Abstract
G-quadruplexes (GQs) are secondary nucleic acid structures that play regulatory roles in various cellular processes. G-quadruplex-forming sequences present within the 5′ UTR of mRNAs can function not only as repressors of translation but also as elements required for optimum function. Based upon previous reports, the majority of the 5′ UTR GQ structures inhibit translation, presumably by blocking the ribosome scanning process that is essential for detection of the initiation codon. However, there are certain mRNAs containing GQs that have been identified as positive regulators of translation, as they are needed for translation initiation. While most cellular mRNAs utilize the 5′ cap structure to undergo cap-dependent translation initiation, many rely on cap-independent translation under certain conditions in which the cap-dependent initiation mechanism is not viable or slowed down, for example, during development, under stress and in many diseases. Cap-independent translation mainly occurs via Internal Ribosomal Entry Sites (IRESs) that are located in the 5′ UTR of mRNAs and are equipped with structural features that can recruit the ribosome or other factors to initiate translation without the need for a 5′ cap. In this review, we will focus only on the role of RNA GQs present in the 5′ UTR of mRNAs, where they play a critical role in translation initiation, and discuss the potential mechanism of this phenomenon, which is yet to be fully delineated.
1. Introduction
A G-quadruplex (GQ) is a secondary structure adopted by both DNA and RNA, and is formed by the stacking of G-tetrad units. G-tetrads are assembled by the combination of four guanines via the Hoogsteen hydrogen bonds, and the GQ structures are stabilized in the presence of metal cations bound in a plane or in between two tetrads (Figure 1) [1,2,3,4]. It has been found that DNA GQs play regulatory roles in replication, transcription, cell proliferation, genome recombination and telomere maintenance [5,6,7,8,9,10]. On the other hand RNA GQs were found to be involved in translational regulation [11,12,13,14,15,16,17,18,19,20,21,22,23,24], 3′-end processing [25,26], transcription termination [27], alternative splicing [28,29,30,31,32], mRNA localization [33,34,35], protein binding [36,37,38] and telomeric RNA biology [39,40,41,42,43]. Compared with their DNA counterparts, RNA GQs are easier to form, since they do not have to compete with a complementary strand. RNA GQs have been found to be more stable in the folded form, presumably because of the 2′-OH group’s ability to participate in hydrogen bonding [18,44,45,46].
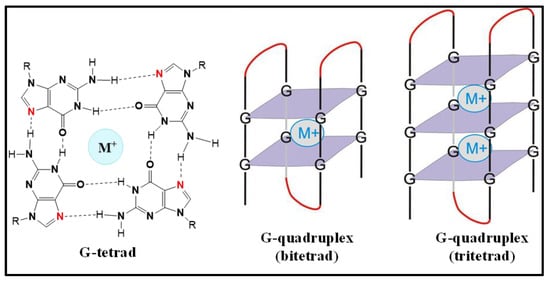
Figure 1.
The chemical structure of a guanine tetrad featuring a central metal cation (G-tetrad), two-tier (bi-tetrad) and three-tier (tri-tetrad) G-quadruplexes.
Bioinformatic analysis has revealed more than 4000 GQ motifs in the 5′ UTR (5′ untranslated region) of human mRNAs [47,48,49,50]. The functional consequences of GQ have been experimentally validated in more than 30 5′ UTR sequences from human mRNAs [20,24]. It has been demonstrated that GQ-forming sequences present within the 5′ UTR can function both as vital elements and as repressors of in vitro and in vivo translation [14,18,21,22,51,52]. More interestingly, the role of GQ structures in the modulation of translation depends on the context in which the GQ structure exists [53,54]. It is well known that GQ structures modulate translation in several clinically important mRNAs. Detailed studies of GQ-forming sequences in the 5′ UTR region of various mRNAs, including NRAS [18], ZIC-1 [11], MT3-MMP [22] and many others [13,15,16,19,23,24,25,36,37,55,56,57,58,59,60,61,62,63,64], have shown that GQ structures usually serve as repressors of translation and, in most cases, they drive mRNA translation through the cap-dependent mechanism [20,65,66]. The inhibitory effect is often a function of the stability and location of the GQ within the 5′ UTR. The most commonly accepted mechanism of inhibition is GQ-mediated hindrance of ribosomal scanning as it marches toward finding the initiation codon. Other mechanisms of inhibition have also been reported. For example, in case of P1-HNF4A, the two long side chains within the 5′ UTR GQ recruit RNA binding proteins that stabilize the GQ and result in strong repression of translation [67]. In another case, the NRAS GQ was able to inhibit in vitro translation only when it was located in close proximity to the 5′ cap [54], indicating that position matters in the case of the inhibitory effects of the GQ when present within the 5′ UTR of mRNAs. In contrast to the widely reported inhibitory role of the RNA GQs in translation, other reports have demonstrated that the RNA GQs in the 5′ UTR of FGF2 [14], VEGF [21], ARPC2 [38,68], TGFβ2 [51], α-synuclein (SNCA) [69], NRF2 [70,71], BAG-1 [72] and mTOR [73] mRNAs are essential for optimal translation. Interestingly, several of these mRNAs harbor Internal Ribosomal Entry Sites (IRESs) that are known to initiate translation in a cap-independent manner. IRES elements are reported to be important during development, stress and many diseases [74,75,76,77,78]. Because IRESs are important in cap-independent translation initiation, GQs embedded in such sequences may play a role in such a process. In fact, in the case of VEGF IRES-A, the 40S ribosomal subunit has been shown to interact with the GQ-containing domain [79]. However, in most cases, the function of GQ structures in the IRES-driven cap-independent translation initiation remains to be uncovered Although initially observed in viral mRNAs, IRES-mediated translation initiation has also been identified in many cellular mRNAs [80,81,82]. Generally, cellular IRESs contain fewer RNA structures than viral IRESs and share little sequence conservation among them, making it difficult to classify and predict novel endogenous IRESs in eukaryotic mRNAs. A recent screen using an in vivo translation reporter assay has demonstrated that about 10% of mammalian mRNAs contain certain elements that are involved in IRES function [83]. However, compared with the viral IRESs, the mechanistic aspects of cellular IRES function are poorly understood [66,84,85,86]. Most studies have shown that cap-independent initiation mechanism utilizes IRES elements in case of impaired m7G cap structure recognition at the 5′-end of mRNAs or non-canonical scanning models of translation initiation in eukaryotes [80,85]. However, cap-independent translation initiation can also occur in the absence of an IRES. Some cellular mRNAs use a separate mechanism, known as ‘cap-independent translation enhancer (CITE)-mediated translation’ under apoptotic conditions, which relies on ribosomal scanning of the 5′ UTR [78,87]. Recently, it has also been mentioned that mRNAs containing N6-methyladenosine (m6A) in their 5′ UTR may also be translated cap-independently [88,89,90]. In this mini review, we will primarily concentrate on the role of GQ structures in the context of IRES-mediated cap-independent translation initiation, along with the experimental systems used to investigate them. Related topics such as the regulatory roles of 5′ UTR mRNAs in translation can be found in many recent reviews [20,24,54,91,92], and the biological functions of IRES-mediated cap-independent translation and regulation have also been reviewed recently [65,66,93,94].
2. The Role of RNA G-Quadruplexes on IRES-Driven Cap-Independent Translation
The traditional translation initiation mechanism relied on eIF4E binding to the 5′ cap of mRNAs for cap-dependent translation until 1988, when Pelletier and Sonenberg [95] showed that some mRNAs have a mechanism for circumventing the need for eIF4E binding, which was termed IRES-mediated translation initiation. This mode of initiation of translation is usually independent of the identification of 5′ cap structure but may include scanning in a search for the AUG start codon or recruiting the 40S ribosomal subunit directly in the vicinity of the start codon. Recruitment of the 40S subunit may take place either in the complete absence of any other protein factors or with the assistance of specific combinations of canonical initiation factors (such as eIF4G and eIF3) and IRES trans-acting factors (ITAFs) (Figure 2) [96,97]. ITAFs are known to help recruit the 40S ribosomal subunit on the mRNA by different interactions or by stabilizing specific active IRES conformations [81,98,99]. Recently, several studies demonstrated the effect of RNA GQ structures on IRES-mediated cap-independent translation when these structures were located either in the vicinity of the IRES elements or as a part of them [14,21,68,72,73]. Below, we review the known role of GQ structures on IRES activity during cap-independent translation initiation mechanisms.
