Alcohol and Prostate Cancer: Time to Draw Conclusions
Abstract
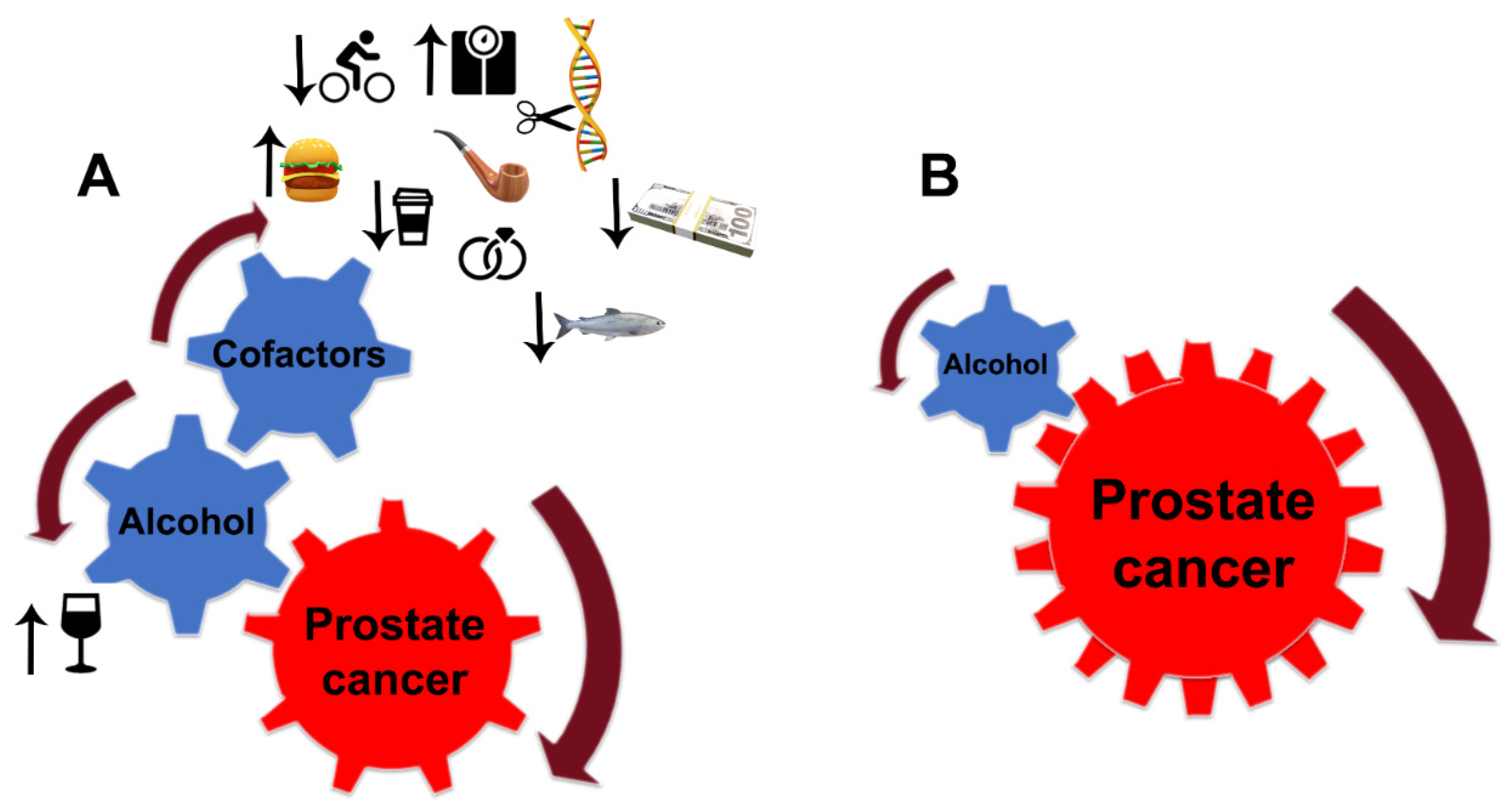
1. Introduction
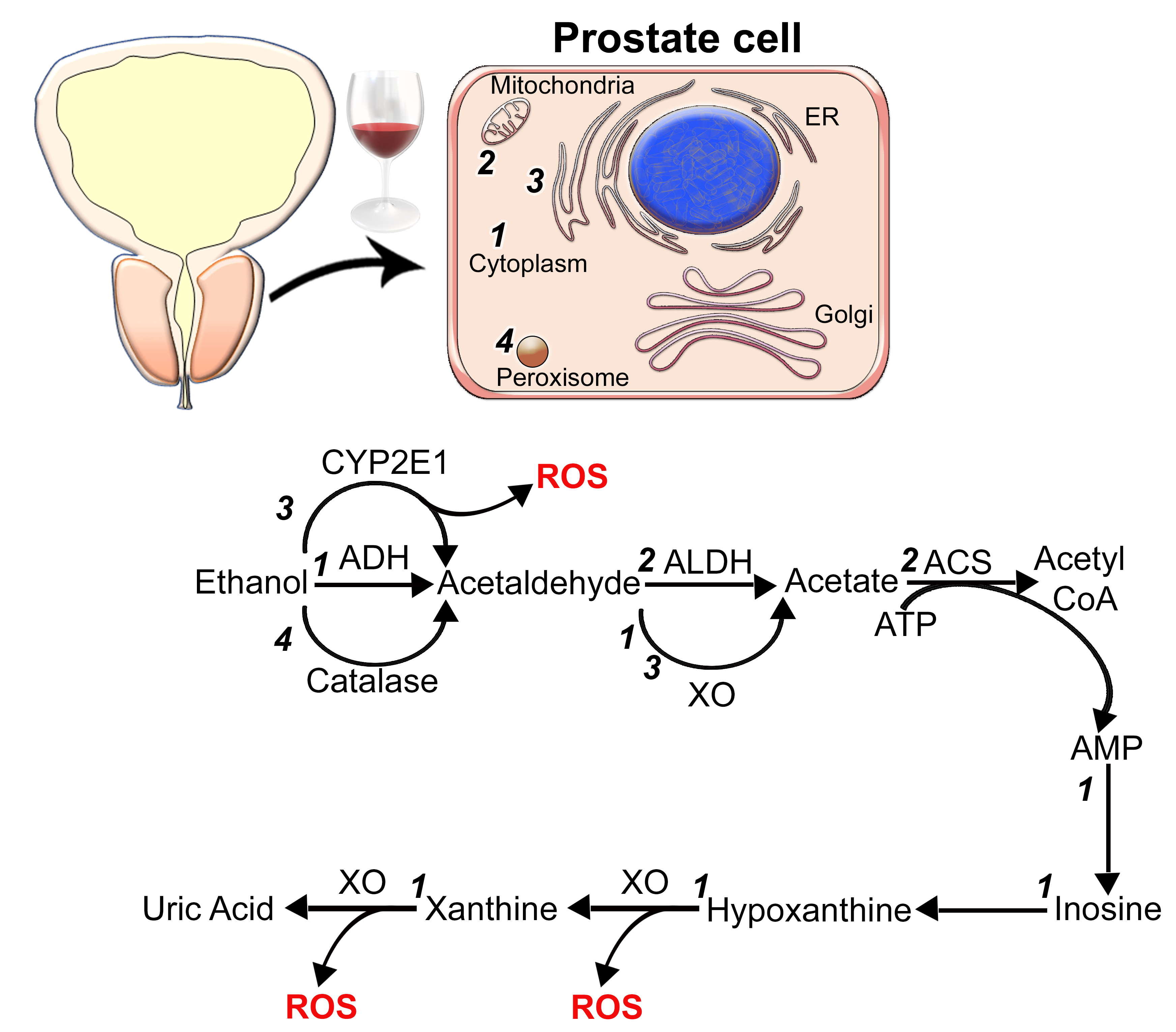
2. The Metabolic Role of Alcohol in PCa
3. Alcohol’s Influence on the Hormonal Status and Prostate Epithelium
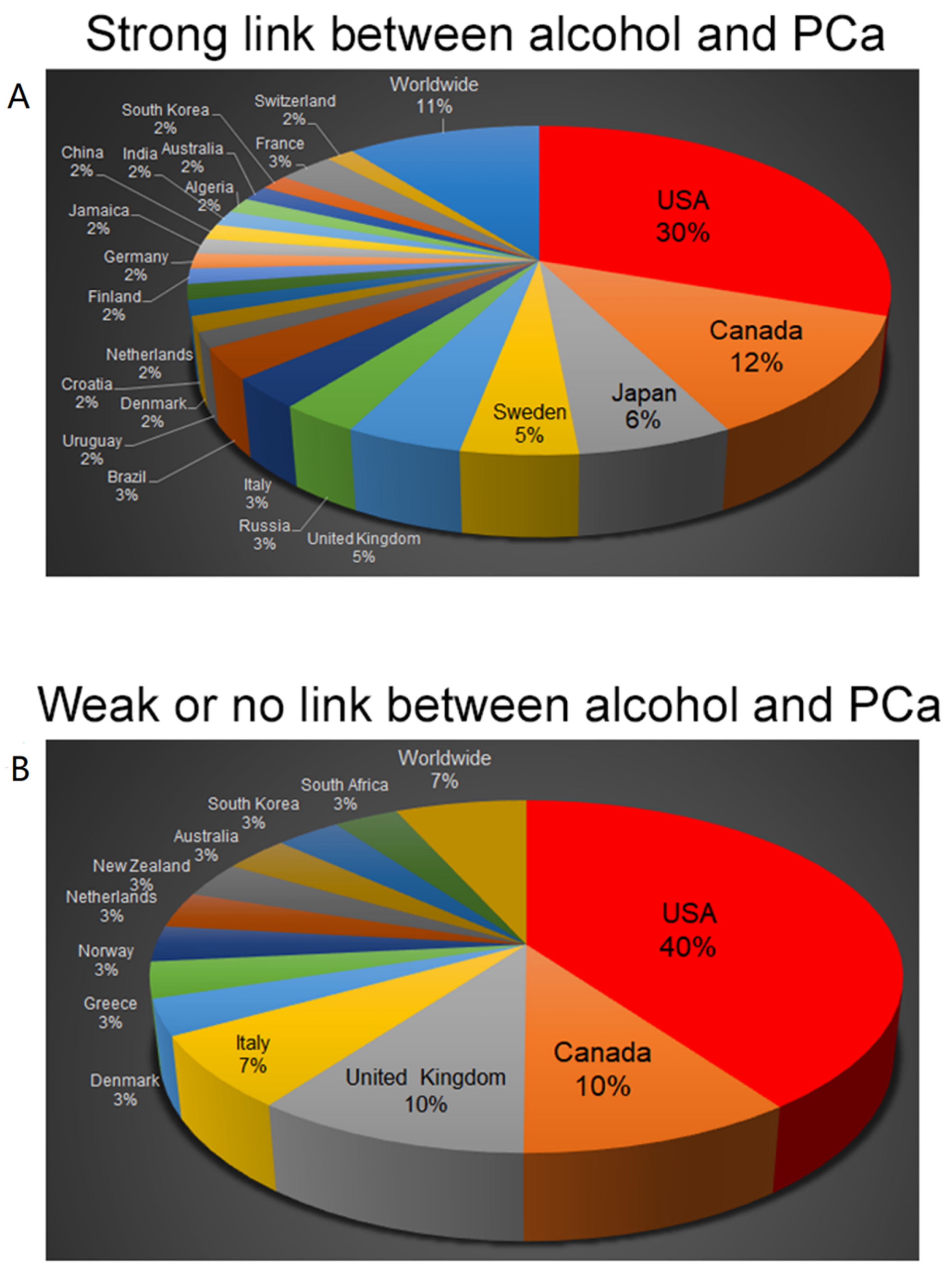
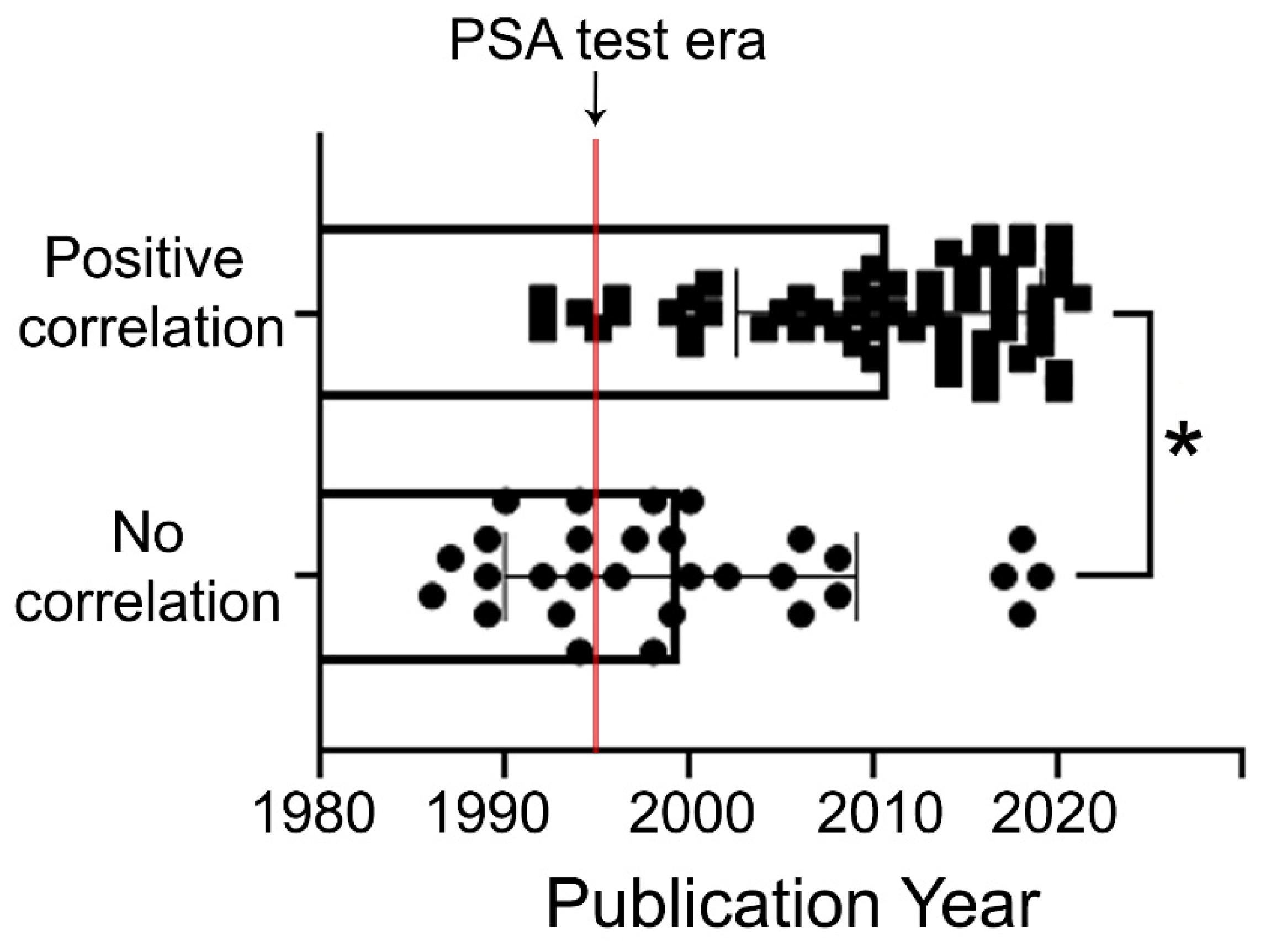
4. Epidemiological Studies Indicating the Link between Alcohol Consumption and the Risk for PCa
5. The Impact of Alcohol on the Aggressiveness of Prostate Tumors and PCa-Associated Death
6. Epidemiological Studies Indicating No Link between Alcohol Consumption and the Risk for PCa
7. Influence of Drinking Alcohol on PSA Levels and Risk of BPH
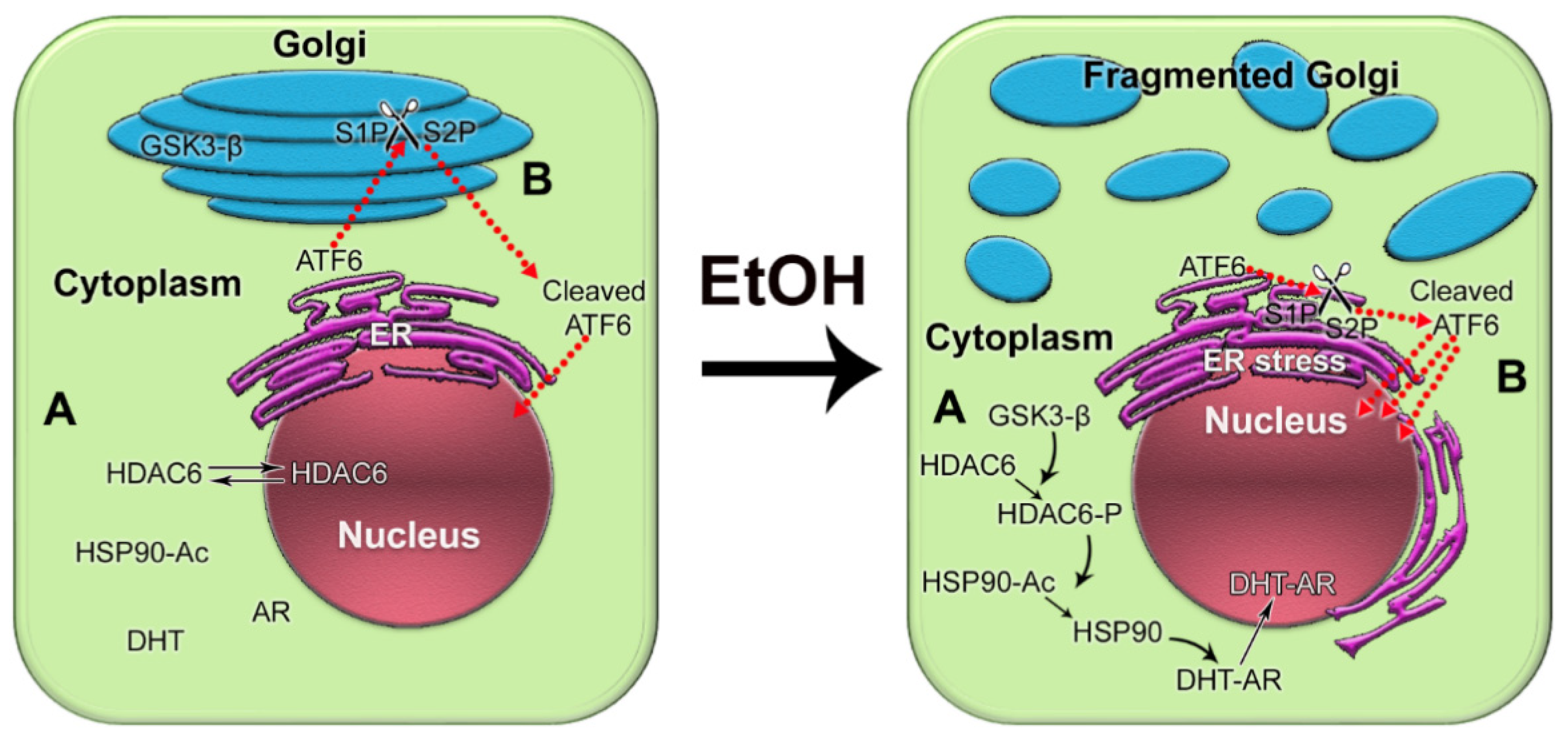
8. The Cellular Mechanisms of Alcohol’s Effect on the Progression of PCa
9. Alcohol’s Interference with Androgen Deprivation Therapy
10. Conclusions
Funding
Conflicts of Interest
Abbreviations
References
- Teissedre, P.-L.; Rasines-Perea, Z.; Ruf, J.-C.; Stockley, C.; Antoce, A.O.; Romano, R.; Fradera, U.; Kosti, R.I. Effects of alcohol consumption in general, and wine in particular, on the risk of cancer development: A review. OENO One 2020, 54, 813–832. [Google Scholar] [CrossRef]
- Agarwal, S.K. Alcohol and noncommunicable diseases: Part ii cancer, diabetes mellitus, kidney diseases, Alzheimer’s disease, arthritis. CMI 2021, 2, 185–200. [Google Scholar]
- Stockwell, T.; Solomon, R.; O’Brien, P.; Vallance, K.; Hobin, E. Cancer warning labels on alcohol containers: A consumer’s right to know, a government’s responsibility to inform, and an industry’s power to thwart. J. Stud. Alcohol. Drugs 2020, 81, 284–292. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Substance Abuse and Mental Health Services Administration (Samhsa). Table 2.1b—Tobacco Product and Alcohol Use in Lifetime, Past Year, and Past Month among Persons Aged 12 or Older, by Age Group: Percentages, 2018 and 2019; National Survey on Drug Use and Health (NSDUH). 2019. Available online: https://www.samhsa.gov/data/sites/default/files/reports/rpt29394/NSDUHDetailedTabs2019/NSDUHDetTabsSect2pe2019.htm#tab2-1b (accessed on 12 January 2022).
- NIH. Understanding Alcohol Use Disorder. 2021. Available online: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/understanding-alcohol-use-disorder (accessed on 12 January 2022).
