Dynamical Analysis of a Boolean Network Model of the Oncogene Role of lncRNA ANRIL and lncRNA UFC1 in Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
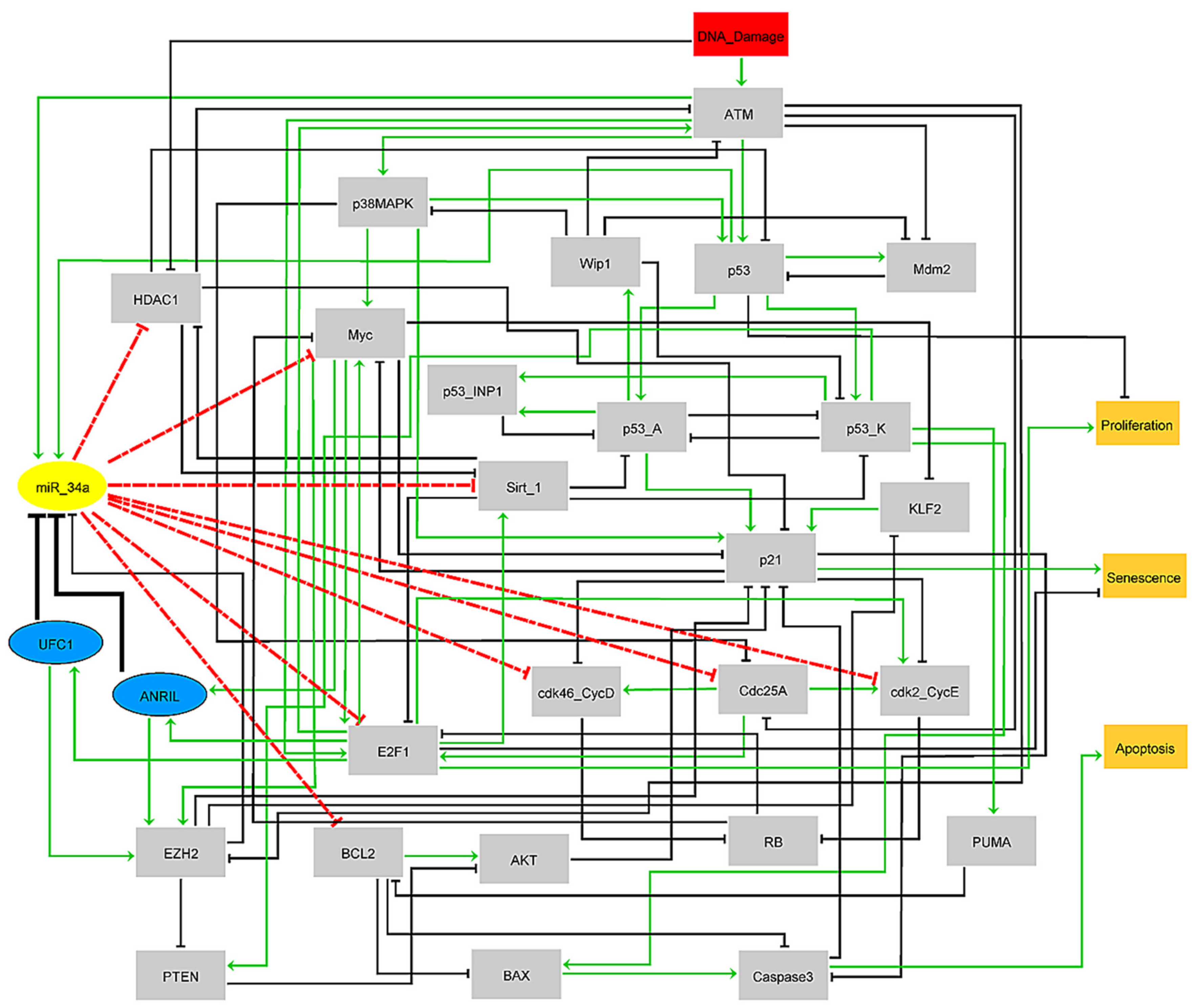
2.1. Collection of the Public Databases/Tools and Development of the Gene Regulatory Network in NSCLC
2.2. Transformation of the PubMed Literature into a Boolean Network, Rules and Simulations
2.3. The Expected Role of These ncRNA in the Boolean Model
2.4. Molecular Mechanisms Mediating ncRNAs at the G1/S Checkpoint in NSCLC
3. Results
3.1. Boolean Model and Its Wild-Type Case Attractors
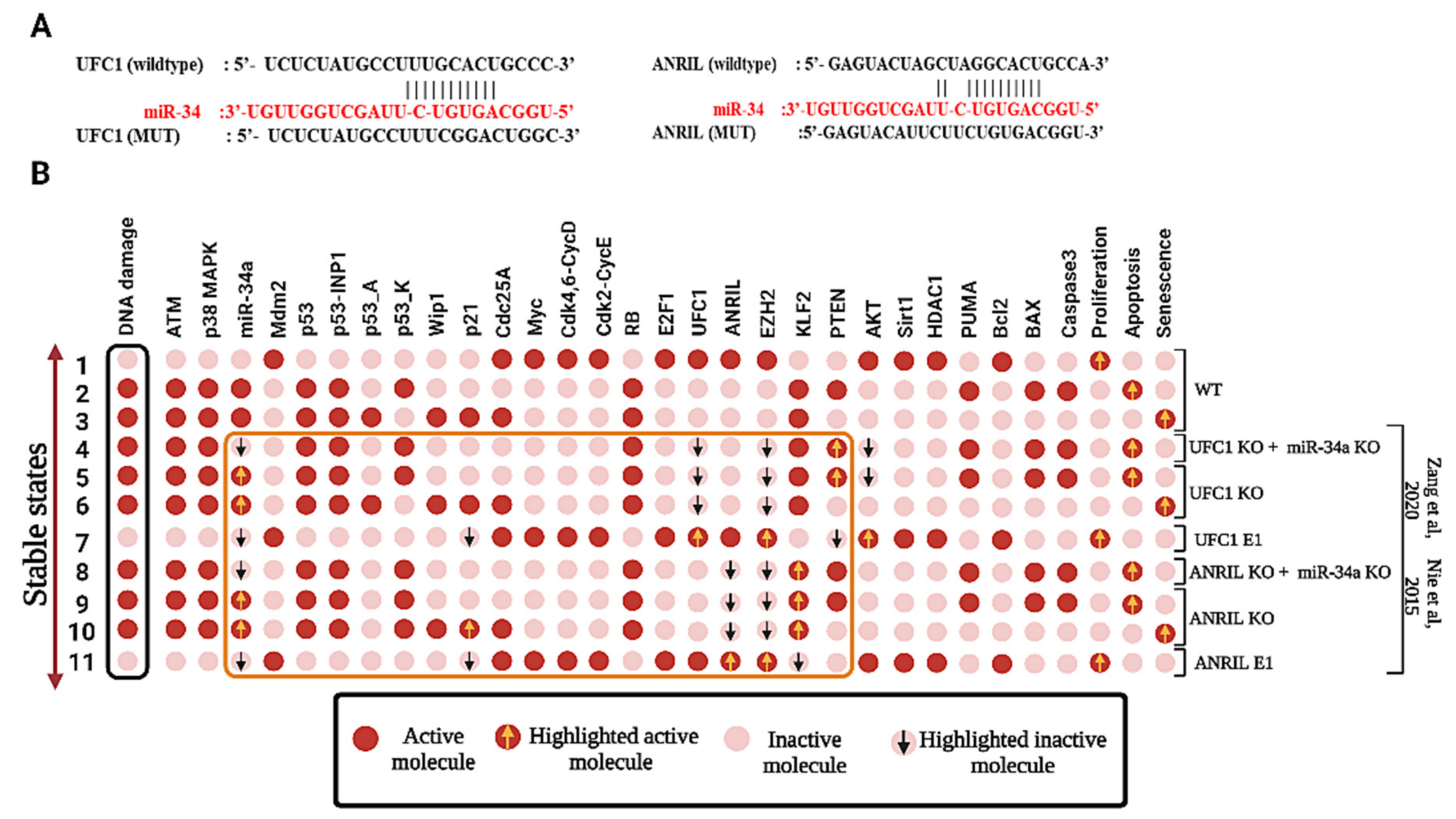
3.2. miR-34a Is a Downstream Target of ANRIL and/or UFC1 in NSCLC
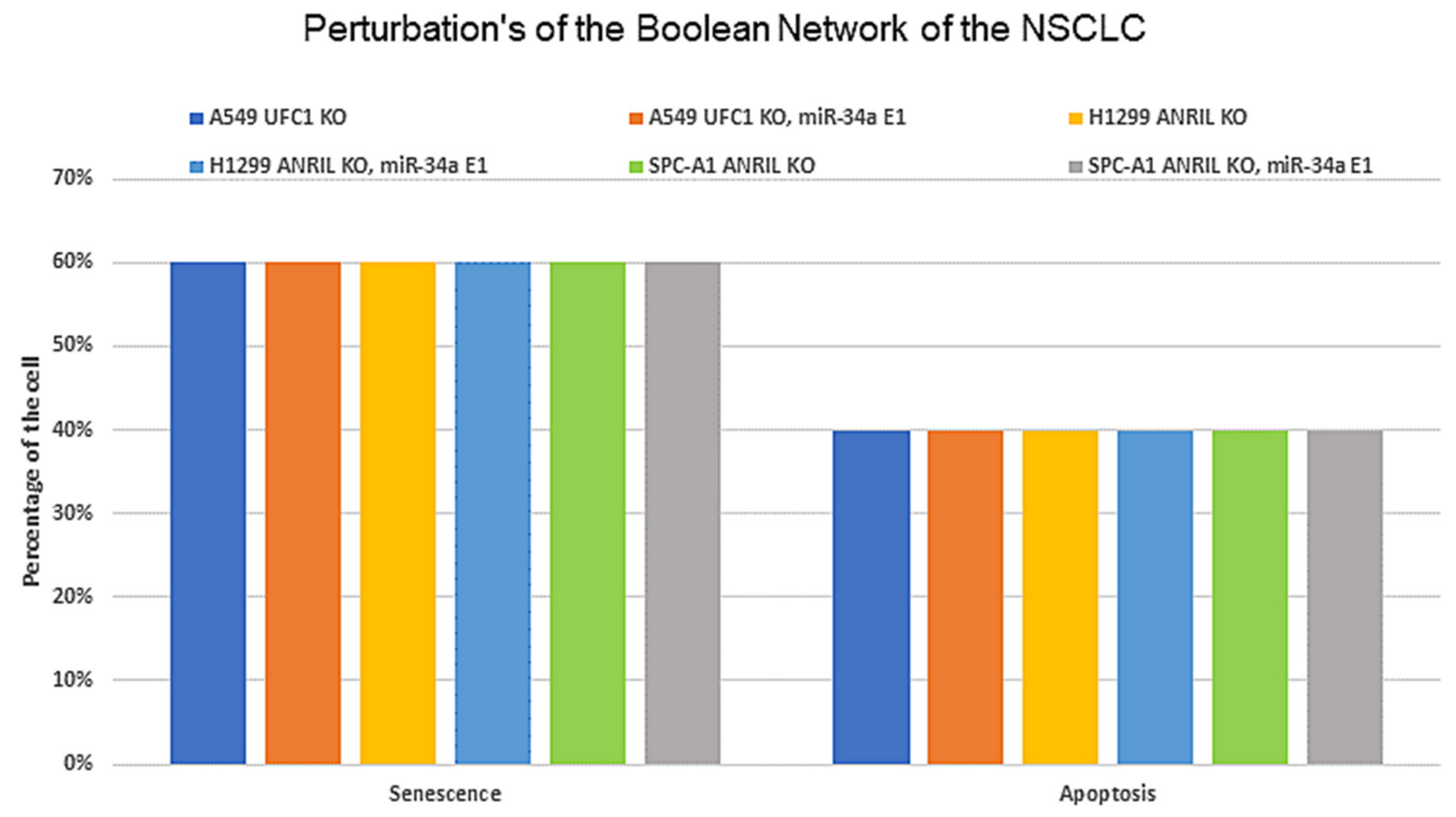
3.3. Calibration between In-Silico Perturbations versus Experimental Studies
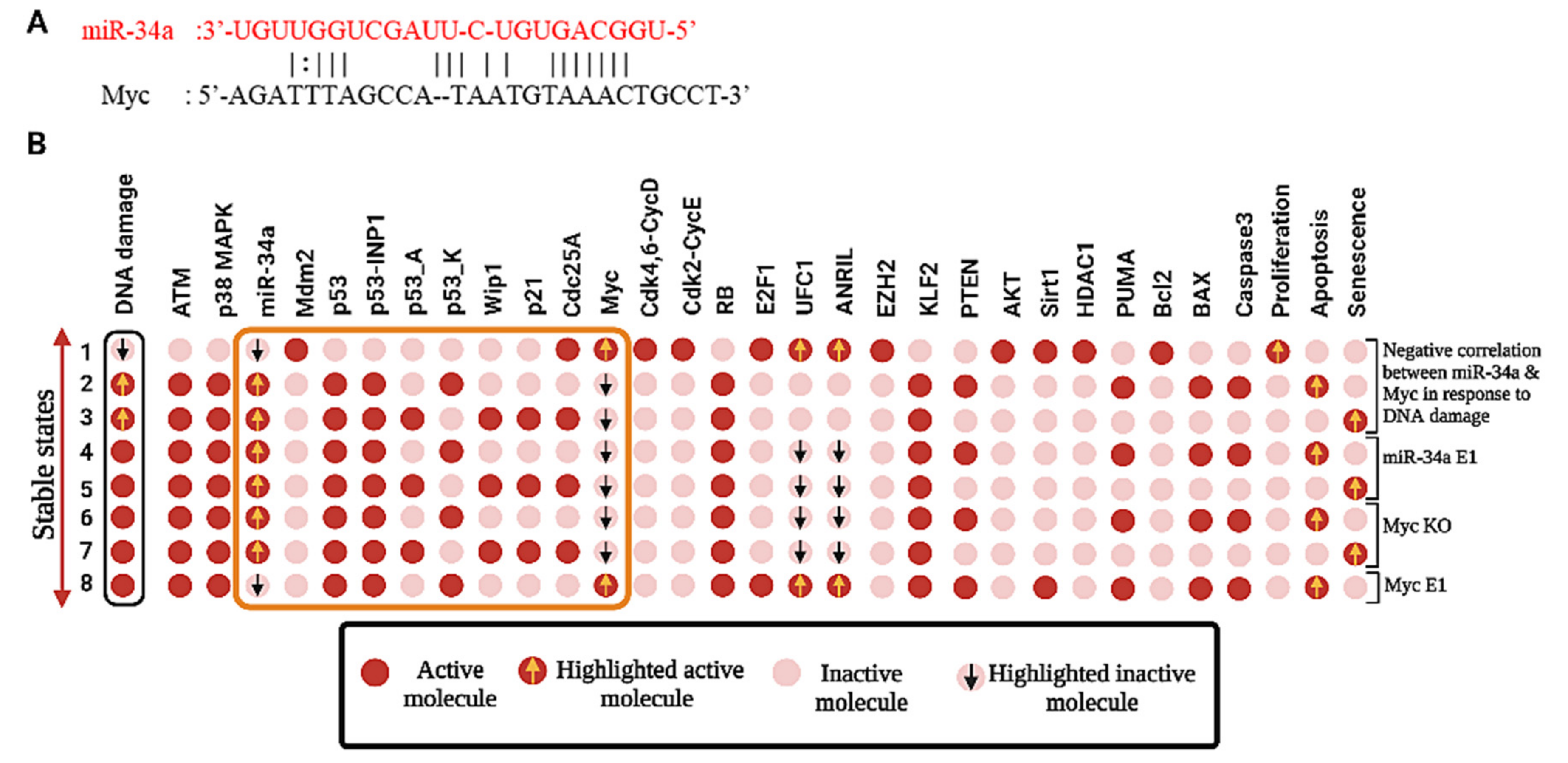
3.4. Activation of miR-34a Inhibits NSCLC Progression through the Targeting of Myc
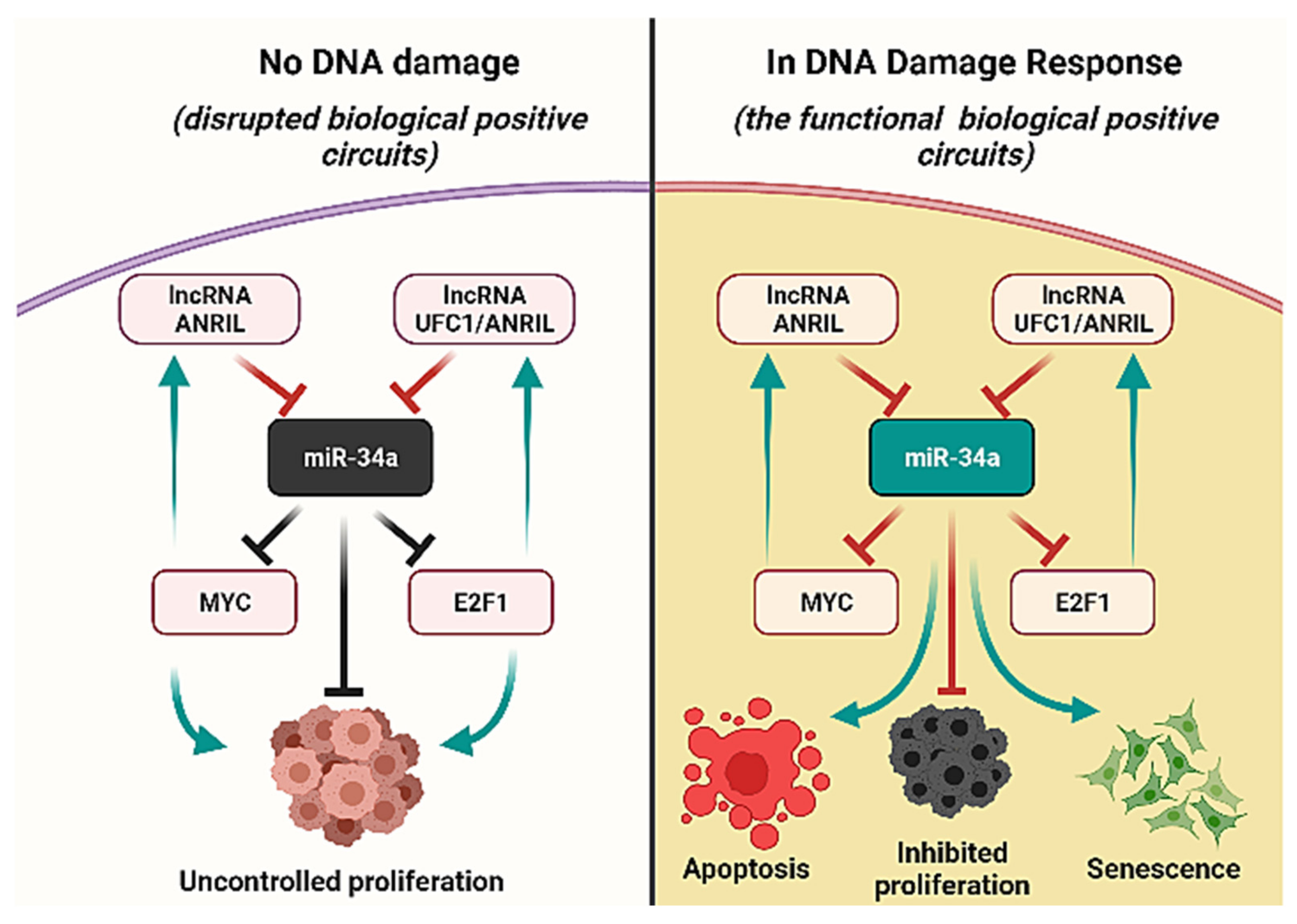
3.5. Biological Circuits and Perturbation Analysis
3.6. Workflow of the Boolean Network Construction and Validation
- UFC1 (KO), miR-34a (E1) → Apoptosis and Senescence;
- ANRIL (KO), miR-34a (E1) → Apoptosis and Senescence;
- ANRIL (KO), UFC1 (KO) → Increased expression of miR-34a.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ginn, L.; Shi, L.; Montagna, M.L.; Garofalo, M. LncRNAs in Non-Small-Cell Lung Cancer. Noncoding RNA 2020, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.; Sun, M.; Yang, J.; Xie, M.; Xu, T.; Xia, R.; Liu, Y.; Liu, X.; Zhang, E.; Lu, K.; et al. Long Noncoding RNA ANRIL Promotes Non-Small Cell Lung Cancer Cell Proliferation and Inhibits Apoptosis by Silencing KLF2 and P21 Expression. Mol. Cancer Ther. 2015, 14, 268–277. [Google Scholar] [CrossRef]
- Zang, X.; Gu, J.; Zhang, J.; Shi, H.; Hou, S.; Xu, X.; Chen, Y.; Zhang, Y.; Mao, F.; Qian, H.; et al. Exosome-Transmitted LncRNA UFC1 Promotes Non-Small-Cell Lung Cancer Progression by EZH2-Mediated Epigenetic Silencing of PTEN Expression. Cell Death Dis. 2020, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-L.; Tsai, Y.-M.; Lien, C.-T.; Kuo, P.-L.; Hung, J.-Y. The Roles of MicroRNA in Lung Cancer. Int. J. Mol. Sci. 2019, 20, 1611. [Google Scholar] [CrossRef]
- Visser, H.; Thomas, A.D. MicroRNAs and the DNA Damage Response: How Is Cell Fate Determined? DNA Repair 2021, 108, 103245. [Google Scholar] [CrossRef]