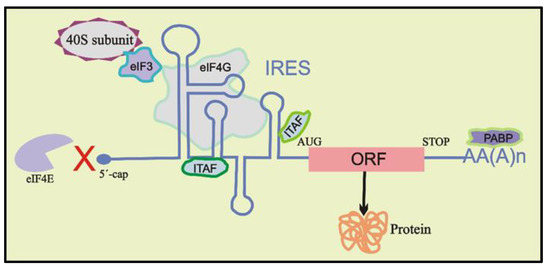
Figure 2.
Schematic representation of cap-independent translation. Stem–loop secondary structures within the IRES in 5′ UTR may recruit the 40S ribosomal subunit directly to the start codon (AUG) of the open reading frame (ORF) or its vicinity through direct or indirect interactions, requiring the aid of certain canonical initiation factors (eIFs) and/or IRES trans-acting factors (ITAFs).
The role of RNA GQ in the 5′ UTR of mRNAs is mostly described as a repressor of translation, leading to the suggestion that RNA GQ is an inhibitory element for gene expression [17]. However, RNA GQ formation has been shown to promote translation initiation in several cases in a cap-independent manner. Several IRES elements in the 5′ UTRs of cellular mRNAs have been reported to contain GQ motifs. In 2003, Bonnal et al. [14] reported an IRES element within the 5′ UTR of the human fibroblast growth factor 2 (FGF2) mRNA from an analysis of the cis-acting elements. They demonstrated that IRES harbors a GQ motif, which is responsible for IRES activity in the translation initiation. They identified the five G-quartets’ RNA GQ structure in the 5′ UTR of FGF2 mRNA by chemical probing and enzymatic footprinting experiments. Moreover, the mutation and deletion studies revealed that a single 176-nucleotide-long IRES contains two RNA stem–loops and a GQ motif, and the contribution of these structures on IRES activity was able to control translation initiation at four downstream initiation codons within the FGF2 mRNA. In a recent study, IRES-mediated translation control was demonstrated for the differential expression of FGF9 protein in response to the metastasis of colon cancer cells [100]. Reports indicated that FGF9 protein synthesis is normally low due to upstream open reading frame (uORF)-mediated translational repression; however, it is upregulated in response to hypoxia via an IRES-dependent translational control.
Morris et al. [21] reported that the RNA GQ structure is essential for IRES-mediated translation initiation in the human vascular endothelial growth factor (hVEGF) mRNA. The hVEGF mRNA possesses a relatively long (1038 nt) 5′ UTR and harbors two separate IRESs, which are independently capable of initiating cap-independent translation [52,101]. RNase T1 and DMS footprinting assays revealed that a 17-nt (GGAGGAGGGGGAGGAGG) guanine-rich sequence of VEGF IRES-A (293 nt) has a GQ structure. To investigate the roles of GQ on IRESs, we performed a dual-luciferase reporter assay in HeLa cells by introducing mutations in the IRES-A. It was found that the mutation of one of the minimal GQ forming segments had no effect on IRES activity, whereas the quadruple mutant (lacking sufficient Gs to adopt an intramolecular G-quadruplex structure) had lost its activity. We proposed that the double mutant that retained the activity has accommodated an alternate GQ structure by which it can maintain an activity similar to the wild-type. Furthermore, it was proven that the IRES-A forms a switchable, two-tier GQ structure, which is essential for its functional role. The different double mutants analyzed in this study were able to utilize different G-stretches and form alternate quadruplex structures (possible by using different combinations of the five G-stretches) with varying levels of activity. We proposed that the structural flexibility and malleability of the VEGF IRES-A GQ is crucial for its unique function that helps it bind to the factors necessary for translation initiation in a cap-independent manner.
In another study, a subsequent mechanistic analysis by using in vitro RNA footprinting showed that the GQ located in the IRES element of the VEGF mRNA interacts directly with the 40S ribosomal subunit in the absence of other protein factors [79]. In 2015, Cammas et al. [102] also analyzed the effect of GQs in VEGF mRNA translation. They increased GQ stability by inserting or replacing the sequence to form a 3-G-quartet quadruplex structure and further stabilized it by utilizing GQ ligands. These led to an inhibitory effect on translation, similar to the previously characterized GQs in the 5′ UTR of several mRNAs.
In a recent study, Al-Zeer et al. [68] provided structural and functional evidence that a GQ structure within the 5′ UTR of the actin-related protein 2/3 complex subunit 2 (ARPC2) mRNA is essential for IRES-mediated cap-independent translation. The 5′ UTR of ARPC2 mRNA exists in two variants; the longer variant adopts IRES, which harbors a GQ motif in its central stem-loop element. They investigated the cellular function of the IRES element by measuring the ARPC2 expression levels as a function of increased cell density-related stress. They observed that the relative ARPC2 protein level increased with cell density, thus suggesting the expected role of IRES elements in the expression of certain genes that represses cap-dependent translation under stressful conditions [76]. Furthermore, they proposed a model for IRES element folding in the 5′ UTR of the ARPC2 mRNA based on structural probing experiments and bioinformatic prediction programs. They demonstrated that RNA folds into three hairpins, the middle of which includes an exposed GQ motif on top of the stem–loop structure. Disruption of the GQ structures by site-directed mutagenesis led to an approximately 30% decrease in translational efficiency. The decreased translation efficiency was less pronounced than the value reported previously for the GQ-containing IRES element in the 5′ UTR of VEGF; nevertheless, it showed the functional effect of GQ in IRES-mediated translation initiation [21]. These results indicated that the GQ in the ARPC2 IRES is at least partly required for its full functionality. In contrast, another study demonstrated that the GQ motif located in the 5′ UTR region of ARPC2 mRNA plays an inhibitory role in translation. They utilized biophysical characterization for GQ structure confirmation and luciferase reporter assays for translational inhibitory activity, but the exact mechanism of GQ’s roles in the 5′ UTR of ARPC2 mRNA was not demonstrated [38,103].
Koukouraki and Doxakis [69] reported another IRES element that harbors a GQ motif in the 5′ UTR of the α-synuclein (SNCA) mRNA. SNCA is a neuronal protein which is likely to be involved in the modulation of synaptic neurotransmission. Several approaches revealed that the 5′ UTR of SNCA mRNA has an internal ribosome entry site (IRES) element, which encompasses most of the 5′ UTR. They observed that different cellular conditions, such as depolarization of the plasma membrane, serum malnutrition and oxidative stress, stimulated the translation of α-synuclein protein through its IRES activity. The increased IRES activity not only enhanced expression of a luciferase reporter but also showed a significant increase in endogenous α-synuclein expression [69]. It was shown that the 5′ UTR initiates SNCA mRNA translation in a cap-independent process when cap-dependent translation was diminished by rapamycin treatment. The human SNCA mRNA has a moderately long 5′ UTR of 264 nt with 66% of GC content that, as expected, has stable stem–loop structures and also includes a GQ motif. To investigate the role of GQ structures in SNCA translation or IRES activity, the authors used mutagenized reporter constructs and performed a dual luciferase assay. Functional studies using dual luciferase and real time RT-PCR assays suggested that the GQ in the 5′ UTR of α-synuclein mRNA was not absolutely required for translational activity or IRES functionality, but certainly increased its efficiency. This study serves as another example of GQs’ role in optimal IRES activity.
Recently, Jodoin et al. [72] showed that an RNA GQ located towards the 5′ end of the BAG-1 5′ UTR influences both cap-dependent as well as cap-independent mRNA translation, making it the first report of the involvement of a quadruplex in both mechanisms of translation initiation. In a previous study, the same group identified that the GQ structure within 6 and 35 nt of the BAG-1 5′ UTR repressed the translation of the luciferase gene [63]. They confirmed that this particular quadruplex represses the cap-dependent translation of the main BAG-1 isoform, which agrees with the role of the several previously identified 5′ UTR GQs. Furthermore, they proved that a mutation in the GQ-forming sequence led to the inhibition of cap-independent translation as well, even though it was not present within the IRES. In order to elucidate this phenomenon, they analyzed the effect of deleting the GQ structure in other secondary structural elements in the 5′ UTR, including its IRES, using the selective 2′-hydroxyl acylation analyzed by primer extension (SHAPE) technique. They found that disruption of the GQ structure created base pairing differences mainly in the IRES region of the 5′ UTR, thereby affecting the global folded secondary structure of the 5′ UTR, which, in turn, created the more stable minimal IRES subdomain in the quadruplex mutant compared with the wild-type. On the basis of this observation, they proposed that the structural stability altered because the quadruplex disruption made it more difficult for the IRES to bind to the ITAFs that are essential for 40S subunit recruitment. In conclusion, the study provided an example of a GQ that is essential for maintaining a specific IRES secondary structure to aid cap-independent translation.
