Biomarkers in Patients with Left Ventricular Assist Device: An Insight on Current Evidence
Abstract
1. Introduction
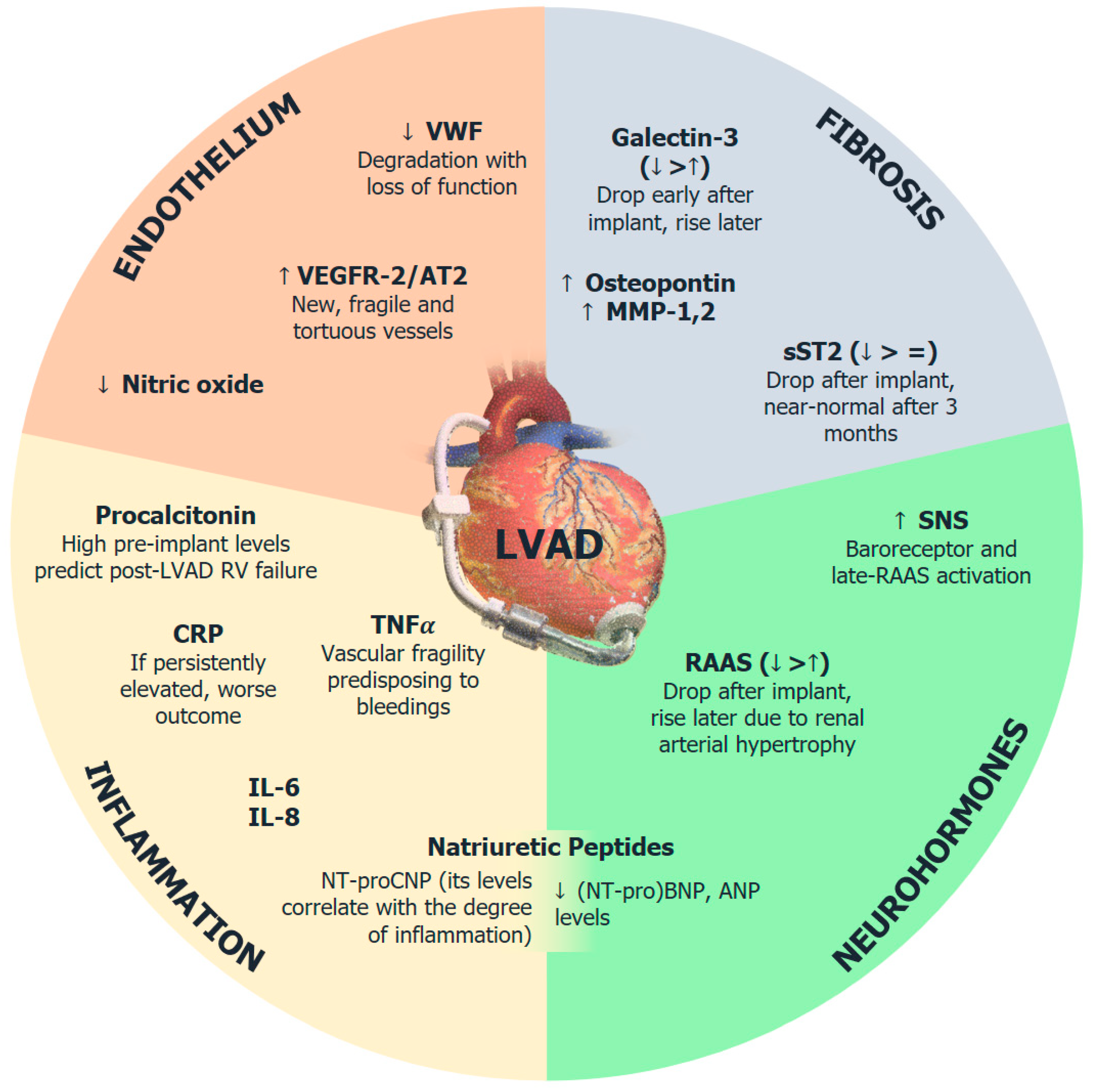
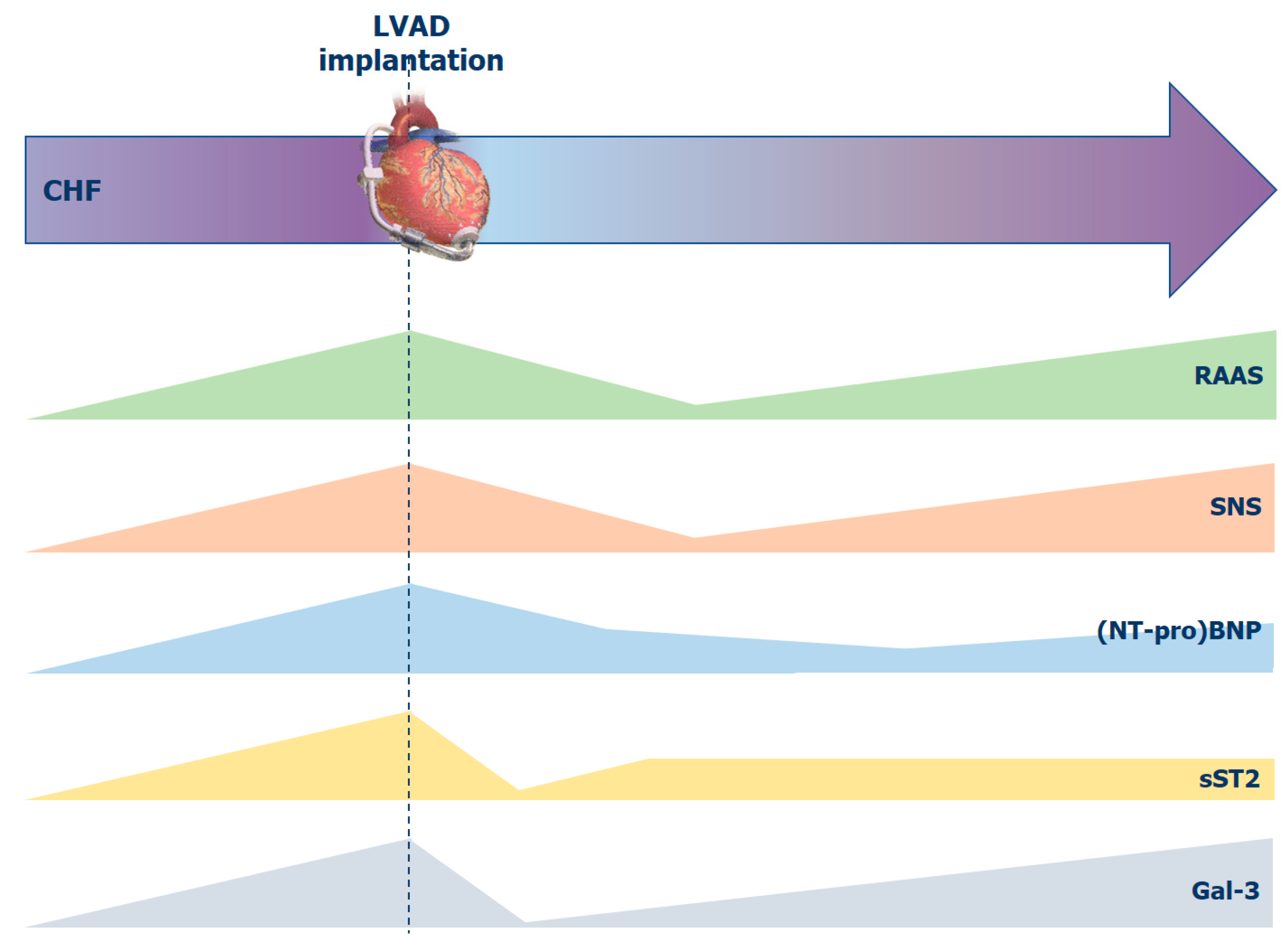
2. Neurohormonal Activation
2.1. Sympathetic Nervous System
2.2. Renin-Angiotensin-Aldosterone System
2.3. Natriuretic Peptide System
3. Markers of Myocardial Fibrosis
4. Endothelial Dysfunction and Neoangiogenesis
5. Systemic Inflammation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, J.J.; Acker, M.A.; Atluri, P. Left Ventricular Assist Devices. Circulation 2018, 138, 2841–2851. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R. The burden of haemocompatibility with left ventricular assist systems: A complex weave. Eur. Heart J. 2019, 40, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Pagani, F.D. Right Heart Failure After Left Ventricular Assist Device Placement: Medical and Surgical Management Considerations. Cardiol. Clin. 2020, 38, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Klotz, S.; Burkhoff, D.; Garrelds, I.M.; Boomsma, F.; Danser, A.J. The impact of left ventricular assist device-induced left ventricular unloading on the myocardial renin-angiotensin-aldosterone system: The rapeutic consequences? Eur. Heart J. 2009, 30, 805–812. [Google Scholar] [CrossRef]
- Long, J.W.; Kfoury, A.G.; Slaughter, M.S.; Silver, M.; Milano, C.; Rogers, J.; Delgado, R.; Frazier, O.H. Long-term destination therapy with the HeartMate XVE left ventricular assist device: Improved outcomes since the REMATCH study. Congest Heart Fail. 2005, 11, 133–138. [Google Scholar] [CrossRef]
- Markham, D.W.; Fu, Q.; Palmer, M.D.; Drazner, M.H.; Meyer, D.M.; Bethea, B.T.; Hastings, J.L.; Fujimoto, N.; Shibata, S.; Levine, B.D. Sympathetic Neural and Hemodynamic Responses to Upright Tilt in Patients with Pulsatile and Nonpulsatile Left Ventricular Assist Devices. Circ. Heart Fail. 2013, 6, 293–299. [Google Scholar] [CrossRef]
- Cowger, J.; Pagani, F.D.; Haft, J.W.; Romano, M.A.; Aaronson, K.D.; Kolias, T.J. The development of aortic insufficiency in left ventricular assist device-supported patients. Circ Heart Fail. 2010, 3, 668–674. [Google Scholar] [CrossRef]
- Ohnishi, H.; Itoh, T.; Nishinaka, T.; Tatsumi, E.; Fukuda, T.; Oshikawa, M.; Shioya, K.; Tsukiya, T.; Takewa, Y.; Homma, A.; et al. Morphological changes of the arterial systems in the kidney under prolonged continuous flow left heart bypass. Artif. Organs. 2002, 26, 974–979. [Google Scholar] [CrossRef]
- George, R.; Birks, E.J.; Cheetham, A.; Webb, C.; Smolenski, R.; Khaghani, A.; Yacoub, M.H.; Kelion, A. The effect of long-term left ventricular assist device support on myocardial sympathetic activity in patients with non-ischaemic dilated cardiomyopathy. Eur. J. Heart Fail. 2013, 15, 1035–1043. [Google Scholar] [CrossRef]
