The Sarcoplasmic Reticulum of Skeletal Muscle Cells: A Labyrinth of Membrane Contact Sites
Abstract
1. Introduction
sAnk1.5 and Obscurin Stabilize the SR around the Myofibrils
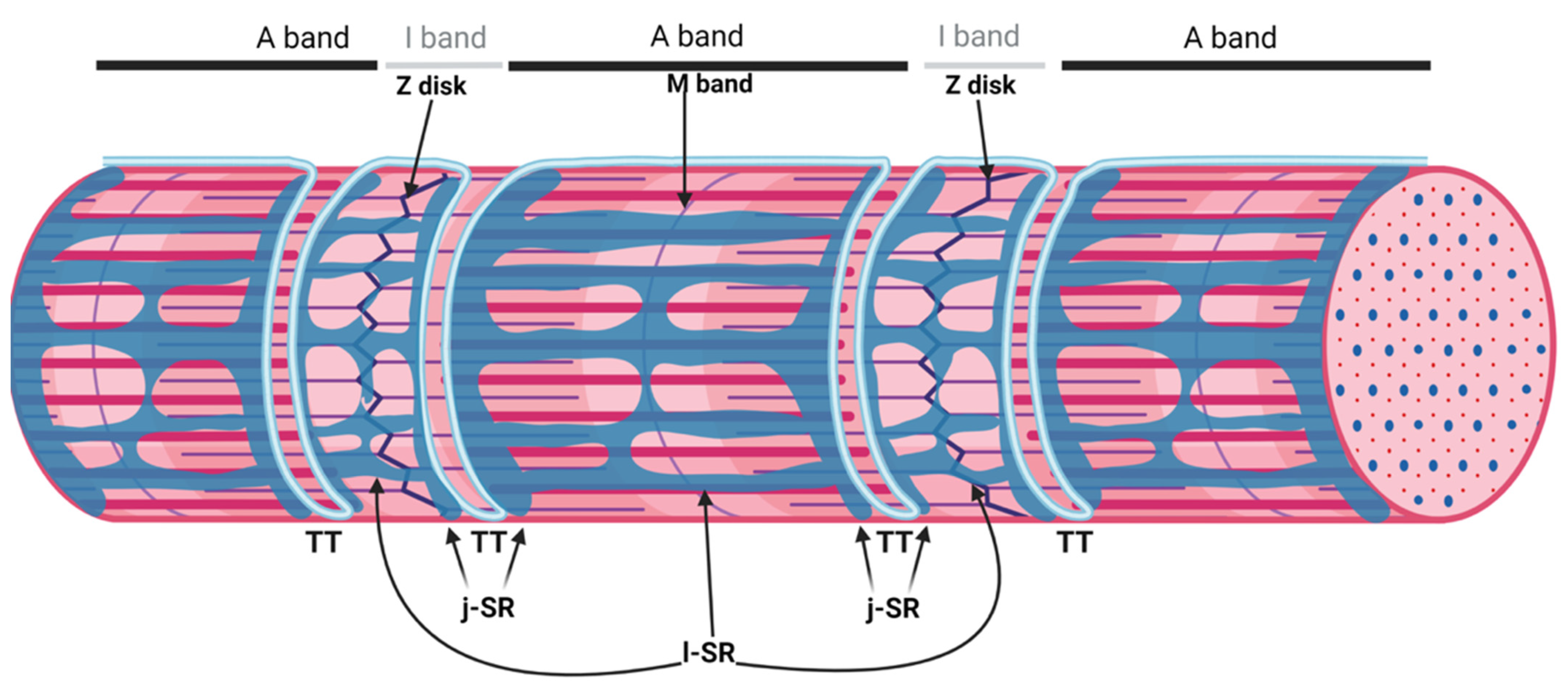
2. The Triad, a Unique Membrane System of the Skeletal Muscle
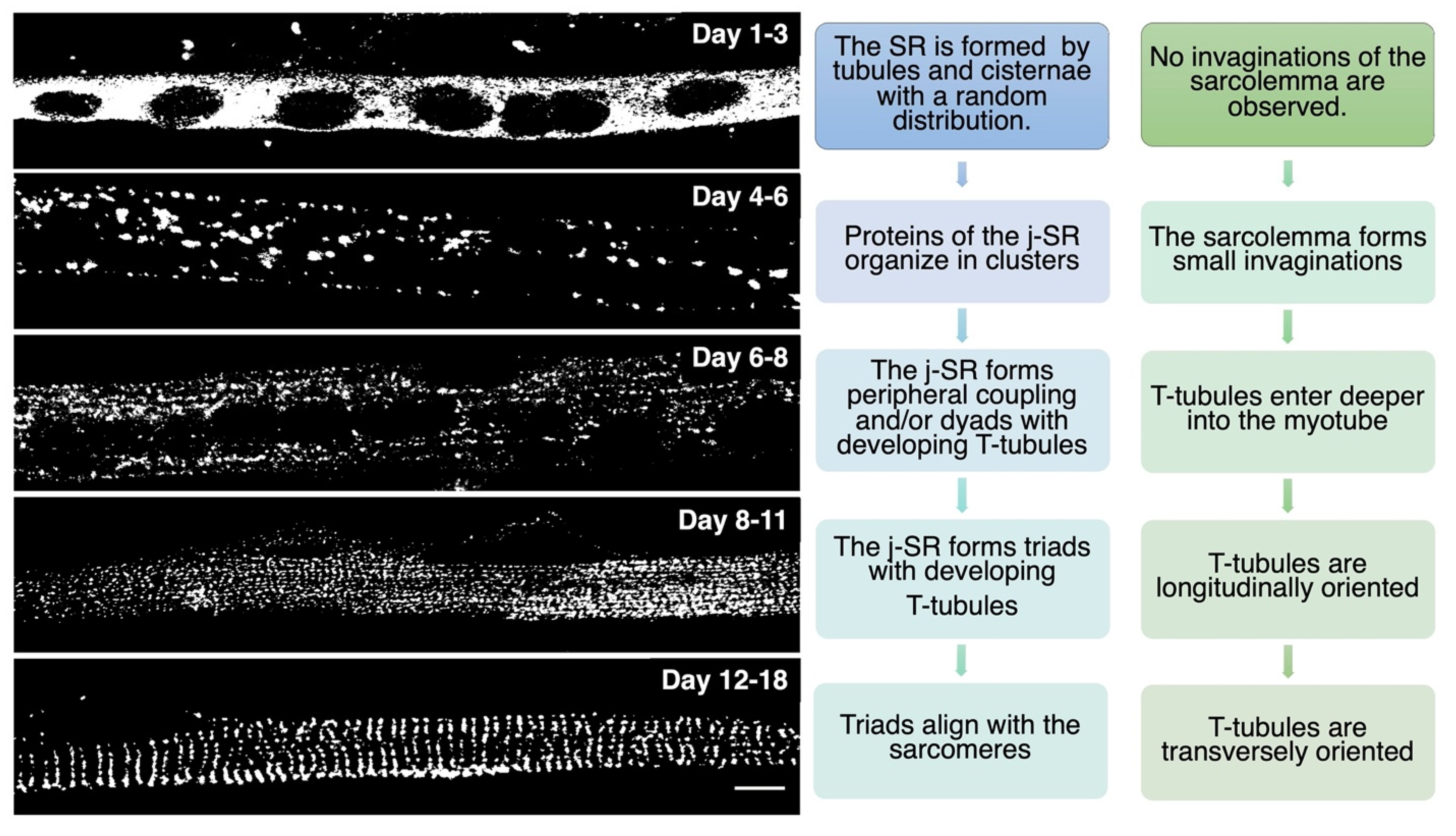
Triad Biogenesis, Repair, and Maintenance
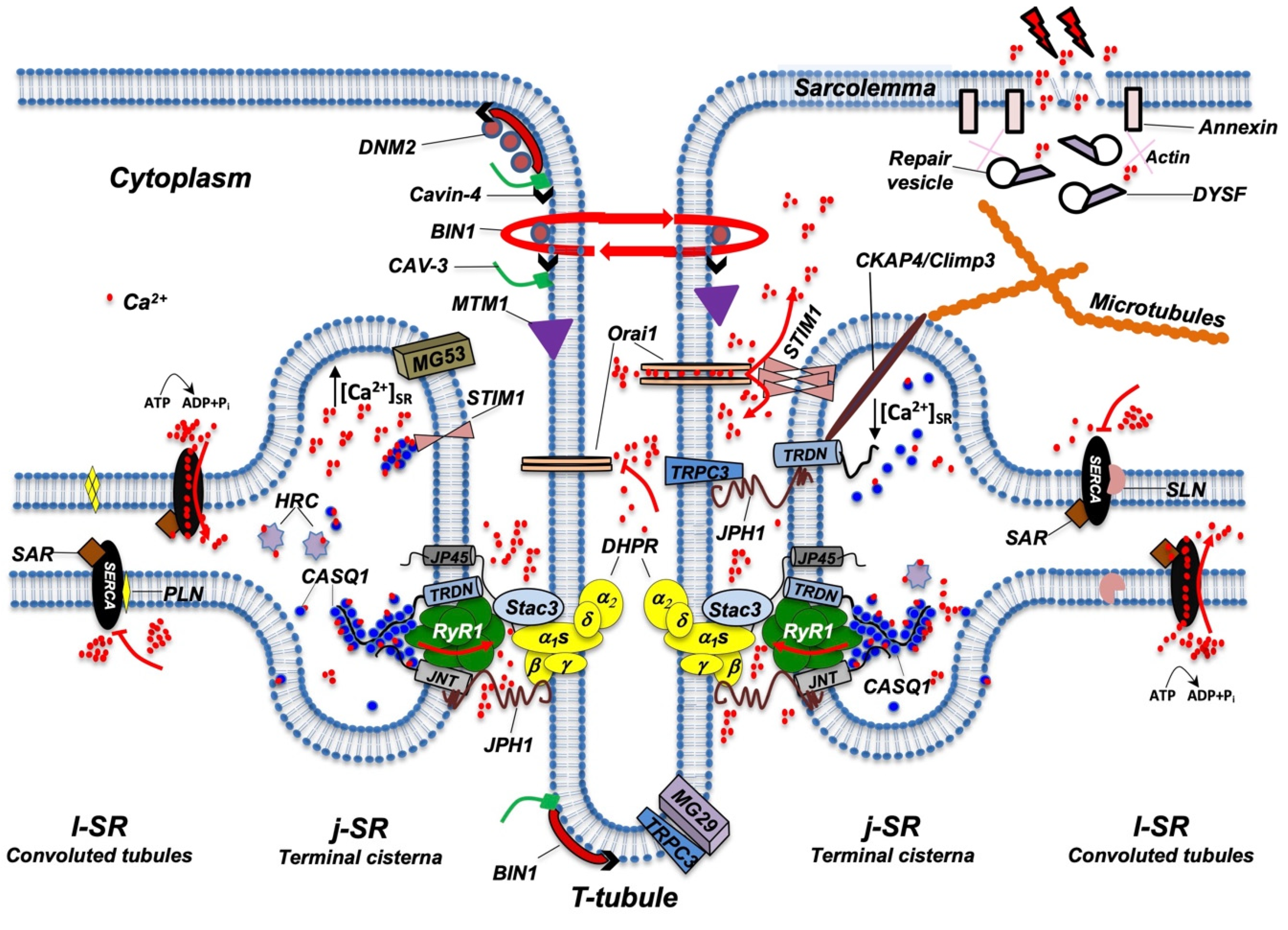
3. The Protein Complex of the ECC
3.1. RyR1, DHPR, and STAC3 Are Essential for ECC
3.2. Triadin and Junctin (JNT), Two Integral Membrane Proteins That Regulate ECC
3.3. Calsequestrin- and Histidine-Rich Calcium-Binding Protein Store Ca2+ in the SR Lumen
4. SERCA Pumps at the l-SR Are Responsible for Ca2+ Re-Uptake from the Sarcoplasm
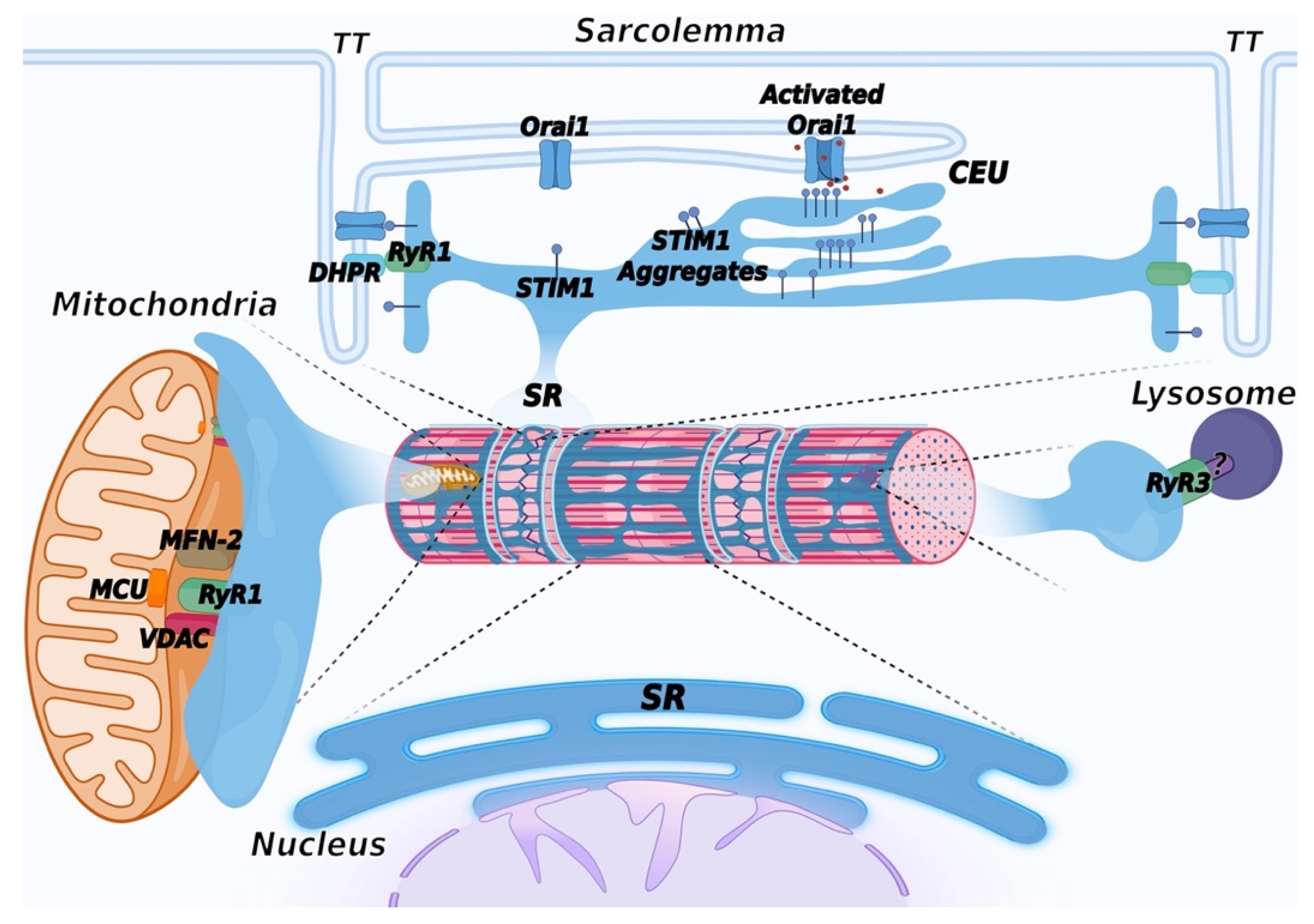
5. Ca2+ Entry Units (CEU): Novel SR/Plasma Membrane Contact Sites to Refill Intracellular Ca2+ Stores
6. Mitochondria-Associated Membranes (MAM)
7. The SR and the ER: Two Faces of a Single Organelle
Additional Contact Sites Contributed by the ER/SR
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Franzini-Armstrong, C. The membrane systems of muscle cells. In Miology, 3rd ed.; Mc Graw-Hill: New York, NY, USA, 2004; pp. 232–256. [Google Scholar]
- Franzini-Armstrong, C. The relationship between form and function throughout the history of excitation-contraction coupling. J. Gen. Physiol. 2018, 150, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, P.; Barone, V.; Giacomello, E.; Rossi, D.; Sorrentino, V. Binding of an ankyrin-1 isoform to obscurin suggests a molecular link between the sarcoplasmic reticulum and myofibrils in striated muscles. J. Cell Biol. 2003, 160, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Kontrogianni-Konstantopoulos, A.; Jones, E.M.; van Rossum, D.B.; Bloch, R.J. Obscurin is a ligand for small ankyrin 1 in skeletal muscle. Mol. Biol. Cell 2003, 14, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Kontrogianni-Konstantopoulos, A.; Catino, D.H.; Strong, J.C.; Sutter, S.; Borisov, A.B.; Pumplin, D.W.; Russell, M.K.; Bloch, R.J. Obscurin modulates the assembly and organization of sarcomeres and the sarcoplasmic reticulum. FASEB J. 2006, 20, 2102–2111. [Google Scholar] [CrossRef]
- Giacomello, E.; Sorrentino, V. Localization of ank1.5 in the sarcoplasmic reticulum precedes that of SERCA and RyR: Relationship with the organization of obscurin in developing sarcomeres. Histochem. Cell Biol. 2009, 131, 371–382. [Google Scholar] [CrossRef]
- Lange, S.; Ouyang, K.; Meyer, G.; Cui, L.; Cheng, H.; Lieber, R.L.; Chen, J. Obscurin determines the architecture of the longitudinal sarcoplasmic reticulum. J. Cell Sci. 2009, 122, 2640–2650. [Google Scholar] [CrossRef]
- Giacomello, E.; Quarta, M.; Paolini, C.; Squecco, R.; Fusco, P.; Toniolo, L.; Blaauw, B.; Formoso, L.; Rossi, D.; Birkenmeier, C.; et al. Deletion of small ankyrin 1 (sAnk1) isoforms results in structural and functional alterations in aging skeletal muscle fibers. Am. J. Physiol.-Cell Physiol. 2015, 308, C123–C138. [Google Scholar] [CrossRef]
- Armani, A.; Galli, S.; Giacomello, E.; Bagnato, P.; Barone, V.; Rossi, D.; Sorrentino, V. Molecular interactions with obscurin are involved in the localization of muscle-specific small ankyrin1 isoforms to subcompartments of the sarcoplasmic reticulum. Exp. Cell Res. 2006, 312, 3546–3558. [Google Scholar] [CrossRef]
- Randazzo, D.; Giacomello, E.; Lorenzini, S.; Rossi, D.; Pierantozzi, E.; Blaauw, B.; Reggiani, C.; Lange, S.; Peter, A.K.; Chen, J.; et al. Obscurin is required for ankyrinB-dependent dystrophin localization and sarcolemma integrity. J. Cell Biol. 2013, 200, 523–536. [Google Scholar] [CrossRef]
- Randazzo, D.; Blaauw, B.; Paolini, C.; Pierantozzi, E.; Spinozzi, S.; Lange, S.; Chen, J.; Protasi, F.; Reggiani, C.; Sorrentino, V. Exercise-induced alterations and loss of sarcomeric M-line organization in the diaphragm muscle of obscurin knockout mice. Am. J. Physiol.-Cell Physiol. 2017, 312, C16–C28. [Google Scholar] [CrossRef]
- Dulhunty, A.F. Excitation-contraction coupling from the 1950s into the new millennium. Clin. Exp. Pharmacol. Physiol. 2006, 33, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Rios, E. Calcium-induced release of calcium in muscle: 50 years of work and the emerging consensus. J. Gen. Physiol. 2018, 150, 521–537. [Google Scholar] [CrossRef] [PubMed]
- Takekura, H.; Flucher, B.E.; Franzini-Armstrong, C. Sequential docking, molecular differentiation, and positioning of T-Tubule/SR junctions in developing mouse skeletal muscle. Dev. Biol. 2001, 239, 204–214. [Google Scholar] [CrossRef]
- Flucher, B.E.; Takekura, H.; Franzini-Armstrong, C. Development of the excitation-contraction coupling apparatus in skeletal muscle: Association of sarcoplasmic reticulum and transverse tubules with myofibrils. Dev. Biol. 1993, 160, 135–147. [Google Scholar] [CrossRef]
- Luff, A.R.; Atwood, H.L. Changes in the sarcoplasmic reticulum and transverse tubular system of fast and slow skeletal muscles of the mouse during postnatal development. J. Cell Biol. 1971, 51, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.E.; Martel, N.; Ariotti, N.; Xiong, Z.; Lo, H.P.; Ferguson, C.; Rae, J.; Lim, Y.W.; Parton, R.G. In Vivo cell biological screening identifies an endocytic capture mechanism for T-tubule formation. Nat. Commun. 2020, 11, 3711. [Google Scholar] [CrossRef]
- Yuan, S.H.; Arnold, W.; Jorgensen, A.O. Biogenesis of transverse tubules and triads: Immunolocalization of the 1,4-dihydropyridine receptor, TS28, and the ryanodine receptor in rabbit skeletal muscle developing in situ. J. Cell Biol. 1991, 112, 289–301. [Google Scholar] [CrossRef]
- Franzini-Armstrong, C.; Pincon-Raymond, M.; Rieger, F. Muscle fibers from dysgenic mouse In Vivo lack a surface component of peripheral couplings. Dev. Biol. 1991, 146, 364–376. [Google Scholar] [CrossRef]
- Powell, J.A.; Petherbridge, L.; Flucher, B.E. Formation of triads without the dihydropyridine receptor alpha subunits in cell lines from dysgenic skeletal muscle. J. Cell Biol. 1996, 134, 375–387. [Google Scholar] [CrossRef]
- Felder, E.; Protasi, F.; Hirsch, R.; Franzini-Armstrong, C.; Allen, P.D. Morphology and molecular composition of sarcoplasmic reticulum surface junctions in the absence of DHPR and RyR in mouse skeletal muscle. Biophys. J. 2002, 82, 3144–3149. [Google Scholar] [CrossRef]
- Al-Qusairi, L.; Laporte, J. T-tubule biogenesis and triad formation in skeletal muscle and implication in human diseases. Skelet. Muscle 2011, 1, 26. [Google Scholar] [CrossRef] [PubMed]
- Barone, V.; Randazzo, D.; Del Re, V.; Sorrentino, V.; Rossi, D. Organization of junctional sarcoplasmic reticulum proteins in skeletal muscle fibers. J. Muscle Res. Cell Motil. 2015, 36, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Peter, B.J.; Kent, H.M.; Mills, I.G.; Vallis, Y.; Butler, P.J.; Evans, P.R.; McMahon, H.T. BAR domains as sensors of membrane curvature: The amphiphysin BAR structure. Science 2004, 303, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Marcucci, M.; Daniell, L.; Pypaert, M.; Weisz, O.A.; Ochoa, G.C.; Farsad, K.; Wenk, M.R.; De Camilli, P. Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science 2002, 297, 1193–1196. [Google Scholar] [CrossRef] [PubMed]
- Wechsler-Reya, R.J.; Elliott, K.J.; Prendergast, G.C. A role for the putative tumor suppressor Bin1 in muscle cell differentiation. Mol. Cell Biol. 1998, 18, 566–575. [Google Scholar] [CrossRef]
- Cowling, B.S.; Prokic, I.; Tasfaout, H.; Rabai, A.; Humbert, F.; Rinaldi, B.; Nicot, A.S.; Kretz, C.; Friant, S.; Roux, A.; et al. Amphiphysin (BIN1) negatively regulates dynamin 2 for normal muscle maturation. J. Clin. Investig. 2017, 127, 4477–4487. [Google Scholar] [CrossRef]
- Fujise, K.; Okubo, M.; Abe, T.; Yamada, H.; Nishino, I.; Noguchi, S.; Takei, K.; Takeda, T. Mutant BIN1-Dynamin 2 complexes dysregulate membrane remodeling in the pathogenesis of centronuclear myopathy. J. Biol. Chem. 2020, 296, 100077. [Google Scholar] [CrossRef]
- Silva-Rojas, R.; Nattarayan, V.; Jaque-Fernandez, F.; Gomez-Oca, R.; Menuet, A.; Reiss, D.; Goret, M.; Messaddeq, N.; Lionello, V.M.; Kretz, C.; et al. Mice with muscle-specific deletion of Bin1 recapitulate centronuclear myopathy and acute downregulation of dynamin 2 improves their phenotypes. Mol. Ther. 2022, 30, 868–880. [Google Scholar] [CrossRef]
- Toussaint, A.; Cowling, B.S.; Hnia, K.; Mohr, M.; Oldfors, A.; Schwab, Y.; Yis, U.; Maisonobe, T.; Stojkovic, T.; Wallgren-Pettersson, C.; et al. Defects in amphiphysin 2 (BIN1) and triads in several forms of centronuclear myopathies. Acta Neuropathol. 2011, 121, 253–266. [Google Scholar] [CrossRef]
- Jungbluth, H.; Gautel, M. Pathogenic Mechanisms in Centronuclear Myopathies. Front. Aging Neurosci. 2014, 6, 339. [Google Scholar] [CrossRef]