- He, X.; Yang, A.; McDonald, D.G.; Riemer, E.C.; Vanek, K.N.; Schulte, B.A.; Wang, G.Y. MiR-34a Modulates Ionizing Radiation-Induced Senescence in Lung Cancer Cells. Oncotarget 2017, 8, 69797–69807. [Google Scholar] [CrossRef]
- Rokavec, M.; Li, H.; Jiang, L.; Hermeking, H. The P53/MiR-34 Axis in Development and Disease. J. Mol. Cell. Biol. 2014, 6, 214–230. [Google Scholar] [CrossRef]
- Neaga, A.; Bagacean, C.; Tempescul, A.; Jimbu, L.; Mesaros, O.; Blag, C.; Tomuleasa, C.; Bocsan, C.; Gaman, M.; Zdrenghea, M. MicroRNAs Associated with a Good Prognosis of Acute Myeloid Leukemia and Their Effect on Macrophage Polarization. Front. Immunol. 2021, 11, 3402. [Google Scholar] [CrossRef]
- Pardini, B.; De Maria, D.; Francavilla, A.; Di Gaetano, C.; Ronco, G.; Naccarati, A. MicroRNAs as Markers of Progression in Cervical Cancer: A Systematic Review. BMC Cancer 2018, 18, 696. [Google Scholar] [CrossRef]
- Wang, C.-H.; Li, Q.-Y.; Nie, L.; Ma, J.; Yao, C.-J.; Chen, F.-P. LncRNA ANRIL Promotes Cell Proliferation, Migration and Invasion during Acute Myeloid Leukemia Pathogenesis via Negatively Regulating MiR-34a. Int. J. Biochem. Cell Biol. 2020, 119, 105666. [Google Scholar] [CrossRef]
- Xi, J.; Feng, J.; Zeng, S.; Huang, P. Long Noncoding RNA UFC1 Is Activated by E2F1 and Exerts Oncogenic Properties by Functioning as a CeRNA of FOXP3. Cancer Med. 2018, 7, 3301–3310. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Silveira, D.A.; Mombach, J.C.M. ATM/MiR-34a-5p Axis Regulates a P21-Dependent Senescence-Apoptosis Switch in Non-Small Cell Lung Cancer: A Boolean Model of G1/S Checkpoint Regulation. FEBS Lett. 2020, 594, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Barbuti, R.; Gori, R.; Milazzo, P.; Nasti, L. A Survey of Gene Regulatory Networks Modelling Methods: From Differential Equations, to Boolean and Qualitative Bioinspired Models. J. Membr. Comput. 2020, 2, 207–226. [Google Scholar] [CrossRef]
- Wang, R.-S.; Saadatpour, A.; Albert, R. Boolean Modeling in Systems Biology: An Overview of Methodology and Applications. Phys. Biol. 2012, 9, 055001. [Google Scholar] [CrossRef]
- Schwab, J.D.; Kühlwein, S.D.; Ikonomi, N.; Kühl, M.; Kestler, H.A. Concepts in Boolean Network Modeling: What Do They All Mean? Comput. Struct. Biotechnol. J. 2020, 18, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Silveira, D.A.; Mombach, J.C.M. Towards DNA-Damage Induced Autophagy: A Boolean Model of P53-Induced Cell Fate Mechanisms. DNA Repair 2020, 96, 102971. [Google Scholar] [CrossRef]