- Lin, H.-Y.; Fisher, P.; Harris, D.; Tseng, T.-S. Alcohol Intake Patterns for Cancer and Non-Cancer Individuals: A population study. Transl. Cancer Res. 2019, 8, S334–S345. [Google Scholar] [CrossRef] [PubMed]
- Druesne-Pecollo, N.; Tehard, B.; Mallet, Y.; Gerber, M.; Norat, T.; Hercberg, S.; Latino-Martel, P. Alcohol and genetic polymorphisms: Effect on risk of alcohol-related cancer. Lancet Oncol. 2009, 10, 173–180. [Google Scholar] [CrossRef]
- Van Roekel, E.H.; Trijsburg, L.; Assi, N.; Carayol, M.; Achaintre, D.; Murphy, N.; Rinaldi, S.; Schmidt, J.A.; Stępień, M.; Kaaks, R.; et al. Circulating Metabolites Associated with Alcohol Intake in the European Prospective Investigation into Cancer and Nutrition Cohort. Nutrients 2018, 10, 654. [Google Scholar] [CrossRef] [PubMed]
- Dellarco, V.L. A mutagenicity assessment of acetaldehyde. Mutat. Res. Genet. Toxicol. 1988, 195, 1–20. [Google Scholar] [CrossRef]
- Vaca, C.E.; Fang, J.-L.; Schweda, E.K. Studies of the reaction of acetaldehyde with deoxynucleosides. Chem. Interact. 1995, 98, 51–67. [Google Scholar] [CrossRef]
- Diaz Gomez, M.I.; Fanelli, S.L.; Castro, G.D.; Costantini, M.H.; Castro, J.A. A liver nuclear ethanol metabolizing system. Formation of metabolites that bind covalently to macromolecules and lipids. Toxicology 1999, 138, 19–28. [Google Scholar] [CrossRef]
- Garro, A.J.; Espina, N.; Farinati, F.; Salvagnini, M. The effects of chronic ethanol consumption on carcinogen metabolism and on O6-methylguanine transferase-mediated repair of alkylated DNA. Alcohol. Clin. Exp. Res. 1986, 10, 73S–77S. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S. Alcohol and the liver: 1994 update. Gastroenterology 1994, 106, 1085–1105. [Google Scholar] [CrossRef]
- Wu, D.; Cederbaum, A.I. Alcohol, oxidative stress, and free radical damage. Alcohol Res. Health 2003, 27, 277–284. [Google Scholar]
- Baan, R.; Straif, K.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Altieri, A.; Cogliano, V. Carcinogenicity of alcoholic beverages. Lancet Oncol. 2007, 8, 292–293. [Google Scholar] [CrossRef]
- Orywal, K.; Jelski, W.; Werel, T.; Szmitkowski, M. The Diagnostic Significance of Serum Alcohol Dehydrogenase Isoenzymes and Aldehyde Dehydrogenase Activity in Prostate Cancer Patients. Anticancer Res. 2017, 37, 4961–4965. [Google Scholar] [CrossRef]
- Jelski, W.; Szmitkowski, M. Alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) in the cancer diseases. Clin. Chim. Acta 2008, 395, 1–5. [Google Scholar] [CrossRef]
- Van den Hoogen, C.; van der Horst, G.; Cheung, H.; Buijs, J.T.; Pelger, R.C.M.; van der Pluijm, G. The aldehyde dehydrogenase enzyme 7A1 is functionally involved in prostate cancer bone metastasis. Clin. Exp. Metastasis 2011, 28, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Orywal, K.; Jelski, W.; Werel, T.; Szmitkowski, M. The Diagnostic Significance of Serum Alcohol Dehydrogenase Isoenzymes and Aldehyde Dehydrogenase Activity in Urinary Bladder Cancer Patients. Anticancer Res. 2017, 37, 3537–3541. [Google Scholar] [CrossRef]
- Yan, J.; De Melo, J.; Cutz, J.-C.; Aziz, T.; Tang, D. Aldehyde dehydrogenase 3A1 associates with prostate tumorigenesis. Br. J. Cancer 2014, 110, 2593–2603. [Google Scholar] [CrossRef]
- Le Magnen, C.; Bubendorf, L.; Rentsch, C.A.; Mengus, C.; Gsponer, J.; Zellweger, T.; Rieken, M.; Thalmann, G.N.; Cecchini, M.G.; Germann, M.; et al. Characterization and Clinical Relevance of ALDHbright Populations in Prostate Cancer. Clin. Cancer Res. 2013, 19, 5361–5371. [Google Scholar] [CrossRef]
- Doherty, R.; Haywood-Small, S.; Sisley, K.; Cross, N. Aldehyde dehydrogenase activity selects for the holoclone phenotype in prostate cancer cells. Biochem. Biophys. Res. Commun. 2011, 414, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liang, C.; Bao, M.; Li, X.; Zhang, L.; Li, S.; Qin, C.; Shao, P.; Li, J.; Hua, L.; et al. ALDH1A3 correlates with luminal phenotype in prostate cancer. Tumor Biol. 2017, 39, 1010428317703652. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Salas, I.; Masiá, E.; Armiñán, A.; Calatrava, A.; Mancarella, C.; Rubio-Briones, J.; Scotlandi, K.; Vicent, M.J.; López-Guerrero, J.A. MiR-187 Targets the Androgen-Regulated Gene ALDH1A3 in Prostate Cancer. PLoS ONE 2015, 10, e0125576. [Google Scholar] [CrossRef] [PubMed]
- Trasino, S.E.; Harrison, E.H.; Wang, T.T.Y. Androgen regulation of aldehyde dehydrogenase 1A3 (ALDH1A3) in the androgen-responsive human prostate cancer cell line LNCaP. Exp. Biol. Med. 2007, 232, 762–771. [Google Scholar]
- Li, T.; Su, Y.; Mei, Y.; Leng, Q.; Leng, B.; Liu, Z.; Stass, S.A.; Jiang, F. ALDH1A1 is a marker for malignant prostate stem cells and predictor of prostate cancer patients’ outcome. Lab. Investig. 2009, 90, 234–244. [Google Scholar] [CrossRef]
- Quattrini, L.; Sadiq, M.; Petrarolo, G.; Maitland, N.J.; Frame, F.M.; Pors, K.; La Motta, C. Aldehyde Dehydrogenases and Prostate Cancer: Shedding Light on Isoform Distribution to Reveal Druggable Target. Biomedicines 2020, 8, 569. [Google Scholar] [CrossRef]
- van den Hoogen, C.; van der Horst, G.; Cheung, H.; Buijs, J.T.; Lippitt, J.M.; Guzmán-Ramírez, N.; Hamdy, F.C.; Eaton, C.L.; Thalmann, G.N.; Cecchini, M.G.; et al. High Aldehyde Dehydrogenase Activity Identifies Tumor-Initiating and Metastasis-Initiating Cells in Human Prostate Cancer. Cancer Res. 2010, 70, 5163–5173. [Google Scholar] [CrossRef]
- Kim, H.; Lapointe, J.; Kaygusuz, G.; Ong, D.E.; Li, C.; van de Rijn, M.; Brooks, J.D.; Pollack, J.R. The Retinoic Acid Synthesis GeneALDH1a2Is a Candidate Tumor Suppressor in Prostate Cancer. Cancer Res. 2005, 65, 8118–8124. [Google Scholar] [CrossRef]
- Brunner, C.; Davies, N.M.; Martin, R.M.; Eeles, R.; Easton, D.; Kote-Jarai, Z.; Al Olama, A.A.; Benlloch, S.; Muir, K.; Giles, G.; et al. Alcohol consumption and prostate cancer incidence and progression: A Mendelian randomisation study. Int. J. Cancer 2016, 140, 75–85. [Google Scholar] [CrossRef]
- Clark, D.W.; Palle, K. Aldehyde dehydrogenases in cancer stem cells: Potential as therapeutic targets. Ann. Transl. Med. 2016, 4, 518. [Google Scholar] [CrossRef]
- Chen, M.-C.; Hsu, S.-L.; Lin, H.; Yang, T.-Y. Retinoic acid and cancer treatment. BioMedicine 2014, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Brocker, C.; Koppaka, V.; Chen, Y.; Jackson, B.C.; Matsumoto, A.; Thompson, D.C.; Vasiliou, V. Aldehyde dehydrogenases in cellular responses to oxidative/electrophilic stress. Free Radic. Biol. Med. 2013, 56, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Cojoc, M.; Peitzsch, C.; Kurth, I.; Trautmann, F.; Kunz-Schughart, L.A.; Telegeev, G.D.; Stakhovsky, E.A.; Walker, J.R.; Simin, K.; Lyle, S.; et al. Aldehyde dehydrogenase is regulated by beta-catenin/tcf and promotes radioresistance in prostate cancer progenitor cells. Cancer Res. 2015, 75, 1482–1494. [Google Scholar] [CrossRef]
- Khandrika, L.; Kumar, B.; Koul, S.; Maroni, P.; Koul, H.K. Oxidative stress in prostate cancer. Cancer Lett. 2009, 282, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Castro, G.D.; Delgado de Layno, A.M.; Costantini, M.H.; Castro, J.A. Rat ventral prostate xanthine oxidase bioactivation of ethanol to acetaldehyde and 1-hydroxyethyl free radicals: Analysis of its potential role in heavy alcohol drinking tumor-promoting effects. Teratog. Carcinog. Mutagen. 2001, 21, 109–119. [Google Scholar] [CrossRef]
- Castro, G.; de Layño, A.D.; Costantini, M.; Castro, J. Rat ventral prostate microsomal biotransformation of ethanol to acetaldehyde and 1-hydroxyethyl radicals: Its potential contribution to prostate tumor promotion. Teratog. Carcinog. Mutagen. 2002, 22, 335–341. [Google Scholar] [CrossRef]
- Castro, G.D.; Costantini, M.H.; Castro, J.A. Rat ventral prostate xanthine oxidase–mediated metabolism of acetaldehyde to acetyl radical. Hum. Exp. Toxicol. 2009, 28, 203–208. [Google Scholar] [CrossRef]
- Veljković, A.; Hadži-Dokić, J.; Sokolović, D.; Bašić, D.; Veličković-Janković, L.; Stojanović, M.; Popović, D.; Kocić, G. Xanthine Oxidase/Dehydrogenase Activity as a Source of Oxidative Stress in Prostate Cancer Tissue. Diagnostics 2020, 10, 668. [Google Scholar] [CrossRef]
- Kato, S.; Kawase, T.; Alderman, J.; Inatomi, N.; Lieber, C.S. Role of xanthine oxidase in ethanol-induced lipid peroxidation in rats. Gastroenterology 1990, 98, 203–210. [Google Scholar] [CrossRef]
- Paschos, A.; Pandya, R.; Duivenvoorden, W.C.M.; Pinthus, J.H. Oxidative stress in prostate cancer: Changing research concepts towards a novel paradigm for prevention and therapeutics. Prostate Cancer Prostatic Dis. 2013, 16, 217–225. [Google Scholar] [CrossRef]
- Biri, H.; Öztürk, H.S.; Kaçmaz, M.; Karaca, K.; Tokuçoglu, H.; Durak, I. Activities of DNA Turnover and Free Radical Metabolizing Enzymes in Cancerous Human Prostate Tissue. Cancer Investig. 1999, 17, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Strmiska, V.; Michalek, P.; Eckschlager, T.; Stiborova, M.; Adam, V.; Krizkova, S.; Heger, Z. Prostate cancer-specific hallmarks of amino acids metabolism: Towards a paradigm of precision medicine. Biochim. Biophys. Acta 2019, 1871, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Cherukuri, M.K.; Choyke, P.L. Metabolic reprogramming in prostate cancer. Br. J. Cancer 2021, 125, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Gann, P.H.; Hennekens, C.H.; Ma, J.; Longcope, C.; Stampfer, M.J. Prospective Study of Sex Hormone Levels and Risk of Prostate Cancer. JNCI J. Natl. Cancer Inst. 1996, 88, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.A.; Kovi, J.; Heshmat, M.Y.; Jones, G.W.; Rao, M.S.; Ahluwalia, B.S. Factors involved in the high incidence of prostatic cancer among American blacks. Prog. Clin. Biol. Res. 1981, 53, 111–132. [Google Scholar]
- Andersson, S.O.; Baron, J.; Bergström, R.; Lindgren, C.; Wolk, A.; Adami, H.O. Lifestyle factors and prostate cancer risk: A case-control study in Sweden. Cancer Epidemiol. Biomark. Prev. 1996, 5, 509–513. [Google Scholar]
- Sarkola, T.; Eriksson, C.J. Testosterone increases in men after a low dose of alcohol. Alcohol. Clin. Exp. Res. 2003, 27, 682–685. [Google Scholar] [CrossRef]
- Gordon, G.G.; Altman, K.; Southren, A.L.; Rubin, E.; Lieber, C.S. Effect of Alcohol (Ethanol) Administration on Sex-Hormone Metabolism in Normal Men. N. Engl. J. Med. 1976, 295, 793–797. [Google Scholar] [CrossRef]
- Rao, M.; Zuo, L.-D.; Fang, F.; Martin, K.; Zheng, Y.; Zhang, H.-P.; Li, H.-G.; Zhu, C.-H.; Xiong, C.-L.; Guan, H.-T. Association of Alcohol Consumption with Markers of Prostate Health and Reproductive Hormone Profiles: A Multi-Center Study of 4535 Men in China. PLoS ONE 2015, 10, e0142780. [Google Scholar] [CrossRef]
- Stålenheim, E.; Eriksson, E.; Von Knorring, L.; Wide, L. Testosterone as a biological marker in psychopathy and alcoholism. Psychiatry Res. 1998, 77, 79–88. [Google Scholar] [CrossRef]