Based on a bioinformatics analysis, it has been revealed that transforming growth factor β2 (TGFβ2) mRNA has a 23-nt putative GQ-forming sequence in its 5′ UTR. Agarwala et al. performed spectroscopic studies and a luciferase reporter assay to characterize the thermodynamic stability of the GQ and its role in modulating gene expression at the translational level [51]. They found that a construct with the GQ forming sequence together with the flanking sequences, as in the context of the entire 5′ UTR of TGFβ2, significantly increased the luciferase expression levels compared with its corresponding mutant. This observation indicated that the GQ within the 5′ UTR of the TGFβ2 mRNA has a context-dependent effect on translation in which the GQ structure acts as an enhancer of gene expression [51]. The enhancing effect of the GQ structure could be attributed to its location in the 5′ UTR, i.e., it lies further away from both the 5′ end of the UTR and from the translation start site. Unlike the GQ structure in VEGF 5′ UTR, which has been previously referred to as a switchable quadruplex that positively regulates cap-independent translation initiation, the TGFβ2 quadruplex is a stable quadruplex within an unusually long 5′ UTR that positively regulates translation in cap-dependent translation. Although there is an IRES element present in the 5′ UTR of TGFβ2, there is no correlation between the role of GQ and IRES functionality. Further study is needed to delineate the precise role of GQ in the context of IRES activity in TGFβ2 translation initiation.
Human nuclear factor erythroid 2–related factor 2 (NRF2) mRNA contains a 555-nt sequence of the 5′ UTR with 70% GC content, which has a 31-nt putative GQ-forming sequence. In 2017, Lee et al. [70] demonstrated the presence of a GQ structure in the 5′ UTR of NRF2 mRNA by utilizing biophysical and biochemical methods, and found that the GQ structure is important for 5′ UTR activity. To identify the exact role of the GQ structure in NRF2 protein translation under oxidative stress, they used proteomics to reveal that elongation factor 1 alpha (EF1a) binds to the GQ sequence. Furthermore, they measured the binding interaction of the EF1a protein with the GQ-forming sequence of 5′ UTR by electrophoretic mobility shift assays (EMSAs) along with RNA–protein interaction assays for cells treated with H2O2. In addition, their use of small interfering RNA (siRNA) to knock down EF1a by using a reporter assay suggested that the presence of the GQ is important for cellular-level activation of NRF2 5′ UTR under oxidative stress. In 2010, Li et al. [104] studied cap-independent translation initiation in NRF2 mRNA via an IRES-mediated mechanism under oxidative stress. They discovered that translation initiation was induced by the binding of 18S rRNA of the ribosome to a highly conserved RNA binding site located within the IRES. According to these two studies, there are two mechanisms through which cap-independent translation is mediated in NRF2 mRNA, via either the IRES or the GQ. Therefore, it would be interesting to study collectively whether there is any influence of the GQ on IRES-mediated translation initiation, since they both are located in close proximity to each other.
3. Mechanism of the Role of G-Quadruplexes in IRES-Mediated Translation Initiation
The initiation of protein synthesis in eukaryotes needs the recruitment of the 40S ribosomal subunit to eventually recognize the start codon (AUG) for translation of the mRNA [105,106,107]. Canonical eukaryotic cap-dependent translation initiation incorporates a complex mechanism for the recruitment and positioning of ribosomes at the start sites, during which, many factors interact with the ribosome [106,108,109]. Alternatively, several viruses and some eukaryotic mRNAs use a cap-independent pathway through highly structured IRES sequences present in the 5′ UTR of mRNAs, which drives pre-initiation complex formation by either positioning the ribosome on or just upstream of the translation start site [66,88,110] (Figure 2). Several studies have shown that GQ structures reside in proximity to the IRES element in 5′ UTR of mRNAs and modulate IRES activity [21,70,71,73,79]. For example, the presence of a switchable GQ structure in the IRES-A of human vascular growth factor (hVEGF) mRNA was deemed to be essential for optimum cap-independent translation initiation [21]. To identify the exact role of GQ in IRES-A-mediated translation initiation of hVEGF, we utilized structure mapping analyses [79]. Our findings indicated that a 17-nucleotide independently folding RNA GQ domain within the 294-nucleotide IRES-A interacted directly with the 40S ribosomal subunit without the aid of other protein factors (Figure 3). In addition, we showed that the GQ-forming domain particularly dictates the function and binding affinity of IRES-A towards the 40S subunit compared with the bacterial 30S subunit. Further, we proved that GQ domain deletion hindered the 40S subunit from binding to the IRES and deteriorated cap-independent translation initiation, indicating the necessity of the GQ structure in IRES function. Similarly, Marques-Ramos et al. [73] also demonstrated the direct binding interactions of GC-rich secondary structures of mTOR 5′ UTR with the 40S ribosomal subunit without the involvement of any initiation factors. They revealed that the mTOR 5′ UTR binds to the ribosomal subunit with a similar binding affinity to the CFSV viral IRES by using a filter binding assay.
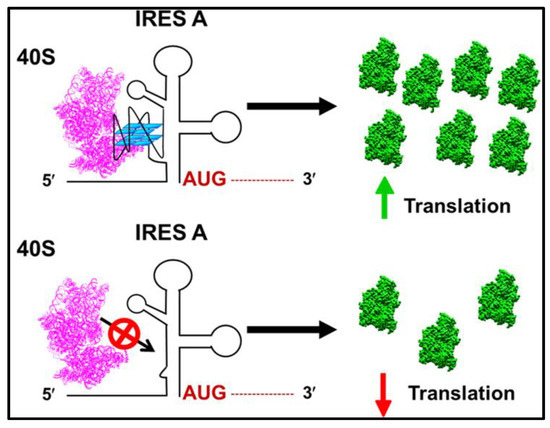
Figure 3.
Schematic representation of direct recruitment of the 40S ribosomal subunit by G-quadruplex structures to drive cap-independent translation initiation. Adapted with permission from Bhattacharyya et al., ACS Biochemistry [79].
In another study, indirect evidence of GQ’s role in the binding of IRES to the 40S ribosomal subunit has been investigated [72]. The authors analyzed the effects of GQ structure disruption on other secondary structural elements in the 5′ UTR, including its IRES using SHAPE and found that GQ disruption made it more difficult for the IRES to bind to ITAFs, which is necessary for 40S subunit recruitment. In 2016, Lee et al. [70] reported that a GQ-forming sequence was important for the 5′ UTR activity of NRF2 mRNA through structural mapping experiments [111]. To investigate the exact role of GQ structure in NRF2 protein translation under oxidative stress, they utilized proteomics and EMSA, and revealed the binding interaction of the elongation factor 1 alpha (EF1a) protein with the GQ-forming sequence within the 5′ UTR. Their siRNA-mediated knockdown of EF1α by using a reporter assay suggested that the presence of the GQ is important for NRF2 5′ UTR activation under oxidative stress. Although some studies have demonstrated the direct and indirect role of GQs in IRES activity in the cap-independent translation of various mRNAs, the exact mechanism and potential variations in GQs’ role in ITAF binding in IRES function still need to be adequately deciphered.
4. Future Perspectives of G-Quadruplexes’ Effects on IRES-Mediated Translation
G-quadruplexes present within the 5′ UTR modulate translation in both a cap-dependent and cap-independent fashion. IRESs are segments located in 5′ UTR of mRNAs that are capable of recruiting the ribosome and initiating translation independently of the well-understood 5′ cap-dependent initiation mechanism [105,107]. IRESs generally function when 5′ cap-dependent translation initiation has been repressed due to an impaired m7G cap structure recognition during development, stress and in many diseases [73,74,79], thereby mediating translation initiation in a cap-independent pathway. There is evidence that the cap-independent mechanism is regulated through RNA secondary structures such as the GQs present within IRESs. However, a limited number of experimentally verified IRES elements have been reported due to the absence of structural and sequence similarities, which make it harder to predict new IRESs in human mRNAs. In 2016, Kwok et al. [112] reported that out of the 3383 RNA GQs observed in the mRNAs, 540 were found in the 5′ UTR. They also identified that in the presence of an rG4 constraint, most of the RNA secondary structures differed extensively, proposing that it not only yields different RNA conformations but can also influence the folding of distal elements. On the basis of these facts and the examples that have been discussed above, we propose that the GQs present within or in the vicinity of an IRES have the potential to either influence IRESs’ functionality or folding [21,51,70,72,104].