- Thieffry, D. Dynamical Roles of Biological Regulatory Circuits. Brief. Bioinform. 2007, 8, 220–225. [Google Scholar] [CrossRef]
- Silveira, D.A.; Gupta, S.; Mombach, J.C.M. Systems Biology Approach Suggests New MiRNAs as Phenotypic Stability Factors in the Epithelial-Mesenchymal Transition. J. R Soc. Interface 2020, 17, 20200693. [Google Scholar] [CrossRef]
- Deritei, D.; Rozum, J.; Ravasz Regan, E.; Albert, R. A Feedback Loop of Conditionally Stable Circuits Drives the Cell Cycle from Checkpoint to Checkpoint. Sci. Rep. 2019, 9, 16430. [Google Scholar] [CrossRef]
- Wooten, D.J.; Zañudo, J.G.T.; Murrugarra, D.; Perry, A.M.; Dongari-Bagtzoglou, A.; Laubenbacher, R.; Nobile, C.J.; Albert, R. Mathematical Modeling of the Candida Albicans Yeast to Hyphal Transition Reveals Novel Control Strategies. PLoS Comput. Biol. 2021, 17, e1008690. [Google Scholar] [CrossRef]
- Borriello, E.; Daniels, B.C. The Basis of Easy Controllability in Boolean Networks. Nat. Commun 2021, 12, 5227. [Google Scholar] [CrossRef] [PubMed]
- Guberman, E.; Sherief, H.; Regan, E.R. Boolean Model of Anchorage Dependence and Contact Inhibition Points to Coordinated Inhibition but Semi-Independent Induction of Proliferation and Migration. Comput. Struct. Biotechnol. J. 2020, 18, 2145–2165. [Google Scholar] [CrossRef] [PubMed]
- Oughtred, R.; Stark, C.; Breitkreutz, B.-J.; Rust, J.; Boucher, L.; Chang, C.; Kolas, N.; O’Donnell, L.; Leung, G.; McAdam, R.; et al. The BioGRID Interaction Database: 2019 Update. Nucleic Acids Res. 2019, 47, D529–D541. [Google Scholar] [CrossRef]
- Kern, F.; Aparicio-Puerta, E.; Li, Y.; Fehlmann, T.; Kehl, T.; Wagner, V.; Ray, K.; Ludwig, N.; Lenhof, H.-P.; Meese, E.; et al. MiRTargetLink 2.0—Interactive MiRNA Target Gene and Target Pathway Networks. Nucleic Acids Res. 2021, 49, W409–W416. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting Effective MicroRNA Target Sites in Mammalian MRNAs. Elife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, T.; Iwakiri, J.; Ono, Y.; Hamada, M. LncRRIsearch: A Web Server for LncRNA-RNA Interaction Prediction Integrated With Tissue-Specific Expression and Subcellular Localization Data. Front. Genet. 2019, 10, 462. [Google Scholar] [CrossRef]
- Naldi, A.; Hernandez, C.; Abou-Jaoudé, W.; Monteiro, P.T.; Chaouiya, C.; Thieffry, D. Logical Modeling and Analysis of Cellular Regulatory Networks With GINsim 3.0. Front. Physiol. 2018, 9, 646. [Google Scholar] [CrossRef]