- Drakos, S.G.; Athanasoulis, T.; Malliaras, K.G.; Terrovitis, J.V.; Diakos, N.; Koudoumas, D.; Ntalianis, A.S.; Theodoropoulos, S.P.; Yacoub, M.H.; Nanas, J.N. Myocardial Sympathetic Innervation and Long-Term Left Ventricular Mechanical Unloading. JACC Cardiovasc. Imaging 2010, 3, 64–70. [Google Scholar] [CrossRef]
- Grosman-Rimon, L.; Kachel, E.; McDonald, M.A.; LaLonde, S.D.; Yip, P.; Ribeiro, R.V.; Adamson, M.B.; Cherney, D.Z.; Rao, V. Association Between Neurohormone Levels and Exercise Testing Measures in Patients with Mechanical Circulatory Supports. ASAIO J. 2019, 66, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Sailer, C.; Edelmann, H.; Buchanan, C.; Giro, P.; Babcock, M.; Swanson, C.; Spotts, M.; Schulte, M.; Pratt-Cordova, A.; Coe, G.; et al. Impairments in Blood Pressure Regulation and Cardiac Baroreceptor Sensitivity Among Patients with Heart Failure Supported With Continuous-Flow Left Ventricular Assist Devices. Circ. Heart Fail. 2021, 14, e007448. [Google Scholar] [CrossRef] [PubMed]
- Moss, N.; Rakita, V.; Lala, A.; Parikh, A.; Roldan, J.; Mitter, S.S.; Anyanwu, A.; Campoli, M.; Burkhoff, D.; Mancini, D.M. Hemodynamic response to exercise in patients supported by continuous flow left ventricular assist devices. JACC Heart Fail. 2020, 8, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Denfeld, Q.E.; Lee, C.S.; Woodward, W.R.; Hiatt, S.O.; Mudd, J.O.; Habecker, B.A. Sympathetic Markers are Different between Clinical Responders and Nonresponders after Left Ventricular Assist Device Implantation. J. Cardiovasc. Nurs. 2019, 34, E1–E10. [Google Scholar] [CrossRef] [PubMed]
- Klotz, S.; Danser, A.J.; Foronjy, R.F.; Oz, M.C.; Wang, J.; Mancini, D.; D’Armiento, J.; Burkhoff, D. The impact of angiotensin-converting enzyme inhibitor therapy on the extracellular collagen matrix during left ventricular assist device support in patients with end-stage heart failure. J. Am. Coll. Cardiol. 2007, 49, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Brinkley, D.M., Jr.; Wang, L.; Yu, C.; Grandin, E.W.; Kiernan, M.S. Impact of renin-angiotensin-aldosterone system inhibition on morbidity and mortality during long-term continuous-flow left ventricular assist device support: An IMACS report. J. Heart Lung Transplant. 2021, 40, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.; Caraballo, C.; Ravindra, N.G.; Miller, P.E.; Mezzacappa, C.; Levin, A.; Gruen, J.; Rodwin, B.; Reinhardt, S.; Van Dijk, D.; et al. Neurohormonal Blockade and Clinical Outcomes in Patients with Heart Failure Supported by Left Ventricular Assist Devices. JAMA Cardiol. 2020, 5, 175–182. [Google Scholar] [CrossRef]