Now the question arises as how to predict the putative IRES structures using the mRNA 5′ UTR sequence amid the lack of structural and sequence similarities among the IRESs. Very recently, Tzu-Hsien et al. [113] retrieved 659 human IRESs with experimental evidence based on analyses of databases including IRESite [114,115] and IRESbase [116] together with published literature. Though the number is 659, many of them are from the transcript variants of a particular gene. Combining all the previously identified databases (for example, IRESsite and IRESbase) and available tools, including IRESPred [117], IRESfinder [118] and IRESpy [119], together with the recent reports published by Tzu-Hsien et al. [113] and Bohálová et al. [120], we can find out the experimentally validated IRES elements as well the mRNAs that have the putative IRES-forming sequence in the 5′ UTR. Given the sequences of these selected mRNAs, we can easily predict the presence of GQ sequences within their IRES-forming motifs or in the 5′ UTR in close proximity to the IRES. Currently, GQ detection software such as QGRS Mapper [50], G4 Hunter [121] and QuadBase [122] are available.
We analyzed the 5′ UTR sequences of 659 previously identified human IRESs to check individually whether any of them contained potential GQ-forming sequences. Our analysis using QGRS Mapper revealed that there are 51 5′ UTR sequences corresponding to the mRNAs of 51 different genes that have potential GQ-forming regions with a G score of 20 and above. Among the 51, there are 14 sequences with G scores of above 30 which can form either a three-tiered or a four-tiered GQ, including PTCH1, which had a very high score of 60 (Table 1).

Table 1.
List of mRNAs comprising high G scores (≥30) at the 5′ UTR according to QGRS Mapper.
The targets identified using QGRS Mapper were also analyzed using the G4 RNA screener tool in order to find other scores such the G4 Hunter score (G4H), the cGcC score and the neural network score (G4NN). G4 Hunter (G4H) [123] predicts a G quadruplex propensity score based on the G richness and G skewness in a given sequence. G4H uses an optimal threshold score of 0.9 to classify an RNA sequence as a G4 RNA. Bedrat et al. [121] proposed that a G4H score above the threshold of 1.2 would be a good measure for the identification of G4-forming potential in a given sequence, though there will be some false positives and false negatives. All the targets given in Table 1 except two have a G4H score above 1.2. In the case of G4NN, the optimal threshold for G4RNA is 0.5, as it assigns scores between 1 and 0 for G4 and non-G4 RNAs, respectively [124]. Most of our predicted targets have a score that is very close to 1, except for insulin receptor (INSR). The cGcC score is used to evaluate the competition between the formation of GQ and Watson–Crick rule-based structures, as the G stretches involved in the formation of the GQ can base-pair with the nearby C stretches and inhibit GQ formation [125]. The given threshold for the cGcC score in G4 Screener is 4.5. We evaluated the GQ-forming sequences given in Table 1, accompanied by an additional 30 nucleotides upstream and downstream to predict the cGcC score. Beaudoin et al. [125], based on their analysis using 12 potential GQ-forming motifs, suggested that C runs that are present as far as 20 to 50 nucleotides from the GQ motif can influence its folding. They also predicted that candidates above the threshold score of 2.05 can fold into a GQ structure [125]. Among the targets that we analyzed in Table 1, all except FBXW7 and RUNX1 satisfied this particular threshold score.
5. Conclusions
The essential role of GQs within a set of 5′ UTR mRNA in upregulating cap-independent translation is still not fully delineated. Unlike the inhibitory effect of GQ within the 5′ UTR, the ones that are involved in the positive regulation are few and far between. G-quadruplexes present in VEGF, alpha synuclein, ARPC2 and BAG-1 were found to be involved in cap-independent translation that occurs via 5′ UTR IRESs. Although, the abovementioned GQs positively regulate IRES-mediated cap-independent translation initiation, only the GQs of VEGF, alpha synuclein and ARPC2 are present within the IRES, whereas BAG-1 is located upstream of the IRES. G-quadruplexes in the 5′ UTR of TGFβ2 and NRF2 can also positively influence translation, but their role in the context of IRES is yet to be reported. According to our analysis, the highly structured nature of the IRES is important in modulating cap-independent translation, especially in binding to ITAFs and recruiting the 40S ribosomal subunit. The GQs, when present within or in the vicinity of IRESs, may help display such structural features in order to recruit trans-acting factors. We believe that, considering the context in which GQs are located and their interactions with translation initiation factors as well as ribosomal subunits, experimental analysis of more mRNAs would help us to better understand the role of GQs in IRES-mediated cap-independent translation initiation. Such efforts are poised to be more successful as more IRES databases and GQ prediction tools become available and their findings are coupled with experimental approaches.
Author Contributions
Conceptualization, S.B., M.E.H. and T.M.; writing—original draft preparation, M.E.H. and T.M.; writing—review and editing, S.B., M.E.H. and T.M.; funding acquisition, S.B. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by funding from Kent State University to S.B.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bhattacharyya, D.; Mirihana Arachchilage, G.; Basu, S. Metal Cations in G-Quadruplex Folding and Stability. Front. Chem. 2016, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, D.E.; Feigon, J. Multistranded DNA Structures. Curr. Opin. Struct. Biol. 1999, 9, 305–314. [Google Scholar] [CrossRef]
- Henderson, E.; Hardin, C.C.; Walk, S.K.; Tinoco, I.; Blackburn, E.H. Telomeric DNA Oligonucleotides Form Novel Intramolecular Structures Containing Guanine-Guanine Base Pairs. Cell 1987, 51, 899–908. [Google Scholar] [CrossRef]
- Kim, J.; Cheong, C.; Moore, P.B. Tetramerization of an RNA Oligonucleotide Containing a GGGG Sequence. Nature 1991, 351, 331–332. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Hurley, L.H.; Neidle, S. Targeting G-Quadruplexes in Gene Promoters: A Novel Anticancer Strategy? Nat. Rev. Drug Discov. 2011, 10, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Brooks, T.A.; Kendrick, S.; Hurley, L. Making Sense of G-Quadruplex and i-Motif Functions in Oncogene Promoters. FEBS J. 2010, 277, 3459–3469. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Zhao, Y.; Li, N. Genome-Wide Analysis Reveals Regulatory Role of G4 DNA in Gene Transcription. Genome Res. 2008, 18, 233–241. [Google Scholar] [CrossRef]
