Hyperbaric Oxygen Therapy Improves Parkinson’s Disease by Promoting Mitochondrial Biogenesis via the SIRT-1/PGC-1α Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals and MPTP-Treated Mice
2.3. Locomotor Activity Test
2.4. Grip Strength Test
2.5. Rotarod Performance Assessment
2.6. Immunohistochemistry
2.7. Western Blot Analysis
2.8. SH-SY5Y Cell Culture
2.9. Immunofluorescence Staining
2.10. Statistical Analysis
3. Results
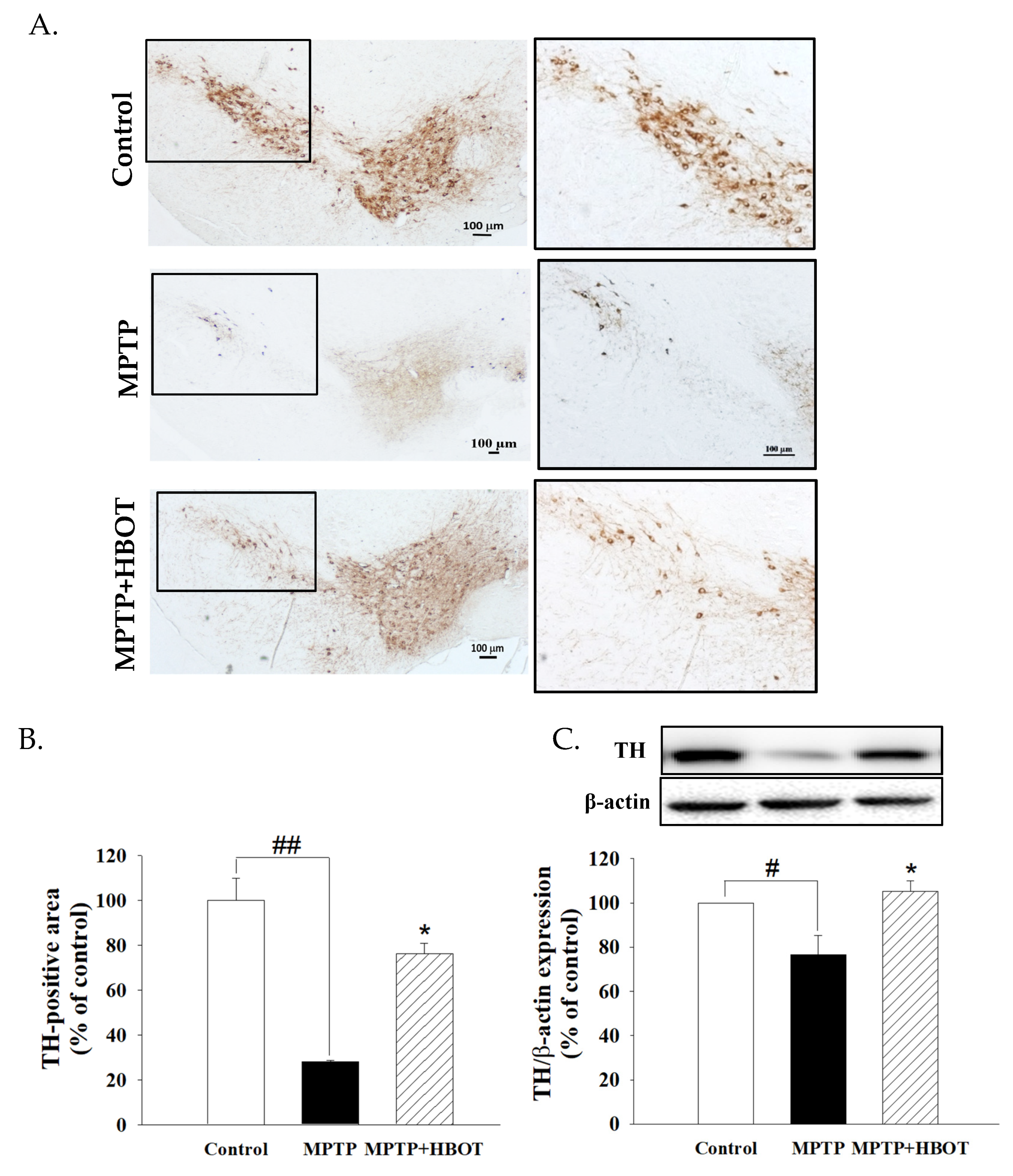
3.1. Hyperbaric Oxygen Therapy Attenuated Substantia Nigra Dopaminergic Neuronal Loss in MPTP-Treated Mice
3.2. Hyperbaric Oxygen Therapy Improves Motor Activity and Grip Strength in MPTP-Treated Mice
3.3. Hyperbaric Oxygen Therapy Inhibits Neuroinflammation in the Brain of MPTP-Treated Mice
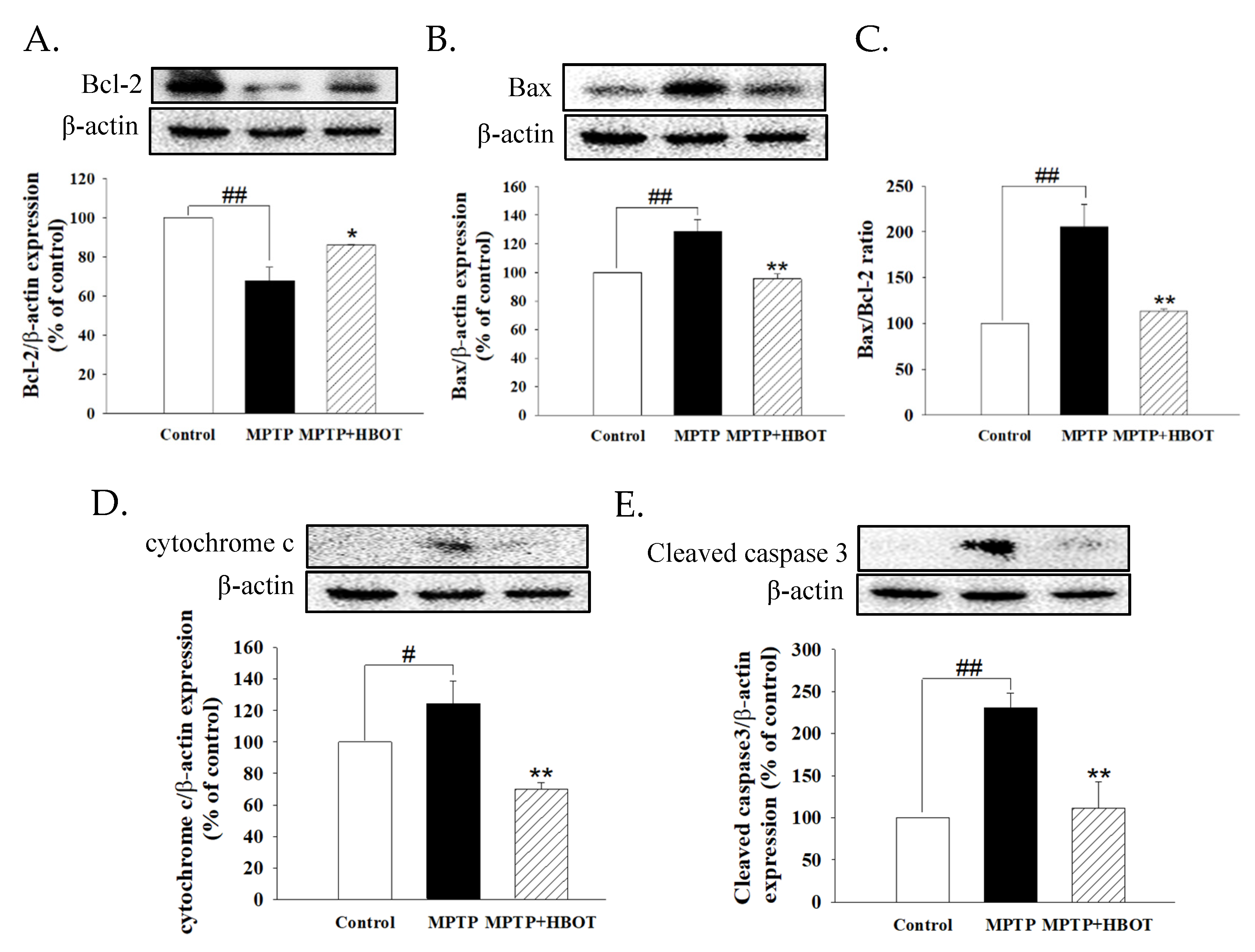
3.4. Hyperbaric Oxygen Therapy Attenuates Apoptosis of Midbrain Tissue in MPTP-Treated Mice
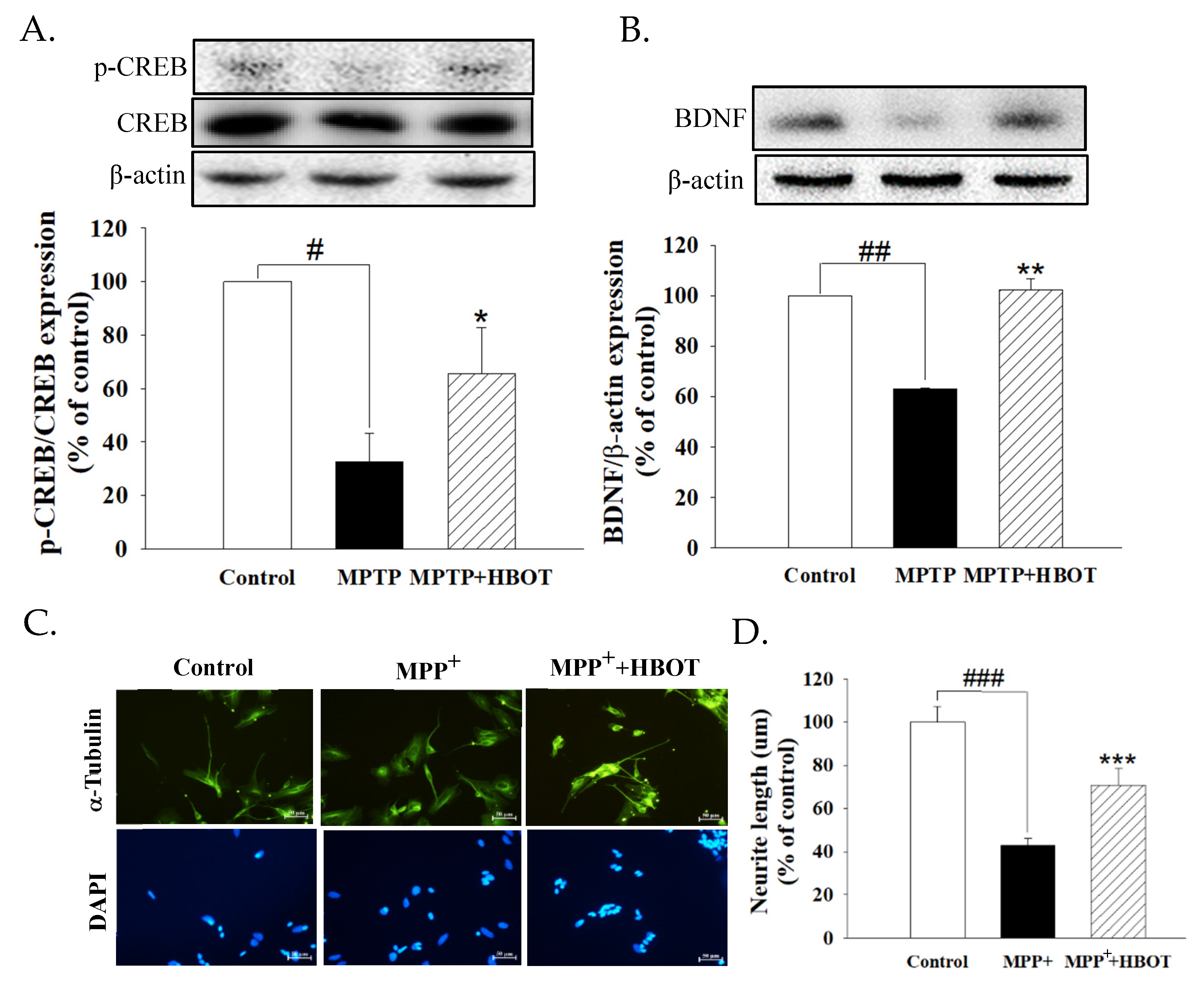
3.5. Hyperbaric Oxygen Therapy Activates Neurotrophic Factor in MPTP-Treated Mice and Promotes the Neurite Outgrowth of MPP+-Treated SH-SY5Y Cells
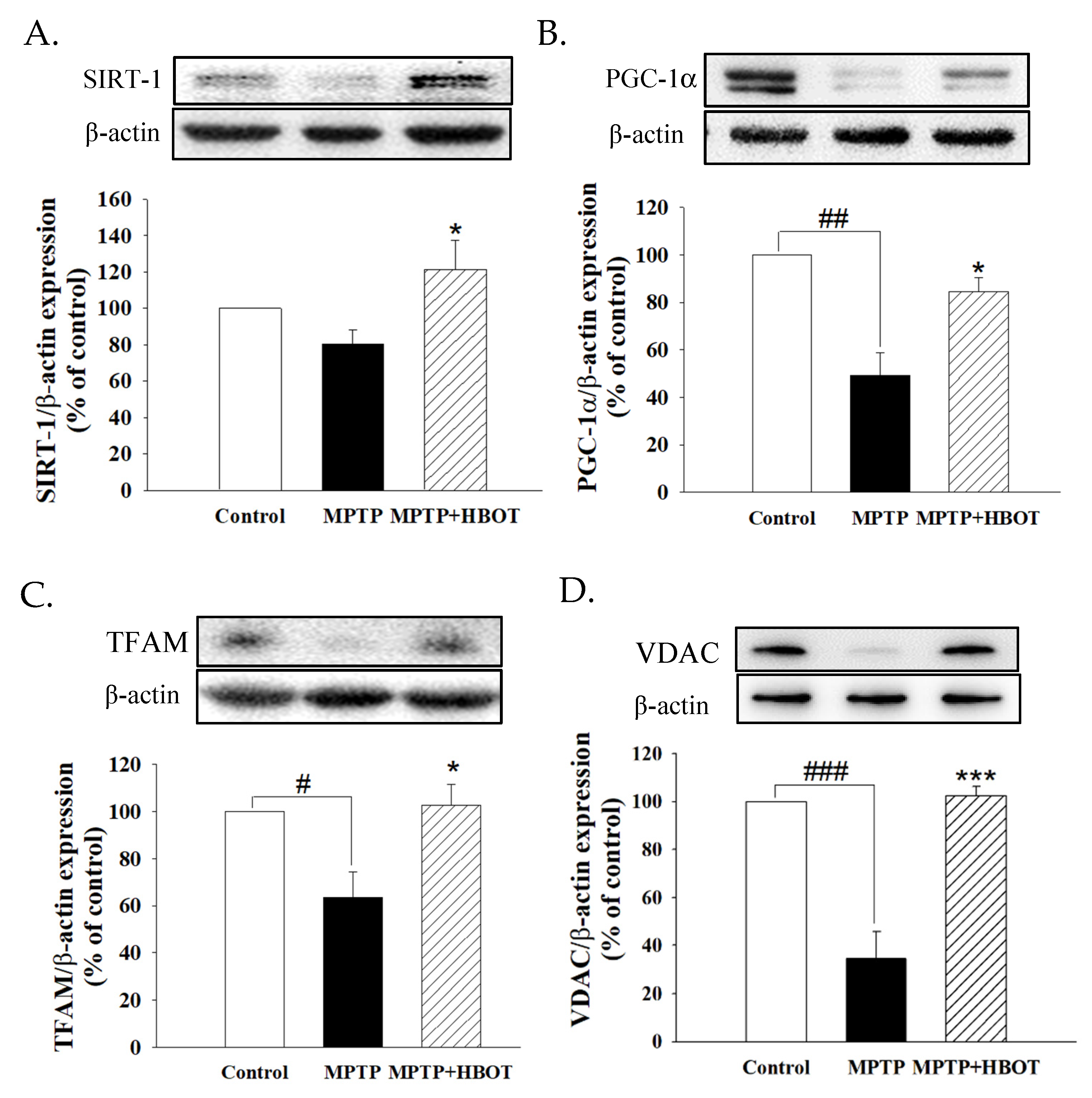
3.6. Hyperbaric Oxygen Therapy Promotes Mitochondrial Biogenesis through the SIRT-1/PGC-1α Pathway in MPTP-Treated Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tysnes, O.B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Siciliano, M.; Trojano, L.; Santangelo, G.; De Micco, R.; Tedeschi, G.; Tessitore, A. Fatigue in Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. Off. J. Mov. Disord. Soc. 2018, 33, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Dick, F.D. Parkinson’s disease and pesticide exposures. Br. Med. Bull. 2006, 79–80, 219–231. [Google Scholar] [CrossRef]