- Ida, Y.; Tsujimaru, S.; Nakamaura, K.; Shirao, I.; Mukasa, H.; Egami, H.; Nakazawa, Y. Effects of acute and repeated alcohol ingestion on hypothalamic-pituitary-gonadal and hypothalamic-pituitary-adrenal functioning in normal males. Drug Alcohol Depend. 1992, 31, 57–64. [Google Scholar] [CrossRef]
- Kapcala, L.P. Alcohol-induced pseudo-cushing’s syndrome mimicking cushing’s disease in a patient with an adrenal mass. Am. J. Med. 1987, 82, 849–856. [Google Scholar] [CrossRef]
- Bannister, P.; Losowsky, M.S.; Lowosky, M.S. Ethanol and hypogonadism. Alcohol Alcohol. 1987, 22, 213–217. [Google Scholar] [PubMed]
- Sinha, R.; Fox, H.C.; Hong, K.-I.A.; Hansen, J.; Tuit, K.; Kreek, M.J. Effects of Adrenal Sensitivity, Stress- and Cue-Induced Craving, and Anxiety on Subsequent Alcohol Relapse and Treatment Outcomes. Arch. Gen. Psychiatry 2011, 68, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Green, G.R. Mechanism of hypogonadism in cirrhotic males. Gut 1977, 18, 843–853. [Google Scholar] [CrossRef]
- Frea, B.; Annoscia, S.; Stanta, G.; Lozzi, C.; Carmignani, G. Correlation between liver cirrhosis and benign prostatic hyperplasia: A morphological study. Urol. Res. 1987, 15, 843–853. [Google Scholar] [CrossRef]
- Salonen, I.; Huhtaniemi, I. Effects of Chronic Ethanol Diet on Pituitary-Testicular Function of the Rat1. Biol. Reprod. 1990, 42, 55–62. [Google Scholar] [CrossRef]
- Fávaro, W.J.; Cagnon, V.H.A. Immunolocalization of androgen and oestrogen receptors in the ventral lobe of rat (Rattus norvegicus) prostate after long-term treatment with ethanol and nicotine. Int. J. Androl. 2008, 31, 609–618. [Google Scholar] [CrossRef]
- Tadic, S.D.; Elm, M.S.; Subbotin, V.M.; Eagon, P.K. Hypogonadism precedes liver feminization in chronic alcohol-fed male rats. Hepatology 2000, 31, 1135–1140. [Google Scholar] [CrossRef]
- Martinez, F.E.; Laura, I.A.; Martinez, M.; Padovani, C.R.; Bustos-Obregón, E. Morphology of the ventral lobe of the prostate and seminal vesicles in an ethanol-drinking strain of rats (UChA and UChB). J. Submicrosc. Cytol. Pathol. 2001, 33, 99–106. [Google Scholar]
- Anderson, R.A.; Phillips, J.F.; Zaneveld, L.J. Chronic Ethanol Ingestion During Puberty Alters the Transient Increase in Testicular 5α-Reductase in the Swiss-Webster Mouse. J. Androl. 1989, 10, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Endogenous Hormones and Prostate Cancer Collaborative Group; Roddam, A.W.; Allen, N.E.; Appleby, P.; Key, T.J. Endogenous sex hormones and prostate cancer: A collaborative analysis of 18 prospective studies. J. Natl. Cancer Inst. 2008, 100, 170–183. [Google Scholar] [PubMed]
- Michaud, J.E.; Billups, K.L.; Partin, A.W. Testosterone and prostate cancer: An evidence-based review of pathogenesis and oncologic risk. Ther. Adv. Urol. 2015, 7, 378–387. [Google Scholar] [CrossRef]
- Shiels, M.S.; Rohrmann, S.; Menke, A.; Selvin, E.; Crespo, C.J.; Rifai, N.; Dobs, A.; Feinleib, M.; Guallar, E.; Platz, E.A. Association of cigarette smoking, alcohol consumption, and physical activity with sex steroid hormone levels in US men. Cancer Causes Control 2009, 20, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Cagnon, V.H.; Tomazini, F.M.; Garcia, P.J.; Martinez, M.; Padovani, C.R.; Martinez, F.E. Structure and ultrastructure of the ventral prostate of isogenic mice (c57b1/6j) submitted to chronic alcohol ingestion. Tissue Cell 2001, 33, 354–360. [Google Scholar] [CrossRef]
- Gumus, E.; Solakoglu, S.; Mutus, R.; Altay, B.; Kiziler, A.R.; Miroglu, C. Effect of Acute Alcohol Intake on Prostate Tissue and Serum PSA-Like Protein Levels in Rats. Urol. Int. 2005, 75, 50–56. [Google Scholar] [CrossRef]
- Cagnon, V.; Garcia, P.J.; Filho, J.G.; Martinez, F.E.; Mello, W.; Martinez, M. Ultrastructural study of the lateral lobe of the prostate of Wistar rats submitted to experimental chronic alcohol ingestion. J. Submicrosc. Cytol. Pathol. 1998, 30, 77–84. [Google Scholar]
- Gómez, M.I.D.; De Castro, C.R.; Fanelli, S.L.; Quintans, L.; Costantini, M.H.; Castro, J.A.; Castro, G.D. Biochemical and ultrastructural alterations in the rat ventral prostate due to repetitive alcohol drinking. J. Appl. Toxicol. 2007, 27, 391–398. [Google Scholar] [CrossRef]
- Liu, F.; Liu, L.; Wang, Z.; Chen, L.; Yu, J.; Xu, X. The role of ethanol in the pathogenesis of non-bacterial prostatitis. Mol. Med. Rep. 2019, 19, 3848–3854. [Google Scholar] [CrossRef]
- Murugan, S.; Zhang, C.; Mojtahedzadeh, S.; Sarkar, D.K. Alcohol Exposure in Utero Increases Susceptibility to Prostate Tumorigenesis in Rat Offspring. Alcohol. Clin. Exp. Res. 2013, 37, 1901–1909. [Google Scholar] [CrossRef]
- Adami, H.-O.; McLaughlin, J.K.; Hsing, A.W.; Wolk, A.; Ekbom, A.; Holmberg, L.; Persson, I. Alcoholism and cancer risk: A population-based cohort study. Cancer Causes Control 1992, 3, 419–425. [Google Scholar] [CrossRef]
- Ellison, L.F. Tea and other beverage consumption and prostate cancer risk: A canadian retrospective cohort study. Eur. J. Cancer Prev. 2000, 9, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, T. Life-style and cancer: From epidemiological evidence to public behavior change to mortality reduction of target cancers. J. Natl. Cancer Inst. Monogr. 1992, 12, 65–74. [Google Scholar]
- Tønnesen, H.; Moller, H.; Andersen, J.; Jensen, E.; Juel, K. Cancer morbidity in alcohol abusers. Br. J. Cancer 1994, 69, 327–332. [Google Scholar] [CrossRef]
- Sharpe, C.R.; Siemiatycki, J. Case-control study of alcohol consumption and prostate cancer risk in Montreal, Canada. Cancer Causes Control 2001, 12, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.B.; Brown, L.M.; Schoenberg, J.B.; Greenberg, R.S.; Silverman, D.T.; Schwartz, A.G.; Swanson, G.M.; Benichou, J.; Liff, J.M.; Hoover, R.N.; et al. Alcohol Use and Prostate Cancer Risk in US Blacks and Whites. Am. J. Epidemiol. 1996, 143, 692–697. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dotson, S.; Howard, M.D.; Aung, M.; Keenan, J.; Jolly, P. The Epidemiology of Prostate Cancer in Western Jamaica: Risk Factors, Knowledge, Attitudes and Practices. West Indian Med. J. 2015, 65, 60–66. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jia, Y.; Sun, X.-Q.; Gao, J.-G.; Zhu, L.-Y.; Weng, B.-W.; Liu, Z.-J.; Zhu, H.; Qiu, Z.-L.; Hou, S.-C. Risk factors of prostate cancer in urban Qingdao: A case-control study. Zhonghua Nan Ke Xue Natl. J. Androl. 2013, 19, 694–698. [Google Scholar]
- Galić, J.; Simunović, D. Prostate disease prevalence with epidemiological and hormonal analysis in randomly selected male population in Croatia. Coll. Antropol. 2008, 32, 1195–1202. [Google Scholar]
- Ziouziou, I.; Touzani, A.M.; Lahlou, L.; Shariat, S.F.; Sanguedolce, F.; Neuzillet, Y.; Ajdi, F.; Khabbal, Y. Association of Prostate Cancer with Nuts, Seeds, Alcohol and Processed Meats: A Worldwide Population-Based Study. Nutr. Cancer 2020, 73, 2538–2545. [Google Scholar] [CrossRef]
- Dennis, L.K. Meta-analysis for combining relative risks of alcohol consumption and prostate cancer. Prostate 2000, 42, 56–66. [Google Scholar] [CrossRef]
- Sesso, H.D.; Paffenbarger, R.S.; Lee, I.-M. Alcohol consumption and risk of prostate cancer: The Harvard Alumni Health Study. Int. J. Epidemiol. 2001, 30, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Sarich, P.; Canfell, K.; Banks, E.; Paige, E.; Egger, S.; Joshy, G.; Korda, R.; Weber, M. A Prospective Study of Health Conditions Related to Alcohol Consumption Cessation Among 97,852 Drinkers Aged 45 and Over in Australia. Alcohol. Clin. Exp. Res. 2019, 43, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Stockwell, T.; Roemer, A.; Chikritzhs, T. Is alcohol consumption a risk factor for prostate cancer? A systematic review and meta–analysis. BMC Cancer 2016, 16, 845. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Ko, M.J.; Han, J.T. Alcohol consumption and mortality from all-cause and cancers among 1.34 million koreans: The results from the korea national health insurance corporation’s health examinee cohort in 2000. Cancer Causes Control 2010, 21, 2295–2302. [Google Scholar] [CrossRef] [PubMed]
- Caprio, G.G.; Picascia, D.; Dallio, M.; Vitiello, P.P.; Giunta, E.F.; De Falco, V.; Abenavoli, L.; Procopio, A.C.; Famiglietti, V.; Martinelli, E.; et al. Light Alcohol Drinking and the Risk of Cancer Development: A Controversial Relationship. Rev. Recent Clin. Trials 2020, 15, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Zaitsu, M.; Takeuchi, T.; Kobayashi, Y.; Kawachi, I. Light to moderate amount of lifetime alcohol consumption and risk of cancer in Japan. Cancer 2019, 126, 1031–1040. [Google Scholar] [CrossRef]
- Azevedo e Silva, G.; de Rezende, L.F.; Gomes Fda, S.; de Souza Junior, P.R.; Szwarcwald, C.L.; Eluf Neto, J. Lifestyle among former cancer patients in brazil in 2013. Cien. Saude Colet. 2016, 21, 379–388. [Google Scholar]
- Lassed, S.; Deus, C.; Lourenço, N.; Dahdouh, A.; Rizvanov, A.A.; Oliveira, P.J.; Zama, D. Diet, Lifestyles, Family History, and Prostate Cancer Incidence in an East Algerian Patient Group. BioMed Res. Int. 2016, 2016, 5730569. [Google Scholar] [CrossRef]
- Watters, J.L.; Park, Y.; Hollenbeck, A.; Schatzkin, A.; Albanes, D. Alcoholic Beverages and Prostate Cancer in a Prospective US Cohort Study. Am. J. Epidemiol. 2010, 172, 773–780. [Google Scholar] [CrossRef]
- Putnam, S.D.; Cerhan, J.R.; Parker, A.S.; Bianchi, G.D.; Wallace, R.B.; Cantor, K.P.; Lynch, C.F. Lifestyle and Anthropometric Risk Factors for Prostate Cancer in a Cohort of Iowa Men. Ann. Epidemiol. 2000, 10, 361–369. [Google Scholar] [CrossRef]
- Platz, E.A.; Leitzmann, M.F.; Rimm, E.B.; Willett, W.C.; Giovannucci, E. Alcohol Intake, Drinking Patterns, and Risk of Prostate Cancer in a Large Prospective Cohort Study. Am. J. Epidemiol. 2004, 159, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Lindström, S.; Schumacher, F.; Siddiq, A.; Travis, R.C.; Campa, D.; Berndt, S.I.; Diver, W.R.; Severi, G.; Allen, N.; Andriole, G.; et al. Characterizing Associations and SNP-Environment Interactions for GWAS-Identified Prostate Cancer Risk Markers—Results from BPC3. PLoS ONE 2011, 6, e17142. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, D.; Li, Y.; Stockwell, T.; Ma, J. Alcohol consumption and incidence of prostate cancer among Chinese people: A systematic review and meta-analysis. Int. J. Alcohol Drug Res. 2020, 8, 12–28. [Google Scholar] [CrossRef]
- Tyagi, B.; Manoharan, N.; Raina, V. A case control study on prostate cancer in Delhi. Asian Pac. J. Cancer Prev. 2010, 11, 397–401. [Google Scholar]
- Kovshik, Y.B.; Savrova, O.B. Prostate Cancer Risk Factor Comparative Analysis. 2017. Available online: https://elibrary.ru/item.asp?id=32338061 (accessed on 19 February 2022).