- Moore, M.J.B.; Schultes, C.M.; Cuesta, J.; Cuenca, F.; Gunaratnam, M.; Tanious, F.A.; Wilson, W.D.; Neidle, S. Trisubstituted Acridines as G-Quadruplex Telomere Targeting Agents. Effects of Extensions of the 3,6- and 9-Side Chains on Quadruplex Binding, Telomerase Activity, and Cell Proliferation. J. Med. Chem. 2006, 49, 582–599. [Google Scholar] [CrossRef]
- Rizzo, A.; Salvati, E.; Porru, M.; D’Angelo, C.; Stevens, M.F.; D’Incalci, M.; Leonetti, C.; Gilson, E.; Zupi, G.; Biroccio, A. Stabilization of Quadruplex DNA Perturbs Telomere Replication Leading to the Activation of an ATR-Dependent ATM Signaling Pathway. Nucleic Acids Res. 2009, 37, 5353–5364. [Google Scholar] [CrossRef]
- Siddiqui-Jain, A.; Grand, C.L.; Bearss, D.J.; Hurley, L.H. Direct Evidence for a G-Quadruplex in a Promoter Region and Its Targeting with a Small Molecule to Repress c-MYC Transcription. Proc. Natl. Acad. Sci. USA 2002, 99, 11593–11598. [Google Scholar] [CrossRef]
- Arora, A.; Dutkiewicz, M.; Scaria, V.; Hariharan, M.; Maiti, S.; Kurreck, J. Inhibition of Translation in Living Eukaryotic Cells by an RNA G-Quadruplex Motif. RNA 2008, 14, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Suess, B. An RNA G-Quadruplex in the 3’ UTR of the Proto-Oncogene PIM1 Represses Translation. RNA Biol. 2011, 8, 802–805. [Google Scholar] [CrossRef]
- Balkwill, G.D.; Derecka, K.; Garner, T.P.; Hodgman, C.; Flint, A.P.F.; Searle, M.S. Repression of Translation of Human Estrogen Receptor Alpha by G-Quadruplex Formation. Biochemistry 2009, 48, 11487–11495. [Google Scholar] [CrossRef] [PubMed]
- Bonnal, S.; Schaeffer, C.; Créancier, L.; Clamens, S.; Moine, H.; Prats, A.-C.; Vagner, S. A Single Internal Ribosome Entry Site Containing a G Quartet RNA Structure Drives Fibroblast Growth Factor 2 Gene Expression at Four Alternative Translation Initiation Codons. J. Biol. Chem. 2003, 278, 39330–39336. [Google Scholar] [CrossRef] [PubMed]
- Derecka, K.; Balkwill, G.D.; Garner, T.P.; Hodgman, C.; Flint, A.P.F.; Searle, M.S. Occurrence of a Quadruplex Motif in a Unique Insert within Exon C of the Bovine Estrogen Receptor α Gene (ESR1). Biochemistry 2010, 49, 7625–7633. [Google Scholar] [CrossRef]
- Gomez, D.; Guédin, A.; Mergny, J.-L.; Salles, B.; Riou, J.-F.; Teulade-Fichou, M.-P.; Calsou, P. A G-Quadruplex Structure within the 5′-UTR of TRF2 mRNA Represses Translation in Human Cells. Nucleic Acids Res. 2010, 38, 7187–7198. [Google Scholar] [CrossRef]
- Halder, K.; Wieland, M.; Hartig, J.S. Predictable Suppression of Gene Expression by 5′-UTR-Based RNA Quadruplexes. Nucleic Acids Res. 2009, 37, 6811–6817. [Google Scholar] [CrossRef]
- Kumari, S.; Bugaut, A.; Huppert, J.L.; Balasubramanian, S. An RNA G-Quadruplex in the 5′ UTR of the NRAS Proto-Oncogene Modulates Translation. Nat. Chem. Biol. 2007, 3, 218–221. [Google Scholar] [CrossRef]
- Lammich, S.; Kamp, F.; Wagner, J.; Nuscher, B.; Zilow, S.; Ludwig, A.-K.; Willem, M.; Haass, C. Translational Repression of the Disintegrin and Metalloprotease ADAM10 by a Stable G-Quadruplex Secondary Structure in Its 5′-Untranslated Region. J. Biol. Chem. 2011, 286, 45063–45072. [Google Scholar] [CrossRef]
- Leppek, K.; Das, R.; Barna, M. Functional 5′ UTR mRNA Structures in Eukaryotic Translation Regulation and How to Find Them. Nat. Rev. Mol. Cell Biol. 2018, 19, 158–174. [Google Scholar] [CrossRef]
- Morris, M.J.; Negishi, Y.; Pazsint, C.; Schonhoft, J.D.; Basu, S. An RNA G-Quadruplex Is Essential for Cap-Independent Translation Initiation in Human VEGF IRES. J. Am. Chem. Soc. 2010, 132, 17831–17839. [Google Scholar] [CrossRef]
- Morris, M.J.; Basu, S. An Unusually Stable G-Quadruplex within the 5′-UTR of the MT3 Matrix Metalloproteinase mRNA Represses Translation in Eukaryotic Cells. Biochemistry 2009, 48, 5313–5319. [Google Scholar] [CrossRef]
- Shahid, R.; Bugaut, A.; Balasubramanian, S. The BCL-2 5′ Untranslated Region Contains an RNA G-Quadruplex-Forming Motif That Modulates Protein Expression. Biochemistry 2010, 49, 8300–8306. [Google Scholar] [CrossRef]
- Song, J.; Perreault, J.-P.; Topisirovic, I.; Richard, S. RNA G-Quadruplexes and Their Potential Regulatory Roles in Translation. Translation 2016, 4, e1244031. [Google Scholar] [CrossRef]
- Christiansen, J.; Kofod, M.; Nielsen, F.C. A Guanosine Quadruplex and Two Stable Hairpins Flank a Major Cleavage Site in Insulin-like Growth Factor II mRNA. Nucleic Acids Res. 1994, 22, 5709–5716. [Google Scholar] [CrossRef] [PubMed]
- Decorsière, A.; Cayrel, A.; Vagner, S.; Millevoi, S. Essential Role for the Interaction between HnRNP H/F and a G Quadruplex in Maintaining P53 Pre-mRNA 3’-End Processing and Function during DNA Damage. Genes Dev. 2011, 25, 220–225. [Google Scholar] [CrossRef]
- Wanrooij, P.H.; Uhler, J.P.; Simonsson, T.; Falkenberg, M.; Gustafsson, C.M. G-Quadruplex Structures in RNA Stimulate Mitochondrial Transcription Termination and Primer Formation. Proc. Natl. Acad. Sci. USA 2010, 107, 16072–16077. [Google Scholar] [CrossRef]
- Didiot, M.-C.; Tian, Z.; Schaeffer, C.; Subramanian, M.; Mandel, J.-L.; Moine, H. The G-Quartet Containing FMRP Binding Site in FMR1 mRNA Is a Potent Exonic Splicing Enhancer. Nucleic Acids Res. 2008, 36, 4902–4912. [Google Scholar] [CrossRef]
- Gomez, D.; Lemarteleur, T.; Lacroix, L.; Mailliet, P.; Mergny, J.-L.; Riou, J.-F. Telomerase Downregulation Induced by the G-Quadruplex Ligand 12459 in A549 Cells Is Mediated by HTERT RNA Alternative Splicing. Nucleic Acids Res. 2004, 32, 371–379. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, J.; Harvey, S.E.; Hu, X.; Cheng, C. RNA G-Quadruplex Secondary Structure Promotes Alternative Splicing via the RNA-Binding Protein HnRNPF. Genes Dev. 2017, 31, 2296–2309. [Google Scholar] [CrossRef]
- Marcel, V.; Tran, P.L.T.; Sagne, C.; Martel-Planche, G.; Vaslin, L.; Teulade-Fichou, M.-P.; Hall, J.; Mergny, J.-L.; Hainaut, P.; Van Dyck, E. G-Quadruplex Structures in TP53 Intron 3: Role in Alternative Splicing and in Production of P53 MRNA Isoforms. Carcinogenesis 2011, 32, 271–278. [Google Scholar] [CrossRef]
- Verma, S.P.; Das, P. Novel Splicing in IGFN1 Intron 15 and Role of Stable G-Quadruplex in the Regulation of Splicing in Renal Cell Carcinoma. PLoS ONE 2018, 13, e0205660. [Google Scholar] [CrossRef]
- Imperatore, J.A.; McAninch, D.S.; Valdez-Sinon, A.N.; Bassell, G.J.; Mihailescu, M.R. FUS Recognizes G Quadruplex Structures Within Neuronal mRNAs. Front. Mol. Biosci. 2020, 7, 6. [Google Scholar] [CrossRef]