- Abou-Jaoudé, W.; Traynard, P.; Monteiro, P.T.; Saez-Rodriguez, J.; Helikar, T.; Thieffry, D.; Chaouiya, C. Logical Modeling and Dynamical Analysis of Cellular Networks. Front. Genet. 2016, 7, 94. [Google Scholar] [CrossRef]
- Gupta, S.; Silveira, D.A.; Barbé-Tuana, F.M.; Mombach, J.C.M. Integrative Data Modeling from Lung and Lymphatic Cancer Predicts Functional Roles for MiR-34a and MiR-16 in Cell Fate Regulation. Sci. Rep. 2020, 10, 2511. [Google Scholar] [CrossRef]
- Gupta, S.; Silveira, D.A.; Mombach, J.C.M. Modeling the Role of MicroRNA-449a in the Regulation of the G2/M Cell Cycle Checkpoint in Prostate LNCaP Cells under Ionizing Radiation. PLoS ONE 2018, 13, e0200768. [Google Scholar] [CrossRef]
- Ito, T.; Teo, Y.V.; Evans, S.A.; Neretti, N.; Sedivy, J.M. Regulation of Cellular Senescence by Polycomb Chromatin Modifiers through Distinct DNA Damage- and Histone Methylation-Dependent Pathways. Cell Rep. 2018, 22, 3480–3492. [Google Scholar] [CrossRef] [PubMed]
- Mirza-Aghazadeh-Attari, M.; Mohammadzadeh, A.; Yousefi, B.; Mihanfar, A.; Karimian, A.; Majidinia, M. 53BP1: A Key Player of DNA Damage Response with Critical Functions in Cancer. DNA Repair 2019, 73, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Bakkenist, C.J.; Kastan, M.B. DNA Damage Activates ATM through Intermolecular Autophosphorylation and Dimer Dissociation. Nature 2003, 421, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, R.L.; Maya, R.; Segel, L.A.; Alon, U.; Levine, A.J.; Oren, M. Generation of Oscillations by the P53-Mdm2 Feedback Loop: A Theoretical and Experimental Study. PNAS 2000, 97, 11250–11255. [Google Scholar] [CrossRef]
- Zhang, X.-P.; Liu, F.; Wang, W. Two-Phase Dynamics of P53 in the DNA Damage Response. Proc. Natl. Acad. Sci. USA 2011, 108, 8990–8995. [Google Scholar] [CrossRef]
- Vousden, K.H.; Lu, X. Live or Let Die: The Cell’s Response to P53. Nat. Rev. Cancer 2002, 2, 594–604. [Google Scholar] [CrossRef]
- Salzman, D.W.; Nakamura, K.; Nallur, S.; Dookwah, M.T.; Metheetrairut, C.; Slack, F.J.; Weidhaas, J.B. MiR-34 Activity Is Modulated through 5′-End Phosphorylation in Response to DNA Damage. Nat. Commun. 2016, 7, 10954. [Google Scholar] [CrossRef]
- Suzuki, H.I.; Yamagata, K.; Sugimoto, K.; Iwamoto, T.; Kato, S.; Miyazono, K. Modulation of MicroRNA Processing by P53. Nature 2009, 460, 529–533. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, Y.; Tang, L. MicroRNA-34 Family: A Potential Tumor Suppressor and Therapeutic Candidate in Cancer. J. Exp. Clin. Cancer Res. 2019, 38, 53. [Google Scholar] [CrossRef]