- Zabarovskaja, S.; Hage, C.; Linde, C.; Daubert, J.-C.; Donal, E.; Gabrielsen, A.; Mellbin, L.; Lund, L.H. Adaptive cardiovascular hormones in a spectrum of heart failure phenotypes. Int. J. Cardiol. 2015, 189, 6–11. [Google Scholar] [CrossRef]
- Milting, H.; Banayosy, A.E.; Kassner, A.; Fey, O.; Sarnowski, P.; Arusoglu, L.; Thieleczek, R.; Brinkmann, T.; Kleesiek, K.; Körfer, R. The time course of natriuretic hormones as plasma markers of myocardial recovery in heart transplant candidates during ventricular assist device support reveals differ- ences among device types. J. Heart Lung Transplant. 2001, 20, 949–955. [Google Scholar] [CrossRef]
- Wagner, F.D.; Buz, S.; Zais, H.; Stasch, J.P.; Hetzer, R.; Hocher, B. Humoralandhemody- namic responses after left ventricular assist device implantation and heart trans- plantation. Exp. Biol. Med. 2006, 231, 861–864. [Google Scholar]
- Knudsen, M.S.S.; Eismark, F.; Goetze, J.P.; Gustafsson, F.; Wolsk, E. The contribution of cardiac and extracardiac factors to NT-proBNP concentrations in patients with advanced heart failure before and after left ventricular assist device implantation. Peptides 2021, 135, 170420. [Google Scholar] [CrossRef] [PubMed]
- Taenaka, Y.; Yagura, A.; Takano, H.; Matsuda, T.; Noda, H.; Kinoshita, M.; Takatani, S.; Akutsu, T. Altered humoral control of circulating volume during artificial circulation. ASAIO Trans. 1988, 34, 692–695. [Google Scholar]
- Alishetti, S.; Braghieri, L.; Jennings, D.L.; Uriel, N.; Colombo, P.C.; Yuzefpolskaya, M. Angiotensin receptor neprilysin inhibitor use in patients with left ventricular assist devices: A single-center experience. Int. J. Artif. Organs. 2022, 45, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Cabiati, M.; Caruso, R.; Caselli, C.; Frigerio, M.; Prescimone, T.; Parodi, O.; Giannessi, D.; Del Ry, S. The natriuretic peptide time-course in end-stage heart failure patients supported by left ventricular assist device implant: Focus on NT-proCNP. Peptides 2012, 36, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Hellman, Y.; Malik, A.S.; Lin, H.; Shen, C.; Wang, I.W.; Wozniak, T.C.; Hashmi, Z.A.; Pickrell, J.; Jani, M.; Caccamo, M.A.; et al. B-Type Natriuretic Peptide Levels Predict Ventricular Arrhythmia Post Left Ventricular Assist Device Implantation. Artif. Organs. 2015, 39, 1051–1055. [Google Scholar] [CrossRef]
- Imamura, T.; Kinugawa, K.; Nitta, D.; Kinoshita, O.; Nawata, K.; Ono, M. Preoperative iodine-123 meta-iodobenzylguanidine imaging is a novel predictor of left ventricular reverse remodeling during treatment with a left ventricular assist device. J. Artif. Organs. 2016, 19, 29–36. [Google Scholar] [CrossRef]