- Maltby, C.J.; Schofield, J.P.R.; Houghton, S.D.; O’Kelly, I.; Vargas-Caballero, M.; Deinhardt, K.; Coldwell, M.J. A 5′ UTR GGN Repeat Controls Localisation and Translation of a Potassium Leak Channel mRNA through G-Quadruplex Formation. Nucleic Acids Res. 2020, 48, 9822–9839. [Google Scholar] [CrossRef]
- Subramanian, M.; Rage, F.; Tabet, R.; Flatter, E.; Mandel, J.-L.; Moine, H. G-Quadruplex RNA Structure as a Signal for Neurite MRNA Targeting. EMBO Rep. 2011, 12, 697–704. [Google Scholar] [CrossRef]
- Darnell, J.C.; Jensen, K.B.; Jin, P.; Brown, V.; Warren, S.T.; Darnell, R.B. Fragile X Mental Retardation Protein Targets G Quartet mRNAs Important for Neuronal Function. Cell 2001, 107, 489–499. [Google Scholar] [CrossRef]
- Schaeffer, C.; Bardoni, B.; Mandel, J.L.; Ehresmann, B.; Ehresmann, C.; Moine, H. The Fragile X Mental Retardation Protein Binds Specifically to Its mRNA via a Purine Quartet Motif. EMBO J. 2001, 20, 4803–4813. [Google Scholar] [CrossRef]
- Serikawa, T.; Spanos, C.; von Hacht, A.; Budisa, N.; Rappsilber, J.; Kurreck, J. Comprehensive Identification of Proteins Binding to RNA G-Quadruplex Motifs in the 5′ UTR of Tumor-Associated mRNAs. Biochimie 2018, 144, 169–184. [Google Scholar] [CrossRef]
- Deng, Z.; Norseen, J.; Wiedmer, A.; Riethman, H.; Lieberman, P.M. TERRA RNA Binding to TRF2 Facilitates Heterochromatin Formation and ORC Recruitment at Telomeres. Mol. Cell 2009, 35, 403–413. [Google Scholar] [CrossRef]
- Huppert, J.L.; Bugaut, A.; Kumari, S.; Balasubramanian, S. G-Quadruplexes: The Beginning and End of UTRs. Nucleic Acids Res. 2008, 36, 6260–6268. [Google Scholar] [CrossRef]
- López de Silanes, I.; Stagno d’Alcontres, M.; Blasco, M.A. TERRA Transcripts Are Bound by a Complex Array of RNA-Binding Proteins. Nat. Commun. 2010, 1, 33. [Google Scholar] [CrossRef]
- Patel, D.J.; Phan, A.T.; Kuryavyi, V. Human Telomere, Oncogenic Promoter and 5′-UTR G-Quadruplexes: Diverse Higher Order DNA and RNA Targets for Cancer Therapeutics. Nucleic Acids Res. 2007, 35, 7429–7455. [Google Scholar] [CrossRef]
- Xu, Y.; Suzuki, Y.; Ito, K.; Komiyama, M. Telomeric Repeat-Containing RNA Structure in Living Cells. Proc. Natl. Acad. Sci. USA 2010, 107, 14579–14584. [Google Scholar] [CrossRef]
- Cheong, C.; Moore, P.B. Solution Structure of an Unusually Stable RNA Tetraplex Containing G- and U-Quartet Structures. Biochemistry 1992, 31, 8406–8414. [Google Scholar] [CrossRef]
- Liu, H.; Matsugami, A.; Katahira, M.; Uesugi, S. A Dimeric RNA Quadruplex Architecture Comprised of Two G:G(:A):G:G(:A) Hexads, G:G:G:G Tetrads and UUUU Loops. J. Mol. Biol. 2002, 322, 955–970. [Google Scholar] [CrossRef]
- Saccà, B.; Lacroix, L.; Mergny, J.-L. The Effect of Chemical Modifications on the Thermal Stability of Different G-Quadruplex-Forming Oligonucleotides. Nucleic Acids Res. 2005, 33, 1182–1192. [Google Scholar] [CrossRef]
- Cobbold, L.C.; Spriggs, K.A.; Haines, S.J.; Dobbyn, H.C.; Hayes, C.; de Moor, C.H.; Lilley, K.S.; Bushell, M.; Willis, A.E. Identification of Internal Ribosome Entry Segment (IRES)-Trans-Acting Factors for the Myc Family of IRESs. Mol. Cell. Biol. 2008, 28, 40–49. [Google Scholar] [CrossRef]
- Garant, J.-M.; Perreault, J.-P.; Scott, M.S. G4RNA Screener Web Server: User Focused Interface for RNA G-Quadruplex Prediction. Biochimie 2018, 151, 115–118. [Google Scholar] [CrossRef]
- Garst, A.D.; Batey, R.T. A Switch in Time: Detailing the Life of a Riboswitch. Biochim. Biophys. Acta 2009, 1789, 584–591. [Google Scholar] [CrossRef]
- Kikin, O.; D’Antonio, L.; Bagga, P.S. QGRS Mapper: A Web-Based Server for Predicting G-Quadruplexes in Nucleotide Sequences. Nucleic Acids Res. 2006, 34, W676–W682. [Google Scholar] [CrossRef]
- Agarwala, P.; Pandey, S.; Mapa, K.; Maiti, S. The G-Quadruplex Augments Translation in the 5′ Untranslated Region of Transforming Growth Factor Β2. Biochemistry 2013, 52, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Bornes, S.; Boulard, M.; Hieblot, C.; Zanibellato, C.; Iacovoni, J.S.; Prats, H.; Touriol, C. Control of the Vascular Endothelial Growth Factor Internal Ribosome Entry Site (IRES) Activity and Translation Initiation by Alternatively Spliced Coding Sequences. J. Biol. Chem. 2004, 279, 18717–18726. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Morris, M.J.; Kharel, P.; Mirihana Arachchilage, G.; Fedeli, K.M.; Basu, S. Engineered Domain Swapping Indicates Context Dependent Functional Role of RNA G-Quadruplexes. Biochimie 2017, 137, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Bugaut, A.; Balasubramanian, S. 5′-UTR RNA G-Quadruplexes: Translation Regulation and Targeting. Nucleic Acids Res. 2012, 40, 4727–4741. [Google Scholar] [CrossRef]
- Beaudoin, J.-D.; Perreault, J.-P. 5′-UTR G-Quadruplex Structures Acting as Translational Repressors. Nucleic Acids Res. 2010, 38, 7022–7036. [Google Scholar] [CrossRef] [PubMed]
- Serikawa, T.; Eberle, J.; Kurreck, J. Effects of Genomic Disruption of a Guanine Quadruplex in the 5′ UTR of the Bcl-2 MRNA in Melanoma Cells. FEBS Lett. 2017, 591, 3649–3659. [Google Scholar] [CrossRef]
- Rouleau, S.G.; Beaudoin, J.-D.; Bisaillon, M.; Perreault, J.-P. Small Antisense Oligonucleotides against G-Quadruplexes: Specific mRNA Translational Switches. Nucleic Acids Res. 2015, 43, 595–606. [Google Scholar] [CrossRef]
- Menon, L.; Mader, S.A.; Mihailescu, M.-R. Fragile X Mental Retardation Protein Interactions with the Microtubule Associated Protein 1B RNA. RNA 2008, 14, 1644–1655. [Google Scholar] [CrossRef]
- Melko, M.; Bardoni, B. The Role of G-Quadruplex in RNA Metabolism: Involvement of FMRP and FMR2P. Biochimie 2010, 92, 919–926. [Google Scholar] [CrossRef]
- Wolfe, A.L.; Singh, K.; Zhong, Y.; Drewe, P.; Rajasekhar, V.K.; Sanghvi, V.R.; Mavrakis, K.J.; Jiang, M.; Roderick, J.E.; Van der Meulen, J.; et al. RNA G-Quadruplexes Cause EIF4A-Dependent Oncogene Translation in Cancer. Nature 2014, 513, 65–70. [Google Scholar] [CrossRef]
- Weng, H.-Y.; Huang, H.-L.; Zhao, P.-P.; Zhou, H.; Qu, L.-H. Translational Repression of Cyclin D3 by a Stable G-Quadruplex in Its 5′ UTR. RNA Biol. 2012, 9, 1099–1109. [Google Scholar] [CrossRef][Green Version]
- Murat, P.; Marsico, G.; Herdy, B.; Ghanbarian, A.; Portella, G.; Balasubramanian, S. RNA G-Quadruplexes at Upstream Open Reading Frames Cause DHX36- and DHX9-Dependent Translation of Human MRNAs. Genome Biol. 2018, 19, 229. [Google Scholar] [CrossRef]
- Jodoin, R.; Bauer, L.; Garant, J.-M.; Mahdi Laaref, A.; Phaneuf, F.; Perreault, J.-P. The Folding of 5′-UTR Human G-Quadruplexes Possessing a Long Central Loop. RNA 2014, 20, 1129–1141. [Google Scholar] [CrossRef]
- Jodoin, R.; Perreault, J.-P. G-Quadruplexes Formation in the 5′UTRs of mRNAs Associated with Colorectal Cancer Pathways. PLoS ONE 2018, 13, e0208363. [Google Scholar] [CrossRef]