- Vlahovich, N.; Kee, A.J.; Van der Poel, C.; Kettle, E.; Hernandez-Deviez, D.; Lucas, C.; Lynch, G.S.; Parton, R.G.; Gunning, P.W.; Hardeman, E.C. Cytoskeletal Tropomyosin Tm5NM1 Is Required for Normal Excitation–Contraction Coupling in Skeletal Muscle. Mol. Biol. Cell. 2009, 20, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Falcone, S.; Roman, W.; Hnia, K.; Gache, V.; Didier, N.; Lainé, J.; Auradé, F.; Marty, I.; Nishino, I.; Charlet-Berguerand, N.; et al. N-WASP is required for Amphiphysin-2/BIN1-dependent nuclear positioning and triad organization in skeletal muscle and is involved in the pathophysiology of centronuclear myopathy. EMBO Mol. Med. 2014, 6, 1455–1475. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Scherer, P.E.; Okamoto, T.; Song, K.; Chu, C.; Kohtz, D.S.; Nishimoto, I.; Lodish, H.F.; Lisanti, M.P. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J. Biol. Chem. 1996, 271, 2255–2261. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G.; Way, M.; Zorzi, N.; Stang, E. Caveolin-3 associates with developing T-tubules during muscle differentiation. J. Cell Biol. 1997, 136, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, F.; Engelman, J.A.; Volonte, D.; Zhang, X.L.; Minetti, C.; Li, M.; Hou, H., Jr.; Kneitz, B.; Edelmann, W.; Lisanti, M.P. Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophinglycoprotein complex, and t-tubule abnormalities. J. Biol. Chem. 2001, 276, 21425–21433. [Google Scholar] [CrossRef]
- Corrotte, M.; Almeida, P.E.; Tam, C.; Castro-Gomes, T.; Fernandes, M.C.; Millis, B.A.; Cortez, M.; Miller, H.; Song, W.; Maugel, T.K.; et al. Caveolae internalization repairs wounded cells and muscle fibers. eLife 2013, 2, e00926. [Google Scholar] [CrossRef]
- Minetti, C.; Sotgia, F.; Bruno, C.; Scartezzini, P.; Broda, P.; Bado, M.; Masetti, E.; Mazzocco, M.; Egeo, A.; Donati, M.A.; et al. Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy. Nat. Genet. 1998, 18, 365–368. [Google Scholar] [CrossRef]
- Betz, R.C.; Schoser, B.G.; Kasper, D.; Ricker, K.; Ramirez, A.; Stein, V.; Torbergsen, T.; Lee, Y.A.; Nothen, M.M.; Wienker, T.F.; et al. Mutations in CAV3 cause mechanical hyperirritability of skeletal muscle in rippling muscle disease. Nat. Genet. 2001, 28, 218–219. [Google Scholar] [CrossRef]
- Woodman, S.E.; Sotgia, F.; Galbiati, F.; Minetti, C.; Lisanti, M.P. Mutations in caveolin-3 cause four distinct autosomal dominant muscle diseases. Neurology 2004, 62, 538–543. [Google Scholar] [CrossRef]
- Kovtun, O.; Tillu, V.A.; Ariotti, N.; Parton, R.G.; Collins, B.M. Cavin family proteins and the assembly of caveolae. J. Cell Sci. 2015, 128, 1269–1278. [Google Scholar] [CrossRef]
- Bastiani, M.; Liu, L.; Hill, M.M.; Jedrychowski, M.P.; Nixon, S.J.; Lo, H.P.; Abankwa, D.; Luetterforst, R.; Fernandez-Rojo, M.; Breen, M.R.; et al. MURC/Cavin-4 and cavin family members form tissue-specific caveolar complexes. J. Cell Biol. 2009, 185, 1259–1273. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Lin, P.; De, G.; Choi, K.H.; Takeshima, H.; Weisleder, N.; Ma, J. Polymerase transcriptase release factor (PTRF) anchors MG53 protein to cell injury site for initiation of membrane repair. J. Biol. Chem. 2011, 286, 12820–12824. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Deviez, D.J.; Martin, S.; Laval, S.H.; Lo, H.P.; Cooper, S.T.; North, K.N.; Bushby, K.; Parton, R.G. Aberrant dysferlin trafficking in cells lacking caveolin or expressing dystrophy mutants of caveolin-3. Hum. Mol. Genet. 2006, 15, 129–142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsuda, C.; Hayashi, Y.K.; Ogawa, M.; Aoki, M.; Murayama, K.; Nishino, I.; Nonaka, I.; Arahata, K.; Brown, R.H., Jr. The sarcolemmal proteins dysferlin and caveolin-3 interact in skeletal muscle. Hum. Mol. Genet. 2001, 10, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Masumiya, H.; Weisleder, N.; Matsuda, N.; Nishi, M.; Hwang, M.; Ko, J.K.; Lin, P.; Thornton, A.; Zhao, X.; et al. MG53 nucleates assembly of cell membrane repair machinery. Nat. Cell Biol. 2009, 11, 56–64. [Google Scholar] [CrossRef]
- Anderson, L.V.; Davison, K.; Moss, J.A.; Young, C.; Cullen, M.J.; Walsh, J.; Johnson, M.A.; Bashir, R.; Britton, S.; Keers, S.; et al. Dysferlin is a plasma membrane protein and is expressed early in human development. Hum. Mol. Genet. 1999, 8, 855–861. [Google Scholar] [CrossRef]
- Bansal, D.; Miyake, K.; Vogel, S.S.; Groh, S.; Chen, C.C.; Williamson, R.; McNeil, P.L.; Campbell, K.P. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature 2003, 423, 168–172. [Google Scholar] [CrossRef]
- Klinge, L.; Harris, J.; Sewry, C.; Charlton, R.; Anderson, L.; Laval, S.; Chiu, Y.H.; Hornsey, M.; Straub, V.; Barresi, R.; et al. Dysferlin associates with the developing T-tubule system in rodent and human skeletal muscle. Muscle Nerve. 2010, 41, 166–173. [Google Scholar] [CrossRef]
- Bashir, R.; Britton, S.; Strachan, T.; Keers, S.; Vafiadaki, E.; Lako, M.; Richard, I.; Marchand, S.; Bourg, N.; Argov, Z.; et al. A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat. Genet. 1998, 20, 37–42. [Google Scholar] [CrossRef]
- Liu, J.; Aoki, M.; Illa, I.; Wu, C.; Fardeau, M.; Angelini, C.; Serrano, C.; Urtizberea, J.A.; Hentati, F.; Hamida, M.B.; et al. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat. Genet. 1998, 20, 31–36. [Google Scholar] [CrossRef]
- Illa, I.; Serrano-Munuera, C.; Gallardo, E.; Lasa, A.; Rojas-García, R.; Palmer, J.; Gallano, P.; Baiget, M.; Matsuda, C.; Brown, R.H. Distal anterior compartment myopathy: A dysferlin mutation causing a new muscular dystrophy phenotype. Ann. Neurol. 2001, 49, 130–134. [Google Scholar] [CrossRef]
- Roostalu, U.; Strähle, U. In Vivo imaging of molecular interactions at damaged sarcolemma. Dev. Cell. 2012, 22, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Di Zanni, E.; Gradogna, A.; Scholz-Starke, J.; Boccaccio, A. Gain of function of TMEM16E/ANO5 scrambling activity caused by a mutation associated with gnathodiaphyseal dysplasia. Cell Mol. Life Sci. 2018, 75, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, V.; Marlow, G.; Boycott, K.M.; Saleki, K.; Inoue, H.; Kroon, J.; Itakura, M.; Robitaille, Y.; Parent, L.; Baas, F.; et al. Recessive mutations in the putative calcium-activated chloride channel Anoctamin 5 cause proximal LGMD2L and distal MMD3 muscular dystrophies. Am. J. Hum. Genet. 2010, 86, 213–221. [Google Scholar] [CrossRef]
- Foltz, S.J.; Cui, Y.Y.; Choo, H.J.; Hartzell, H.C. ANO5 ensures trafficking of annexins in wounded myofibers. J. Cell Biol. 2021, 220, e202007059. [Google Scholar] [CrossRef]
- Chandra, G.; Sreetama, S.C.; Mázala, D.A.G.; Charton, K.; VanderMeulen, J.H.; Richard, I.; Jaiswal, J.K. Endoplasmic reticulum maintains ion homeostasis required for plasma membrane repair. J. Cell Biol. 2021, 220, e202006035. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Yue, H.; Whitson, B.A.; Haggard, E.; Xu, X.; Ma, J. MG53, A Tissue Repair Protein with Broad Applications in Regenerative Medicine. Cells 2021, 10, 122. [Google Scholar] [CrossRef]
- McNeil, P.L.; Kirchhausen, T. An emergency response team for membrane repair. Nat. Rev. Mol. Cell Biol. 2005, 6, 499–505. [Google Scholar] [CrossRef]
- Cai, C.; Weisleder, N.; Ko, J.K.; Komazaki, S.; Sunada, Y.; Nishi, M.; Takeshima, H.; Ma, J. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J. Biol. Chem. 2009, 284, 15894–15902. [Google Scholar] [CrossRef]
- Takeshima, H.; Shimuta, M.; Komazaki, S.; Ohmi, K.; Nishi, M.; Iino, M.; Miyata, A.; Kangawa, K. Mitsugumin29, a novel synaptophysin family member from the triad junction in skeletal muscle. Biochem. J. 1998, 331, 317–322. [Google Scholar] [CrossRef]
- Shimuta, M.; Komazaki, S.; Nishi, M.; Iino, M.; Nakagawara, K.-I.; Takeshima, H. Structure and expression of mitsugumin29 gene. FEBS Lett. 1998, 431, 263–267. [Google Scholar] [CrossRef]
- Komazaki, S.; Nishi, M.; Kangawa, K.; Takeshima, H. Immunolocalization of mitsugumin29 in developing skeletal muscle and effects of the protein expressed in amphibian embryonic cells. Dev. Dyn. 1999, 215, 87–95. [Google Scholar] [CrossRef]
- Nishi, M.; Komazaki, S.; Kurebayashi, N.; Ogawa, Y.; Noda, T.; Iino, M.; Takeshima, H. Abnormal features in skeletal muscle from mice lacking mitsugumin29. J. Cell Biol. 1999, 147, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Komazaki, S.; Nishi, M.; Takeshima, H.; Nakamura, H. Abnormal formation of sarcoplasmic reticulum networks and triads during early development of skeletal muscle cells in mitsugumin 29-deficient mice. Dev. Growth Differ. 2001, 43, 717–723. [Google Scholar] [CrossRef]
- Woo, J.S.; Hwang, J.H.; Huang, M.; Ahn, M.K.; Cho, C.H.; Ma, J.; Lee, E.H. Interaction between mitsugumin 29 and TRPC3 participates in regulating Ca2+ transients in skeletal muscle. Biochem. Biophys. Res. Commun. 2015, 464, 133–139. [Google Scholar] [CrossRef]
- Raess, M.A.; Friant, S.; Cowling, B.S.; Laporte, J. WANTED-Dead or alive: Myotubularins, a large disease-associated protein family. Adv. Biol. Regul. 2017, 63, 49–58. [Google Scholar] [CrossRef]
- Al-Qusairi, L.; Weiss, N.; Toussaint, A.; Berbey, C.; Messaddeq, N.; Kretz, C.; Sanoudou, D.; Beggs, A.H.; Allard, B.; Mandel, J.L.; et al. T-tubule disorganization and defective excitation-contraction coupling in muscle fibers lacking myotubularin lipid phosphatase. Proc. Natl. Acad. Sci. USA 2009, 106, 18763–18768. [Google Scholar] [CrossRef]