- Wan, G.; Mathur, R.; Hu, X.; Liu, Y.; Zhang, X.; Peng, G.; Lu, X. Long Non-Coding RNA ANRIL (CDKN2B-AS) Is Induced by the ATM-E2F1 Signaling Pathway. Cell Signal. 2013, 25, 1086–1095. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, X.; Xu, L.; Rong, C.; Shen, C.; Bian, W. Long Noncoding RNA ANRIL Could Be Transactivated by C-Myc and Promote Tumor Progression of Non-Small-Cell Lung Cancer. Onco. Targets Ther. 2016, 9, 3077–3084. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ren, J.; Ding, L.; Xu, Q.; Shi, G.; Li, X.; Li, X.; Ji, J.; Zhang, D.; Wang, Y.; Wang, T.; et al. LF-MF Inhibits Iron Metabolism and Suppresses Lung Cancer through Activation of P53-MiR-34a-E2F1/E2F3 Pathway. Sci. Rep. 2017, 7, 749. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Song, K.; Han, C.; Zhang, J.; Lu, L.; Chen, W.; Wu, T. Epigenetic Silencing of MiRNA-34a in Human Cholangiocarcinoma via EZH2 and DNA Methylation: Impact on Regulation of Notch Pathway. Am. J. Pathol. 2017, 187, 2288–2299. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-X.; Cao, L.-Y.; Chen, X.; Xiao, J.; Zou, Y.; Chen, Q. PTEN Inhibits Cell Proliferation, Promotes Cell Apoptosis, and Induces Cell Cycle Arrest via Downregulating the PI3K/AKT/HTERT Pathway in Lung Adenocarcinoma A549 Cells. BioMed. Res. Inter. 2016, 2016, e2476842. [Google Scholar] [CrossRef]
- Taniguchi, H.; Jacinto, F.V.; Villanueva, A.; Fernandez, A.F.; Yamamoto, H.; Carmona, F.J.; Puertas, S.; Marquez, V.E.; Shinomura, Y.; Imai, K.; et al. Silencing of Kruppel-like Factor 2 by the Histone Methyltransferase EZH2 in Human Cancer. Oncogene 2012, 31, 1988–1994. [Google Scholar] [CrossRef]
- Silveira, D.A.; Gupta, S.; Mombach, J.C.M. P53/E2F1/MiR-25 Axis Regulates Apoptosis Induction in Glioblastoma Cells: A Qualitative Model. J. Phys. Complex. 2020, 1, 035001. [Google Scholar] [CrossRef]
- Xie, R.; Wang, M.; Zhou, W.; Wang, D.; Yuan, Y.; Shi, H.; Wu, L. Long Non-Coding RNA (LncRNA) UFC1/MiR-34a Contributes to Proliferation and Migration in Breast Cancer. Med. Sci. Monit. 2019, 25, 7149–7157. [Google Scholar] [CrossRef]
- Zhao, J.; Lammers, P.; Torrance, C.J.; Bader, A.G. TP53-Independent Function of MiR-34a via HDAC1 and P21(CIP1/WAF1.). Mol. Ther. 2013, 21, 1678–1686. [Google Scholar] [CrossRef]
- Pomerening, J.R. Positive Feedback Loops in Cell Cycle Progression. FEBS Lett. 2009, 583, 3388–3396. [Google Scholar] [CrossRef]
- Zhang, Y.; Fujita, N.; Tsuruo, T. Caspase-Mediated Cleavage of P21Waf1/Cip1 Converts Cancer Cells from Growth Arrest to Undergoing Apoptosis. Oncogene 1999, 18, 1131–1138. [Google Scholar] [CrossRef]