- Block, M.L.; Hong, J.S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog. Neurobiol. 2005, 76, 77–98. [Google Scholar] [CrossRef]
- Nambu, H.; Takada, S.; Fukushima, A.; Matsumoto, J.; Kakutani, N.; Maekawa, S.; Shirakawa, R.; Nakano, I.; Furihata, T.; Katayama, T.; et al. Empagliflozin restores lowered exercise endurance capacity via the activation of skeletal muscle fatty acid oxidation in a murine model of heart failure. Eur. J. Pharmacol. 2020, 866, 172810. [Google Scholar] [CrossRef]
- Lu, Z.; Ma, J.; Liu, B.; Dai, C.; Xie, T.; Ma, X.; Li, M.; Dong, J.; Lan, Q.; Huang, Q. Hyperbaric oxygen therapy sensitizes nimustine treatment for glioma in mice. Cancer Med. 2016, 5, 3147–3155. [Google Scholar] [CrossRef] [Green Version]
- Vadas, D.; Kalichman, L.; Hadanny, A.; Efrati, S. Hyperbaric Oxygen Environment Can Enhance Brain Activity and Multitasking Performance. Front. Integr. Neurosci. 2017, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, E.C. Hyperbaric oxygenation in peripheral nerve repair and regeneration. Neurol. Res. 2007, 29, 184–198. [Google Scholar] [CrossRef]
- Xu, J.J.; Yang, S.T.; Sha, Y.; Ge, Y.Y.; Wang, J.M. Hyperbaric oxygen treatment for Parkinson’s disease with severe depression and anxiety: A case report. Medicine 2018, 97, e0029. [Google Scholar] [CrossRef]
- Kusuda, Y.; Takemura, A.; Nakano, M.; Ishihara, A. Mild hyperbaric oxygen inhibits the decrease of dopaminergic neurons in the substantia nigra of mice with MPTP-induced Parkinson’s disease. Neurosci. Res. 2018, 132, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Langston, J.W.; Ballard, P.A., Jr. Parkinson’s disease in a chemist working with 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. N. Engl. J. Med. 1983, 309, 310. [Google Scholar] [PubMed]