- Kaprin, A.D.; Aleksandrovich, A.A.; Kulchenko, N.G.; Tolkachev, A.O. Prostate Cancer Screening Based on Multivariant Analysis. 2014. Available online: https://www.elibrary.ru/item.asp?id=23611200 (accessed on 19 February 2022).
- Russo, G.I.; Solinas, T.; Urzì, D.; Privitera, S.; Campisi, D.; Cocci, A.; Carini, M.; Madonia, M.; Cimino, S.; Morgia, G. Adherence to Mediterranean diet and prostate cancer risk in Sicily: Population-based case–control study. Int. J. Impot. Res. 2018, 31, 269–275. [Google Scholar] [CrossRef] [PubMed]
- de Menezes, R.F.; Bergmann, A.; de Aguiar, S.S.; Thuler, L.C. Alcohol consumption and the risk of cancer in brazil: A study involving 203,506 cancer patients. Alcohol 2015, 49, 747–751. [Google Scholar] [CrossRef]
- Sutcliffe, S.; Giovannucci, E.; Leitzmann, M.F.; Rimm, E.B.; Stampfer, M.J.; Willett, W.C.; Platz, E.A. A prospective cohort study of red wine consumption and risk of prostate cancer. Int. J. Cancer 2007, 120, 1529–1535. [Google Scholar] [CrossRef]
- Lall, R.K.; Syed, D.N.; Adhami, V.M.; Khan, M.I.; Mukhtar, H. Dietary Polyphenols in Prevention and Treatment of Prostate Cancer. Int. J. Mol. Sci. 2015, 16, 3350–3376. [Google Scholar] [CrossRef]
- Kampa, M.; Hatzoglou, A.; Notas, G.; Damianaki, A.; Bakogeorgou, E.; Gemetzi, C.; Kouroumalis, E.; Martin, P.-M.; Castanas, E. Wine Antioxidant Polyphenols Inhibit the Proliferation of Human Prostate Cancer Cell Lines. Nutr. Cancer 2000, 37, 223–233. [Google Scholar] [CrossRef]
- Schuurman, A.G.; Goldbohm, R.A.; Brandt, P.A.V.D. A prospective cohort study on consumption of alcoholic beverages in relation to prostate cancer incidence (The Netherlands). Cancer Causes Control 1999, 10, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Velicer, C.M.; Kristal, A.; White, E. Alcohol Use and the Risk of Prostate Cancer: Results from the VITAL Cohort Study. Nutr. Cancer 2006, 56, 50–56. [Google Scholar] [CrossRef]
- Schoonen, W.M.; Salinas, C.A.; Kiemeney, L.A.; Stanford, J.L. Alcohol consumption and risk of prostate cancer in middle-aged men. Int. J. Cancer 2004, 113, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Downer, M.K.; Kenfield, S.A.; Stampfer, M.J.; Wilson, K.M.; Dickerman, B.A.; Giovannucci, E.L.; Rimm, E.B.; Wang, M.; Mucci, L.A.; Willett, W.C.; et al. Alcohol Intake and Risk of Lethal Prostate Cancer in the Health Professionals Follow-Up Study. J. Clin. Oncol. 2019, 37, 1499–1511. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Haque, R.; Van Den Eeden, S.K.; Caan, B.J.; Poon, K.Y.; Quinn, V.P. Red wine consumption and risk of prostate cancer: The california men’s health study. Int. J. Cancer 2010, 126, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Vartolomei, M.D.; Kimura, S.; Ferro, M.; Foerster, B.; Abufaraj, M.; Briganti, A.; Karakiewicz, P.I.; Shariat, S. The impact of moderate wine consumption on the risk of developing prostate cancer. Clin. Epidemiol. 2018, 10, 431–444. [Google Scholar] [CrossRef]
- Demoury, C.; Karakiewicz, P.; Parent, M.-E. Association between lifetime alcohol consumption and prostate cancer risk: A case-control study in Montreal, Canada. Cancer Epidemiol. 2016, 45, 11–17. [Google Scholar] [CrossRef]
- De Stéfani, E.; Fierro, L.; Barrios, E.; Ronco, A. Tobacco, Alcohol, Diet and Risk of prostate Cancer. Tumori J. 1995, 81, 315–320. [Google Scholar] [CrossRef]
- Gong, Z.; Kristal, A.R.; Schenk, J.M.; Tangen, C.M.; Goodman, P.J.; Thompson, I.M. Alcohol consumption, finasteride, and prostate cancer risk: Results from the prostate cancer prevention trial. Cancer 2009, 115, 3661–3669. [Google Scholar] [CrossRef]
- Dickerman, B.A.; Markt, S.C.; Koskenvuo, M.; Pukkala, E.; Mucci, L.A.; Kaprio, J. Alcohol intake, drinking patterns, and prostate cancer risk and mortality: A 30-year prospective cohort study of Finnish twins. Cancer Causes Control 2016, 27, 1049–1058. [Google Scholar] [CrossRef]
- Zuccolo, L.; Lewis, S.J.; Donovan, J.L.; Hamdy, F.C.; Neal, D.E.; Smith, G.D. Alcohol consumption and psa-detected prostate cancer risk—A case-control nested in the protect study. Int. J. Cancer 2013, 132, 2176–2185. [Google Scholar] [CrossRef] [PubMed]
- Fowke, J.H.; Howard, L.; Andriole, G.L.; Freedland, S.J. Alcohol Intake Increases High-grade Prostate Cancer Risk Among Men Taking Dutasteride in the REDUCE Trial. Eur. Urol. 2014, 66, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Sawada, N.; Inoue, M.; Iwasaki, M.; Sasazuki, S.; Yamaji, T.; Shimazu, T.; Tsugane, S. Alcohol and smoking and subsequent risk of prostate cancer in Japanese men: The Japan Public Health Center-based prospective study. Int. J. Cancer 2013, 134, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Farris, M.S.; Courneya, K.S.; Kopciuk, K.A.; McGregor, S.E.; Friedenreich, C.M. Post-diagnosis alcohol intake and prostate cancer survival: A population-based cohort study. Int. J. Cancer 2018, 143, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Breslow, R.A.; Chen, C.M.; Graubard, B.I.; Mukamal, K.J. Prospective Study of Alcohol Consumption Quantity and Frequency and Cancer-Specific Mortality in the US Population. Am. J. Epidemiol. 2011, 174, 1044–1053. [Google Scholar] [CrossRef]
- Papa, N.P.; MacInnis, R.J.; Jayasekara, H.; English, D.R.; Bolton, D.; Davis, I.D.; Lawrentschuk, N.; Millar, J.L.; Pedersen, J.; Severi, G.; et al. Total and beverage-specific alcohol intake and the risk of aggressive prostate cancer: A case–control study. Prostate Cancer Prostatic Dis. 2017, 20, 305–310. [Google Scholar] [CrossRef]
- Sawada, N. Risk and preventive factors for prostate cancer in Japan: The Japan Public Health Center-based prospective (JPHC) study. J. Epidemiol. 2017, 27, 2–7. [Google Scholar] [CrossRef]
- Penuelas, J.; Krisztin, T.; Obersteiner, M.; Huber, F.; Winner, H.; Janssens, I.A.; Ciais, P.; Sardans, J. Country-Level Relationships of the Human Intake of N and P, Animal and Vegetable Food, and Alcoholic Beverages with Cancer and Life Expectancy. Int. J. Environ. Res. Public Health 2020, 17, 7240. [Google Scholar] [CrossRef]
- Michael, J.; Howard, L.E.; Markt, S.; De Hoedt, A.; Bailey, C.; Mucci, L.A.; Freedland, S.J.; Allott, E.H. Early-Life Alcohol Intake and High-Grade Prostate Cancer: Results from an Equal-Access, Racially Diverse Biopsy Cohort. Cancer Prev. Res. 2018, 11, 621–628. [Google Scholar] [CrossRef]
- Alattas, M.; Ross, C.S.; Henehan, E.R.; Naimi, T.S. Alcohol policies and alcohol-attributable cancer mortality in U.S. States. Chem. Interact. 2019, 315, 108885. [Google Scholar] [CrossRef]
- Kunzmann, A.T.; Coleman, H.G.; Huang, W.-Y.; Berndt, S.I. The association of lifetime alcohol use with mortality and cancer risk in older adults: A cohort study. PLoS Med. 2018, 15, e1002585. [Google Scholar] [CrossRef]
- Grundmann, E. Cancer morbidity and mortality in USA Mormons and Seventh-day Adventists. Arch Anat. Cytol. Pathol. 1992, 40, 73–78. [Google Scholar]
- Hassanipour, S.; Mohammadian-Hafshejani, A.; Ghoncheh, M.; Towhidi, F.; Jamehshorani, S.; Salehiniya, H. Incidence and mortality of prostate cancer and their relationship with the Human Development Index worldwide. Prostate Int. 2016, 4, 118–124. [Google Scholar] [CrossRef]
- WHO. Alcohol, Recorded Per Capita (15+) Consumption (in Litres of Pure Alcohol). Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/alcohol-recorded-per-capita-(15-)-consumption-(in-litres-of-pure-alcohol) (accessed on 19 February 2022).