- Kishimoto, I.; Rossi, K.; Garbers, D.L. A genetic model provides evidence that the receptor for atrial natriuretic peptide (guanylyl cyclase-A) inhibits cardiac ventricular myocyte hypertrophy. Proc. Natl. Acad. Sci. USA 2001, 98, 2703–2706. [Google Scholar] [CrossRef]
- Tamura, N.; Ogawa, Y.; Chusho, H.; Nakamura, K.; Nakao, K.; Suda, M.; Kasahara, M.; Hashimoto, R.; Katsuura, G.; Mukoyama, M.; et al. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc. Natl. Acad. Sci. USA 2000, 97, 4239–4244. [Google Scholar] [CrossRef]
- Kuhn, M.; Voß, M.; Mitko, D.; Stypmann, J.; Schmid, C.; Kawaguchi, N.; Grabellus, F.; Baba, H.A. Left ventricular assist device support reverses altered cardiac expression and function of natriuretic peptides and receptors in end-stage heart failure. Cardiovasc. Res. 2004, 64, 308–314. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Zhang, Y.; Ky, B. ST2 and Patient Prognosis in Chronic Heart Failure. Am. J. Cardiol. 2015, 115, 64B–69B. [Google Scholar] [CrossRef]
- Holzhauser, L.; Kim, G.; Sayer, G.; Uriel, N. The Effect of Left Ventricular Assist Device Therapy on Cardiac Biomarkers: Implications for the Identification of Myocardial Recovery. Curr. Hear. Fail. Rep. 2018, 15, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; Botta, L.; Verde, A.; Milazzo, F.; Vecchi, I.; Trivella, M.G.; Martinelli, L.; Paino, R.; Frigerio, M.; Parodi, O. Relationship between Pre-Implant Interleukin-6 Levels, Inflammatory Response, and Early Outcome in Patients Supported by Left Ventricular Assist Device: A Prospective Study. PLoS ONE 2014, 9, e90802. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.C.S.; Huibers, M.M.H.; Gaykema, L.H.; Koning, E.S.-D.; Ramjankhan, F.Z.; Maisel, A.S.; de Jonge, N. Soluble ST2 in end-stage heart failure, before and after support with a left ventricular assist device. Eur. J. Clin. Investig. 2018, 48, e12886. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.C.; Sethi, T. The regulation of inflammation by galectin-3. Immunol. Rev. 2009, 230, 160–171. [Google Scholar] [CrossRef]
- A McCullough, P.; Olobatoke, A.; E Vanhecke, T. Galectin-3: A novel blood test for the evaluation and management of patients with heart failure. Rev. Cardiovasc. Med. 2011, 12, 200–210. [Google Scholar]
- Coromilas, E.; Que-Xu, E.-C.; Moore, D.; Kato, T.S.; Wu, C.; Ji, R.; Givens, R.; Jorde, U.P.; Takayama, H.; Naka, Y.; et al. Dynamics and prognostic role of galectin-3 in patients with advanced heart failure, during left ventricular assist device support and following heart transplantation. BMC Cardiovasc. Disord. 2016, 16, 138. [Google Scholar] [CrossRef]
- Lok, S.I.; Nous, F.M.; Van Kuik, J.; Van Der Weide, P.; Winkens, B.; Kemperman, H.; Huisman, A.; Lahpor, J.R.; De Weger, R.A.; De Jonge, N. Myocardial fibrosis and pro-fibrotic markers in end-stage heart failure patients during continuous-flow left ventricular assist device support. Eur. J. Cardio-Thoracic. Surg. 2015, 48, 407–415. [Google Scholar] [CrossRef]