- Jänicke, R.U.; Sohn, D.; Essmann, F.; Schulze-Osthoff, K. The Multiple Battles Fought by Anti-Apoptotic P21. Cell Cycle 2007, 6, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Coller, H.A.; Forman, J.J.; Legesse-Miller, A. “Myc’ed Messages”: Myc Induces Transcription of E2F1 While Inhibiting Its Translation via a MicroRNA Polycistron. PLoS Genet. 2007, 3, e146. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Lin, F.T.; Nevins, J.R. Selective Induction of E2F1 in Response to DNA Damage, Mediated by ATM-Dependent Phosphorylation. Genes Dev. 2001, 15, 1833–1844. [Google Scholar] [PubMed]
- Wang, C.; Chen, L.; Hou, X.; Li, Z.; Kabra, N.; Ma, Y.; Nemoto, S.; Finkel, T.; Gu, W.; Cress, W.D.; et al. Interactions between E2F1 and SirT1 Regulate Apoptotic Response to DNA Damage. Nat. Cell Biol. 2006, 8, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Ma, O.; Nguyen, T.-A.; Jones, S.N.; Oren, M.; Donehower, L.A. The Wip1 Phosphatase Acts as a Gatekeeper in the P53-Mdm2 Autoregulatory Loop. Cancer Cell 2007, 12, 342–354. [Google Scholar] [CrossRef]
- Dong, X.; Jin, Z.; Chen, Y.; Xu, H.; Ma, C.; Hong, X.; Li, Y.; Zhao, G. Knockdown of Long Non-Coding RNA ANRIL Inhibits Proliferation, Migration, and Invasion but Promotes Apoptosis of Human Glioma Cells by Upregulation of MiR-34a. J. Cell. Biochem. 2018, 119, 2708–2718. [Google Scholar] [CrossRef]
- Lu, T.; Wang, Y.; Chen, D.; Liu, J.; Jiao, W. Potential Clinical Application of LncRNAs in Non-Small Cell Lung Cancer. Onco Targets Ther. 2018, 11, 8045–8052. [Google Scholar] [CrossRef]
- Zupkovitz, G.; Grausenburger, R.; Brunmeir, R.; Senese, S.; Tischler, J.; Jurkin, J.; Rembold, M.; Meunier, D.; Egger, G.; Lagger, S.; et al. The Cyclin-Dependent Kinase Inhibitor P21 Is a Crucial Target for Histone Deacetylase 1 as a Regulator of Cellular Proliferation. Mol. Cell Biol. 2010, 30, 1171–1181. [Google Scholar] [CrossRef]
- Fiorentino, F.P.; Tokgün, E.; Solé-Sánchez, S.; Giampaolo, S.; Tokgün, O.; Jauset, T.; Kohno, T.; Perucho, M.; Soucek, L.; Yokota, J. Growth Suppression by MYC Inhibition in Small Cell Lung Cancer Cells with TP53 and RB1 Inactivation. Oncotarget 2016, 7, 31014–31028. [Google Scholar] [CrossRef]
- Christoffersen, N.R.; Shalgi, R.; Frankel, L.B.; Leucci, E.; Lees, M.; Klausen, M.; Pilpel, Y.; Nielsen, F.C.; Oren, M.; Lund, A.H. P53-Independent Upregulation of MiR-34a during Oncogene-Induced Senescence Represses MYC. Cell Death Differ. 2010, 17, 236–245. [Google Scholar] [CrossRef]