- Trancikova, A.; Tsika, E.; Moore, D.J. Mitochondrial dysfunction in genetic animal models of Parkinson’s disease. Antioxid. Redox Signal. 2012, 16, 896–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stajic, D.; Selakovic, D.; Jovicic, N.; Joksimovic, J.; Arsenijevic, N.; Lukic, M.L.; Rosic, G. The role of galectin-3 in modulation of anxiety state level in mice. Brain Behav. Immun. 2019, 78, 177–187. [Google Scholar] [CrossRef]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef]
- Chinta, S.J.; Andersen, J.K. Redox imbalance in Parkinson’s disease. Biochim. Et Biophys. Acta 2008, 1780, 1362–1367. [Google Scholar] [CrossRef] [Green Version]
- Uittenbogaard, M.; Chiaramello, A. Mitochondrial biogenesis: A therapeutic target for neurodevelopmental disorders and neurodegenerative diseases. Curr. Pharm. Des. 2014, 20, 5574–5593. [Google Scholar] [CrossRef] [Green Version]
- Daugherty, W.P.; Levasseur, J.E.; Sun, D.; Rockswold, G.L.; Bullock, M.R. Effects of hyperbaric oxygen therapy on cerebral oxygenation and mitochondrial function following moderate lateral fluid-percussion injury in rats. J. Neurosurg. 2004, 101, 499–504. [Google Scholar] [CrossRef]
- Palzur, E.; Zaaroor, M.; Vlodavsky, E.; Milman, F.; Soustiel, J.F. Neuroprotective effect of hyperbaric oxygen therapy in brain injury is mediated by preservation of mitochondrial membrane properties. Brain Res. 2008, 1221, 126–133. [Google Scholar] [CrossRef]