- Kato, T.S.; Chokshi, A.; Singh, P.; Khawaja, T.; Iwata, S.; Homma, S.; Akashi, H.; Cheema, F.H.; Yang, J.; Takayama, H.; et al. Markers of extracellular matrix turnover and the development of right ventricular failure after ventricular assist device implantation in patients with advanced heart failure. J. Hear. Lung Transplant. 2011, 31, 37–45. [Google Scholar] [CrossRef]
- Hennig, F.; Stepanenko, A.V.; Lehmkuhl, H.B.; Kukucka, M.; Dandel, M.; Krabatsch, T.; Hetzer, R.; Potapov, E.V. Neurohumoral and inflammatory markers for prediction of right ventricular failure after implantation of a left ventricular assist device. Gen. Thorac. Cardiovasc. Surg. 2011, 59, 19–24. [Google Scholar] [CrossRef]
- Pronschinske, K.B.; Qiu, S.; Wu, C.; Kato, T.S.; Khawaja, T.; Takayama, H.; Naka, Y.; Templeton, D.L.; George, I.; Farr, M.A.; et al. Neutrophil gelatinase- associated lipocalin and cystatin C for the prediction of clinical events in patients with advanced heart failure and after ventricular assist device placement. J. Heart Lung Transplant. 2014, 33, 1215–1222. [Google Scholar] [CrossRef][Green Version]
- Tabit, C.E.; Chen, P.; Kim, G.H.; Fedson, S.E.; Sayer, G.; Coplan, M.J.; Jeevanandam, V.; Uriel, N.; Liao, J.K. Elevated Angiopoietin-2 level in patients with continuous-flow left ventricular assist devices leads to altered angiogenesis and is associated with higher nonsurgical bleeding. Circulation 2016, 134, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Madan, S.; Saeed, O.; Algodi, M.; Luke, A.; Gibber, M.; Goldstein, D.J.; Jorde, U.P. Association of nasal mucosal vascular alterations, gastrointestinal arteriovenous malformations, and bleeding in patients with continuous-flow left ventricular assist devices. JACC Heart Fail. 2016, 4, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Kihara, S.; Litwak, K.N.; Nichols, L.; Litwak, P.; Kameneva, M.V.; Wu, Z.; Kormos, R.L.; Griffith, B.P. Smooth muscle cell hypertrophy of renal cortex arteries with chronic continuous flow left ventricular assist. Ann. Thorac. Surg. 2003, 75, 178–183. [Google Scholar] [CrossRef]
- Bartoli, C.R.; Restle, D.J.; Zhang, D.M.; Acker, M.A.; Atluri, P. Pathologic von Willebrand factor degradation with a left ventricular assist device occurs via two distinct mechanisms: Mechanical demolition and enzymatic cleavage. J. Thorac. Cardiovas. Surg. 2015, 149, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, C.R.; Zhang, D.M.; Hennessy-Strahs, S.; Kang, J.; Restle, D.J.; Bermudez, C.; Atluri, P.; Acker, M.A. Clinical and In Vitro Evidence That Left Ventricular Assist Device-Induced von Willebrand Factor Degradation Alters Angiogenesis. Circ. Heart Fail. 2018, 11, e004638. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, C.R.; Spence, P.A.; Siess, T.; Raess, D.H.; Koenig, S.C.; Dowling, R.D. Non-physiologic blood flow triggers endothelial and arterial remodeling in vivo: Implications for novel left ventricular assist devices with a peripheral anastomosis. J. Thorac. Cardiovasc. Surg. 2014, 148, 311–321. [Google Scholar] [CrossRef][Green Version]
- Kang, J.; Hennessy-Strahs, S.; Kwiatkowski, P.; Bermudez, C.A.; Acker, M.A.; Atluri, P.; McConnell, P.I.; Bartoli, C.R. Continuous-Flow LVAD Support Causes a Distinct Form of Intestinal Angiodysplasia. Circ. Res. 2017, 121, 963–969. [Google Scholar] [CrossRef]
- Starke, R.D.; Ferraro, F.; Paschalaki, K.; Dryden, N.H.; McKinnon, T.A.J.; Sutton, R.E.; Payne, E.M.; Haskard, D.O.; Hughes, A.; Cutler, D.; et al. Endothelial von Willebrand factor regulates angiogenesis. Blood 2011, 117, 1071–1080. [Google Scholar] [CrossRef]
- Cascone, T.; Heymach, J.V. Targeting the angiopoietin/Tie2 pathway: Cutting tumor vessels with a double-edged sword? J. Clin. Oncol. 2012, 30, 441–444. [Google Scholar] [CrossRef]
- Boyle, A.J.; Russell, S.D.; Teuteberg, J.J.; Slaughter, M.S.; Moazami, N.; Pagani, F.; Frazier, O.H.; Heatley, G.; Farrar, D.J.; John, R. Low Thromboembolism and Pump Thrombosis with the HeartMate II Left Ventricular Assist Device: Analysis of Outpatient Anti-coagulation. J. Heart Lung Transplant. 2009, 28, 881–887. [Google Scholar] [CrossRef]
- Poredos, P.; Jezovnik, M.K.; Radovancevic, R.; Gregoric, I.D. Endothelial Function in Patients with Continuous-Flow Left Ventricular Assist Devices. Angiology 2020, 72, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kalman, J.; Mayer, L.; Fillit, H.M.; Packer, M. Elevated Circulating Levels of Tumor Necrosis Factor in Severe Chronic Heart Failure. N. Engl. J. Med. 1990, 323, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Torre-Amione, G.; Kapadia, S.; Benedict, C.; Oral, H.; Young, J.B.; Mann, D.L. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: A report from the Studies of Left Ventricular Dysfunction (SOLVD). J. Am. Coll. Cardiol. 1996, 27, 1201–1206. [Google Scholar] [CrossRef]
- Markousis-Mavrogenis, G.; Tromp, J.; Ouwerkerk, W.; Devalaraja, M.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.S.; Van Der Harst, P.; Lang, C.C.; et al. The clinical significance of interleukin-6 in heart failure: Results from the BIOSTAT-CHF study. Eur. J. Heart Fail. 2019, 21, 965–973. [Google Scholar] [CrossRef]
- Masai, T.; Sawa, Y.; Ohtake, S.; Nishida, T.; Nishimura, M.; Fukushima, N.; Yamaguchi, T.; Matsuda, H. Hepatic dysfunction after left ventricular mechanical assist in patients with end-stage heart failure: Role of inflammatory response and hepatic microcirculation. Ann. Thorac. Surg. 2002, 73, 549–555. [Google Scholar] [CrossRef]