- Hnia, K.; Vaccari, I.; Bolino, A.; Laporte, J. Myotubularin phosphoinositide phosphatases: Cellular functions and disease pathophysiology. Trends Mol. Med. 2012, 18, 317–327. [Google Scholar] [CrossRef]
- Amoasii, L.; Bertazzi, D.L.; Tronchère, H.; Hnia, K.; Chicanne, G.; Rinaldi, B.; Cowling, B.S.; Ferry, A.; Klaholz, B.; Payrastre, B.; et al. Phosphatase-dead myotubularin ameliorates X-linked centronuclear myopathy phenotypes in mice. PLoS Genet. 2012, 8, e1002965. [Google Scholar] [CrossRef]
- Buj-Bello, A.; Fougerousse, F.; Schwab, Y.; Messaddeq, N.; Spehner, D.; Pierson, C.R.; Durand, M.; Kretz, C.; Danos, O.; Douar, A.M.; et al. AAV-mediated intramuscular delivery of myotubularin corrects the myotubular myopathy phenotype in targeted murine muscle and suggests a function in p.plasma membrane homeostasis. Hum. Mol. Genet. 2008, 17, 2132–2143. [Google Scholar] [CrossRef]
- Amoasii, L.; Hnia, K.; Chicanne, G.; Brech, A.; Cowling, B.S.; Muller, M.M.; Schwab, Y.; Koebel, P.; Ferry, A.; Payrastre, B.; et al. Myotubularin and PtdIns3P remodel the sarcoplasmic reticulum in muscle In Vivo. J. Cell Sci. 2013, 126, 1806–1819. [Google Scholar] [CrossRef] [PubMed]
- Royer, B.; Hnia, K.; Gavriilidis, C.; Tronchère, H.; Tosch, V.; Laporte, J. The myotubularin-amphiphysin 2 complex in membrane tubulation and centronuclear myopathies. EMBO Rep. 2013, 14, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.B.; Pierson, C.R.; Joshi, M.; Liu, X.; Ravenscroft, G.; Moghadaszadeh, B.; Talabere, T.; Viola, M.; Swanson, L.C.; Haliloğlu, G.; et al. SPEG interacts with myotubularin, and its deficiency causes centronuclear myopathy with dilated cardiomyopathy. Am. J. Hum. Genet. 2014, 95, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Rosen, S.M.; Li, Q.; Agrawal, P.B. Striated Preferentially Expressed Protein Kinase (SPEG) in Muscle Development, Function, and Disease. Int. J. Mol. Sci. 2021, 22, 5732. [Google Scholar] [CrossRef] [PubMed]
- Garbino, A.; van Oort, R.J.; Dixit, S.S.; Landstrom, A.P.; Ackerman, M.J.; Wehrens, X.H.T. Molecular evolution of the junctophilin gene family. Physiol. Genomics 2009, 37, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, H.; Komazaki, S.; Nishi, M.; Iino, M.; Kangawa, K. Junctophilins: A novel family of junctional membrane complex proteins. Mol. Cell 2000, 6, 11–22. [Google Scholar] [CrossRef]
- Nishi, H.; Sakagami, S.; Komazaki, H.; Kondo, H.; Takeshima, H. Coexpression of junctophilin type 3 and type 4 in brain. Mol. Brain Res. 2003, 118, 102–110. [Google Scholar] [CrossRef]
- Landstrom, A.P.; Beavers, D.L.; Wehrens, X.H.T. The junctophilin family of proteins: From bench to bedside. Trends Mol. Med. 2014, 20, 353–362. [Google Scholar] [CrossRef]
- Takeshima, H.; Hoshijima, M.; Song, L.S. Ca²⁺ microdomains organized by junctophilins. Cell Calcium 2015, 58, 349–356. [Google Scholar] [CrossRef]
- Rossi, D.; Scarcella, A.M.; Liguori, E.; Lorenzini, S.; Pierantozzi, E.; Kutchukian, C.; Jacquemond, V.; Messa, M.; De Camilli, P.; Sorrentino, V. Molecular determinants of homo- and heteromeric interactions of Junctophilin-1 at triads in adult skeletal muscle fibers. Proc. Natl. Acad. Sci. USA 2019, 116, 15716–15724. [Google Scholar] [CrossRef]
- Perni, S.; Lavorato, M.; Beam, K.G. De novo reconstitution reveals the proteins required for skeletal muscle voltage-induced Ca2+ release. Proc. Natl. Acad. Sci. USA 2017, 114, 13822–13827. [Google Scholar] [CrossRef] [PubMed]
- Perni, S.; Beam, K.G. Voltage-induced Ca2+ release is supported by junctophilins 1, 2 and 3, and not by junctophilin 4. J. Gen. Physiol. 2022, 154, e2021ecc22. [Google Scholar] [CrossRef]
- Hirata, Y.; Brotto, M.; Weisleder, N.; Chu, Y.; Lin, P.; Zhao, X.; Thornton, A.; Komazaki, S.; Takeshima, H.; Ma, J.; et al. Uncoupling store-operated Ca2+ entry and altered Ca2+ release from sarcoplasmic reticulum through silencing of junctophilin genes. Biophys. J. 2006, 90, 4418–4427. [Google Scholar] [CrossRef] [PubMed]
- Golini, L.; Chouabe, C.; Berthier, C.; Cusimano, V.; Fornaro, M.; Bonvallet, R.; Formoso, L.; Giacomello, E.; Jacquemond, V.; Sorrentino, V. Junctophilin 1 and 2 proteins interact with the L-type Ca2+ channel dihydropyridine receptors (DHPRs) in skeletal muscle. J. Biol. Chem. 2011, 286, 43717–43725. [Google Scholar] [CrossRef]
- van Oort, R.J.; Garbino, A.; Wang, W.; Dixit, S.S.; Landstrom, A.P.; Gaur, N.; De Almeida, A.C.; Skapura, D.G.; Rudy, Y.; Burns, A.R.; et al. Disrupted junctional membrane complexes and hyperactive ryanodine receptors after acute junctophilin knockdown in mice. Circulation 2011, 123, 979–988. [Google Scholar] [CrossRef]
- Phimister, A.J.; Lango, J.; Lee, E.H.; Ernst-Russell, M.A.; Takeshima, H.; Ma, J.; Allen, P.D.; Pessah, I.N. Conformation-dependent stability of junctophilin 1 (JP1) and ryanodine receptor type 1 (RyR1) channel complex is mediated by their hyper-reactive thiols. J. Biol. Chem. 2007, 282, 8667–8677. [Google Scholar] [CrossRef]
- Nakada, T.; Kashihara, T.; Komatsu, M.; Kojima, K.; Takeshita, T.; Yamada, M. Physical interaction of junctophilin and the CaV1.1 C terminus is crucial for skeletal muscle contraction. Proc. Natl. Acad. Sci. USA 2018, 115, 4507–4512. [Google Scholar] [CrossRef]
- Gross, P.; Johnson, J.; Romero, C.M.; Eaton, D.M.; Poulet, C.; Sanchez-Alonso, J.; Lucarelli, C.; Ross, J.; Gibb, A.A.; Garbincius, J.F.; et al. Interaction of the Joining Region in Junctophilin-2 With the L-Type Ca2+ Channel Is Pivotal for Cardiac Dyad Assembly and Intracellular Ca2+ Dynamics. Circ. Res. 2021, 128, 92–114. [Google Scholar] [CrossRef]
- Poulet, C.; Sanchez-Alonso, J.; Swiatlowska, P.; Mouy, F.; Lucarelli, C.; Alvarez-Laviada, A.; Gross, P.; Terracciano, C.; Houser, S.; Gorelik, J. Junctophilin-2 tethers T-tubules and recruits functional L-type calcium channels to lipid rafts in adult cardiomyocytes. Cardiovasc. Res. 2021, 117, 149–161. [Google Scholar] [CrossRef]
- Woo, J.S.; Hwang, J.H.; Ko, J.K.; Kim, D.H.; Ma, J.; Lee, E.H. Glutamate at position 227 of junctophilin-2 is involved in binding to TRPC3. Mol. Cell Biochem. 2009, 328, 25–32. [Google Scholar] [CrossRef]
- Li, H.; Ding, X.; Lopez, J.R.; Takeshima, H.; Ma, J.; Allen, P.D.; Eltit, J.M. Impaired Orai1-mediated resting Ca2+ entry reduces the cytosolic [Ca2+] and sarcoplasmic reticulum Ca2+ loading in quiescent junctophilin 1 knock-out myotubes. J. Biol. Chem. 2010, 285, 39171–39179. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Komazaki, S.; Sasamoto, K.; Yoshida, M.; Nishi, M.; Kitamura, K.; Takeshima, H. Deficiency of triad junction and contraction in mutant skeletal muscle lacking junctophilin type 1. J. Cell Biol. 2001, 154, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Komazaki, S.; Ito, K.; Takeshima, H.; Nakamura, H. Deficiency of triad formation in developing skeletal muscle cells lacking junctophilin type 1. FEBS Lett. 2002, 524, 225–229. [Google Scholar] [CrossRef]
- Beavers, D.L.; Landstrom, A.P.; Chiang, D.Y.; Wehrens, X.H.T. Emerging roles of junctophilin-2 in the heart and implications for cardiac diseases. Cardiovasc. Res. 2014, 103, 198–205. [Google Scholar] [CrossRef]
- Bennett, H.J.; Davenport, J.B.; Collins, R.F.; Trafford, A.W.; Pinali, C.; Kitmitto, A. Human junctophilin-2 undergoes a structural rearrangement upon binding PtdIns(3,4,5)P3 and the S101R mutation identified in hypertrophic cardiomyopathy obviates this response. Biochem. J. 2013, 456, 205–217. [Google Scholar] [CrossRef]
- Quick, A.P.; Landstrom, A.P.; Wang, Q.; Beavers, D.L.; Reynolds, J.O.; Barreto-Torres, G.; Tran, V.; Showell, J.; Philippen, L.E.; Morris, S.A.; et al. Novel junctophilin-2 mutation A405S is associated with basal septal hypertrophy and diastolic dysfunction. JACC Basic Transl. Sci. 2017, 2, 56–67. [Google Scholar] [CrossRef]
- Vanninen, S.U.M.; Leivo, K.; Seppälä, E.H.; Aalto-Setälä, K.; Pitkänen, O.; Suursalmi, P.; Annala, A.P.; Anttila, I.; Alastalo, T.P.; Myllykangas, S.; et al. Heterozygous junctophilin-2 (JPH2) p.(Thr161Lys) is a monogenic cause for HCM with heart failure. PLoS ONE 2018, 13, e0203422. [Google Scholar] [CrossRef]
- Lyu, Y.; Verma, V.K.; Lee, Y.; Taleb, I.; Badolia, R.; Shankar, T.S.; Kyriakopoulos, C.P.; Selzman, C.H.; Caine, W.; Alharethi, R.; et al. Remodeling of t-system and proteins underlying excitation-contraction coupling in aging versus failing human heart. NPJ Aging Mech. Dis. 2021, 7, 16. [Google Scholar] [CrossRef]
- Minamisawa, S.; Oshikawa, J.; Takeshima, H.; Hoshijima, M.; Wang, Y.; Chien, K.R.; Ishikawa, Y.; Matsuoka, R. Junctophilin type 2 is associated with caveolin-3 and is down-regulated in the hypertrophic and dilated cardiomyopathies. Biochem. Biophys. Res. Commun. 2004, 325, 852–856. [Google Scholar] [CrossRef]
- Murphy, R.M.; Dutka, T.L.; Horvath, D.; Bell, J.R.; Delbridge, L.M.; Lamb, G.D. Ca2+-dependent proteolysis of junctophilin-1 and junctophilin-2 in skeletal and cardiac muscle. J. Physiol. 2013, 591, 719–729. [Google Scholar] [CrossRef]