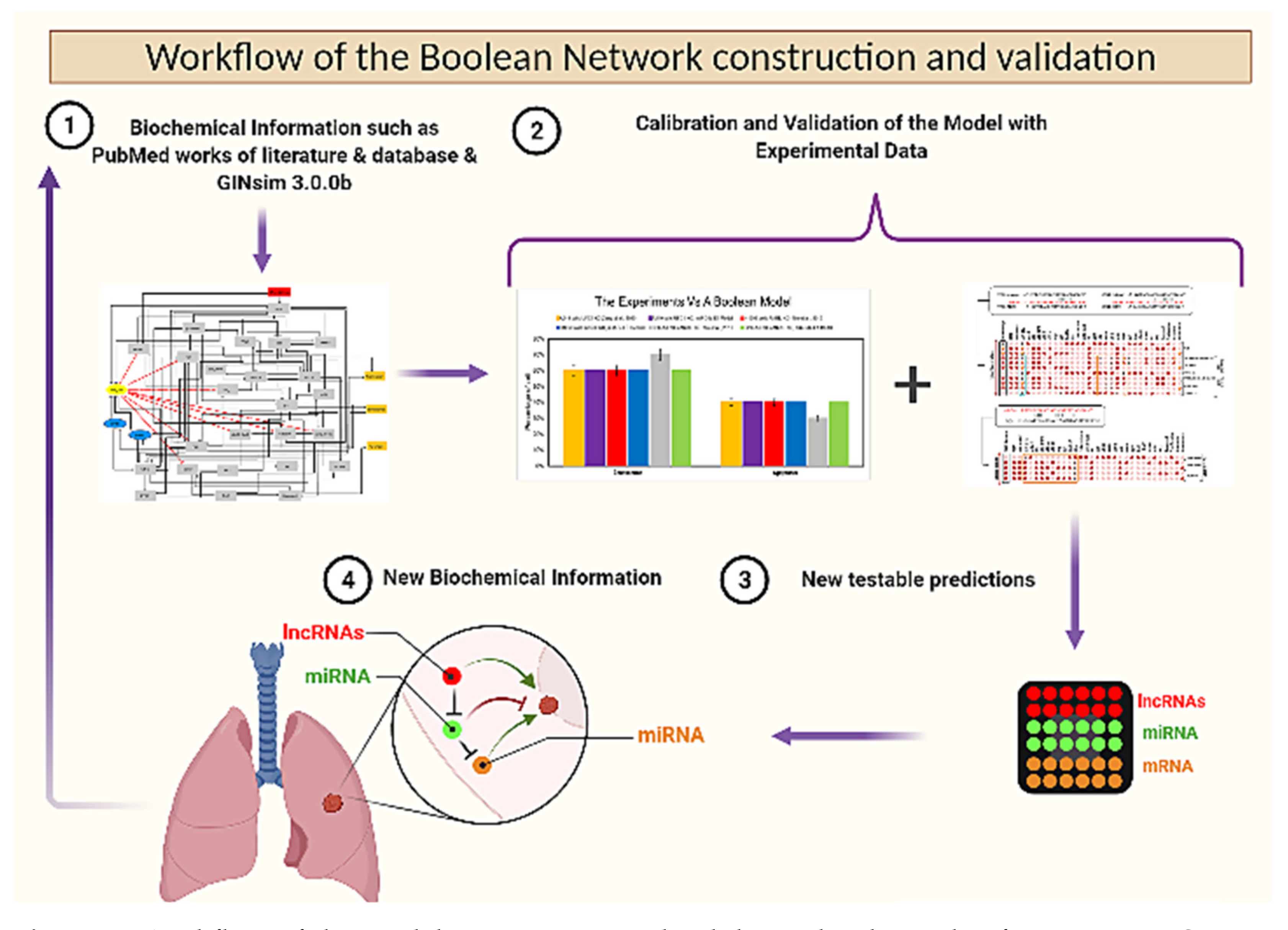
| Stimulus/Perturbations | Model Response | Experimental Observations | Agreement | Cell Lines | References |
|---|---|---|---|---|---|
| In response to DNA damage, miR-34a vs. Myc in NSCLC | Negative correlation | Negative correlation | Yes | A549, H460 | [6] |
| In response to DNA damage, miR-34a E1 in NSCLC | Inhibits the activity of Myc, HDAC1, and BCL2, and induces Senescence and Apoptosis | Represses Myc, and BCL2 inducing Senescence and Apoptosis | Yes | A549, H460 | [6] |
| In response to DNA damage, Myc KO in NSCLC | Induces the activity of miR-34a activating Senescence and Apoptosis | Overexpression of miR-34a induces Senescence and Apoptosis | Yes | A549, H460 | [6] |
| In response to DNA damage, Myc E1 in NSCLC | Inhibits miR-34a expression and induces apoptosis | Represses miR-34a expression and induces apoptotic cell death | Yes | A549, H460 | [6] |
| In response to DNA damage, ANRIL KO in NSCLC | Inactivation of ANRIL activating senescence and Apoptosis | Knockdown (KO) of ANRIL Inhibits proliferation and induced apoptosis and cell cycle arrest | Yes | A549, H1299 | [2] |
| In response to DNA damage, UFC1 KO in NSCLC | Inactivation of UFC1 induces senescence and Apoptosis | Knockdown (KO) of UFC1 Inhibits proliferation and induced apoptosis and cell cycle arrest | Yes | A549, H1299 | [3] |
| In response to DNA damage, ANRIL/UFC1 vs. miR-34a in NSCLC | Negative correlation | Negative correlation | - | A549, H460, H1299 | ? |
| In response to DNA damage, UFC1 KO in NSCLC | Inactivation of UFC1 triggers miR-34a activity induces Senescence and Apoptosis | Inhibits proliferation and induced apoptosis and senescence | - | A549, H460, H1299 | ? |
| In response to DNA damage, ANRIL KO in NSCLC | Inactivation of UFC1 stimulates miR-34a activity induces Senescence and Apoptosis | Inhibits proliferation and induced apoptosis and senescence | - | A549, H460, H1299 | ? |
| Biological Circuits | References |
|---|---|
| Positive | |
| p53/miR-34a/HDAC1 | [48] |
| E2F1/CDK2-CycE/RB | [49] |
| p21/Caspase3 | [50] |
| Myc/p21 | [51] |
| ATM/miR-34a/HDAC1 | [12] |
| p53-A/p53-K | [35] |
| E2F1/Myc | [52] |
| miR-34a/E2F1/ANRIL | ? |
| miR-34a/E2F1/UFC1 | ? |
| miR-34a/Myc/ANRIL | ? |
| E2F1/ATM | [53] |
| Negative | |
| p53/Mdm2 | [34] |
| E2F1/Sirt1 | [54] |
| p53-A/p53-INP1 | [35] |
| E2F1/ATM/miR-34a | [12] |