- Caruso, R.; Trunfio, S.; Milazzo, F.; Campolo, J.; De Maria, R.; Colombo, T.; Parolini, M.; Cannata, A.; Russo, C.; Paino, R. Early expression of pro- and anti-inflammatory cytokines in left ventricular assist device recipients with multiple organ failure syndrome. ASAIO J. 2010, 56, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Lappegard, K.T.; Bergseth, G.; Riesenfeld, J.; Pharo, A.; Magotti, P.; Lambris, J.D.; Mollnes, T.E. The artificial surface-induced whole blood inflammatory reaction revealed by increases in a series of chemokines and growth factors is largely complement dependent. J. Biomed Mater. Res. A 2008, 87, 129–135. [Google Scholar] [CrossRef]
- Takahashi, T.; Anzai, T.; Yoshikawa, T.; Maekawa, Y.; Asakura, Y.; Satoh, T.; Mitamura, H.; Ogawa, S. Serum C-reactive protein elevation in left ventricular remodeling after acute myocardial infarction--role of neurohormones and cytokines. Int. J. Cardiol. 2003, 88, 257–265. [Google Scholar] [CrossRef]
- Tabit, C.E.; Coplan, M.J.; Chen, P.; Jeevanandam, V.; Uriel, N.; Liao, J.K. Tumor necrosis factor-alpha levels and non-surgical bleeding in continuous-flow left ventricular assist devices. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2018, 37, 107–115. [Google Scholar] [CrossRef]
- Rondina, M.T.; Weyrich, A.S.; Zimmerman, G.A. Platelets as cellular effectors of inflammation in vascular diseases. Circ. Res. 2013, 112, 1506–1519. [Google Scholar] [CrossRef]
- Ahmad, T.; Wang, T.; O’Brien, E.C.; Samsky, M.D.; Pura, J.A.; Lokhnygina, Y.; Rogers, J.G.; Hernandez, A.F.; Craig, D.; Bowles, D.E.; et al. Effects of Left Ventricular Assist Device Support on Biomarkers of Cardiovascular Stress, Fibrosis, Fluid Homeostasis, Inflammation, and Renal Injury. JACC Heart Fail. 2015, 3, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Westaby, S.; Piggot, D.; Dudnikov, S.; Robson, D.; Catarino, P.A.; Clelland, C.; Nojiri, C. End-organ function during chronic nonpulsatile circulation. Ann. Thorac. Surg. 2002, 74, 1080–1085. [Google Scholar] [CrossRef]
- Welp, H.; Rukosujew, A.; Tjan, T.D.; Hoffmeier, A.; Kösek, V.; Scheld, H.H.; Drees, G. Effect of pulsatile and non-pulsatile left ventricular assist devices on the renin-angiotensin system in patients with end-stage heart failure. Thorac. Cardiovasc. Surg. 2010, 58 (Suppl. 2), S185–S188. [Google Scholar] [CrossRef]