- Guo, A.; Wang, Y.; Chen, B.; Wang, Y.; Yuan, J.; Zhang, L.; Hall, D.; Wu, J.; Shi, Y.; Zhu, Q.; et al. E-C coupling structural protein junctophilin-2 encodes a stress-adaptive transcription regulator. Science 2018, 362, eaan3303. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Hall, D.; Zhang, C.; Peng, T.; Miller, J.D.; Kutschke, W.; Grueter, C.E.; Johnson, F.L.; Lin, R.Z.; Song, L.S. Molecular Determinants of Calpain-dependent Cleavage of Junctophilin-2 Protein in Cardiomyocytes. J. Biol. Chem. 2015, 290, 17946–17955. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, S.K.; Quick, A.P.; Samson-Couterie, B.; Hulsurkar, M.; Elzenaar, I.; van Oort, R.J.; Wehrens, X.H.T. Nuclear localization of a novel calpain-2 mediated junctophilin-2 C-terminal cleavage peptide promotes cardiomyocyte remodeling. Basic Res. Cardiol. 2020, 115, 49. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Fang, W.; Gibson, S. Sequence determinants of human junctophilin-2 protein nuclear localization and phase separation. Biochem. Biophys. Res. Commun. 2021, 563, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Barone, V.; Giacomello, E.; Cusimano, V.; Sorrentino, V. The sarcoplasmic reticulum: An organized patchwork of specialized domains. Traffic 2008, 9, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Simeoni, I.; Micheli, M.; Bootman, M.; Lipp, P.; Allen, P.D.; Sorrentino, V. RyR1 and RyR3 isoforms provide distinct intracellular Ca2+ signals in HEK 293 cells. J. Cell Sci. 2002, 115, 2497–2504. [Google Scholar] [CrossRef]
- Rossi, D.; Sorrentino, V. Molecular genetics of ryanodine receptors Ca2+-release channels. Cell Calcium 2002, 32, 307–319. [Google Scholar] [CrossRef]
- Van Petegem, F. Ryanodine Receptors: Structure and Function. J. Biol. Chem. 2012, 287, 31624–31632. [Google Scholar] [CrossRef]
- Des Georges, A.; Clarke, O.B.; Zalk, R.; Yuan, Q.; Condon, K.J.; Grassucci, R.A.; Frank, J. Structural basis for gating and activation of RyR1. Cell 2016, 167, 145–157. [Google Scholar] [CrossRef]
- Caprara, G.A.; Morabito, C.; Perni, S.; Navarra, R.; Guarnieri, S.; Mariggiò, M.A. Evidence for Altered Ca2+ Handling in Growth Associated Protein 43-Knockout Skeletal Muscle. Front. Physiol. 2016, 7, 493. [Google Scholar] [CrossRef]
- Gong, D.; Chi, X.; Wei, J.; Zhou, G.; Huang, G.; Zhang, L.; Wang, R.; Lei, J.; Chen, S.R.W.; Yan, N. Modulation of cardiac ryanodine receptor 2 by calmodulin. Nature 2019, 572, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Prosser, B.L.; Hernández-Ochoa, E.O.; and Schneider, M.F. S100A1 and calmodulin regulation of ryanodine receptor in striated muscle. Cell Calcium 2011, 50, 323–331. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brillantes, A.B.; Ondrias, K.; Scott, A.; Kobrinsky, E.; Ondriašová, E.; Moschella, M.C.; Jayaraman, T.; Landers, M.; Ehrlich, B.E.; Marks, A.R. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell 1994, 77, 513–523. [Google Scholar] [CrossRef]
- Bultynck, G.; De Smet, P.; Rossi, D.; Callewaert, G.; Missiaen, L.; Sorrentino, V.; De Smedt, H.; Parys, J.B. Characterization and mapping of the 12 kDa FK506-binding protein (FKBP12)-binding site on different isoforms of the ryanodine receptor and of the inositol 1,4,5-trisphosphate receptor. Biochem. J. 2001, 354, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Pessah, I.N.; Cherednichenko, G.; Lein, P.J. Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol. Ther. 2010, 125, 260–285. [Google Scholar] [CrossRef] [PubMed]
- Gurrola, G.B.; Capes, E.M.; Zamudio, F.Z.; Possani, L.D.; Valdivia, H.H. Imperatoxin A, a Cell-Penetrating Peptide from Scorpion Venom, as a Probe of Ca-Release Channels/Ryanodine Receptors. Pharmaceuticals 2010, 3, 1093–1107. [Google Scholar] [CrossRef]
- Santulli, G.; Lewis, D.; des Georges, A.; Marks, A.R.; Frank, J. Ryanodine Receptor Structure and Function in Health and Disease. Subcell Biochem. 2018, 87, 329–352. [Google Scholar] [CrossRef]
- Hopkins, P.M. Malignant hyperthermia: Pharmacology of triggering. Br. J. Anaesth. 2011, 107, 48–56. [Google Scholar] [CrossRef]
- Hu, H.; Wang, Z.; Wei, R.; Fan, G.; Wang, Q.; Zhang, K.; Yin, C.C. The molecular architecture of dihydropyrindine receptor/L-type Ca2+ channel complex. Sci. Rep. 2015, 5, 8370. [Google Scholar] [CrossRef]
- Flucher, B.E. Skeletal muscle CaV1.1 channelopathies. Pflügers Arch.-Eur. J. Physiol. 2020, 472, 739–754. [Google Scholar] [CrossRef]
- Van Petegem, F.; Clark, K.A.; Chatelain, F.C.; Minor, D.L. Structure of a complex between a voltage-gated calcium channel b-subunit and an a-subunit domain. Nature 2004, 429, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Buraei, Z.; Yang, J. The β Subunit of Voltage-Gated Ca2+ Channels. Physiol. Rev. 2010, 90, 1461–1506. [Google Scholar] [CrossRef] [PubMed]
- Gregg, R.G.; Messing, A.; Strube, C.; Beurg, M.; Moss, R.; Behan, M.; Sukhareva, M.; Haynes, S.; Powell, J.A.; Coronado, R.; et al. Absence of the beta subunit (cchb1) of the skeletal muscle dihydropyridine receptor alters expression of the alpha 1 subunit and eliminates excitation-contraction coupling. Proc. Natl. Acad. Sci. USA 1996, 93, 13961–13966. [Google Scholar] [CrossRef]
- Tanabe, T.; Beam, K.G.; Powell, J.A.; Numa, S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature 1988, 336, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Bannister, R.A. Bridging the myoplasmic gap II: More recent advances in skeletal muscle excitation-contraction coupling. J. Exp. Biol. 2016, 219, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Nakai, J.; Dirksen, R.T.; Nguyen, H.T.; Pessah, I.N.; Beam, K.G.; Allen, P.D. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature 1996, 380, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Horstick, E.J.; Linsley, J.W.; Dowling, J.J.; Hauser, M.A.; McDonald, K.K.; Ashley-Koch, A.; Saint-Amant, L.; Satish, A.; Cui, W.W.; Zhou, W.; et al. Stac3 is a component of the excitation–contraction coupling machinery and mutated in Native American myopathy. Nat. Commun. 2013, 4, 1952. [Google Scholar] [CrossRef]
- Polster, A.; Nelson, B.R.; Olson, E.N.; Beam, K.G. Stac3 has a direct role in skeletal muscle-type excitation-contraction coupling that is disrupted by a myopathy-causing mutation. Proc. Natl. Acad. Sci. USA 2016, 113, 10986–10991. [Google Scholar] [CrossRef]
- Flucher, B.E.; Campiglio, M. STAC proteins: The missing link in skeletal muscle EC coupling and new regulators of calcium channel function. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1101–1110. [Google Scholar] [CrossRef]
- Rufenach, B.; Van Petegem, F. Structure and function of STAC proteins: Calcium channel modulators and critical components of muscle excitation-contraction coupling. J. Biol. Chem. 2021, 297, 100874. [Google Scholar] [CrossRef]
- Nelson, B.R.; Wu, F.; Liu, Y.; Anderson, D.M.; McAnally, J.; Lin, W.; Cannon, S.C.; Bassel-Duby, R.; Olson, E.N. Skeletal muscle-specific T-tubule protein STAC3 mediates voltage-induced Ca2+ release and contractility. Proc. Natl. Acad. Sci. USA 2013, 110, 11881–11886. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, M.; Nishino, I. A review of core myopathy: Central core disease, multiminicore disease, dusty core disease, and core-rod myopathy. Neuromuscul. Disord. 2021, 31, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Abath Neto, O.; Moreno, C.A.M.; Malfatti, E.; Donkervoort, S.; Böhm, J.; Guimarães, J.B.; Foley, A.R.; Mohassel, P.; Dastgir, J.; Bharucha-Goebel, D.X.; et al. Common and variable clinical, histological, and imaging findings of recessive RYR1-related centronuclear myopathy patients. Neuromuscul. Disord. 2017, 27, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Lawal, T.A.; Todd, J.J.; Witherspoon, J.W.; Bönnemann, C.G.; Dowling, J.J.; Hamilton, S.L.; Meilleur, K.G.; Dirksen, R.T. Ryanodine receptor 1-related disorders: An historical perspective and proposal for a unified nomenclature. Skelet. Muscle 2020, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- North, K.N.; Wang, C.H.; Clarke, N.; Jungbluth, H.; Vainzof, M.; Dowling, J.J.; Amburgey, K.; Quijano-Roy, S.; Beggs, A.H.; Sewry, C.; et al. International Standard of Care Committee for Congenital Myopathies. Approach to the diagnosis of congenital myopathies. Neuromuscul. Disord. 2014, 24, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.M.; Jones, L.R. Identification of triadin 1 as the predominant triadin isoform expressed in mammalian myocardium. J. Biol. Chem. 1999, 274, 28660–28668. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vassilopoulos, S.; Thevenon, D.; Rezgui, S.S.; Brocard, J.; Chapel, A.; Lacampagne, A.; Lunardi, J.; Dewaard, M.; Marty, I. Triadins are not triad-specific proteins: Two new skeletal muscle triadins possibly involved in the architecture of sarcoplasmic reticulum. J. Biol. Chem. 2005, 280, 28601–28609. [Google Scholar] [CrossRef]
- Marty, I. Triadin regulation of the ryanodine receptor complex. J. Physiol. 2015, 593, 3261–3266. [Google Scholar] [CrossRef]
- Fourest-Lieuvin, A.; Rendu, J.; Osseni, A.; Pernet-Gallay, K.; Rossi, D.; Oddoux, S.; Brocard, J.; Sorrentino, V.; Marty, I.; Fauré, J. Role of triadin in the organization of reticulum membrane at the muscle triad. J. Cell Sci. 2012, 125, 3443–3453. [Google Scholar] [CrossRef]
