17 (***) | +17 (***) | +25 (***) |
| IC AFP vs. EGFP | +21 (***) | +38 (***) | +19 (***) | +15 (***) | +18 (***) |
| 0% DMSO | 5% DMSO | 10% DMSO | 15% DMSO | 20% DMSO | |
|---|---|---|---|---|---|
| Untransfected | 80 | 47 | 29 | 42 | 46 |
| EGFP | 81 | 45 | 30 | 40 | 42 |
| IC AFP | 66 | 25 | 16 | 25 | 32 |
| IC AFP vs. Untransfected | −14 (*) | −22 (***) | −13 (*) | −17 (**) | −14 (ns) |
| IC AFP vs. EGFP | −15 (*) | −20 (***) | −14 (*) | −15 (**) | −10 (ns) |
| 5 µM EC AFP | 15 µM EC AFP | IC AFP | 5 µM EC and IC AFP | 15 µM EC and IC AFP | |
|---|---|---|---|---|---|
| 0% DMSO | +12 (***) | +16 (***) | +24 (***) | +24 (***) | +32 (***) |
| 5% DMSO | +19 (***) | +18 (***) | +27 (***) | +29 (***) | +34 (***) |
| 10% DMSO | +14 (***) | +20 (***) | +20 (***) | +23 (***) | +26 (***) |
| 5 µM EC AFP | 15 µM EC AFP | IC AFP | 5 µM EC and IC AFP | 15 µM EC and IC AFP | |
|---|---|---|---|---|---|
| 0% DMSO | −15 (ns) | −13 (ns) | −23 (*) | −22 (*) | −24 (**) |
| 5% DMSO | −17 (*) | −15 (*) | −31 (***) | −32 (***) | −34 (***) |
| 10% DMSO | −14 (*) | −13 (ns) | −20 (***) | −19 (***) | −20 (***) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sreter, J.A.; Foxall, T.L.; Varga, K. Intracellular and Extracellular Antifreeze Protein Significantly Improves Mammalian Cell Cryopreservation. Biomolecules 2022, 12, 669. https://doi.org/10.3390/biom12050669
Sreter JA, Foxall TL, Varga K. Intracellular and Extracellular Antifreeze Protein Significantly Improves Mammalian Cell Cryopreservation. Biomolecules. 2022; 12(5):669. https://doi.org/10.3390/biom12050669
Chicago/Turabian StyleSreter, Jonathan A., Thomas L. Foxall, and Krisztina Varga. 2022. "Intracellular and Extracellular Antifreeze Protein Significantly Improves Mammalian Cell Cryopreservation" Biomolecules 12, no. 5: 669. https://doi.org/10.3390/biom12050669
APA StyleSreter, J. A., Foxall, T. L., & Varga, K. (2022). Intracellular and Extracellular Antifreeze Protein Significantly Improves Mammalian Cell Cryopreservation. Biomolecules, 12(5), 669. https://doi.org/10.3390/biom12050669