- Grosman-Rimon, L.; McDonald, M.A.; Jacobs, I.; Tumiati, L.C.; Pollock Bar-Ziv, S.; Shogilev, D.J.; Mociornita, A.G.; Ghashghai, A.; Chruscinski, A.; Cherney, D.Z.; et al. Markers of inflammation in recipients of continuous-flow left ventricular assist devices. ASAIO J. 2014, 60, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; Garcia-Gonzalez, M.; Ferrer, J. Prognostic value of interleukin-8 as a predictor of heart failure in patients with myocardial infarction and percutaneous intervention. Int. J. Cardiol. 2006, 111, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, Y.; Anzai, T.; Yoshikawa, T.; Sugano, Y.; Mahara, K.; Kohno, T.; Takahashi, T.; Ogawa, S. Effect of granulocyte-macrophage colony-stimulating factor inducer on left ventricular remodeling after acute myocardial infarction. J. Am. Coll. Cardiol. 2004, 44, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Hein, T.W.; Singh, U.; Vasquez-Vivar, J.; Devaraj, S.; Kuo, L.; Jialal, I. Human C-reactive protein induces endothelial dysfunction and uncoupling of eNOS In Vivo. Atherosclerosis 2009, 206, 61–68. [Google Scholar] [CrossRef]
- Wassmann, S.; Stumpf, M.; Strehlow, K.; Schmid, A.; Schieffer, B.; Böhm, M.; Nickenig, G. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ. Res. 2004, 94, 534–541. [Google Scholar] [CrossRef]
- Walenga, J.M.; Torres, T.A.; Jeske, W.P.; Schwartz, J.; Escalante, V.; Newman, J.D.; Bakhos, M. Protein C Pathway, Inflammation, and Pump Thrombosis in Patients with Left Ventricular Assist Devices. Clin. Appl. Thromb. 2020, 26, 1076029620959724. [Google Scholar] [CrossRef]
- Grosman-Rimon, L.; Jacobs, I.; Tumiati, L.C.; McDonald, M.A.; Bar-Ziv, S.P.; Fuks, A.; Kawajiri, H.; Lazarte, J.; Ghashghai, A.; Shogilev, D.J.; et al. Longitudinal assessment of inflammation in recipients of continuous-flow left ventricular assist devices. Can. J. Cardiol. 2015, 31, 34. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciaccaluga, C.; Ghionzoli, N.; Mandoli, G.E.; D’Ascenzi, F.; Focardi, M.; Valente, S.; Cameli, M. Biomarkers in Patients with Left Ventricular Assist Device: An Insight on Current Evidence. Biomolecules 2022, 12, 334. https://doi.org/10.3390/biom12020334
Sciaccaluga C, Ghionzoli N, Mandoli GE, D’Ascenzi F, Focardi M, Valente S, Cameli M. Biomarkers in Patients with Left Ventricular Assist Device: An Insight on Current Evidence. Biomolecules. 2022; 12(2):334. https://doi.org/10.3390/biom12020334
Chicago/Turabian StyleSciaccaluga, Carlotta, Nicolò Ghionzoli, Giulia Elena Mandoli, Flavio D’Ascenzi, Marta Focardi, Serafina Valente, and Matteo Cameli. 2022. "Biomarkers in Patients with Left Ventricular Assist Device: An Insight on Current Evidence" Biomolecules 12, no. 2: 334. https://doi.org/10.3390/biom12020334
APA StyleSciaccaluga, C., Ghionzoli, N., Mandoli, G. E., D’Ascenzi, F., Focardi, M., Valente, S., & Cameli, M. (2022). Biomarkers in Patients with Left Ventricular Assist Device: An Insight on Current Evidence. Biomolecules, 12(2), 334. https://doi.org/10.3390/biom12020334