- Rossi, D.; Bencini, C.; Maritati, M.; Benini, F.; Lorenzini, S.; Pierantozzi, E.; Scarcella, A.M.; Paolini, C.; Protasi, F.; Sorrentino, V. Distinct regions of triadin are required for targeting and retention at the junctional domain of the sarcoplasmic reticulum. Biochem. J. 2014, 458, 407–417. [Google Scholar] [CrossRef]
- Caswell, H.; Motoike, H.K.; Fan, H.; Brandt, N.R. Location of ryanodine receptor binding site on skeletal muscle triadin. Biochemistry 1999, 38, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Groh, S.; Marty, I.; Ottolia, M.; Prestipino, G.; Chapel, A.; Villaz, M.; Ronjat, M. Functional interaction of the cytoplasmic domain of triadin with the skeletal ryanodine receptor. J. Biol. Chem. 1999, 274, 12278–12283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kelley, J.; Schmeisser, G.; Kobayashi, Y.M.; Jones, L.R. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J. Biol. Chem. 1997, 272, 23389–23397. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Lorenzini, S.; Pierantozzi, E.; Van Petegem, F.; Amadsun, D.O.; Sorrentino, V. Multiple regions within junctin drive its interaction with calsequestrin-1 and its localization to triads in skeletal muscle. J. Cell Sci. 2022, 135, jcs259185. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Campbell, K.P. Association of triadin with the ryanodine receptor and calsequestrin in the lumen of the sarcoplasmic reticulum. J. Biol. Chem. 1995, 270, 9027–9030. [Google Scholar] [CrossRef]
- Kobayashi, Y.M.; Alseikhan, B.A.; Jones, L.R. Localization and characterization of the calsequestrin-binding domain of triadin 1. Evidence for a charged beta-strand in mediating the protein-protein interaction. J. Biol. Chem. 2000, 275, 17639–17646. [Google Scholar] [CrossRef]
- Shin, D.W.; Ma, J.; Kim, D.H. The asp-rich region at the carboxyl-terminus of calsequestrin binds to Ca2+ and interacts with triadin. FEBS Lett. 2000, 486, 178–182. [Google Scholar] [CrossRef]
- Lee, H.G.; Kang, H.; Kim, D.H.; Park, W.J. Interaction of HRC (histidine-rich Ca2+-binding protein) and triadin in the lumen of sarcoplasmic reticulum. J. Biol. Chem. 2001, 276, 39533–39538. [Google Scholar] [CrossRef]
- Osseni, A.; Sébastien, M.; Sarrault, O.; Baudet, M.; Couté, Y.; Fauré, J.; Fourest-Lieuvin, A.; Marty, I. Triadin and CLIMP-63 form a link between triads and microtubules in muscle cells. J. Cell Sci. 2016, 129, 3744–3755. [Google Scholar] [CrossRef]
- Shen, X.; Franzini-Armstrong, C.; Lopez, J.R.; Jones, L.R.; Kobayashi, Y.M.; Wang, Y.; Kerrick, W.G.L.; Caswell, A.H.; Potter, J.D.; Miller, T.; et al. Triadins modulate intracellular Ca2+ homeostasis but are not essential for excitation-contraction coupling in skeletal muscle. J. Biol. Chem. 2007, 282, 37864–37874. [Google Scholar] [CrossRef]
- Oddoux, S.; Brocard, J.; Schweitzer, A.; Szentesi, P.; Giannesini, B.; Brocard, J.; Fauré, J.; Pernet-Gallay, K.; Bendahan, D.; Lunardi, J.; et al. Triadin deletion induces impaired skeletal muscle function. J. Biol. Chem. 2009, 284, 34918–34929. [Google Scholar] [CrossRef] [PubMed]
- Dulhunty, A.; Wei, L.; Beard, N. Junctin—The quiet achiever. J. Physiol. 2009, 587, 3135–3137. [Google Scholar] [CrossRef] [PubMed]
- Dulhunty, A.F.; Wei-LaPierre, L.; Casarotto, M.G.; Beard, N.A. Core skeletal muscle ryanodine receptor calcium release complex. Clin. Exp. Pharmacol. Physiol. 2017, 44, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mirza, S.; Richardson, S.J.; Gallant, E.M.; Thekkedam, C.; Pace, S.M.; Zorzato, F.; Liu, D.; Beard, N.A.; Dulhunty, A.F. A new cytoplasmic interaction between junctin and ryanodine receptor Ca2+ release channels. J. Cell Sci. 2015, 128, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Fan, G.C.; Dong, M.; Altschafl, B.; Diwan, A.; Ren, X.; Hahn, H.H.; Zhao, W.; Waggoner, J.R.; Jones, L.R.; et al. Sarcoplasmic reticulum calcium overloading in junctin deficiency enhances cardiac contractility but increases ventricular automaticity. Circulation 2007, 115, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Boncompagni, S.; Thomas, M.; Lopez, J.R.; Allen, P.D.; Yuan, Q.; Kranias, E.G.; Franzini-Armstrong, C.; Perez, C.F. Triadin/junctin double null mouse reveals a differential role for triadin and junctin in anchoring CASQ to the jSR and regulating Ca2+ homeostasis. PLoS ONE 2012, 7, e3962. [Google Scholar] [CrossRef]
- Fan, G.C.; Yuan, Q.; Kranias, E.G. Regulatory roles of junctin in sarcoplasmic reticulum calcium cycling and myocardial function. Trends Cardiovasc. Med. 2008, 18, 1–5. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Duan, H.; Fulton, T.R.; Eu, J.P.; Meissner, G. Altered stored calcium release in skeletal myotubes deficient of triadin and junctin. Cell Calcium 2009, 45, 29–37. [Google Scholar] [CrossRef]
- Biral, D.; Volpe, P.; Damiani, E.; Margreth, A. Coexistence of two calsequestrin isoforms in rabbit slow-twitch skeletal muscle fibers. FEBS Lett. 1992, 299, 175–178. [Google Scholar] [CrossRef]
- Beard, N.A.; Laver, D.R.; Dulhunty, A.F. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog. Biophys. Mol. Biol. 2004, 85, 33–69. [Google Scholar] [CrossRef]
- MacLennan, D.H.; Wong, P.T. Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc. Natl. Acad. Sci. USA 1971, 68, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.M.; Larkins, N.T.; Mollica, J.P.; Beard, N.A.; Lamb, G.D. Calsequestrin content and SERCA determine normal and maximal Ca2+ storage levels in sarcoplasmic reticulum of fast- and slow-twitch fibres of rat. J. Physiol. 2009, 587, 443–460. [Google Scholar] [CrossRef] [PubMed]
- Maguire, P.B.; Briggs, F.N.; Lennon, N.J.; Ohlendieck, K. Oligomerization is an intrinsic property of calsequestrin in normal and transformed skeletal muscle. Biochem. Biophys. Res. Commun. 1997, 240, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Youn, B.; Kemper, L.; Campbell, C.; Milting, H.; Varsanyi, M.; Kang, C. Characterization of human cardiac calsequestrin and its deleterious mutants. J. Mol. Biol. 2007, 373, 1047–1057. [Google Scholar] [CrossRef]
- Wang, S.; Trumble, W.R.; Liao, H.; Wesson, C.R.; Dunker, A.K.; Kang, C.H. Crystal structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum. Nat. Struct. Biol. 1998, 5, 476–483. [Google Scholar] [CrossRef]
- Park, H.; Wu, S.; Dunker, A.K.; Kang, C. Polymerization of calsequestrin. Implications for Ca2+ regulation. J. Biol. Chem. 2003, 278, 16176–16182. [Google Scholar] [CrossRef]
- Cala, S.E.; Jones, L.R. Phosphorylation of cardiac and skeletal muscle calsequestrin isoforms by casein kinase II. Demonstration of a cluster of unique rapidly phosphorylated sites in cardiac calsequestrin. J. Biol. Chem. 1991, 266, 391–398. [Google Scholar] [CrossRef]
- Beard, N.A.; Wei, L.; Cheung, S.N.; Kimura, T.; Varsányi, M.; Dulhunty, A.F. Phosphorylation of skeletal muscle calsequestrin enhances its Ca2+ binding capacity and promotes its association with junctin. Cell Calcium 2008, 44, 363–373. [Google Scholar] [CrossRef]
- Kumar, A.; Chakravarty, H.; Bal, N.C.; Balaraju, T.; Jena, N.; Misra, G.; Bal, C.; Pieroni, E.; Periasamy, M.; Sharon, A. Identification of calcium binding sites on calsequestrin 1 and their implications for polymerization. Mol. Biosyst. 2013, 9, 1949–1957. [Google Scholar] [CrossRef]
- Mitchell, R.D.; Simmerman, H.K.; Jones, L.R. Ca2+ binding effects on protein conformation and protein interactions of canine cardiac calsequestrin. J. Biol. Chem. 1988, 263, 1376–1381. [Google Scholar] [CrossRef]
- Manno, C.; Figueroa, L.C.; Gillespie, D.; Fitts, R.; Kang, C.; Franzini-Armstrong, C.; Rios, E. Calsequestrin depolymerizes when calcium is depleted in the sarcoplasmic reticulum of working muscle. Proc. Natl. Acad. Sci. USA 2017, 114, E638–E647. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.R.; Zhang, L.; Sanborn, K.; Jorgensen, A.O.; Kelley, J. Purification, primary structure, and immunological characterization of the 26-kDa calsequestrin binding protein (junctin) from cardiac junctional sarcoplasmic reticulum. J. Biol. Chem. 1995, 270, 30787–30796. [Google Scholar] [CrossRef] [PubMed]
- Herzog, A.; Szegedi, C.; Jona, I.; Herberg, F.W.; Varsanyi, M. Surface plasmon resonance studies prove the interaction of skeletal muscle sarcoplasmic reticular Ca2+ release channel/ryanodine receptor with calsequestrin. FEBS Lett. 2000, 472, 73–77. [Google Scholar] [CrossRef]
- Wei, L.; Varsányi, M.; Dulhunty, A.F.; Beard, N.A. The conformation of calsequestrin determines its ability to regulate skeletal ryanodine receptors. Biophys. J. 2006, 91, 1288–1301. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kasai, M. Regulation of calcium channel in sarcoplasmic reticulum by calsequestrin. Biochem. Biophys. Res. Commun. 1994, 199, 1120–1127. [Google Scholar] [CrossRef]
- Beard, N.A.; Sakowska, M.M.; Dulhunty, A.F.; Laver, D.R. Calsequestrin is an inhibitor of skeletal muscle ryanodine receptor calcium release channels. Biophys. J. 2002, 82, 310–320. [Google Scholar] [CrossRef]