- Huang, L.; Obenaus, A. Hyperbaric oxygen therapy for traumatic brain injury. Med. Gas Res. 2011, 1, 21. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Huang, L.; Nong, Z.; Li, Y.; Chen, W.; Huang, J.; Pan, X.; Wu, G.; Lin, Y. Hyperbaric Oxygen Prevents Cognitive Impairments in Mice Induced by D-Galactose by Improving Cholinergic and Anti-apoptotic Functions. Neurochem. Res. 2017, 42, 1240–1253. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, A.; Kawano, F.; Okiura, T.; Morimatsu, F.; Ohira, Y. Hyperbaric exposure with high oxygen concentration enhances oxidative capacity of neuromuscular units. Neurosci. Res. 2005, 52, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Okiura, T.; Morimatsu, F.; Ohira, Y.; Ishihara, A. Effects of hyperbaric exposure with high oxygen concentration on the physical activity of developing rats. Dev. Neurosci. 2007, 29, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Nutt, J.G.; Carter, J.H.; Sexton, G.J. The dopamine transporter: Importance in Parkinson’s disease. Ann. Neurol. 2004, 55, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Schintu, N.; Frau, L.; Ibba, M.; Garau, A.; Carboni, E.; Carta, A.R. Progressive dopaminergic degeneration in the chronic MPTPp mouse model of Parkinson’s disease. Neurotox. Res. 2009, 16, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Arsenijevic, N.; Selakovic, D.; Katanic Stankovic, J.S.; Mihailovic, V.; Mitrovic, S.; Milenkovic, J.; Milanovic, P.; Vasovic, M.; Nikezic, A.; Milosevic-Djordjevic, O.; et al. Variable neuroprotective role of Filipendula ulmaria extract in rat hippocampus. J. Integr. Neurosci. 2021, 20, 871–883. [Google Scholar] [CrossRef]