- Colli, J.L.; Colli, A. International comparisons of prostate cancer mortality rates with dietary practices and sunlight levels. Urol. Oncol. Semin. Orig. Investig. 2006, 24, 184–194. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Wang, X.; Tseng, T.-S.; Kao, Y.-H.; Fang, Z.; Molina, P.E.; Cheng, C.-H.; Berglund, A.E.; Eeles, R.A.; Muir, K.R.; et al. Alcohol Intake and Alcohol–SNP Interactions Associated with Prostate Cancer Aggressiveness. J. Clin. Med. 2021, 10, 553. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, J.; Li, T.; He, Y.; Wang, J.; Xie, L.; Qin, X.; Li, S. Association between Tumor necrosis factor-alpha gene polymorphisms and prostate cancer risk: A meta-analysis. Diagn. Pathol. 2014, 9, 74. [Google Scholar] [CrossRef]
- Shao, N.; Xu, B.; Mi, Y.-Y.; Hua, L.-X. IL-10 polymorphisms and prostate cancer risk: A meta-analysis. Prostate Cancer Prostatic Dis. 2011, 14, 129–135. [Google Scholar] [CrossRef][Green Version]
- Bandil, K.; Singhal, P.; Dogra, A.; Bharadwaj, M.; Rawal, S.K.; Doval, D.C.; Varshney, A.K. Association of SNPs/haplotypes in promoter of TNF A and IL-10 gene together with life style factors in prostate cancer progression in Indian population. Agents Actions 2017, 66, 1085–1097. [Google Scholar] [CrossRef]
- Cerhan, J.; Torner, J.C.; Lynch, C.F.; Rubenstein, L.M.; Lemke, J.H.; Cohen, M.B.; Lubaroff, D.M.; Wallace, R.B. Association of smoking, body mass, and physical activity with risk of prostate cancer in the Iowa 65+ Rural Health Study (United States). Cancer Causes Control 1997, 8, 229–238. [Google Scholar] [CrossRef]
- Le Marchand, L.; Kolonel, L.N.; Wilkens, L.R.; Myers, B.C.; Hirohata, T. Animal fat consumption and prostate cancer: A prospective study in Hawaii. Epidemiology 1994, 5, 276–282. [Google Scholar] [CrossRef]
- Jain, M.G.; Hislop, G.T.; Howe, G.R.; Burch, J.D.; Ghadirian, P. Alcohol and other beverage use and prostate cancer risk among Canadian men. Int. J. Cancer 1998, 78, 707–711. [Google Scholar] [CrossRef]
- Villeneuve, P.; Johnson, K.C.; Kreiger, N.; Mao, Y. Risk factors for prostate cancer: Results from the Canadian National Enhanced Cancer Surveillance System. The Canadian cancer registries epidemiology research group. Cancer Causes Control 1999, 10, 355–367. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Thanos, A.; Mitropoulos, D.; Deliveliotis, C.; Mantzoros, C.S.; Trichopoulos, D. Risk factors for prostate cancer: A case-control study in Greece. Int. J. Cancer 1999, 80, 699–703. [Google Scholar] [CrossRef]
- Mettlin, C.; Selenskas, S.; Natarajan, N.; Huben, R. Beta-carotene and animal fats and their relationship to prostate cancer risk. A case—control study. Cancer 1989, 64, 605–612. [Google Scholar] [CrossRef]
- Checkoway, H.; Diferdinando, G.; Hulka, B.S.; Mickey, D.D. Medical, life-style, and occupational risk factors for prostate cancer. Prostate 1987, 10, 79–88. [Google Scholar] [CrossRef]
- Rohrmann, S.; Linseisen, J.; Key, T.J.; Jensen, M.K.; Overvad, K.; Johnsen, N.F.; Tjonneland, A.; Kaaks, R.; Bergmann, M.M.; Weikert, C.; et al. Alcohol Consumption and the Risk for Prostate Cancer in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1282–1287. [Google Scholar] [CrossRef]
- Albertsen, K.; Grønbaek, M. Does amount or type of alcohol influence the risk of prostate cancer? Prostate 2002, 52, 297–304. [Google Scholar] [CrossRef]
- Nilsen, T.I.L.; Johnsen, R.; Vatten, L.J. Socio-economic and lifestyle factors associated with the risk of prostate cancer. Br. J. Cancer 2000, 82, 1358–1363. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, S.; Joung, J.Y.; Kwon, W.-A.; Seo, H.K.; Chung, J.; Nam, B.-H.; Lee, K.H. Lifestyle Risk Prediction Model for Prostate Cancer in a Korean Population. Cancer Res. Treat. 2018, 50, 1194–1202. [Google Scholar] [CrossRef]
- Betts, G.; Ratschen, E.; Breton, M.O.; Grainge, M.J. Alcohol consumption and risk of common cancers: Evidence from a cohort of adults from the UK. J. Public Health 2017, 40, 540–548. [Google Scholar] [CrossRef]
- Severson, R.K.; Nomura, A.M.; Grove, J.S.; Stemmermann, G.N. A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res. 1989, 49, 1857–1860. [Google Scholar] [PubMed]
- Tavani, A.; Negri, E.; Franceschi, S.; Talamini, R.; La Vecchia, C. Alcohol consumption and risk of prostate cancer. Nutr. Cancer 1994, 21, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Ewings, P.; Bowie, C. A case-control study of cancer of the prostate in Somerset and east Devon. Br. J. Cancer 1996, 74, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.; Walker, B.; Tsotetsi, N.; Sebitso, C.; Siwedi, D.; Walker, A. Case-control study of prostate cancer in black patients in Soweto, South Africa. Br. J. Cancer 1992, 65, 438–441. [Google Scholar] [CrossRef]
- Talamini, R.; La Vecchia, C.; DeCarli, A.; Negri, E.; Franceschi, S. Nutrition, social factors and prostatic cancer in a Northern Italian population. Br. J. Cancer 1986, 53, 817–821. [Google Scholar] [CrossRef]
- Van der Gulden, J.W.J.; Verbeek, A.L.M.; Kolk, J.J. Smoking and drinking habits in relation to prostate cancer. Br. J. Urol. 1994, 73, 382–389. [Google Scholar] [CrossRef]
- Lane, J.A.; Oliver, S.E.; Appleby, P.N.; Lentjes, M.A.H.; Emmett, P.; Kuh, D.; Stephen, A.; Brunner, E.; Shipley, M.J.; Hamdy, F.C.; et al. Prostate cancer risk related to foods, food groups, macronutrients and micronutrients derived from the UK Dietary Cohort Consortium food diaries. Eur. J. Clin. Nutr. 2016, 71, 274–283. [Google Scholar] [CrossRef]
- Rogers, L.Q.; Courneya, K.S.; Paragi-Gururaja, R.; Markwell, S.J.; Imeokparia, R. Lifestyle behaviors, obesity, and perceived health among men with and without a diagnosis of prostate cancer: A population-based, cross-sectional study. BMC Public Health 2008, 8, 23. [Google Scholar] [CrossRef]
- Cox, B.; Sneyd, M.J.; Paul, C.; Skegg, D.C.G. Risk factors for prostate cancer: A national case-control study. Int. J. Cancer 2006, 119, 1690–1694. [Google Scholar] [CrossRef]
- Baglietto, L.; Severi, G.; English, D.; Hopper, J.L.; Giles, G.G. Alcohol consumption and prostate cancer risk: Results from the Melbourne collaborative cohort study. Int. J. Cancer 2006, 119, 1501–1504. [Google Scholar] [CrossRef]
- Lumey, L.H.; Pittman, B.; Wynder, E.L. Alcohol use and prostate cancer in U.S. Whites: No association in a confirmatory study. Prostate 1998, 36, 250–255. [Google Scholar] [CrossRef]
- Hiatt, R.A.; Armstrong, M.A.; Klatsky, A.L.; Sidney, S. Alcohol consumption, smoking, and other risk factors and prostate cancer in a large health plan cohort in California (United States). Cancer Causes Control 1994, 5, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; West, D.W. Smoking, alcohol, coffee, tea, caffeine, and theobromine: Risk of prostate cancer in Utah (United States). Cancer Causes Control 1993, 4, 559–563. [Google Scholar] [CrossRef]
- Mills, P.K.; Beeson, W.L.; Phillips, R.L.; Fraser, G.E. Cohort study of diet, lifestyle, and prostate cancer in adventist men. Cancer 1989, 64, 598–604. [Google Scholar] [CrossRef]
- Hsing, A.W.; McLaughlin, J.K.; Schuman, L.M.; Bjelke, E.; Gridley, G.; Wacholder, S.; Chien, H.T.; Blot, W.J. Diet, tobacco use, and fatal prostate cancer: Results from the Lutheran Brotherhood Cohort Study. Cancer Res. 1990, 50, 6836–6840. [Google Scholar] [PubMed]
- Catalona, W.J. Prostate cancer screening. Med. Clin. N. Am. 2018, 102, 199–214. [Google Scholar] [CrossRef]
- Littlejohns, T.J.; Travis, R.C.; Key, T.J.; Allen, N.E. Lifestyle factors and prostate-specific antigen (PSA) testing in UK Biobank: Implications for epidemiological research. Cancer Epidemiol. 2016, 45, 40–46. [Google Scholar] [CrossRef]
- Harris, A.; Gray, M.A.; Slaney, D.P.; Turley, M.L.; Fowles, J.R.; Weinstein, P. Ethnic differences in diet and associations with clinical markers of prostate disease in New Zealand men. Anticancer Res. 2004, 24, 2551–2556. [Google Scholar]
- Nackauzi, J.D.E.; Colla, R.H.; Ravazzani, G.R.; Gaido, M.I.; Bertolotto, P.; Actis, A.B. Prostate-specific antigen: Its relationship with alcohol intake and tobacco. Med. Oncol. 2011, 29, 823–826. [Google Scholar] [CrossRef]
- Zlotta, A.R.; Mari, A.; Erlich, A.; Kuk, C.; Misurka, J.; Marzario, S.; Zisman, A.; Nesbit, M.; Carlsson, S.; Perlis, N.; et al. Pt109-the effect of lifestyle changes on serum psa levels in a cohort of asymptomatic men with psa between 2-10 ng/ml and normal dre. Eur. Urol. Suppl. 2019, 18, e1814. [Google Scholar] [CrossRef]
- Pizent, A.; Čolak, B.; Kljaković, Z.; Telišman, S. Prostate-Specific Antigen (PSA) in Serum in Relation to Blood Lead Concentration and Alcohol Consumption in Men. Arch. Ind. Hyg. Toxicol. 2009, 60, 69–78. [Google Scholar] [CrossRef]