- Beard, N.A.; Casarotto, M.G.; Wei, L.; Varsányi, M.; Laver, D.R.; Dulhunty, A.F. Regulation of ryanodine receptors by calsequestrin: Effect of high luminal Ca2+ and phosphorylation. Biophys. J. 2005, 88, 3444–3454. [Google Scholar] [CrossRef]
- Qin, J.; Valle, G.; Nani, A.; Chen, H.; Ramos-Franco, J.; Nori, A.; Volpe, P.; Fill, M. Ryanodine receptor luminal Ca2+ regulation: Swapping calsequestrin and channel isoforms. Biophys. J. 2009, 97, 1961–1970. [Google Scholar] [CrossRef]
- Beard, N.A.; Dulhunty, A.F. C-terminal residues of skeletal muscle calsequestrin are essential for calcium binding and for skeletal ryanodine receptor inhibition. Skelet. Muscle 2015, 22, 5–6. [Google Scholar] [CrossRef]
- Chen, H.; Valle, G.; Furlan, S.; Nani, A.; Gyorke, S.; Fill, M.; Volpe, P. Mechanism of calsequestrin regulation of single cardiac ryanodine receptor in normal and pathological conditions. J. Gen. Physiol. 2013, 142, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Canato, M.; Scorzeto, M.; Giacomello, M.; Protasi, F.; Reggiani, C.; Stienen, G.J.M. Massive alterations of sarcoplasmic reticulum free calcium in skeletal muscle fibers lacking calsequestrin revealed by a genetically encoded probe. Proc. Natl. Acad. Sci. USA 2010, 107, 22326–22331. [Google Scholar] [CrossRef] [PubMed]
- Protasi, F.; Paolini, C.; Canato, M.; Reggiani, C.; Quarta, M. Lessons from calsequestrin-1 ablation In Vivo: Much more than a Ca2+ buffer after all. J. Muscle Res. Cell Motil. 2011, 32, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W.; Pan, Z.; Kim, E.K.; Lee, J.M.; Bhat, M.B.; Parness, J.; Kim, D.H.; Ma, J. A retrograde signal from calsequestrin for the regulation of store-operated Ca2+ entry in skeletal muscle. J. Biol. Chem. 2003, 278, 3286–3292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, L.; Li, S.; Xue, J.; Luo, D. Calsequestrin-1 Regulates Store-Operated Ca2+ Entry by Inhibiting STIM1 Aggregation. Cell Physiol. Biochem. 2016, 38, 2183–2193. [Google Scholar] [CrossRef]
- Zhao, X.; Min, C.K.; Ko, J.; Parness, J.; Kim, D.H.; Weisleder, N.; Ma, J. Increased store-operated Ca2+ entry in skeletal muscle with reduced calsequestrin-1 expression. Biophys. J. 2010, 99, 1556–1564. [Google Scholar] [CrossRef]
- Michelucci, A.; Boncompagni, S.; Pietrangelo, L.; Takano, T.; Protasi, F.; Dirksen, R.T. Pre-assembled Ca2+ entry units and constitutively active Ca2+ entry in skeletal muscle of calsequestrin-1 knockout mice. J. Gen. Physiol. 2020, 152, e202012617. [Google Scholar] [CrossRef]
- Rossi, D.; Vezzani, B.; Galli, L.; Paolini, C.; Toniolo, L.; Pierantozzi, E.; Spinozzi, S.; Barone, V.; Pegoraro, E.; Bello, L.; et al. A mutation in the CASQ1 gene causes a vacuolar myopathy with accumulation of sarcoplasmic reticulum protein aggregates. Hum. Mutat. 2014, 35, 1163–1170. [Google Scholar] [CrossRef]
- D’Adamo, M.C.; Sforna, L.; Visentin, S.; Grottesi, A.; Servettini, L.; Guglielmi, L.; Macchioni, L.; Saredi, S.; Curcio, M.; De Nuccio, C.; et al. A Calsequestrin-1 Mutation Associated with a Skeletal Muscle Disease Alters Sarcoplasmic Ca2+ Release. PLoS ONE 2016, 11, e0155516. [Google Scholar] [CrossRef]
- Barone, V.; Del Re, V.; Gamberucci, A.; Polverino, V.; Galli, L.; Rossi, D.; Costanzi, E.; Toniolo, L.; Berti, G.; Malandrini, A.; et al. Identification and characterization of three novel mutations in the CASQ1 gene in four patients with tubular aggregate myopathy. Hum. Mutat. 2017, 38, 1761–1773. [Google Scholar] [CrossRef]
- Böhm, J.; Lornage, X.; Chevessier, F.; Birck, C.; Zanotti, S.; Cudia, P.; Bulla, M.; Granger, F.; Bui, M.T.; Sartori, M.; et al. CASQ1 mutations impair calsequestrin polymerization and cause tubular aggregate myopathy. Acta Neuropathol. 2018, 135, 149–151. [Google Scholar] [CrossRef]
- Arvanitis, D.A.; Vafiadaki, E.; Sanoudou, D.; Kranias, E.G. Histidine-rich calcium binding protein: The new regulator of sarcoplasmic reticulum calcium cycling. J. Mol. Cell Cardiol. 2011, 50, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.L.; Goldstein, J.L.; Orth, K.; Moomaw, C.R.; Slaughter, C.A.; Brown, M.S. Molecular cloning of a histidine-rich Ca2+-binding protein of sarcoplasmic reticulum that contains highly conserved repeated elements. J. Biol. Chem. 1989, 264, 18083–18090. [Google Scholar] [CrossRef]
- Sacchetto, R.; Damiani, E.; Turcato, F.; Nori, A.; Margreth, A. Ca2+-dependent interaction of triadin with histidine-rich Ca2+-binding protein carboxyl-terminal region. Biochem. Biophys. Res. Commun. 2001, 289, 1125–1134. [Google Scholar] [CrossRef]
- Suk, J.Y.; Kim, Y.S.; Park, W.J. HRC (histidine-rich Ca2+ binding protein) resides in the lumen of sarcoplasmic reticulum as a multimer. Biochem. Biophys. Res. Commun. 1999, 263, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Orr, I.; Shoshan-Barmatz, V. Modulation of the skeletal muscle ryanodine receptor by endogenous phosphorylation of 160/150-kDa proteins of the sarcoplasmic reticulum. Biochim. Biophys. Acta-Biomembr. 1996, 1283, 80–88. [Google Scholar] [CrossRef]
- Park, C.S.; Chen, S.; Lee, H.; Cha, H.; Oh, J.G.; Hong, S.; Han, P.; Ginsburg, K.S.; Jin, S.; Park, I.; et al. Targeted ablation of the histidine-rich Ca2+-binding protein (HRC) gene is associated with abnormal SR Ca2+-cycling and severe pathology under pressure-overload stress. Basic Res. Cardiol. 2013, 108, 344. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salanova, M.; Priori, G.; Barone, V.; Intravaia, E.; Flucher, B.; Ciruela, F.; McIlhinney, R.A.J.; Parys, J.B.; Mikoshiba, K.; Sorrentino, V. Homer proteins and InsP(3) receptors co-localise in the longitudinal sarcoplasmic reticulum of skeletal muscle fibres. Cell Calcium 2002, 32, 193–200. [Google Scholar] [CrossRef] [PubMed]
- López-Marqués, R.L.; Gourdon, P.; Günther Pomorski, T.; Palmgren, M. The transport mechanism of P4 ATPase lipid flippases. Biochem. J. 2020, 477, 3769–3790. [Google Scholar] [CrossRef]
- Thever, M.D.; Saier, M.H., Jr. Bioinformatic characterization of p-type ATPases encoded within the fully sequenced genomes of 26 eukaryotes. J. Membr. Biol. 2009, 229, 115–130. [Google Scholar] [CrossRef][Green Version]
- Periasamy, M.; Kalyanasundaram, A. SERCA pump isoforms: Their role in calcium transport and disease. Muscle Nerve. 2007, 35, 430–442. [Google Scholar] [CrossRef]
- Brini, M.; Calì, T.; Ottolini, D.; Carafoli, E. Calcium pumps: Why so many? Compr. Physiol. 2012, 2, 1045–1060. [Google Scholar] [CrossRef] [PubMed]
- Brandl, C.J.; de Leon, S.; Martin, D.R.; MacLennan, D.H. Adult forms of the Ca2+-ATPase of sarcoplasmic reticulum. Expression in developing skeletal muscle. J. Biol. Chem. 1987, 262, 3768–3774. [Google Scholar] [CrossRef]
- Zádor, E.; Vangheluwe, P.; Wuytack, F. The expression of the neonatal sarcoplasmic reticulum Ca2+ pump (SERCA1b) hints to a role in muscle growth and development. Cell Calcium 2007, 41, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Kósa, M.; Brinyiczki, K.; van Damme, P.; Goemans, N.; Hancsák, K.; Mendler, L.; Zádor, E. The neonatal sarcoplasmic reticulum Ca2+-ATPase gives a clue to development and pathology in human muscles. J. Muscle Res. Cell Motil. 2015, 36, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Gélébart, P.; Martin, V.; Enouf, J.; Papp, B. Identification of a new SERCA2 splice variant regulated during monocytic differentiation. Biochem. Biophys. Res. Commun. 2003, 303, 676–684. [Google Scholar] [CrossRef]
- Vandecaetsbeek, I.; Vangheluwe, P.; Raeymaekers, L.; Wuytack, F.; Vanoevelen, J. The Ca2+ pumps of the endoplasmic reticulum and Golgi apparatus. Cold Spring Harb. Perspect. Biol. 2011, 1, a004184. [Google Scholar] [CrossRef]
- Wu, K.D.; Lee, W.S.; Wey, J.; Bungard, D.; Lytton, J. Localization and quantification of endoplasmic reticulum Ca2+-ATPase isoform transcripts. Am. J. Physiol. 1995, 269, C775–C784. [Google Scholar] [CrossRef]
- Dally, S.; Corvazier, E.; Bredoux, R.; Bobe, R.; Enouf, J. Multiple and diverse coexpression, location, and regulation of additional SERCA2 and SERCA3 isoforms in nonfailing and failing human heart. J. Mol. Cell Cardiol. 2010, 48, 633–644. [Google Scholar] [CrossRef]
- Wuytack, F.; Dode, L.; Baba-Aissa, F.; Raeymaekers, L. The SERCA3-type of organellar Ca2+ pumps. Biosci. Rep. 1995, 15, 299–306. [Google Scholar] [CrossRef]
- Sumbilla, C.; Cavagna, M.; Zhong, L.; Ma, H.; Lewis, D.; Farrance, I.; Inesi, G. Comparison of SERCA1 and SERCA2a expressed in COS-1 cells and cardiac myocytes. Am. J. Physiol. 1999, 277, H2381–H2391. [Google Scholar] [CrossRef]
- Lytton, J.; Westlin, M.; Burk, S.E.; Shull, G.E.; MacLennan, D.H. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J. Biol. Chem. 1992, 267, 14483–14489. [Google Scholar] [CrossRef]
- Kirchberber, M.A.; Tada, M.; Katz, A.M. Phospholamban: A regulatory protein of the cardiac sarcoplasmic reticulum. Recent Adv. Stud. Card. Struct. Metab. 1975, 5, 103–115. [Google Scholar]
- Kranias, E.G.; Hajjar, R.J. Modulation of cardiac contractility by the phospholamban/SERCA2a regulatome. Circ. Res. 2012, 110, 1646–1660. [Google Scholar] [CrossRef] [PubMed]
- Slack, J.P.; Grupp, I.L.; Luo, W.; Kranias, E.G. Phospholamban ablation enhances relaxation in the murine soleus. Am. J. Physiol. 1997, 273, C1–C6. [Google Scholar] [CrossRef] [PubMed]