- Tieu, K. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2011, 1, a009316. [Google Scholar] [CrossRef]
- Lofrumento, D.D.; Saponaro, C.; Cianciulli, A.; De Nuccio, F.; Mitolo, V.; Nicolardi, G.; Panaro, M.A. MPTP-induced neuroinflammation increases the expression of pro-inflammatory cytokines and their receptors in mouse brain. Neuroimmunomodulation 2011, 18, 79–88. [Google Scholar] [CrossRef]
- Rai, S.N.; Birla, H.; Singh, S.S.; Zahra, W.; Patil, R.R.; Jadhav, J.P.; Gedda, M.R.; Singh, S.P. Mucuna pruriens Protects against MPTP Intoxicated Neuroinflammation in Parkinson’s Disease through NF-κB/pAKT Signaling Pathways. Front. Aging Neurosci. 2017, 9, 421. [Google Scholar] [CrossRef] [Green Version]
- Mu, J.; Krafft, P.R.; Zhang, J.H. Hyperbaric oxygen therapy promotes neurogenesis: Where do we stand? Med. Gas Res. 2011, 1, 14. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Xu, J.; Lazarovici, P.; Quirion, R.; Zheng, W. cAMP Response Element-Binding Protein (CREB): A Possible Signaling Molecule Link in the Pathophysiology of Schizophrenia. Front. Mol. Neurosci. 2018, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Landeira, B.S.; Santana, T.; Araújo, J.A.M.; Tabet, E.I.; Tannous, B.A.; Schroeder, T.; Costa, M.R. Activity-Independent Effects of CREB on Neuronal Survival and Differentiation during Mouse Cerebral Cortex Development. Cereb. Cortex 2018, 28, 538–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.Z.; Nusslock, R. Exercise-Mediated Neurogenesis in the Hippocampus via BDNF. Front. Neurosci. 2018, 12, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haigis, M.C.; Sinclair, D.A. Mammalian sirtuins: Biological insights and disease relevance. Annu. Rev. Pathol. 2010, 5, 253–295. [Google Scholar] [CrossRef] [Green Version]