- Woo, H.-Y.; Park, H.; Kwon, M.-J.; Chang, Y.; Ryu, S. Association of prostate specific antigen concentration with lifestyle characteristics in Korean men. Asian Pac. J. Cancer Prev. 2012, 13, 5695–5699. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parsons, J.K.; Im, R. Alcohol Consumption is Associated with a Decreased Risk of Benign Prostatic Hyperplasia. J. Urol. 2009, 182, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.; Erickson, B.; Messersmith, E.E.; Pelletier-Cameron, A.; Lai, H.H.; Kreder, K.J.; Yang, C.C.; Merion, R.M.; Bavendam, T.G.; Kirkali, Z.; et al. Evidence of the Impact of Diet, Fluid Intake, Caffeine, Alcohol and Tobacco on Lower Urinary Tract Symptoms: A Systematic Review. J. Urol. 2017, 198, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Oh, S.; Park, J.; Cho, S.Y.; Cho, M.C.; Jeong, H.; Son, H. The impacts of metabolic syndrome and lifestyle on the prevalence of benign prostatic hyperplasia requiring treatment: Historical cohort study of 130 454 men. Br. J. Urol. 2018, 123, 140–148. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, Y.; Li, X.; Qin, F.; Yuan, J. The prevalence and associated factors of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in aging males. Aging Male 2020, 23, 1432–1439. [Google Scholar] [CrossRef]
- Jang, H.S.; Kim, J.S.; Kim, S.S.; Jung, J.-G.; Yoon, S.-J.; Yang, H.; Joung, H.C. Relationship Between Alcohol Consumption and Prostatic Hyperplasia According to Facial Flushing After Drinking in Korean Men. Korean J. Fam. Med. 2017, 38, 93–98. [Google Scholar] [CrossRef]
- Lu, C.-B.; Qiu, P.-L.; Kong, Q.-J.; Zhu, B.-B.; Li, C.-M.; Liu, B. Effects of alcohol on benign prostate hyperplasia induced by testosterone propionate in mice. Zhongguo Yingyong Shenglixue Zazhi Chin. J. Appl. Physiol. 2018, 34, 501–506. [Google Scholar]
- McConnell, J.D.; Wilson, J.D.; George, F.W.; Geller, J.; Pappas, F.; Stoner, E. Finasteride, an inhibitor of 5 alpha-reductase, suppresses prostatic dihydrotestosterone in men with benign prostatic hyperplasia. J. Clin. Endocrinol. Metab. 1992, 74, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.M.; Goodman, P.J.; Tangen, C.M.; Lucia, M.S.; Miller, G.J.; Ford, L.G.; Lieber, M.M.; Cespedes, R.D.; Atkins, J.N.; Lippman, S.M.; et al. The influence of finasteride on the development of prostate cancer. N. Engl. J. Med. 2003, 349, 215–224. [Google Scholar] [CrossRef]
- Thompson, I.M.; Goodman, P.J.; Tangen, C.M.; Parnes, H.L.; Minasian, L.M.; Godley, P.A.; Lucia, M.S.; Ford, L.G. Long-Term Survival of Participants in the Prostate Cancer Prevention Trial. N. Engl. J. Med. 2013, 369, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Shepard, B.D.; Fernandez, D.J.; Tuma, P.L. Alcohol consumption impairs hepatic protein trafficking: Mechanisms and consequences. Genes Nutr. 2009, 5, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Renau-Piqueras, J.; Gómez-Perretta, C.; Guerri, C.; Sanchis, R. Qualitative and quantitative ultrastructural alterations in hepatocytes of rats prenatally exposed to ethanol with special reference to mitochondria, golgi apparatus and peroxisomes. Virchows Arch. 1985, 405, 237–251. [Google Scholar] [CrossRef]
- Petrosyan, A.; Cheng, P.-W.; Clemens, D.L.; Casey, C.A. Downregulation of the small GTPase SAR1A: A key event underlying alcohol-induced Golgi fragmentation in hepatocytes. Sci. Rep. 2015, 5, 17127. [Google Scholar] [CrossRef]
- Romero, A.M.; Renau-Piqueras, J.; Marín, M.P.; Esteban-Pretel, G. Chronic Alcohol Exposure Affects the Cell Components Involved in Membrane Traffic in Neuronal Dendrites. Neurotox. Res. 2014, 27, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Renau-Piqueras, J.; Miragall, F.; Guerri, C.; Baguena-Cervellera, R. Prenatal exposure to alcohol alters the Golgi apparatus of newborn rat hepatocytes: A cytochemical study. J. Histochem. Cytochem. 1987, 35, 221–228. [Google Scholar] [CrossRef]
- Pandol, S.J.; Gorelick, F.; Gerloff, A.; Lugea, A. Alcohol Abuse, Endoplasmic Reticulum Stress and Pancreatitis. Dig. Dis. 2010, 28, 776–782. [Google Scholar] [CrossRef]
- Ji, C. Mechanisms of Alcohol-Induced Endoplasmic Reticulum Stress and Organ Injuries. Biochem. Res. Int. 2011, 2012, 216450. [Google Scholar] [CrossRef]
- Török, N.; Marks, D.; Hsiao, K.; Oswald, B.J.; McNiven, M.A. Vesicle movement in rat hepatocytes is reduced by ethanol exposure: Alterations in microtubule-based motor enzymes. Gastroenterology 1997, 113, 1938–1948. [Google Scholar] [CrossRef]
- Ren, J.-C.; Zhu, Q.; LaPaglia, N.; Emanuele, N.V.; Emanuele, M.A. Ethanol-induced alterations in Rab proteins: Possible implications for pituitary dysfunction. Alcohol 2005, 35, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Rasineni, K.; McVicker, B.L.; Tuma, D.J.; McNiven, M.A.; Casey, C.A. Rab GTPases Associate with Isolated Lipid Droplets (LDs) and Show Altered Content After Ethanol Administration: Potential Role in Alcohol-Impaired LD Metabolism. Alcohol. Clin. Exp. Res. 2013, 38, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Casey, C.A.; Macke, A.J.; Gough, R.R.; Pachikov, A.N.; Morris, M.E.; Thomes, P.G.; Kubik, J.L.; Holzapfel, M.S.; Petrosyan, A. Alcohol-Induced Liver Injury: Down-regulation and Redistribution of Rab3D Results in Atypical Protein Trafficking. Hepatol. Commun. 2021, 6, 374–388. [Google Scholar] [CrossRef] [PubMed]
- Petrosyan, A.; Casey, C.A.; Cheng, P.-W. The role of Rab6a and phosphorylation of non-muscle myosin IIA tailpiece in alcohol-induced Golgi disorganization. Sci. Rep. 2016, 6, 31962. [Google Scholar] [CrossRef] [PubMed]
- Tomás, M.; Marín, M.P.; Martínez-Alonso, E.; Esteban-Pretel, G.; Díaz-Ruiz, A.; Vázquez-Martínez, R.; Malagón, M.M.; Renau-Piqueras, J.; Martínez-Menárguez, J.A. Alcohol induces Golgi fragmentation in differentiated PC12 cells by deregulating Rab1-dependent ER-to-Golgi transport. Histochem. Cell Biol. 2012, 138, 489–501. [Google Scholar] [CrossRef]
- Li, Y.; Ding, W. Impaired Rab7 and dynamin2 block fat turnover by autophagy in alcoholic fatty livers. Hepatol. Commun. 2017, 1, 473–476. [Google Scholar] [CrossRef]
- Tomás, M.; Marín, P.; Megías, L.; Egea, G.; Renau-Piqueras, J. Ethanol perturbs the secretory pathway in astrocytes. Neurobiol. Dis. 2005, 20, 773–784. [Google Scholar] [CrossRef]
- Marmillot, P.; Rao, M.N.; Lakshman, M. Chronic ethanol exposure in rats affects rabs-dependent hepatic trafficking of apolipoprotein E and transferrin. Alcohol 2001, 25, 195–200. [Google Scholar] [CrossRef]
- Larkin, J.M.; Oswald, B.; McNiven, M.A. Ethanol-induced retention of nascent proteins in rat hepatocytes is accompanied by altered distribution of the small GTP-binding protein rab2. J. Clin. Investig. 1996, 98, 2146–2157. [Google Scholar] [CrossRef]
- Casey, C.A.; Bhat, G.; Holzapfel, M.S.; Petrosyan, A. Study of Ethanol-Induced Golgi Disorganization Reveals the Potential Mechanism of Alcohol-Impaired N-Glycosylation. Alcohol. Clin. Exp. Res. 2016, 40, 2573–2590. [Google Scholar] [CrossRef]
- Frisbie, C.P.; Lushnikov, A.Y.; Krasnoslobodtsev, A.V.; Riethoven, J.-J.M.; Clarke, J.L.; Stepchenkova, E.I.; Petrosyan, A. Post-ER Stress Biogenesis of Golgi Is Governed by Giantin. Cells 2019, 8, 1631. [Google Scholar] [CrossRef]
- Petrosyan, A.; Ali, M.F.; Cheng, P.-W. Glycosyltransferase-specific Golgi-targeting Mechanisms. J. Biol. Chem. 2012, 287, 37621–37627. [Google Scholar] [CrossRef] [PubMed]
- Petrosyan, A.; Holzapfel, M.S.; Muirhead, D.E.; Cheng, P.-W. Restoration of Compact Golgi Morphology in Advanced Prostate Cancer Enhances Susceptibility to Galectin-1–Induced Apoptosis by Modifying Mucin O-Glycan Synthesis. Mol. Cancer Res. 2014, 12, 1704–1716. [Google Scholar] [CrossRef]
- Petrosyan, A. Unlocking golgi: Why does morphology matter? Biochemistry 2019, 84, 1490–1501. [Google Scholar] [CrossRef] [PubMed]
- Egea, G.; Franci, C.; Gambus, G.; Lesuffleur, T.; Zweibaum, A.; Real, F.X. cis-Golgi resident proteins and O-glycans are abnormally compartmentalized in the RER of colon cancer cells. J. Cell Sci. 1993, 105, 819–830. [Google Scholar] [CrossRef]
- Kellokumpu, S.; Sormunen, R.; Kellokumpu, I. Abnormal glycosylation and altered Golgi structure in colorectal cancer: Dependence on intra-Golgi pH. FEBS Lett. 2002, 516, 217–224. [Google Scholar] [CrossRef]
- McKinnon, C.M.; Mellor, H. The tumor suppressor RhoBTB1 controls Golgi integrity and breast cancer cell invasion through METTL7B. BMC Cancer 2017, 17, 145. [Google Scholar] [CrossRef]
- Tan, X.; Banerjee, P.; Guo, H.-F.; Ireland, S.; Pankova, D.; Ahn, Y.-H.; Nikolaidis, I.M.; Liu, X.; Zhao, Y.; Xue, Y.; et al. Epithelial-to-mesenchymal transition drives a pro-metastatic Golgi compaction process through scaffolding protein PAQR11. J. Clin. Investig. 2016, 127, 117–131. [Google Scholar] [CrossRef]
- Petrosyan, A. Onco-golgi: Is fragmentation a gate to cancer progression? Biochem. Mol. Biol. J. 2015, 1, 36. [Google Scholar] [CrossRef]
- Manca, S.; Frisbie, C.P.; Lagrange, C.A.; Casey, C.A.; Riethoven, J.-J.M.; Petrosyan, A. The Role of Alcohol-Induced Golgi Fragmentation for Androgen Receptor Signaling in Prostate Cancer. Mol. Cancer Res. 2018, 17, 225–237. [Google Scholar] [CrossRef]
- Kubyshkin, A.V.; Fomochkina, I.I.; Petrosyan, A.M. The impact of alcohol on pro-metastatic n-glycosylation in prostate cancer. Krim Z Eksp Klin Med. 2018, 8, 11–20. [Google Scholar]