- Lacerda, R.; Menezes, J.; Romão, L. More than Just Scanning: The Importance of Cap-Independent mRNA Translation Initiation for Cellular Stress Response and Cancer. Cell. Mol. Life Sci. CMLS 2017, 74, 1659–1680. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z. IRES-Mediated Cap-Independent Translation, a Path Leading to Hidden Proteome. J. Mol. Cell Biol. 2019, 11, 911–919. [Google Scholar] [CrossRef]
- Guo, S.; Lu, H. Conjunction of Potential G-Quadruplex and Adjacent Cis-Elements in the 5′ UTR of Hepatocyte Nuclear Factor 4-Alpha Strongly Inhibit Protein Expression. Sci. Rep. 2017, 7, 17444. [Google Scholar] [CrossRef]
- Al-Zeer, M.A.; Dutkiewicz, M.; von Hacht, A.; Kreuzmann, D.; Röhrs, V.; Kurreck, J. Alternatively Spliced Variants of the 5′-UTR of the ARPC2 mRNA Regulate Translation by an Internal Ribosome Entry Site (IRES) Harboring a Guanine-Quadruplex Motif. RNA Biol. 2019, 16, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Koukouraki, P.; Doxakis, E. Constitutive Translation of Human α-Synuclein Is Mediated by the 5′-Untranslated Region. Open Biol. 2016, 6, 160022. [Google Scholar] [CrossRef]
- Lee, S.C.; Zhang, J.; Strom, J.; Yang, D.; Dinh, T.N.; Kappeler, K.; Chen, Q.M. G-Quadruplex in the NRF2 MRNA 5′ Untranslated Region Regulates De Novo NRF2 Protein Translation under Oxidative Stress. Mol. Cell. Biol. 2016, 37, e00122-16. [Google Scholar] [CrossRef]
- Waller, Z.A.E.; Howell, L.A.; MacDonald, C.J.; O’Connell, M.A.; Searcey, M. Identification and Characterisation of a G-Quadruplex Forming Sequence in the Promoter Region of Nuclear Factor (Erythroid-Derived 2)-like 2 (Nrf2). Biochem. Biophys. Res. Commun. 2014, 447, 128–132. [Google Scholar] [CrossRef]
- Jodoin, R.; Carrier, J.C.; Rivard, N.; Bisaillon, M.; Perreault, J.-P. G-Quadruplex Located in the 5′UTR of the BAG-1 mRNA Affects Both Its Cap-Dependent and Cap-Independent Translation through Global Secondary Structure Maintenance. Nucleic Acids Res. 2019, 47, 10247–10266. [Google Scholar] [CrossRef]
- Marques-Ramos, A.; Candeias, M.M.; Menezes, J.; Lacerda, R.; Willcocks, M.; Teixeira, A.; Locker, N.; Romão, L. Cap-Independent Translation Ensures MTOR Expression and Function upon Protein Synthesis Inhibition. RNA 2017, 23, 1712–1728. [Google Scholar] [CrossRef] [PubMed]
- Brödel, A.K.; Sonnabend, A.; Roberts, L.O.; Stech, M.; Wüstenhagen, D.A.; Kubick, S. IRES-Mediated Translation of Membrane Proteins and Glycoproteins in Eukaryotic Cell-Free Systems. PLoS ONE 2013, 8, e82234. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.D.; Semler, B.L. Bridging IRES Elements in MRNAs to the Eukaryotic Translation Apparatus. Biochim. Biophys. Acta 2009, 1789, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Godet, A.-C.; David, F.; Hantelys, F.; Tatin, F.; Lacazette, E.; Garmy-Susini, B.; Prats, A.-C. IRES Trans-Acting Factors, Key Actors of the Stress Response. Int. J. Mol. Sci. 2019, 20, 924. [Google Scholar] [CrossRef]
- Martínez-Salas, E.; Piñeiro, D.; Fernández, N. Alternative Mechanisms to Initiate Translation in Eukaryotic mRNAs. Comp. Funct. Genomics 2012, 2012, e391546. [Google Scholar] [CrossRef]
- Shatsky, I.N.; Dmitriev, S.E.; Terenin, I.M.; Andreev, D.E. Cap- and IRES-Independent Scanning Mechanism of Translation Initiation as an Alternative to the Concept of Cellular IRESs. Mol. Cells 2010, 30, 285–293. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Diamond, P.; Basu, S. An Independently Folding RNA G-Quadruplex Domain Directly Recruits the 40S Ribosomal Subunit. Biochemistry 2015, 54, 1879–1885. [Google Scholar] [CrossRef]
- Hellen, C.U.; Sarnow, P. Internal Ribosome Entry Sites in Eukaryotic mRNA Molecules. Genes Dev. 2001, 15, 1593–1612. [Google Scholar] [CrossRef]
- Jackson, R.J.; Hellen, C.U.T.; Pestova, T.V. The Mechanism of Eukaryotic Translation Initiation and Principles of Its Regulation. Nat. Rev. Mol. Cell Biol. 2010, 11, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Komar, A.A.; Mazumder, B.; Merrick, W.C. A New Framework for Understanding IRES-Mediated Translation. Gene 2012, 502, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Weingarten-Gabbay, S.; Elias-Kirma, S.; Nir, R.; Gritsenko, A.A.; Stern-Ginossar, N.; Yakhini, Z.; Weinberger, A.; Segal, E. Comparative Genetics. Systematic Discovery of Cap-Independent Translation Sequences in Human and Viral Genomes. Science 2016, 351, aad4939. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, W.V. Alternative Ways to Think about Cellular Internal Ribosome Entry. J. Biol. Chem. 2010, 285, 29033–29038. [Google Scholar] [CrossRef]
- Jackson, R.J. The Current Status of Vertebrate Cellular MRNA IRESs. Cold Spring Harb. Perspect. Biol. 2013, 5, a011569. [Google Scholar] [CrossRef]
- Kozak, M. A Second Look at Cellular mRNA Sequences Said to Function as Internal Ribosome Entry Sites. Nucleic Acids Res. 2005, 33, 6593–6602. [Google Scholar] [CrossRef]
- Terenin, I.M.; Andreev, D.E.; Dmitriev, S.E.; Shatsky, I.N. A Novel Mechanism of Eukaryotic Translation Initiation That Is Neither M7G-Cap-, nor IRES-Dependent. Nucleic Acids Res. 2013, 41, 1807–1816. [Google Scholar] [CrossRef]
- Meyer, K.D.; Patil, D.P.; Zhou, J.; Zinoviev, A.; Skabkin, M.A.; Elemento, O.; Pestova, T.V.; Qian, S.-B.; Jaffrey, S.R. 5′ UTR m(6)A Promotes Cap-Independent Translation. Cell 2015, 163, 999–1010. [Google Scholar] [CrossRef]
- Zhou, J.; Rode, K.A.; Qian, S.-B. M(6)A: A Novel Hallmark of Translation. Cell Cycle Georget. Tex 2016, 15, 309–310. [Google Scholar] [CrossRef][Green Version]
- Zhou, J.; Wan, J.; Gao, X.; Zhang, X.; Jaffrey, S.R.; Qian, S.-B. Dynamic m(6)A MRNA Methylation Directs Translational Control of Heat Shock Response. Nature 2015, 526, 591–594. [Google Scholar] [CrossRef]
- Kharel, P.; Balaratnam, S.; Beals, N.; Basu, S. The Role of RNA G-Quadruplexes in Human Diseases and Therapeutic Strategies. Wiley Interdiscip. Rev. RNA 2020, 11, e1568. [Google Scholar] [CrossRef]
- Varshney, D.; Spiegel, J.; Zyner, K.; Tannahill, D.; Balasubramanian, S. The Regulation and Functions of DNA and RNA G-Quadruplexes. Nat. Rev. Mol. Cell Biol. 2020, 1–16. [Google Scholar] [CrossRef]
- Komar, A.A.; Hatzoglou, M. Cellular IRES-Mediated Translation: The War of ITAFs in Pathophysiological States. Cell Cycle Georget. Tex 2011, 10, 229–240. [Google Scholar] [CrossRef]
- Spriggs, K.A.; Bushell, M.; Willis, A.E. Translational Regulation of Gene Expression during Conditions of Cell Stress. Mol. Cell 2010, 40, 228–237. [Google Scholar] [CrossRef]
- Pelletier, J.; Sonenberg, N. Internal Initiation of Translation of Eukaryotic mRNA Directed by a Sequence Derived from Poliovirus RNA. Nature 1988, 334, 320–325. [Google Scholar] [CrossRef]