- Gamu, D.; Juracic, E.S.; Fajardo, V.A.; Rietze, B.A.; Tran, K.; Bombardier, E.; Tupling, A.R. Phospholamban deficiency does not alter skeletal muscle SERCA pumping efficiency or predispose mice to diet-induced obesity. Am. J. Physiol.-Endocrinol. Metab. 2019, 316, E432–E442. [Google Scholar] [CrossRef]
- Periasamy, M.; Bhupathy, P.; Babu, G.J. Regulation of sarcoplasmic reticulum Ca2+-ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc. Res. 2008, 77, 265–273. [Google Scholar] [CrossRef]
- Bhupathy, P.; Babu, G.J.; Periasamy, M. Sarcolipin and phospholamban as regulators of cardiac sarcoplasmic reticulum Ca2+-ATPase. J. Mol. Cell Cardiol. 2007, 42, 903–911. [Google Scholar] [CrossRef]
- MacLennan, D.H.; Kranias, E.G. Phospholamban: A crucial regulator of cardiac contractility. Na.t Rev. Mol. Cell Biol. 2003, 4, 566–577. [Google Scholar] [CrossRef]
- Wittmann, T.; Lohse, M.J.; Schmitt, J.P. Phospholamban pentamers attenuate PKA-dependent phosphorylation of monomers. J. Mol. Cell Cardiol. 2015, 80, 90–97. [Google Scholar] [CrossRef]
- Shaikh, S.A.; Sahoo, S.K.; Periasamy, M. Phospholamban and sarcolipin: Are they functionally redundant or distinct regulators of the Sarco(Endo)Plasmic Reticulum Calcium ATPase? J. Mol. Cell Cardiol. 2016, 91, 81–91. [Google Scholar] [CrossRef]
- Bal, N.C.; Maurya, S.K.; Sopariwala, D.H.; Sahoo, S.K.; Gupta, S.C.; Shaikh, S.A.; Pant, M.; Rowland, L.A.; Bombardier, E.; Goonasekera, S.A.; et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat. Med. 2012, 18, 1575–1579. [Google Scholar] [CrossRef] [PubMed]
- Maurya, S.K.; Bal, N.C.; Sopariwala, D.H.; Pant, M.; Rowland, L.A.; Shaikh, S.A.; Periasamy, M. Sarcolipin Is a Key Determinant of the Basal Metabolic Rate, and Its Overexpression Enhances Energy Expenditure and Resistance against Diet-induced Obesity. J. Biol. Chem. 2015, 290, 10840–10849. [Google Scholar] [CrossRef] [PubMed]
- Leberer, E.; Timms, B.G.; Campbell, K.P.; MacLennan, D.H. Purification, calcium binding properties, and ultrastructural localization of the 53,000- and 160,000 (sarcalumenin)-dalton glycoproteins of the sarcoplasmic reticulum. J. Biol. Chem. 1990, 265, 10118–10124. [Google Scholar] [CrossRef]
- Yoshida, M.; Minamisawa, S.; Shimura, M.; Komazaki, S.; Kume, H.; Zhang, M.; Matsumura, K.; Nishi, M.; Saito, M.; Saeki, Y.; et al. Impaired Ca2+ store functions in skeletal and cardiac muscle cells from sarcalumenin-deficient mice. J. Biol. Chem. 2005, 280, 3500–3506. [Google Scholar] [CrossRef] [PubMed]
- Shimura, M.; Minamisawa, S.; Takeshima, H.; Jiao, Q.; Bai, Y.; Umemura, S.; Ishikawa, Y. Sarcalumenin alleviates stress-induced cardiac dysfunction by improving Ca2+ handling of the sarcoplasmic reticulum. Cardiovasc. Res. 2008, 77, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Q.; Bai, Y.; Akaike, T.; Takeshima, H.; Ishikawa, Y.; Minamisawa, S. Sarcalumenin is essential for maintaining cardiac function during endurance exercise training. Am. J. Physiol.-Heart Circ. Physiol. 2009, 297, H576–H582. [Google Scholar] [CrossRef]
- MacLennan, D.H. Ca2+ signalling and muscle disease. Eur. J. Biochem. 2000, 267, 5291–5297. [Google Scholar] [CrossRef]
- Molenaar, J.P.; Verhoeven, J.I.; Rodenburg, R.J.; Kamsteeg, E.J.; Erasmus, C.E.; Vicart, S.; Behin, A.; Bassez, G.; Magot, A.; Péréon, Y.; et al. Clinical, morphological and genetic characterization of Brody disease: An international study of 40 patients. Brain 2020, 143, 452–466. [Google Scholar] [CrossRef]
- Close, M.; Perni, S.; Franzini-Armstrong, C.; Cundall, D. Highly extensible skeletal muscle in snakes. J. Exp. Biol. 2014, 217, 2445–2448. [Google Scholar] [CrossRef]
- Tibbits, G.F.; Thomas, M.J. Ca2+ transport across the plasma membrane of striated muscle. Med. Sci. Sports Exerc. 1989, 1, 399–405. [Google Scholar] [CrossRef]
- Krebs, J. The plethora of PMCA isoforms: Alternative splicing and differential expression. Biochim. Biophys. Acta-Mol. Cell Res. 2015, 1853, 2018–2024. [Google Scholar] [CrossRef] [PubMed]
- Greeb, J.; Shull, G.E. Molecular cloning of a third isoform of the calmodulin-sensitive plasma membrane Ca2+-transporting ATPase that is expressed predominantly in brain and skeletal muscle. J. Biol. Chem. 1989, 264, 18569–18576. [Google Scholar] [CrossRef]
- Sacchetto, R.; Margreth, A.; Pelosi, M.; Carafoli, E. Colocalization of the dihydropyridine receptor, the plasma-membrane calcium ATPase isoform 1 and the sodium/calcium exchanger to the junctional-membrane domain of transverse tubules of rabbit skeletal muscle. Eur. J. Biochem. 1996, 237, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Kimura, J.; Noma, A.; Irisawa, H. Na-Ca exchange current in mammalian heart cells. Nature 1986, 319, 596–597. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.Y.M.; Verkaart, S.; Koopman, W.J.H.; Willems, P.H.G.M.; Hoenderop, J.G.J.; Bindels, R.J.M. Function and regulation of the Na+-Ca2+ exchanger NCX3 splice variants in brain and skeletal muscle. J. Biol. Chem. 2014, 289, 11293–11303. [Google Scholar] [CrossRef]
- Fraysse, B.; Rouaud, T.; Millour, M.; Fontaine-Pérus, J.; Gardahaut, M.F.; Levitsky, D.O. Expression of the Na+/Ca2+ exchanger in skeletal muscle. Am. J. Physiol.-Cell Physiol. 2001, 280, C146–C154. [Google Scholar] [CrossRef]
- Reeves, J.P.; Condrescu, M.; Chernaya, G.; Gardner, J.P. Na+/Ca2+ antiport in the mammalian heart. J. Exp. Biol. 1994, 196, 375–388. [Google Scholar] [CrossRef]
- Shigekawa, M.; Iwamoto, T. Cardiac Na+-Ca2+ exchange: Molecular and pharmacological aspects. Circ. Res. 2001, 88, 864–876. [Google Scholar] [CrossRef]
- Shen, Y.; Thillaiappan, N.B.; Taylor, C.W. The store-operated Ca2+ entry complex comprises a small cluster of STIM1 associated with one Orai1 channel. Proc. Natl. Acad. Sci. USA 2021, 118, e2010789118. [Google Scholar] [CrossRef]
- Kurebayashi, N.; Ogawa, Y. Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J. Physiol. 2001, 533, 185–199. [Google Scholar] [CrossRef]
- Boncompagni, S. Discovery of new intracellular junctions: The calcium entry units (CEUs). J. Gen. Physiol. 2022, 154, e2021ecc39. [Google Scholar] [CrossRef]
- Boncompagni, S.; Michelucci, A.; Pietrangelo, P.; Dirksen, R.T.; Protasi, F. Exercise-dependent formation of new junctions that promote STIM1-Orai1 assembly in skeletal muscle. Sci. Rep. 2017, 7, 14286. [Google Scholar] [CrossRef] [PubMed]
- Protasi, F.; Pietrangelo, L.; Boncompagni, S. Calcium entry units (CEUs): Perspectives in skeletal muscle function and disease. J. Muscle Res. Cell Motil. 2021, 42, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Chevessier, F.; Bauché-Godard, S.; Leroy, J.P.; Koenig, J.; Paturneau-Jouas, M.; Eymard, B.; Hantaï, D.; Verdière-Sahuqué, M. The origin of tubular aggregates in human myopathies. J. Pathol. 2015, 207, 313–323. [Google Scholar] [CrossRef]
- Silva-Rojas, R.; Laporte, J.; Böhm, J. STIM1/ORAI1 Loss-of-Function and Gain-of-Function Mutations Inversely Impact on SOCE and Calcium Homeostasis and Cause Multi-Systemic Mirror Diseases. Front. Physiol. 2020, 11, 604941. [Google Scholar] [CrossRef] [PubMed]
- Böhm, J.; Bulla, M.; Urquhart, J.E.; Malfatti, E.; Williams, S.G.; O’Sullivan, J.; Szlauer, A.; Koch, C.; Baranello, G.; Mora, M.; et al. ORAI1 Mutations with Distinct Channel Gating Defects in Tubular Aggregate Myopathy. Hum. Mutat. 2017, 38, 426–438. [Google Scholar] [CrossRef]
- Böhm, J.; Laporte, J. Gain-of-function mutations in STIM1 and ORAI1 causing tubular aggregate myopathy and Stormorken syndrome. Cell Calcium 2018, 76, 1–9. [Google Scholar] [CrossRef]
- Lacruz, R.S.; Feske, S. Diseases caused by mutations in ORAI1 and STIM1. Ann. N. Y. Acad. Sci. 2015, 1356, 45–79. [Google Scholar] [CrossRef]
- Eisner, V.; Csordás, G.; Hajnóczky, G. Interactions between sarco-endoplasmic reticulum and mitochondria in cardiac and skeletal muscle—pivotal roles in Ca²⁺ and reactive oxygen species signaling. J. Cell Sci. 2013, 126, 2965–2978. [Google Scholar] [CrossRef]
- Boncompagni, S.; Rossi, A.E.; Micaroni, M.; Hamilton, S.L.; Dirksen, R.T.; Franzini-Armstrong, C.; Protasi, F. Characterization and temporal development of cores in a mouse model of malignant hyperthermia. Proc. Natl. Acad. Sci. USA 2009, 106, 21996–22001. [Google Scholar] [CrossRef]
- Boncompagni, S.; Pozzer, D.; Viscomi, C.; Ferreiro, A.; Zito, E. Physical and Functional Cross Talk Between Endo-Sarcoplasmic Reticulum and Mitochondria in Skeletal Muscle. Antioxid. Redox Signal. 2020, 32, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Ramesh, V.; Franzini-Armstrong, C.; Sheu, S.S. Transport of Ca2+ from sarcoplasmic reticulum to mitochondria in rat ventricular myocytes. J. Bioenerg. Biomembr. 2000, 32, 97–104. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, C.; Schneider, T.G.; Hajnóczky, G.; Csordás, G. Alignment of sarcoplasmic reticulum-mitochondrial junctions with mitochondrial contact points. Am. J. Physiol.-Heart Circ. Physiol. 2011, 301, H1907–H1915. [Google Scholar] [CrossRef] [PubMed]