- Gerhart-Hines, Z.; Rodgers, J.T.; Bare, O.; Lerin, C.; Kim, S.H.; Mostoslavsky, R.; Alt, F.W.; Wu, Z.; Puigserver, P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007, 26, 1913–1923. [Google Scholar] [CrossRef]
- Cooper, A.A.; Gitler, A.D.; Cashikar, A.; Haynes, C.M.; Hill, K.J.; Bhullar, B.; Liu, K.; Xu, K.; Strathearn, K.E.; Liu, F.; et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science 2006, 313, 324–328. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Nguyen, J.L.; Hulleman, J.D.; Li, L.; Rochet, J.C. Mechanisms of DJ-1 neuroprotection in a cellular model of Parkinson’s disease. J. Neurochem. 2008, 105, 2435–2453. [Google Scholar] [CrossRef]
- Liu, F.; Hindupur, J.; Nguyen, J.L.; Ruf, K.J.; Zhu, J.; Schieler, J.L.; Bonham, C.C.; Wood, K.V.; Davisson, V.J.; Rochet, J.C. Methionine sulfoxide reductase A protects dopaminergic cells from Parkinson’s disease-related insults. Free. Radic. Biol. Med. 2008, 45, 242–255. [Google Scholar] [CrossRef] [Green Version]
- Ekstrand, M.I.; Falkenberg, M.; Rantanen, A.; Park, C.B.; Gaspari, M.; Hultenby, K.; Rustin, P.; Gustafsson, C.M.; Larsson, N.G. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum. Mol. Genet. 2004, 13, 935–944. [Google Scholar] [CrossRef]
- Risiglione, P.; Zinghirino, F.; Di Rosa, M.C.; Magrì, A.; Messina, A. Alpha-Synuclein and Mitochondrial Dysfunction in Parkinson’s Disease: The Emerging Role of VDAC. Biomolecules 2021, 11, 718. [Google Scholar] [CrossRef]