- Lozano, G.; Martínez-Salas, E. Structural Insights into Viral IRES-Dependent Translation Mechanisms. Curr. Opin. Virol. 2015, 12, 113–120. [Google Scholar] [CrossRef]
- Komar, A.A.; Hatzoglou, M. Exploring Internal Ribosome Entry Sites as Therapeutic Targets. Front. Oncol. 2015, 5, 233. [Google Scholar] [CrossRef]
- Hellen, C.U.T. IRES-Induced Conformational Changes in the Ribosome and the Mechanism of Translation Initiation by Internal Ribosomal Entry. Biochim. Biophys. Acta 2009, 1789, 558–570. [Google Scholar] [CrossRef]
- Balvay, L.; Rifo, R.S.; Ricci, E.P.; Decimo, D.; Ohlmann, T. Structural and Functional Diversity of Viral IRESes. Biochim. Biophys. Acta BBA Gene Regul. Mech. 2009, 1789, 542–557. [Google Scholar] [CrossRef]
- Chen, T.-M.; Shih, Y.-H.; Tseng, J.T.; Lai, M.-C.; Wu, C.-H.; Li, Y.-H.; Tsai, S.-J.; Sun, H.S. Overexpression of FGF9 in Colon Cancer Cells Is Mediated by Hypoxia-Induced Translational Activation. Nucleic Acids Res. 2014, 42, 2932–2944. [Google Scholar] [CrossRef]
- Huez, I.; Créancier, L.; Audigier, S.; Gensac, M.C.; Prats, A.C.; Prats, H. Two Independent Internal Ribosome Entry Sites Are Involved in Translation Initiation of Vascular Endothelial Growth Factor mRNA. Mol. Cell. Biol. 1998, 18, 6178–6190. [Google Scholar] [CrossRef]
- Cammas, A.; Dubrac, A.; Morel, B.; Lamaa, A.; Touriol, C.; Teulade-Fichou, M.-P.; Prats, H.; Millevoi, S. Stabilization of the G-Quadruplex at the VEGF IRES Represses Cap-Independent Translation. RNA Biol. 2015, 12, 320–329. [Google Scholar] [CrossRef]
- von Hacht, A.; Seifert, O.; Menger, M.; Schütze, T.; Arora, A.; Konthur, Z.; Neubauer, P.; Wagner, A.; Weise, C.; Kurreck, J. Identification and Characterization of RNA Guanine-Quadruplex Binding Proteins. Nucleic Acids Res. 2014, 42, 6630–6644. [Google Scholar] [CrossRef]
- Li, W.; Thakor, N.; Xu, E.Y.; Huang, Y.; Chen, C.; Yu, R.; Holcik, M.; Kong, A.-N. An Internal Ribosomal Entry Site Mediates Redox-Sensitive Translation of Nrf2. Nucleic Acids Res. 2010, 38, 778–788. [Google Scholar] [CrossRef]
- Kieft, J.S.; Zhou, K.; Jubin, R.; Doudna, J.A. Mechanism of Ribosome Recruitment by Hepatitis C IRES RNA. RNA N. Y. N 2001, 7, 194–206. [Google Scholar] [CrossRef]
- Sachs, A.B.; Sarnow, P.; Hentze, M.W. Starting at the Beginning, Middle, and End: Translation Initiation in Eukaryotes. Cell 1997, 89, 831–838. [Google Scholar] [CrossRef]
- Poulin, F.; Sonenberg, N. Mechanism of Translation Initiation in Eukaryotes; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Merrick, W.C.; Pavitt, G.D. Protein Synthesis Initiation in Eukaryotic Cells. Cold Spring Harb. Perspect. Biol. 2018, 10, a033092. [Google Scholar] [CrossRef]
- Pain, V.M. Initiation of Protein Synthesis in Eukaryotic Cells. Eur. J. Biochem. 1996, 236, 747–771. [Google Scholar] [CrossRef]
- Merrick, W.C. Cap-Dependent and Cap-Independent Translation in Eukaryotic Systems. Gene 2004, 332, 1–11. [Google Scholar] [CrossRef]
- Sun, D.; Hurley, L.H. Biochemical Techniques for the Characterization of G-Quadruplex Structures: EMSA, DMS Footprinting, and DNA Polymerase Stop Assay. Methods Mol. Biol. Clifton NJ 2010, 608, 65–79. [Google Scholar] [CrossRef]
- Kwok, C.K.; Marsico, G.; Sahakyan, A.B.; Chambers, V.S.; Balasubramanian, S. RG4-Seq Reveals Widespread Formation of G-Quadruplex Structures in the Human Transcriptome. Nat. Methods 2016, 13, 841–844. [Google Scholar] [CrossRef]
- Yang, T.-H.; Wang, C.-Y.; Tsai, H.-C.; Liu, C.-T. Human IRES Atlas: An Integrative Platform for Studying IRES-Driven Translational Regulation in Humans. Database J. Biol. Databases Curation 2021, 2021, baab025. [Google Scholar] [CrossRef] [PubMed]
- Mokrejs, M.; Masek, T.; Vopálensky, V.; Hlubucek, P.; Delbos, P.; Pospísek, M. IRESite—A Tool for the Examination of Viral and Cellular Internal Ribosome Entry Sites. Nucleic Acids Res. 2010, 38, D131–D136. [Google Scholar] [CrossRef] [PubMed]
- Mokrejs, M.; Vopálenský, V.; Kolenaty, O.; Masek, T.; Feketová, Z.; Sekyrová, P.; Skaloudová, B.; Kríz, V.; Pospísek, M. IRESite: The Database of Experimentally Verified IRES Structures (Www.Iresite.Org). Nucleic Acids Res. 2006, 34, D125–D130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Y.; Wang, C.; Zhang, H.; Zhang, H.; Jiang, B.; Guo, X.; Song, X. IRESbase: A Comprehensive Database of Experimentally Validated Internal Ribosome Entry Sites. Genom. Proteom. Bioinform. 2020, 18, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Kolekar, P.; Pataskar, A.; Kulkarni-Kale, U.; Pal, J.; Kulkarni, A. IRESPred: Web Server for Prediction of Cellular and Viral Internal Ribosome Entry Site (IRES). Sci. Rep. 2016, 6, 27436. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wu, J.; Xu, T.; Yang, Q.; He, J.; Song, X. IRESfinder: Identifying RNA Internal Ribosome Entry Site in Eukaryotic Cell Using Framed k-Mer Features. J. Genet. Genom. 2018, 45, 403–406. [Google Scholar] [CrossRef]
- Wang, J.; Gribskov, M. IRESpy: An XGBoost Model for Prediction of Internal Ribosome Entry Sites. BMC Bioinformatics 2019, 20, 409. [Google Scholar] [CrossRef]
- Bohálová, N.; Cantara, A.; Bartas, M.; Kaura, P.; Šťastný, J.; Pečinka, P.; Fojta, M.; Mergny, J.-L.; Brázda, V. Analyses of Viral Genomes for G-Quadruplex Forming Sequences Reveal Their Correlation with the Type of Infection. Biochimie 2021, 186, 13–27. [Google Scholar] [CrossRef]
- Bedrat, A.; Lacroix, L.; Mergny, J.-L. Re-Evaluation of G-Quadruplex Propensity with G4Hunter. Nucleic Acids Res. 2016, 44, 1746–1759. [Google Scholar] [CrossRef]
- Yadav, V.K.; Abraham, J.K.; Mani, P.; Kulshrestha, R.; Chowdhury, S. QuadBase: Genome-Wide Database of G4 DNA—Occurrence and Conservation in Human, Chimpanzee, Mouse and Rat Promoters and 146 Microbes. Nucleic Acids Res. 2008, 36, D381–D385. [Google Scholar] [CrossRef] [PubMed]
- Brázda, V.; Kolomazník, J.; Lýsek, J.; Bartas, M.; Fojta, M.; Šťastný, J.; Mergny, J.-L. G4Hunter Web Application: A Web Server for G-Quadruplex Prediction. Bioinformatics 2019, 35, 3493–3495. [Google Scholar] [CrossRef] [PubMed]
- Garant, J.-M.; Perreault, J.-P.; Scott, M.S. Motif Independent Identification of Potential RNA G-Quadruplexes by G4RNA Screener. Bioinformatics 2017, 33, 3532–3537. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, J.-D.; Jodoin, R.; Perreault, J.-P. New Scoring System to Identify RNA G-Quadruplex Folding. Nucleic Acids Res. 2014, 42, 1209–1223. [Google Scholar] [CrossRef]
- Kwok, C.K.; Merrick, C.J. G-Quadruplexes: Prediction, Characterization, and Biological Application. Trends Biotechnol. 2017, 35, 997–1013. [Google Scholar] [CrossRef]
- Puig Lombardi, E.; Londoño-Vallejo, A. A Guide to Computational Methods for G-Quadruplex Prediction. Nucleic Acids Res. 2020, 48, 1–15. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).