| p53/Wip1/ATM | [55] |
| Positive Circuits | Circuit Elements | Target | Direct/Indirect Interaction | References |
|---|---|---|---|---|
| miR-34a/E2F1/ANRIL | miR-34a | E2F1 | Direct inhibition | [42] |
| E2F1 | ANRIL | Direct activation | [40] | |
| ANRIL | miR-34a | Direct inhibition | [10,56] | |
| miR-34a/E2F1/UFC1 | miR-34a | E2F1 | Direct inhibition | [42] |
| E2F1 | UFC1 | Direct activation | [11] | |
| UFC1 | miR-34a | Direct inhibition | [47] | |
| miR-34a/Myc/ANRIL | miR-34a | Myc | Direct inhibition | [6] |
| Myc | ANRIL | Direct activation | [41] | |
| ANRIL | miR-34a | Direct inhibition | [10,56] |
| Positive Circuits | Perturbations | Phenotypes |
|---|---|---|
| miR-34a/E2F1/ANRIL | KO/KO/KO | Apoptosis |
| E1/KO/KO | Senescence and Apoptosis | |
| KO/E1/KO | Apoptosis | |
| KO/KO/E1 | Apoptosis | |
| E1/E1/KO | Apoptosis | |
| E1/KO/E1 | Senescence and Apoptosis | |
| KO/E1/E1 | Apoptosis | |
| E1/E1/E1 | Apoptosis | |
| miR-34a/E2F1/UFC1 | KO/KO/KO | Apoptosis |
| E1/KO/KO | Senescence and Apoptosis | |
| KO/E1/KO | Apoptosis | |
| KO/KO/E1 | Apoptosis | |
| E1/E1/KO | Apoptosis | |
| E1/KO/E1 | Senescence and Apoptosis | |
| KO/E1/E1 | Apoptosis | |
| E1/E1/E1 | Apoptosis | |
| miR-34a/Myc/ANRIL | KO/KO/KO | Apoptosis |
| E1/KO/KO | Senescence and Apoptosis | |
| KO/E1/KO | Apoptosis | |
| KO/KO/E1 | Apoptosis | |
| E1/E1/KO | Apoptosis | |
| E1/KO/E1 | Senescence and Apoptosis | |
| KO/E1/E1 | Apoptosis | |
| E1/E1/E1 | Apoptosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, S.; Hashimoto, R.F. Dynamical Analysis of a Boolean Network Model of the Oncogene Role of lncRNA ANRIL and lncRNA UFC1 in Non-Small Cell Lung Cancer. Biomolecules 2022, 12, 420. https://doi.org/10.3390/biom12030420
Gupta S, Hashimoto RF. Dynamical Analysis of a Boolean Network Model of the Oncogene Role of lncRNA ANRIL and lncRNA UFC1 in Non-Small Cell Lung Cancer. Biomolecules. 2022; 12(3):420. https://doi.org/10.3390/biom12030420
Chicago/Turabian StyleGupta, Shantanu, and Ronaldo F. Hashimoto. 2022. "Dynamical Analysis of a Boolean Network Model of the Oncogene Role of lncRNA ANRIL and lncRNA UFC1 in Non-Small Cell Lung Cancer" Biomolecules 12, no. 3: 420. https://doi.org/10.3390/biom12030420
APA StyleGupta, S., & Hashimoto, R. F. (2022). Dynamical Analysis of a Boolean Network Model of the Oncogene Role of lncRNA ANRIL and lncRNA UFC1 in Non-Small Cell Lung Cancer. Biomolecules, 12(3), 420. https://doi.org/10.3390/biom12030420