- Min, C.K.; Yeom, D.R.; Lee, K.; Kwon, H.; Kang, M.; Kim, Y.; Park, Z.Y.; Jeon, H.; Kim, D.O. Coupling of ryanodine receptor 2 and voltage-dependent anion channel 2 is essential for Ca2+ transfer from the sarcoplasmic reticulum to the mitochondria in the heart. Biochem. J. 2012, 447, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Csordás, G.; Jowdy, C.; Schneider, T.G.; Csordás, N.; Wang, W.; Liu, Y.; Kohlhaas, M.; Meiser, M.; Bergem, S.; et al. Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca2+ crosstalk. Circ. Res. 2012, 111, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Ainbinder, A.; Boncompagni, S.; Protasi, F.; Dirksen, R.T. Role of Mitofusin-2 in mitochondrial localization and calcium uptake in skeletal muscle. Cell Calcium 2015, 57, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Giacomello, M.; Drago, I.; Bortolozzi, M.; Scorzeto, M.; Gianelle, A.; Pizzo, P.; Pozzan, T. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol. Cell 2010, 38, 280–290. [Google Scholar] [CrossRef]
- Penna, E.; Espino, J.; De Stefani, D.; Rizzuto, R. The MCU complex in cell death. Cell Calcium 2018, 69, 73–80. [Google Scholar] [CrossRef]
- Mammucari, C.; Raffaello, A.; Vecellio Reane, D.; Gherardi, G.; De Mario, A.; Rizzuto, R. Mitochondrial calcium uptake in organ physiology: From molecular mechanism to animal models. Pflugers Arch. 2018, 470, 1165–1179. [Google Scholar] [CrossRef]
- Rossi, A.; Pizzo, P.; Filadi, R. Calcium, mitochondria and cell metabolism: A functional triangle in bioenergetics. Biochim. Biophys. Acta.-Mol. Cell Res. 2019, 1866, 1068–1078. [Google Scholar] [CrossRef]
- Wu, Y.; Rasmussen, T.P.; Koval, O.M.; Joiner, M.L.; Hall, D.D.; Chen, B.; Luczak, E.D.; Wang, Q.; Rokita, A.G.; Wehrens, X.H.; et al. The mitochondrial uniporter controls fight or flight heart rate increases. Nat. Commun. 2015, 6, 6081. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, G.; Nogara, L.; Ciciliot, S.; Fadini, G.P.; Blaauw, B.; Braghetta, P.; Bonaldo, P.; De Stefani, D.; Rizzuto, R.; Mammucari, C. Loss of mitochondrial calcium uniporter rewires skeletal muscle metabolism and substrate preference. Cell Death Differ. 2019, 26, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Robert, V.; Massimino, M.L.; Tosello, V.; Marsault, R.; Cantini, M.; Sorrentino, V.; Pozzan, T. Alteration in calcium handling at the subcellular level in mdx myotubes. J. Biol. Chem. 2001, 276, 4647–4651. [Google Scholar] [CrossRef] [PubMed]
- Durham, W.J.; Aracena-Parks, P.; Long, C.; Rossi, A.E.; Goonasekera, S.A.; Boncompagni, S.; Galvan, D.L.; Gilman, C.P.; Baker, M.R.; Shirokova, N.; et al. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell 2008, 133, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.; Terentyeva, R.; Clements, R.T.; Belevych, A.E.; Terentyev, D. Sarcoplasmic reticulum-mitochondria communication; implications for cardiac arrhythmia. J. Mol. Cell Cardiol. 2021, 156, 105–113. [Google Scholar] [CrossRef]
- Lee, C.S.; Hanna, A.D.; Wang, H.; Dagnino-Acosta, A.; Joshi, A.D.; Knoblauch, M.; Xia, Y.; Georgiou, D.K.; Xu, J.; Long, C.; et al. A chemical chaperone improves muscle function in mice with a RyR1 mutation. Nat. Commun. 2017, 8, 14659. [Google Scholar] [CrossRef]
- Volpe, P.; Villa, A.; Podini, P.; Martini, A.; Nori, A.; Panzeri, M.C.; Meldolesi, J. The endoplasmic reticulum-sarcoplasmic reticulum connection: Distribution of endoplasmic reticulum markers in the sarcoplasmic reticulum of skeletal muscle fibers. Proc. Natl. Acad. Sci. USA 1992, 89, 6142–6146. [Google Scholar] [CrossRef]
- Kaisto, T.; Metsikkö, K. Distribution of the endoplasmic reticulum and its relationship with the sarcoplasmic reticulum in skeletal myofibers. Exp. Cell Res. 2003, 289, 47–57. [Google Scholar] [CrossRef]
- Bogdanov, V.; Soltisz, A.M.; Moise, N.; Sakuta, G.; Orengo, B.H.; Janssen, P.M.-L.; Weinberg, S.H.; Davis, J.P.; Veeraraghavan, R.; Györke, S. Distributed synthesis of sarcolemmal and sarcoplasmic reticulum membrane proteins in cardiac myocytes. Basic Res. Cardiol. 2021, 116, 1–16. [Google Scholar] [CrossRef]
- Villa, A.; Podini, P.; Panzeri, M.C.; Söling, H.D.; Volpe, P.; Meldolesi, J. The endoplasmic-sarcoplasmic reticulum of smooth muscle: Immunocytochemistry of vas deferens fibers reveals specialized subcompartments differently equipped for the control of Ca2+ homeostasis. J. Cell Biol. 1993, 121, 1041–1051. [Google Scholar] [CrossRef]
- Prinz, W.A.; Toulmay, A.; Balla, T. The functional universe of membrane contact sites. Nat. Rev. Mol. Cell Biol. 2020, 21, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Kinnear, N.P.; Wyatt, C.N.; Clark, J.H.; Calcraft, P.J.; Fleischer, S.; Jeyakumar, L.H.; Nixon, G.F.; Evans, A.M. Lysosomes co-localize with ryanodine receptor subtype 3 to form a trigger zone for calcium signalling by NAADP in rat pulmonary arterial smooth muscle. Cell Calcium 2008, 44, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Aston, D.; Capel, R.A.; Ford, K.L.; Christian, H.C.; Mirams, G.R.; Rog-Zielinska, E.A.; Kohl, P.; Galione, A.; Burton, R.A.; Terrar, D.A. High resolution structural evidence suggests the Sarcoplasmic Reticulum forms microdomains with Acidic Stores (lysosomes) in the heart. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Capel, R.A.; Bolton, E.L.; Lin, W.K.; Aston, D.; Wang, Y.; Liu, W.; Wang, X.; Burton, R.A.; Bloor-Young, D.; Shade, K.T.; et al. Two-pore Channels (TPC2s) and Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP) at Lysosomal-Sarcoplasmic Reticular Junctions Contribute to Acute and Chronic β-Adrenoceptor Signaling in the Heart. J. Biol. Chem. 2015, 290, 30087–30098. [Google Scholar] [CrossRef]
- Wu, X.; Bers, D.M. Sarcoplasmic reticulum and nuclear envelope are one highly interconnected Ca2+ store throughout cardiac myocyte. Circ. Res. 2006, 99, 283–291. [Google Scholar] [CrossRef]
- Malhas, A.; Goulbourne, C.; Vaux, D.J. The nucleoplasmic reticulum: Form and function. Trends Cell Biol. 2011, 21, 362–373. [Google Scholar] [CrossRef]
- Marius, P.; Guerra, M.T.; Nathanson, M.H.; Ehrlich, B.E.; Leite, M.F. Calcium release from ryanodine receptors in the nucleoplasmic reticulum. Cell Calcium 2006, 39, 65–73. [Google Scholar] [CrossRef]
- Irvine, R.F. Nuclear inositide signalling-expansion, structures and clarification. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2006, 1761, 505–508. [Google Scholar] [CrossRef]
- Avedanian, L.; Jacques, D.; Bkaily, G. Presence of tubular and reticular structures in the nucleus of human vascular smooth muscle cells. J. Mol. Cell Cardiol. 2011, 50, 175–186. [Google Scholar] [CrossRef]
- Lee, S.H.; Hadipour-Lakmehsari, S.; Miyake, T.; Gramolini, A.O. Three-dimensional imaging reveals endo(sarco)plasmic reticulum-containing invaginations within the nucleoplasm of muscle. Am. J. Physiol.-Cell Physiol. 2018, 314, C257–C267. [Google Scholar] [CrossRef]
- Roman, W.; Gomes, E.R. Nuclear positioning in skeletal muscle. Semin. Cell Dev. Biol. 2018, 82, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Reuveny, A.; Shnayder, M.; Lorber, D.; Wang, S.; Volk, T. Ma2/d promotes myonuclear positioning and association with the sarcoplasmic reticulum. Development 2018, 145, dev159558. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.; Baylies, M.K. Getting into Position: Nuclear Movement in Muscle Cells. Trends Cell Biol. 2020, 30, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Reuveny, A.; Volk, T. Nesprin provides elastic properties to muscle nuclei by cooperating with spectraplakin and EB1. J. Cell Biol. 2015, 209, 529–538. [Google Scholar] [CrossRef]
| Causative Gene (S) * | Inheritance | Histological Features | Clinical Features | |
|---|---|---|---|---|
| Central Core Disease (CCD) | RyR1 > 90% CACNA1S | AD or AR |
|
|
| Multiminicore Disease (MMD) | RyR1 CACNA1S | AR |
|
|
| Centronuclear Myopathy (CNM) | RyR1~12% | AR |
|
|
| Congenital Fibre Type Disproportion (CFTD) | RyR1~20% | AR |
|
|
| Dusty Core Disease (DUCD) | RyR1 | AR |
|
|
| Core Rod Myopathy (CRM) | RyR1 | AD or AR |
|
|
| Malignant Hyperthermia (MH) | RyR1 CACNA1S STAC3 | AD |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, D.; Pierantozzi, E.; Amadsun, D.O.; Buonocore, S.; Rubino, E.M.; Sorrentino, V. The Sarcoplasmic Reticulum of Skeletal Muscle Cells: A Labyrinth of Membrane Contact Sites. Biomolecules 2022, 12, 488. https://doi.org/10.3390/biom12040488
Rossi D, Pierantozzi E, Amadsun DO, Buonocore S, Rubino EM, Sorrentino V. The Sarcoplasmic Reticulum of Skeletal Muscle Cells: A Labyrinth of Membrane Contact Sites. Biomolecules. 2022; 12(4):488. https://doi.org/10.3390/biom12040488
Chicago/Turabian StyleRossi, Daniela, Enrico Pierantozzi, David Osamwonuyi Amadsun, Sara Buonocore, Egidio Maria Rubino, and Vincenzo Sorrentino. 2022. "The Sarcoplasmic Reticulum of Skeletal Muscle Cells: A Labyrinth of Membrane Contact Sites" Biomolecules 12, no. 4: 488. https://doi.org/10.3390/biom12040488
APA StyleRossi, D., Pierantozzi, E., Amadsun, D. O., Buonocore, S., Rubino, E. M., & Sorrentino, V. (2022). The Sarcoplasmic Reticulum of Skeletal Muscle Cells: A Labyrinth of Membrane Contact Sites. Biomolecules, 12(4), 488. https://doi.org/10.3390/biom12040488