- Stewart, T.; Yapa, K.T.; Monteith, G.R. Altered calcium signaling in cancer cells. Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1848, 2502–2511. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Moenner, M.; Pluquet, O.; Bouchecareilh, M.; Chevet, E. Integrated Endoplasmic Reticulum Stress Responses in Cancer: Figure 1. Cancer Res. 2007, 67, 10631–10634. [Google Scholar] [CrossRef] [PubMed]
- Oakes, S.A. Endoplasmic Reticulum Stress Signaling in Cancer Cells. Am. J. Pathol. 2020, 190, 934–946. [Google Scholar] [CrossRef]
- Yadav, R.K.; Chae, S.-W.; Kim, H.-R.; Chae, H.J. Endoplasmic Reticulum Stress and Cancer. J. Cancer Prev. 2014, 19, 75–88. [Google Scholar] [CrossRef]
- Mahadevan, N.R.; Rodvold, J.; Sepulveda, H.; Rossi, S.; Drew, A.F.; Zanetti, M. Transmission of endoplasmic reticulum stress and pro-inflammation from tumor cells to myeloid cells. Proc. Natl. Acad. Sci. USA 2011, 108, 6561–6566. [Google Scholar] [CrossRef]
- Hung, J.H.; Su, I.J.; Lei, H.Y.; Wang, H.C.; Lin, W.C.; Chang, W.T.; Huang, W.; Chang, W.C.; Chang, Y.S.; Chen, C.C.; et al. Endoplasmic reticulum stress stimulates the expression of cyclooxygenase-2 through activation of nf-kappab and pp38 mitogen-activated protein kinase. J. Biol. Chem. 2004, 279, 46384–46392. [Google Scholar] [CrossRef]
- Katanasaka, Y.; Ishii, T.; Asai, T.; Naitou, H.; Maeda, N.; Koizumi, F.; Miyagawa, S.; Ohashi, N.; Oku, N. Cancer antineovascular therapy with liposome drug delivery systems targeted to BiP/GRP78. Int. J. Cancer 2010, 127, 2685–2698. [Google Scholar] [CrossRef]
- Storm, M.; Sheng, X.; Arnoldussen, Y.J.; Saatcioglu, F. Prostate cancer and the unfolded protein response. Oncotarget 2016, 7, 54051–54066. [Google Scholar] [CrossRef]
- Guan, M.; Su, L.; Yuan, Y.-C.; Li, H.; Chow, W.A. Nelfinavir and Nelfinavir Analogs Block Site-2 Protease Cleavage to Inhibit Castration-Resistant Prostate Cancer. Sci. Rep. 2015, 5, 9698. [Google Scholar] [CrossRef]
- Sreenath, T.L.; Macalindong, S.; Mikhalkevich, N.; Sharad, S.; Mohamed, A.; Young, D.; Borbiev, T.; Xavier, C.; Gupta, R.; Jamal, M.; et al. ETS Related Gene mediated Androgen Receptor Aggregation and Endoplasmic Reticulum Stress in Prostate Cancer Development. Sci. Rep. 2017, 7, 1109. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, N.R.; Rodvold, J.; Almanza, G.; Perez, A.F.; Wheeler, M.C.; Zanetti, M. Er stress drives lipocalin 2 upregulation in prostate cancer cells in an nf-kappab-dependent manner. BMC Cancer 2011, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Chen, X.; Hendershot, L.; Prywes, R. ER Stress Regulation of ATF6 Localization by Dissociation of BiP/GRP78 Binding and Unmasking of Golgi Localization Signals. Dev. Cell 2002, 3, 99–111. [Google Scholar] [CrossRef]
- Pachikov, A.N.; Gough, R.R.; Christy, C.E.; Morris, M.E.; Casey, C.A.; LaGrange, C.A.; Bhat, G.; Kubyshkin, A.V.; Fomochkina, I.I.; Zyablitskaya, E.Y.; et al. The non-canonical mechanism of ER stress-mediated progression of prostate cancer. J. Exp. Clin. Cancer Res. 2021, 40, 289. [Google Scholar] [CrossRef]
- Morel, K.L.; Ormsby, R.; Solly, E.L.; Tran, L.N.K.; Sweeney, C.J.; Klebe, S.; Cordes, N.; Sykes, P.J. Chronic low dose ethanol induces an aggressive metastatic phenotype in TRAMP mice, which is counteracted by parthenolide. Clin. Exp. Metastasis 2018, 35, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Umathe, S.; Bhutada, P.; Dixit, P.; Jain, N. Leuprolide—A luteinizing hormone releasing hormone agonist attenuates ethanol withdrawal syndrome and ethanol-induced locomotor sensitization in mice. Neuropeptides 2008, 42, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Umathe, S.; Bhutada, P.; Dixit, P.; Shende, V. Increased marble-burying behavior in ethanol-withdrawal state: Modulation by gonadotropin-releasing hormone agonist. Eur. J. Pharmacol. 2008, 587, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Tanvetyanon, T. Physician practices of bone density testing and drug prescribing to prevent or treat osteoporosis during androgen deprivation therapy. Cancer 2005, 103, 237–241. [Google Scholar] [CrossRef]
- Panday, K.; Gona, A.; Humphrey, M.B. Medication-induced osteoporosis: Screening and treatment strategies. Ther. Adv. Musculoskelet. Dis. 2014, 6, 185–202. [Google Scholar] [CrossRef]
- Diamond, T.H.; Bucci, J.; Kersley, J.H.; Aslan, P.; Lynch, W.B.; Bryant, C. Osteoporosis and spinal fractures in men with prostate cancer: Risk factors and effects of androgen deprivation therapy. J. Urol. 2004, 172, 529–532. [Google Scholar] [CrossRef]
- Owen, P.; Daly, R.M.; Livingston, P.M.; Fraser, S.F. Lifestyle guidelines for managing adverse effects on bone health and body composition in men treated with androgen deprivation therapy for prostate cancer: An update. Prostate Cancer Prostatic Dis. 2017, 20, 137–145. [Google Scholar] [CrossRef]
- Thomas, S.A. Can I Drink Alcohol If I’m Taking Prostate Cancer Medications Like Casodex? Available online: https://www.goodrx.com/conditions/prostate-cancer/bicalutamide-prostate-cancer-meds-and-alcohol (accessed on 19 February 2022).
- Wilson, K.M.; Giovannucci, E.L.; Mucci, L.A. Lifestyle and dietary factors in the prevention of lethal prostate cancer. Asian J. Androl. 2012, 14, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Freedland, S.J.; Aronson, W.J. Examining the relationship between obesity and prostate cancer. Rev. Urol. 2004, 6, 73–81. [Google Scholar] [PubMed]
- Makarem, N.; Lin, Y.; Bandera, E.; Jacques, P.F.; Parekh, N. Concordance with World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) guidelines for cancer prevention and obesity-related cancer risk in the Framingham Offspring cohort (1991–2008). Cancer Causes Control 2015, 26, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Graffouillère, L.; Deschasaux, M.; Mariotti, F.; Neufcourt, L.; Shivappa, N.; Hébert, J.R.; Wirth, M.D.; Latino-Martel, P.; Hercberg, S.; Galan, P.; et al. The Dietary Inflammatory Index Is Associated with Prostate Cancer Risk in French Middle-Aged Adults in a Prospective Study. J. Nutr. 2015, 146, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Chhim, A.-S.; Fassier, P.; Latino-Martel, P.; Druesne-Pecollo, N.; Zelek, L.; Duverger, L.; Hercberg, S.; Galan, P.; Deschasaux, M.; Touvier, M. Prospective association between alcohol intake and hormone-dependent cancer risk: Modulation by dietary fiber intake. Am. J. Clin. Nutr. 2015, 102, 182–189. [Google Scholar] [CrossRef]
- Kobayashi, L.C.; Limburg, H.; Miao, Q.; Woolcott, C.; Bedard, L.L.; Massey, T.E.; Aronson, K.J. Folate intake, alcohol consumption, and the methylenetetrahydrofolate reductase (MTHFR) C677T gene polymorphism: Influence on prostate cancer risk and interactions. Front. Oncol. 2012, 2, 100. [Google Scholar] [CrossRef] [PubMed]
- Kenfield, S.A.; Dupre, N.; Richman, E.L.; Stampfer, M.J.; Chan, J.M.; Giovannucci, E.L. Mediterranean Diet and Prostate Cancer Risk and Mortality in the Health Professionals Follow-up Study. Eur. Urol. 2014, 65, 887–894. [Google Scholar] [CrossRef]
- Richard, A.; Faeh, D.; Rohrmann, S.; Braun, J.; Tarnutzer, S.; Bopp, M. Italianity is associated with lower risk of prostate cancer mortality in Switzerland. Cancer Causes Control 2014, 25, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.; Su, L.J.; Arab, L.; Bensen, J.T.; Farnan, L.; Fontham, E.T.H.; Song, L.; Hussey, J.; Merchant, A.T.; Mohler, J.L.; et al. Dietary patterns based on the Mediterranean diet and DASH diet are inversely associated with high aggressive prostate cancer in PCaP. Ann. Epidemiol. 2019, 29, 16–22.e1. [Google Scholar] [CrossRef]
- McGregor, S.E.; Courneya, K.S.; Kopciuk, K.A.; Tosevski, C.; Friedenreich, C.M. Case–control study of lifetime alcohol intake and prostate cancer risk. Cancer Causes Control 2012, 24, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, A.; Parent, M.-E.; Siemiatycki, J. Lifetime consumption of alcoholic beverages and risk of 13 types of cancer in men: Results from a case–control study in Montreal. Cancer Detect. Prev. 2009, 32, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Fillmore, K.M.; Chikritzhs, T.; Stockwell, T.; Bostrom, A.; Pascal, R. Alcohol use and prostate cancer: A meta-analysis. Mol. Nutr. Food Res. 2009, 53, 240–255. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macke, A.J.; Petrosyan, A. Alcohol and Prostate Cancer: Time to Draw Conclusions. Biomolecules 2022, 12, 375. https://doi.org/10.3390/biom12030375
Macke AJ, Petrosyan A. Alcohol and Prostate Cancer: Time to Draw Conclusions. Biomolecules. 2022; 12(3):375. https://doi.org/10.3390/biom12030375
Chicago/Turabian StyleMacke, Amanda J., and Armen Petrosyan. 2022. "Alcohol and Prostate Cancer: Time to Draw Conclusions" Biomolecules 12, no. 3: 375. https://doi.org/10.3390/biom12030375
APA StyleMacke, A. J., & Petrosyan, A. (2022). Alcohol and Prostate Cancer: Time to Draw Conclusions. Biomolecules, 12(3), 375. https://doi.org/10.3390/biom12030375