- Magri, A.; Messina, A. Interactions of VDAC with Proteins Involved in Neurodegenerative Aggregation: An Opportunity for Advancement on Therapeutic Molecules. Curr. Med. Chem. 2017, 24, 4470–4487. [Google Scholar] [CrossRef] [PubMed]
- Shoshan-Barmatz, V.; De Pinto, V.; Zweckstetter, M.; Raviv, Z.; Keinan, N.; Arbel, N. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol. Asp. Med. 2010, 31, 227–285. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, H.-T.; Yang, Y.-L.; Chang, W.-H.; Fang, W.-Y.; Huang, S.-H.; Chou, S.-H.; Lo, Y.-C. Hyperbaric Oxygen Therapy Improves Parkinson’s Disease by Promoting Mitochondrial Biogenesis via the SIRT-1/PGC-1α Pathway. Biomolecules 2022, 12, 661. https://doi.org/10.3390/biom12050661
Hsu H-T, Yang Y-L, Chang W-H, Fang W-Y, Huang S-H, Chou S-H, Lo Y-C. Hyperbaric Oxygen Therapy Improves Parkinson’s Disease by Promoting Mitochondrial Biogenesis via the SIRT-1/PGC-1α Pathway. Biomolecules. 2022; 12(5):661. https://doi.org/10.3390/biom12050661
Chicago/Turabian StyleHsu, Hung-Te, Ya-Lan Yang, Wan-Hsuan Chang, Wei-Yu Fang, Shu-Hung Huang, Shah-Hwa Chou, and Yi-Ching Lo. 2022. "Hyperbaric Oxygen Therapy Improves Parkinson’s Disease by Promoting Mitochondrial Biogenesis via the SIRT-1/PGC-1α Pathway" Biomolecules 12, no. 5: 661. https://doi.org/10.3390/biom12050661
APA StyleHsu, H.-T., Yang, Y.-L., Chang, W.-H., Fang, W.-Y., Huang, S.-H., Chou, S.-H., & Lo, Y.-C. (2022). Hyperbaric Oxygen Therapy Improves Parkinson’s Disease by Promoting Mitochondrial Biogenesis via the SIRT-1/PGC-1α Pathway. Biomolecules, 12(5), 661. https://doi.org/10.3390/biom12050661





